Abstract
Despite aggressive treatment regimes, glioma remains a largely fatal disease. Current treatment limitations are attributed to the precarious locations within the brain where such tumors grow, their highly infiltrative nature precluding complete resection and lack of specificity among agents capable of attenuating their growth. Here, we show that in vitro, glioma cells of diverse origins internalize a peptide encompassing a tubulin-binding site (TBS) on the neurofilament light protein. The internalized peptide disrupts the microtubule network, inhibits migration and proliferation, and leads to apoptosis. Using an intracerebral transplant model, we show that most, if not all, of these responses to peptide exposure also occur in vivo. Notably, a single intratumor injection significantly attenuates tumor growth, while neither peptide uptake nor downstream consequences are observed elsewhere in the host nervous system. Such preferential uptake suggests that the peptide may have potential as a primary or supplementary glioblastoma treatment modality by exploiting its autonomous microtubule-disrupting activity or engaging its capacity to selectively target glioma cells with other cell-disrupting cargos.
Introduction
Malignant glioma is the most prevalent primary tumor of the central nervous system. Despite aggressive therapies including combinations of surgery, radiotherapy, and chemotherapy, median post-diagnostic survival is ~1 year.1,2 The highly infiltrative nature of many gliomas and the lack of specific antiglioblastoma agents are among the factors limiting the effectiveness of current therapies.
In an effort to identify novel therapeutic targets, large-scale genomic, transcriptomic, and proteomic analyses have been applied to glioblastoma and typically reveal upregulated expression of several tubulin isoforms.3,4 Tubulin-binding compounds are a historically important class of anticancer drugs achieving their effects by promoting excessive microtubule stability, as seen for the taxane family,5,6,7,8 or by inducing microtubule depolymerization, as seen for the Vinca alkaloids.9 By modulating microtubule dynamics, these compounds disrupt cell motility, arrest mitosis, and promote apoptosis.10,11 Despite the potential of such microtubule-targeting drugs as chemotherapeutic agents, multiple cellular strategies diminish their long-term effectiveness including upregulation of transmembrane efflux pumps and upregulated expression of tubulin isotypes less affected by such tubulin-binding compounds.12,13,14 Another property affecting their clinical application is high toxicity resulting from their lack of specificity for cancer cells.11,15,16,17,18,19 Consequently, identifying microtubule-interacting agents that demonstrate higher specificity for tumor cells continues to be a proximal objective in the search for more effective cancer treatments.
Previously, we demonstrated that intermediate filament proteins contain short motifs that bind unpolymerized tubulin and that 24-amino acid peptides encompassing these tubulin-binding sites (TBS) maintain tubulin-binding capacity. We also demonstrated that a TBS derived from the neurofilament light subunit (neurofilament light (NFL)-TBS.40-63) inhibited tubulin polymerization in vitro. Moreover, this TBS containing peptide was internalized by human T98G glioblastoma cells in vitro where it led to disruption of their microtubule network and reduced their viability.20 Here, we compared diverse glioma cell lines with various normal cell types for their capacity to internalize NFL-TBS.40-63 in vitro. Although other cell penetrating peptides can be internalized by many cell types, the diverse central nervous system cell types examined here had limited capacity to internalize NFL-TBS.40-63 and were unaffected by in vitro peptide exposure. In contrast, a markedly enhanced ability to internalize the peptide was a prominent feature shared among multiple glioma cell lines, and such internalization was accompanied by major disruptions in their microtubule networks, reduced motility, inhibition of proliferation and apoptosis. Based on these observations, we explored the possibility that infusion of the peptide into gliomas generated in a transplant model would have therapeutic benefit. Following a single intratumor infusion of peptide, faithful glioma targeting specificity was accompanied with pronounced antitumor activity and therapeutic benefit.
Results
We first investigated if peptide uptake in vitro demonstrated cell type specificity and characterized the cellular consequences precipitated by peptide internalization. We next evaluated the in vivo response of intracranial transplanted glioma cells and normal brain to peptide exposure.
The NFL-TBS.40-63 peptide is internalized by malignant glioma cells from diverse origins
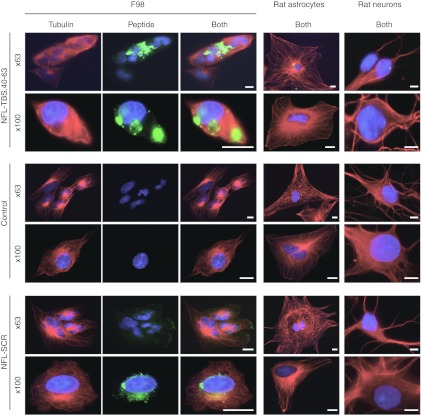
We showed previously that cells of the T98G human glioblastoma line internalize the NFL-TBS.40-63 peptide causing their microtubule network to be destroyed, tubulin aggregates to form around the nucleus, and their cell shape to become spherical. However, when such treated cells have grown in close apposition to each other, they experience a similar destruction in their microtubule networks but their shapes are less affected (Figure 1).
Figure 1.
Effects of the NFL-TBS.40-63 peptide on the microtubule cytoskeleton of rat glioma cells and primary astrocytes and neurons. Rat F98 glioma cells and rat primary astrocytes or neurons were grown in the presence of NFL-TBS.40-63 or NFL-SCR peptides (10 µmol/l) for 6 hours. Microtubules were detected using an antitubulin antibody (red), and the biotinylated NFL-TBS.40-63 peptide was detected using Alexa-labeled avidin (green). Cells were examined with a confocal microscope and numerous fluorescent aggregates, corresponding to the biotinylated peptide typically were observed. Glioma cells containing NFL-TBS.40-63 lack a normal microtubule network and typically assume a spherical shape. Except for rare cells demonstrating one or two relatively weakly labeled aggregates, primary rat astrocytes or neurons cultured under similar conditions remained unlabelled and their microtubule networks appeared unaltered. Incubation with the scrambled NFL-SCR peptide did not alter microtubule organization in astrocytes, neurons or glioma cells. White bars, 10 µm. NFL, neurofilament light; SCR, scrambled; TBS, tubulin-binding site.
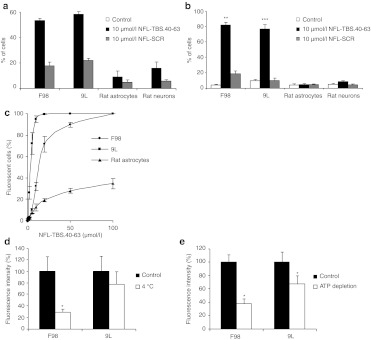
The relative peptide internalizing capacity of F98 and 9L glioma cells was evaluated following a 6-hour incubation in media containing 10 µmol/l of biotin-labeled NFL-TBS.40-63 peptide, or vehicle alone. Cultures were processed for immunofluorescence and the proportion of labeled cells determined by evaluating 200 cells in each of several 40× fields (Figure 1). In both F98 and 9L cultures, more than half of the cells revealed a clearly detectable fluorescent signal (53.5 ± 1.5 % for F98 and 58.2 ± 9 % for 9L) while cells exposed to vehicle alone did not fluoresce (Figure 2a). Similar results were obtained with human U87 and mouse GL261 glioma cells (Supplementary Figure S1) indicating that glioma cell lines arising from different species and from different primary tumors share a capacity to internalize the NFL-TBS.40-63 peptide.
Figure 2.
Internalization of the NFL-TBS.40-63 peptide by different rat cell lines and effects on their microtubule networks. (a,b) Rat F98 and 9L glioma cells, as well as primary astrocytes or neurons, were treated with 10 µmol NFL-TBS.40-63 or NFL-SCR peptide for 6 hours. The number of cells positive for peptide staining (a) and those with disrupted microtubules were counted (b). More than 50% of the cells in both glioma lines contained peptide and displayed a disorganized microtubule cytoskeleton while a much reduced number of primary rat astrocytes and neurons contained peptide or displayed disorganized microtubules. (c) Rat glioma cells or astrocytes were incubated for 1 hour at 37 °C with carboxyfluorescein-labeled NFL-TBS.40-63 at concentrations of 1, 5, 10, 20, 50, and 100 µmol/l. Cellular uptake was assessed by FACS analysis and the percent of fluorescent cells determined. Glioma cells demonstrated a marked preferential uptake of the NFL-TBS.40-63 peptide at all concentrations. (d,e) Uptake of the NFL-TBS.40-63 peptide is energy and temperature-dependant. Glioma cells were incubated for 30 minutes at 37 °C in the presence of 20 µmol/l fluorescein-tagged peptide. Intracellular ATP pools were either unmanipulated (black columns) or depleted by preincubation for 30 minutes with 10 mmol/l sodium azide and 6 mmol/l deoxyglucose (white columns). (d) Glioma cells were incubated for 1 hour at 37 °C (black columns) or at 4 °C (white columns) in the presence of 20 µmol/l fluorescein-tagged NFL-TBS.40-63 peptide. (e) Both ATP depletion and reduced temperature significantly attenuated, but did not abolish peptide uptake. Asterisks indicate significant level versus control *P < 0.05; **P < 0.005; ***P < 0.001. FACS, fluorescence-activated cell sorting; NFL, neurofilament light; SCR, scrambled; TBS, tubulin-binding site.
To determine if the peptide internalizing capacity of glioma cells differed from normal central nervous system cells, we established primary cultures of rat astrocytes and neurons and exposed them to peptide under identical conditions. Although such cells could internalize the peptide, the proportion demonstrating a detectable signal was significantly less than that observed with gliomas (9 ± 4.6 % of astrocytes and 17.9 ± 5.9 % of neurons) (Figure 2a). Additionally, the intensity of the fluorescent signal emitted from labeled glioma cells was higher than that from labeled primary cells. Notably, many fluorescent aggregates were typical of labeled glioma cells while only one or two were observed in the rare primary cells that demonstrated fluorescence (Figure 1). Thus, compared to glial and neuronal primary cell cultures established from rat, five glioma cell lines originating from three different species demonstrated markedly more intense labeling in a larger proportion of cells. Thus, enhanced peptide internalization and/or retention are properties widely shared among glioma cells from diverse origins.
To determine if unlabelled cells in the above fluorescence assay had accumulated peptide below detectable levels, we next evaluated peptide incorporation using the more sensitive fluorescence-activated cell sorting (FACS) technique. Cells were incubated for 1 hour with media containing increasing concentrations of carboxyfluorescein-tagged peptide. To discriminate between potentially nonspecific membrane-bound versus internalized fluorochrome, peptide-treated cells were incubated with trypsin prior to FACS analysis.21 At 10 µmol/l of carboxyfluorescein-tagged peptide, 95.4 ± 3.3 % of 9L and 32.7 ± 4.3 % of F98 cells internalized the peptide, while at 20 µmol/l most glioma cells internalized the peptide (100 ± 0.3 % of 9L and 72.1 ± 6.9 % of F98 glioma cells). In contrast, a markedly lower proportion of primary astrocytes were positive at all concentrations of peptide evaluated (Figure 2c). Neurons were not amenable to FACS analysis because of their adherent properties. Thus, even at a five times higher concentration than for immunocytochemistry, these FACS results show that the peptide preferentially penetrates in glioma cells than in primary cells.
We next sought to gain insight into the mechanism supporting preferential uptake of the NFL-TBS.40-63 peptide by rat F98 and 9L glioma cells. To achieve this, we first evaluated the relative contributions of the two well-characterized mechanisms through which cells are known to internalize peptide, endocytosis and direct translocation. To test the endocytosis pathway, F98 and 9L cells were exposed to peptide and incubated at 4 °C or in an ATP-depleted state achieved through preincubation with sodium azide and deoxyglucose. Under both conditions, significantly reduced uptake was observed indicating that peptide internalization occurs through an energy-dependant mechanism implicating endocytosis (Figure 2d,e). Nonetheless, under these experimental conditions, uptake was not abolished suggesting that direct translocation of the peptide also occur. Similar results were obtained with human (T98G and U87) and mouse (GL261) glioma cells (Supplementary Figure S1).
We showed previously that TBS containing peptides interfere with normal tubulin dynamics.20 To investigate the effects of internalized NFL-TBS.40-63 further, we treated cultured cells with peptide or vehicle alone and evaluated intracellular tubulin distribution, cell shape, migration, and viability in vitro. While F98 and 9L cells, either untreated or treated with vehicle alone, were large, flat, and filled with a dense network of microtubules, cells that internalized detectable levels of NFL-TBS.40-63 typically became spherical, lost their pronounced microtubule network, and developed large aggregates (Figure 1a). As previously shown, some of these aggregates co-label for tubulin and the peptide.20 Obvious microtubular perturbations were observed in 82 ± 3 % of the F98 cells and 76.7 ± 5.8 % of the 9L cells that accumulated detectable peptide. In contrast, only 4.7 ± 0.6 % of astrocytes and 8.5 ± 1.5 % of neurons with detectable peptide demonstrated any obvious microtubular disruptions (Figure 2b). A similarly low incidence of microtubule disruption was observed in glioma cells, primary astrocytes, and neurons following culture in media containing the same concentration of the randomly scrambled NFL-TBS.40-63 peptide designated NFL-SCR. Similar results were obtained with the human T98G and U87 and mouse GL261 glioma cell lines (Supplementary Figure S1a,b).
NFL-TBS.40-63 peptide specifically reduces viability, proliferation, and migration of malignant glioma cells
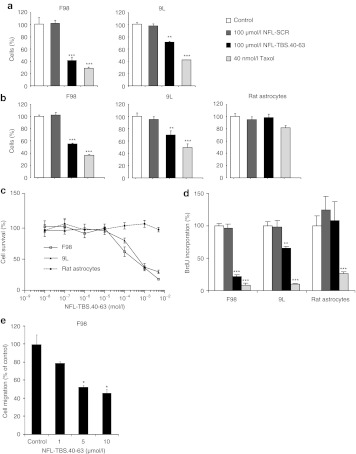
To evaluate cell viability following peptide treatment, F98 and 9L glioma cells and astrocytes were incubated for 72 hours in media containing vehicle alone, NFL-TBS.40-63 or NFL-SCR at 100 µmol/l. Following exposure to media containing the NFL-TBS.40-63 peptide, an 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay revealed that F98 and 9L cells had viability reduced by 60.8 ± 2.8 % and 30.0 ± 4.4 %, respectively. In contrast, exposure to vehicle alone or to NFL-SCR had no measurable effect in either cell type (Figure 3a).
Figure 3.
In vitro effects of NFL-TBS.40-63 peptide on viability, proliferation, and migration of rat malignant glioma cell lines and primary astrocytes. (a) The NFL-TBS.40-63 peptide reduces the viability of rat F98 and 9L glioma cells. Cells were exposed for 72 hours to the NFL-TBS.40-63 peptide (100 µmol/l), NFL-SCR (100 µmol/l), or, as a positive control, Taxol (40 nmol/l). The MTS cytotoxicity assay reveals an increase in cell death in those cells treated with the NFL-TBS.40-63 peptide, or taxol, while NFL-SCR had no similar effect. (b) A trypan blue exclusion assay revealed a similar reduction in the viability of rat F98 and 9L glioma cells incubated with the NFL-TBS.40-63 peptide. Results are expressed as percent of viable cells in treated relative to control cultures. (c) Viability of rat astrocytes, F98, and 9L glioma cells after 72 hours of exposure to different concentrations of NFL-TBS.40-63. The concentration of peptide necessary to reduce viability, evaluated using the MTS cytotoxicity assay, to 50% (IC50) was 17.3 µmol/l for F98 and 23.2 µmol/l for 9L cells. In contrast, peptide exposure at even the highest concentration had no major effect on astrocyte viability. (d) The NFL-TBS.40-63 peptide inhibits DNA synthesis in rat F98 or 9L glioma cells but not in astrocytes. Cells were treated as above and incubated with BrdU (1 mg/ml) for 4 hours. Results are expressed as percent of cells incorporating BrdU in treated versus control cultures. The NFL-TBS.40-63 peptide reduced DNA synthesis in both glioma cell lines but not in astrocytes while NFL-SCR treatment had no effect in any cell type. (e) Migration of F98 glioma cells following 15 hours of exposure to different concentrations of NFL-TBS.40-63 peptide. In a transwell migration assay, peptide treatment caused significant inhibition first recognized at the noncytotoxic concentration of 5 µmol/l. Asterisks indicate significant level versus control **P < 0.005; ***P < 0.001. BrdU, 5-bromodeoxyuridine; MTS, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium; NFL, neurofilament light; SCR, scrambled; TBS, tubulin-binding site.
Cell viability was evaluated next using a trypan blue dye exclusion assay in which only nonviable cells with damaged membranes label. As observed with the MTS assay, exposure to the NFL-TBS.40-63 peptide reduced viability of F98 and 9L gliomas while similarly treated astrocytes were unaffected (Figure 3b).
To evaluate the effect of peptide concentration on viability, we conducted a dose–response study in which cells were exposed to peptide for 72 hours (Figure 3c). The peptide concentration required to reduce viability 50% (IC50) was 17.3 µmol/l for F98 and 23.2 µmol/l for 9L cells. Similar results were obtained with mouse GL261 and human T98G and U87 cell lines (Supplementary Figure S2). In contrast, primary rat astrocytes were unaffected at all concentrations of peptide tested.
We next investigated the effect of peptide exposure on cell proliferation. Treatment of glioma cells with 100 µmol/l of NFL-TBS.40-63 peptide for 72 hours reduced 5-bromodeoxyuridine (BrdU) incorporation in both F98 and 9L cultures by 78.2 ± 3.0 % and 34.8 ± 2.6 %, respectively (Figure 3d). Similar results were obtained with both human and mouse cells (Supplementary Figure S2). In contrast, exposing primary rat astrocytes to a similar peptide concentration had no effect on BrdU incorporation. Similarly, exposure to the NFL-SCR peptide had no effect on either glioma cells or astrocytes.
To determine if the toxic effect of the peptide is mediated through an apoptotic mechanism, as observed for other microtubule-binding drugs, F98, 9L glioma cells and astrocytes were incubated for 72 hours with 100 µmol/l of the peptide or vehicle alone, stained with propidium iodide (PI) and labeled for annexin V. Viable cells with intact membranes exclude PI, while in cells undergoing apoptosis, phospholipid phosphatidylserine (PS) translocates from the inner to the outer membrane leaflet and thus can be labeled with annexin V. FACS analysis revealed that the percentage of peptide-treated F98 cells demonstrating early (annexin V) and late (PI) apoptotic markers was, respectively, 2.3 and 3.4 times higher than in vehicle-treated cultures. Similarly, 9L cells displayed a 7.1-fold increase in the number of cells with the early apoptotic markers and a 2.3-fold increase with the late apoptotic markers. Consistent results were observed with all human and mouse glioma cell lines (data not shown) demonstrating that the toxic effect of the peptide is mediated through apoptosis. In contrast, astrocytes were far less affected by the NFL-TBS.40-63 peptide showing only a 0.1- and 0.5-fold increase, of early and late apoptotic markers, respectively.
As glioblastomas are highly invasive and the tubulin cytoskeleton plays a critical role in multiple aspects of cell movement, we next investigated if NFL-TBS.40-63 peptide exposure affected the capacity of F98 cells to migrate. Cultures were exposed to peptide concentrations of 1, 5, and 10 µmol/l, and the capacity of cells to migrate was assessed using a Transwell assay (Figure 3e). Although a small change in migratory activity was induced by exposure to 1 µmol/l peptide, inhibition ~50% and 60% was observed on exposure to peptide at 5 µmol/l and 10 µmol/l, respectively. Similarly, inhibition of U87-MG human glioma cell migration began at low peptide concentration (5 µmol/l) and was higher at a peptide concentration of 10 µmol/l (Supplementary Figure S2e). As rat 9L glioma cells show limited migratory capacity under normal culture conditions,22,23 they were not similarly evaluated. Notably, chronic exposure of F98 or U87 cells to 5 µmol/l peptide concentration does not lead to obvious disruption of their tubulin cytoskeleton nor cytotoxicity. Thus, apparently subtle peptide-induced perturbations of tubulin cytoskeletal function can significantly attenuate the migratory capacity of glioma cells.
The NFL-TBS.40-63 peptide is internalized by glioma cells and inhibits their proliferation in a transplant model
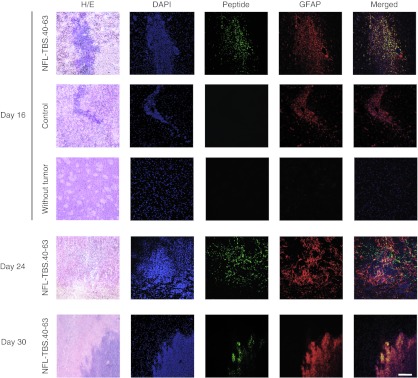
We next used a transplant preparation in which F98 glioma cells were transplanted by stereotaxy to the striatum of rats. Six days following transplantation, animals were injected at the original transplant site with vehicle alone or 60 µl of NFL-TBS.40-63 peptide (5 mmol/l) to determine if peptide could be internalized by tumor cells in vivo. At post-transplant days 16, 24, or 30 (i.e., post-peptide administration days 10, 18, and 24), animals were killed and serial coronal brain sections were analyzed by immunofluorescence for the presence of the NFL-TBS.40-63 peptide (Figure 4).
Figure 4.
In the brain, the NFL-TBS.40-63 peptide is selectively internalized by glioma cells. F98 glioma cells were transplanted to the striatum by stereotaxic injection. Six days later, 60 µm of NFL-TBS.40-63 peptide or PBS were injected at the same stereotaxic coordinates. Normal rats (without tumor) were injected with the peptide according to the same procedure. Animals were killed at 16, 24, and 30 days following tumor transplantation. Coronal brain sections were immunolabeled for glial fibrillary acidic protein (GFAP) (red), to identify glioma cells, and with Alexa-Fluo labeled Avidin to reveal the NFL-TBS.40-63 peptide (green). To evaluate tumor-related changes in cellularity, sections also were stained with DAPI and hematoxylin and eosin (H/E). At all post-transplant times examined, NFL-TBS.40-63 peptide was detected only in GFAP+ glioma cells, demonstrating its selective uptake. In brains without transplanted glioma cells, the injected peptide was undetectable from the earliest postinjection age examined (10 days). White bars, 100 µm. DAPI, 4′6-diaminido-2-phenylindole; NFL, neurofilament light; SCR, scrambled; PBS, phosphate-buffered saline; TBS, tubulin-binding site.
Glioma cells were recognized by intense glial fibrillary acidic protein (GFAP) labeling and, at the multiple post-peptide injection ages examined, most appeared to co-label for peptide. In contrast, no fluorescence nor observable lesion was detectable in the brains of peptide-injected rats that did not first receive a glioma cell transplant (Supplementary Figure S3). These observations indicate that the NFL-TBS.40-63 peptide can be internalized by glioma cells growing in vivo. They also suggest that the peptide is eliminated from the injection site in normal brains, and possibly from normal brain regions in tumor-bearing rats.
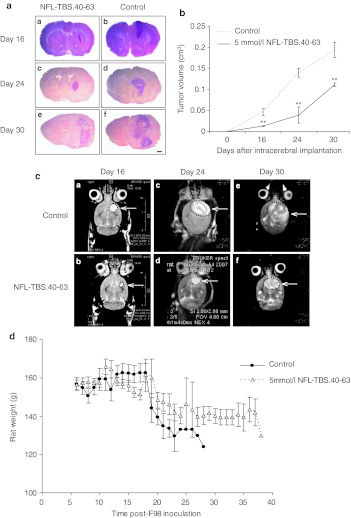
To determine if the NFL-TBS.40-63 peptide inhibited tumor growth in vivo, serial brain sections were stained with hematoxylin and eosin, and the size of the tumor was evaluated by morphometry. Relative to tumors treated with vehicle alone, those treated with NFL-TBS.40-63 were significantly smaller; volume of peptide-treated tumors was reduced at post-transplant days 16 by 71.7 ± 18.9 %, day 24 by 72.0 ± 21.2 %, and day 30 by 42.8 ± 11.3 % (Figure 5a,b).
Figure 5.
Intracerebral administration of the NFL-TBS.40-63 peptide reduces tumor growth. (a) Typical coronal sections of brains stained with hematoxylin and eosin (H/E) from rats treated with the NFL-TBS.40-63 peptide (A, C, E) or saline (B, D, F). Brains were recovered at 16 (A, B), 24 (C, D), and 30 days (E, F) following glioma cell transplantation. Black bars, 1 mm. (b) Tumor volumes in NFL-TBS.40-63-treated and control animals (mean ± SEM of three animals per treatment group at each post-transplant age). Tumor volume was determined by histomorphometry on serial sections. Compared to controls, tumor volume in rats treated with the NFL-TBS.40-63 peptide is reduced by 72% at 16 and 24 days post-transplantation, and by 42.8% at 30 days post-transplantation. Asterisks indicate significant level versus control **P < 0.005. (c): T2-weighted axial magnetic resonance images of rat brains treated with NFL-TBS.40-63 peptide (A, C, E) or vehicle (B, D, F), at days 16, 24, or 30. MRI evaluation revealed a similar reduction in tumor volume following NFL-TBS.40-63 peptide treatment. (d) Mean weights of glioma-bearing animals following peptide or vehicle treatment. MRI, magnetic resonance imagery; NFL, neurofilament light; TBS, tubulin-binding site.
Tumor size also was evaluated using magnetic resonance imagery (MRI) where intense signals emanating from the right striatum were detected (Figure 5c). Consistent with morphometry, MRI analysis at post-transplant day 24 demonstrated that peptide-treated animals had tumor volumes reduced by 63.5 % compared to those treated with vehicle alone (Figure 5b). Recorded tumor volumes of 0.093 ± 0.03 cm3 for peptide-treated and 0.25 ± 0.06 cm3 for vehicle treated animals are slightly larger than those determined by morphometry, and this difference is attributed to an overestimation of tumor size by MRI that also recognizes peritumor edema.24
The clinical condition of the rats was monitored daily and while weight loss was experienced by all tumor-bearing animals, those treated with NFL-TBS.40-63 peptide lost significantly less (Figure 5d). As seen with all parameters evaluated in vitro, treatment with NFL-SCR had no effect and relative weight loss was similar to untreated rats (data not shown). Consistent with the absence of obvious lesions in rats exposed to peptide alone, no weight loss or clinical signs were observed when nontransplanted rats were injected with the NFL-TBS.40-63 peptide (data not shown). Thus, a single intracerebral injection of peptide, at a concentration that significantly attenuates glioma growth, is tolerated by the normal brain.
Discussion
The NFL-TBS.40-63 peptide is derived from the neurofilament light protein and encompasses a TBS. Of all TBS containing peptides so far examined, NFL-TBS.40-63 demonstrates the highest capacity to inhibit microtubule formation in an in vitro polymerization assay. Our previous studies also showed that this peptide is internalized by T98G glioblastoma cells where it disrupts their microtubule cytoskeleton and inhibits proliferation.20 In this investigation, we evaluated the potential glioma specificity of internalization and downstream consequences by comparing the antimitotic and toxic effects of peptide exposure on primary astrocytes and neurons and on multiple malignant gliomas derived from different species. We show that the NFL-TBS.40-63 peptide is internalized more efficiently in vitro by glioma cells than by either primary astrocytes or neurons and that this difference is not species specific. Moreover, once internalized by glioma cells, the peptide strongly affected their microtubule network, attenuated proliferation, and led to apoptosis, a cell death mechanism shared by multiple cancer cells when treated with antimitotic drugs.25 While the mechanism that leads to disruption of the microtubule cytoskeleton remains to be investigated, physical associations between tubulin and the peptide are suggested by the results of the in vitro tubulin polymerization assay and their frequent co-localization when cells containing peptide are observed by confocal microscopy.20 Also, it is thought that glioblastoma motility relies on microtubules, and consistent with this notion, noncytotoxic concentrations of Taxol and Vinca alkaloids can impede their migration.26,27 Similarly, a noncytotoxic concentration of the NFL-TBS.40-63 peptide was found to attenuate glioblastoma migration.
Based on the observations that levels of peptide accumulation could be saturated and uptake was attenuated, but not abolished, at reduced temperature (4 °C) or after ATP depletion, peptide internalization appears to involve both endocytosis and direct translocation. As neither the scrambled peptide (NFL-SCR) nor its D-amino acid analogue were internalized,20 the uptake mechanism appears to display peptide specificity. Also, human prostate, epithelial carcinoma and mouse immortalized fibroblast NIH-3T3, accumulated the NFL-TBS.40-63 peptide to lower levels that had little effect on their microtubule cytoskeletons (Supplementary Figure S4). In further demonstration of peptide-specific properties, a peptide encompasing a vimentin TBS, while readily internalized by glioblastoma cells, had no effect on their microtubule network or proliferation.28 These combined observations illuminate numerous mechanisms through which significant specificity affecting both targeting and downstream activities are realized.
The preferential uptake of peptide by glioma cells demonstrated in vitro also was observed when the peptide was injected into the brains of glioma-bearing animals. Further, the peptide was cleared entirely from the brains of normal animals. Several mechanisms could account for this selectivity including expression by glioma cells of a cell surface receptor recognizing the peptide or different membrane properties associated with rapid proliferation. Persistence of strong fluorescent signals in glioma cells in animals killed up to 24 days following peptide administration suggests that, subsequent to peptide internalization, peptide concentration was not diluted by rapid proliferation. While apoptosis is the common end to most cells treated with microtubule-disrupting agents, including glioma cells treated with the peptide in vitro (shown here), glioma cells internalizing the peptide in vivo appear capable of long-term survival albeit with their proliferative, and presumably migratory, capacities attenuated. The mechanisms conferring such differences are currently unknown but must reflect major differences between the two dimensional microenvironment in vitro and that provided in vivo within a tumor growing in the brain.
In addition to the NFL-TBS.40-63 peptide investigated here, other peptides identified in phage-display peptide libraries have been shown to target malignant glioma cells.29,30,31,32 In an effort to exploit the therapeutic potential of such tropism, several of these have been ligated to anticancer drugs.31 However, such conjugation has the potential to alter biological activity and limit their application as vectors. In contrast, the autonomous tubulin-binding capacity of the unaltered NFL-TBS.40-63 peptide attenuates glioma cell movement and confers antimitotic activity. In support of its potential therapeutic potential, we demonstrate here that a single injection of the peptide into a transplanted F98 glioma markedly attenuates glioma growth and disease course. These results, combined with the observation that a therapeutically effective dose was well tolerated by normal brain tissue, lead to the suggestion that the peptide may have utility as a primary or supplemental treatment modality for a malignant glioma.
Materials and Methods
Synthetic peptides. Biotinylated or carboxyfluorescein-labeled peptides corresponding to the TBS of NFL (NFL-TBS.40-63: YSSYSAPVSSSLSVRRSYSSSSGS) and similarly labeled scrambled peptides (NFL-SCR: SLGSPSSSVRASYSSSRSYVYSSS) were synthesized (more than 95% purity) by Millegen (Toulouse, France) and dissolved in water at a concentration of 1 or 5 mmol/l.
Cell culture. Rat F98 and 9L glioma cell lines, as well as human T98G and U87-MG glioma cell lines, were obtained from ATCC (Manassas, VA). Mouse GL261 glioma cell lines were kindly provided by Dr Paul R Walker (Geneva University Hospital). They were grown in Dulbecco's modified Eagle's medium (DMEM) media with glucose and L-glutamine (Lonza, Levallois-Perret, France), containing 10% fetal calf serum (Lonza), 1% penicillin/ streptomycin (Sigma, Saint-Quentin Fallavier, France) in humidified incubator gassed with 5% CO2 (37 °C) until reaching 80–90% confluence. Normal human astrocytes were obtained from Lonza (Reference CC-2565 NHA-Astrocytes AGM, Levallois-Perret, France) and grown in AGM Astrocyte Growth Medium (Lonza) in humidified incubator gassed with 5% CO2 (37 °C) until reaching 70–80% confluence.
Rat primary astrocytes and mouse primary astrocytes were obtained from cultures of cerebral cortex as originally described.33 Briefly, the cerebral cortex was dissected from newborns, and cells were recovered after tissue homogenization, trypsination, and centrifugation. They were grown during 3 weeks in DMEM media with glucose and L-glutamine (Lonza), containing 10% fetal calf serum (Lonza), 1% penicillin/streptomycin (Sigma) in humidified incubator gassed with 5% CO2 (37 °C).
Hippocampal neuronal cultures were prepared from newborn rat or mouse brains according to the methods of Ray et al. and Kaech and Banker.34,35 Briefly, the hippocampi of animals younger than 2 days were recovered, minced, and digested in 0.01% trypsin for 1 hour at 37 °C. Dissociated cells were plated on coverslips precoated with 5 µg/ml poly-l-lysine and 7 µg/ml collagen at densities of 2 × 105/ml and incubated at 37 °C with a 5% CO2 atmosphere. Twenty-four hours later, the plating solution was replaced by B-27 neurobasal medium, and the second day cytosine arabinoside (20 µmol/l) was added to eliminate non-neural cells. Experiments were performed 7 days after plating.
Analysis of cell viability and proliferation. The effects of peptides on the viability of glioma cells or astrocytes were evaluated by the MTS cytotoxicity assay and by counting directly the number of cells. For the MTS assay (Promega, Charbonnières-les-Bains, France), 500 cells were seeded in 96-well plates, incubated at 37 °C for 24 hours followed by incubation with peptides (100 µmol/l), Taxol (40 nmol/l) or vehicle (DMEM) for 72 hours. Media and peptides were replaced daily. Peptides were prepared in DMEM, and Taxol (Paclitaxel; Sigma) was dissolved in dimethylsulfoxide at a concentration of 2 mmol/l and further diluted into DMEM (40 nmol/l). Viability was also determined by trypan blue staining. For manual counts, cells were treated with 0.25% trypsin/0.53 mmol/l EDTA, centrifuged, and counted with a hemacytometer following addition of trypan blue dye. At each time, 3–6 wells per treatment were counted.
To assess cell proliferation, we used BrdU immunohistochemistry. Cells were platted on coverslips and cultured in media containing biotinylated peptides (100 µmol/l) for 72 hours, and incubated during 4 hours in the presence of 1 mg/ml BrdU (Sigma). Cells were then washed in phosphate-buffered saline (PBS), fixed in 3% paraformaldehyde for 10 minutes, and permeabilized with 1% Triton X-100 in PBS for 10 minutes. Cells were acidified to denature the DNA (2 N HCl, 10 minutes), neutralized (0.1 mol/l sodium borate, 10 minutes) and then rinsed extensively in PBS. After blocking with 10% NGS (10 minutes), the cells were labeled with monoclonal anti-BrdU antibody (1/400) followed by Alexa 568 nm anti-mouse antibody (1/200). Nuclei were stained with 4′6-diaminido-2-phenylindole (Sigma). The stained cells were observed with a Leica DMI6000 inverted microscope. Images were acquired with a CoolSNAP-HQ2 camera and analyzed with Metamorph 7.1.7.0. software. Minimums of 200 cells were scored for BrdU incorporation, and experiments were repeated at least three times.
Flow cytometry. To evaluate the internalization of fluorescein-labeled NFL-TBS.40-63 peptide by FACS, glioma cells or astrocytes were seeded in 35-mm dishes and cultured for 1 hour at 37 °C in media containing fluorescein-labeled NFL-TBS.40-63 peptide at increasing concentrations or with vehicle (PBS). Cells were trypsinized, washed twice in cold PBS before incubation with trypsin (1 mg/ml) during 15 minutes at 37 °C. To investigate the energy-dependant uptake mechanism, cells were incubated at 4 °C with 20 µmol/l fluorescein-labeled NFL-TBS.40-63 peptide (after 15 minutes of 4 °C preincubation), or with 10 mmol/l sodium azide in the presence of 6 mmol/l 2-deoxy-D-glucose for 1 hour to deplete cellular ATP before addition of 20 µmol/l fluorescence-labeled NFL-TBS.40-63 peptide. Cells were then washed once, resuspended in 500 µl containing 50 µg/ml PI (Sigma), and analyzed with a FACSCalibur flow cytometer. Experiment on each cell type was repeated three times; 20,000 events per sample were analyzed in each experiment.
To detect possible apoptotic processes, cells were seeded in 35-mm dishes and cultured in media containing biotinylated peptides (100 µmol/l) or PBS alone for 72 hours. Paclitaxel (40 nmol/l) was used as a positive control to induce apoptosis.36 Cells were then trypsinized, washed in cold PBS, and stained with annexin V-FITC (BD Pharmingen, Le Pont-De-Claix, France) in annexin buffer for 15 minutes at room temperature. Finally, they were counterstained with 50 µg/ml PI (Sigma) and analyzed with a FACSCalibur flow cytometer. Experiment on each cell type was repeated three times; 20,000 events per sample were analyzed in each experiment.
Immunocytochemistry. Cells were plated on coverslips and cultured in media containing biotinylated peptides (10 µmol/l) for 6 hours. Following PBS washing, the cells were fixed for 10 minutes in 4% paraformaldehyde and washed three times in PBS. They were then incubated for 10 minutes in a 0.5% triton X-100 permeabilization solution and washed three times in PBS before incubation in a blocking solution (5% bovine serum albumin) for 15 minutes. Glioma cells and astrocytes were incubated overnight at 4 °C with mouse anti-β-tubulin antibody (Sigma) 1/200, and neurons with mouse anti-βIII-tubulin antibody 1/200. Tubulin and biotinylated peptides were localized using, respectively, Alexa 568 nm anti-mouse antibody and streptavidin Alexa 488 nm (Molecular Probes) 1/200 for 1 hour, followed by washing in PBS. The preparations were counterstained with 3 µmol/l 4′6-diaminido-2-phenylindole (Sigma) for 5 minutes and washed twice with PBS. Coverslips were mounted with an antifading solution.
Observations were carried out with an Olympus confocal microscope (BX50) using Fluoview.3.1. Software or a Leica DMI6000 inverted microscope. Images were acquired with a CoolSNAP-HQ2 camera and analyzed with Metamorph 7.1.7.0. software. We counted cells positive for peptide staining and cells with destroyed microtubule network. To evaluate the number of cells containing the peptide, several fields of the slide were analyzed with a ×40 or a ×63 objective and using a fluorescent microscope. Experiments on each cell type were repeated at least three times, and minimums of 200 cells per slide were analyzed for each experiment.
Transwell migration assay. Cells were seeded on the upper side of 8-µm-pore-size transwell migration chamber (Becton Dickinson, Le Pont de Claix, France) at a concentration of 5 × 104 cells/well in 200 µl of DMEM-free medium. The lower chamber was filled with 700 µl of DMEM medium with 10% fetal bovine serum. After 15 hours of treatment, cells on the topside of the filter were removed by scrubbing twice with a tipped swab, then cells on the lower side were fixed with 1% glutaraldehyde (VWR, Strasbourg, France) for 10 minutes and stained with 1% crystal-violet solution in 20% methanol for 10 minutes. After washing and drying, pictures of the cells were taken using a Leica Macroscope equipped with a Leica DFC420-C (Leica Microsystems, Berlin, Germany). Five fields per condition were imaged, and transmigrated cells were counted. Results were expressed as percent of transmigrated cells compared with no treatment condition. At least three independent experiments were performed for each condition.
Animal studies. Nine- to 10-week-old Female syngeneic Fisher 344 rats were obtained from Charles River Laboratories France (L'Arbresle, France). The animals were housed in a temperature and humidity-controlled room with 12-hour on–off light cycles, and given free access to food and water.
Intracerebral tumor transplantation. All experimental procedures and animal care were carried out in conformity with the guidelines of the French Government and approved by the Regional Committee for Ethics on Animal Experiments. Rat F98 cells at 70% confluency were trypsinized, counted with an hemacytometer, and checked for viability by trypan blue exclusion. Cells were washed twice in DMEM, and a final suspension of 5 × 104 cells/ml in DMEM was obtained. Animals were anesthetized by intraperitoneal injection of a mixture of ketamine 10% (0.8 µl/g) and xylazine 2% (0.5 µl/g). Using a stereotaxic frame (David Kopf instruments, Tujunga, CA), a sagital incision was made through the skin to expose the cranium, and a small dental drill was used to make a burr hole in the skull 1 mm anterior and 2 mm lateral to the bregma. A volume of 10 µl of DMEM alone or containing 500 tumor cells was injected at a flow rate of 2 µl/minute using a 10-µl Hamilton syringe (Hamilton glass syringe 700 series RN) with a 32-G needle (Hamilton, VWR), at a depth of 4 mm deep from the outer border of the cranium into the striatum of the rat. The needle was left in place for an additional 5 minutes to avoid expulsion of the suspension from the brain, and then slowly withdrawn (0.5 mm/minute).
Convection-enhanced delivery procedure. Six days following glioma implantation, 60 µl of peptide or vehicle were injected at the same coordinates using a 10-µl Hamilton syringe with a 32-G needle. This syringe was connected to a 100-µl Hamilton 22-G syringe containing the peptide (Harvard Apparatus, Les Ulis, France) through a cannula (CoExTM PE/PVC tubing, Harvard Apparatus). Slow-infusion convection-enhanced delivery was performed with an osmotic pump (Harvard Apparatus) at a rate of 0.5 µl/minute for 2 hours to achieve a total volume of 60 µl. After infusion, the needle was removed and the wound sutured.
Following intracerebral tumor cell implantation (day 0), rats were randomized into four groups. Six days postimplantation (day 6), the rats were treated by convection-enhanced delivery as follows: group 1: controls (60 µl of vehicle; n = 10); group 2: 60 µl of 1 mmol/l NFL-TBS.40-63 peptide (n = 7); group 3: 60 µl of 1 mmol/l NFL-SCR peptide (n = 7); group 4: 60 µl of 5 mmol/l NFL-TBS.40-63 peptide (n = 7).
The animals were monitored each day for weight loss, ataxia, and periorbital hemorrhage.37 Animals were killed when affected by hemiplegia or 20% weight loss. Animals were killed at post-tumor transplant day 16, 23, and 30 (n = 3/group) and brains removed, frozen in isopentane cooled to −30 °C and stored at −80 °C.
Tumor volume evaluation. Frozen brains were serially sectioned using a Leica cryostat, and 20-µm sections were hematoxylin and eosin-stained for histomorphology and measures of the tumor volume. Images of hematoxylin and eosin-stained sections were captured with a Leica Z16APO macroscope using the Leica Application Suite 2.8.1 Software. The tumor area was manually outlined and measured using Image J software. Knowing the thickness and the number of sections, we calculated the total volume of each tumor. Tumor volumes were measured for three animals per group.
For immunohistochemistry, 12-µm sections were fixed with cold methanol during 10 minutes, washed three times in PBS, before blocking at room temperature for 1 hour with PBS 5% bovine serum albumin. Sections were incubated with mouse anti-GFAP antibody (Sigma) diluted 1/200 in PBS 5% bovine serum albumin overnight, and then rinsed with PBS (3 × 5 minutes). Staining of intermediate filaments such as GFAP, vimentin or synemin can be used as markers for rat or human glioma cells.38,39 GFAP and biotinylated peptides were localized using, respectively, anti-mouse antibody Alexa 568 nm and streptavidin Alexa 488 nm (Molecular Probes, Invitrogen, Villebon sur Yvette, France), diluted 1/200 in PBS 5% bovine serum albumin for 90 minutes, followed by washing in PBS. The preparations were counterstained with 3 µmol/l 4′6-diaminido-2-phenylindole (Sigma) for 5 minutes and washed twice with PBS. Slides were mounted with an antifading solution and observed with a Leica DMR fluorescence microscope and the Leica IM500 software.
MRI was performed with a Bruker Avance DRX 300 (Bruker, Wissembourg, France) apparatus equipped with a vertical superwide-bore magnet of 7T. Qualitative T2-weighted images were obtained using rapid acquisition with relaxation enhancement (RARE) sequence (TR = 2,000 ms; mean echo time (Tem) = 31.7 ms; RARE factor = 8; FOV = 3 × 3 cm; matrix 128 × 128; nine contiguous slices of 1 mm, eight acquisitions). 1H-magnetic resonance spectroscopy was performed using a PRESS sequence with water suppression and cardiac triggering (Bruker). 1H spectra were acquired with the following parameters: TR/TE = 1,500/11 ms; NEX = 128; vowel size 27 µl (3 × 3 × 3 mm), where TR is time of repetation, TE is time of echo, and NEX is number of excitations. The Brucker Paravision 2.1 Software (Bruker) was used to calculate tumor volumes by manual contour analysis on the MRI images. The total tumor volume was calculated as the summed area on all slices, multiplied by the slice thickness (2 mm).
Statistical analysis. Data are presented as mean ± SEM (bars). Cell counting, cellular viability data, and tumor volumes were analyzed by Student's t test using Prism version 3.00 (GraphPad Software, San Diego, CA). Asterisks indicate significant level versus control *P < 0.05; **P < 0.005; ***P < 0.001.
SUPPLEMENTARY MATERIAL Figure S1. NFL-TBS.40-63 peptide internalization and microtubule network disruption in glioma and primary cells from human or mouse. Figure S2. In vitro effects of NFL-TBS.40-63 peptide on viability, proliferation and migration of human and mouse glioma cells and astrocytes. Figure S3. The NFL-TBS.40-63 peptide has no effect on normal brain. Figure S4. Internalization of the NFL-TBS.40-63 peptide by LNCaP, Hela and NIH-3T3 cell lines, effects on their microtubule networks and proliferation.
Acknowledgments
We gratefully acknowledge the Service Commun d'Imagerie et d'Analyses Microscopiques de l'Universite d'Angers for assistance in confocal microscopy, C. Guillet and the Service Commun de Cytometrie et d'Analyse Nucleotidique de l'Université d'Angers, F. Franconi and the Service Commun d'Analyses Spectroscopiques de l'Université d'Angers. We are grateful to E. Garcion for advice in cell culture (INSERM, Angers, France), S. Jouaneton for help with tumor volume evaluation, and H. Friedman for comments on the manuscript. This investigation was supported by the Association Française contre la Myopathie (AFM), Association pour la Recherche sur le Cancer (ARC), Fonds Européens de Développement Régional (FEDER), Institut National du Cancer (INCA), and Ciblage Moléculaire et Applications Thérapeutiques (CIMATH) to J.E. R.B. and J.B. were supported by la Région des Pays de la Loire.
Supplementary Material
NFL-TBS.40-63 peptide internalization and microtubule network disruption in glioma and primary cells from human or mouse.
In vitro effects of NFL-TBS.40-63 peptide on viability, proliferation and migration of human and mouse glioma cells and astrocytes.
The NFL-TBS.40-63 peptide has no effect on normal brain.
Internalization of the NFL-TBS.40-63 peptide by LNCaP, Hela and NIH-3T3 cell lines, effects on their microtubule networks and proliferation.
REFERENCES
- Westphal M, Hilt DC, Bortey E, Delavault P, Olivares R, Warnke PC.et al. (2003A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma Neuro-oncology 579–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- Katsetos CD, Dráberová E, Smejkalová B, Reddy G, Bertrand L, de Chadarévian JP.et al. (2007Class III beta-tubulin and gamma-tubulin are co-expressed and form complexes in human glioblastoma cells Neurochem Res 321387–1398. [DOI] [PubMed] [Google Scholar]
- Collet B, Guitton N, Saïkali S, Avril T, Pineau C, Hamlat A.et al. (2011Differential analysis of glioblastoma multiforme proteome by a 2D-DIGE approach Proteome Sci 916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowinsky EK. The development and clinical utility of the taxane class of antimicrotubule chemotherapy agents. Annu Rev Med. 1997;48:353–374. doi: 10.1146/annurev.med.48.1.353. [DOI] [PubMed] [Google Scholar]
- Schiller JH. Role of taxanes in lung-cancer chemotherapy. Cancer Invest. 1998;16:471–477. doi: 10.3109/07357909809011701. [DOI] [PubMed] [Google Scholar]
- Schrijvers D., and, Vermorken JB. Update on the taxoids and other new agents in head and neck cancer therapy. Curr Opin Oncol. 1998;10:233–241. doi: 10.1097/00001622-199805000-00010. [DOI] [PubMed] [Google Scholar]
- Wiseman LR., and, Spencer CM. Paclitaxel. An update of its use in the treatment of metastatic breast cancer and ovarian and other gynaecological cancers. Drugs Aging. 1998;12:305–334. doi: 10.2165/00002512-199812040-00005. [DOI] [PubMed] [Google Scholar]
- Budman DR. Vinorelbine (Navelbine): a third-generation vinca alkaloid. Cancer Invest. 1997;15:475–490. doi: 10.3109/07357909709047587. [DOI] [PubMed] [Google Scholar]
- Jordan MA., and, Wilson L. Microtubules and actin filaments: dynamic targets for cancer chemotherapy. Curr Opin Cell Biol. 1998;10:123–130. doi: 10.1016/s0955-0674(98)80095-1. [DOI] [PubMed] [Google Scholar]
- Mollinedo F., and, Gajate C. Microtubules, microtubule-interfering agents and apoptosis. Apoptosis. 2003;8:413–450. doi: 10.1023/a:1025513106330. [DOI] [PubMed] [Google Scholar]
- Dumontet C., and, Sikic BI. Mechanisms of action of and resistance to antitubulin agents: microtubule dynamics, drug transport, and cell death. J Clin Oncol. 1999;17:1061–1070. doi: 10.1200/JCO.1999.17.3.1061. [DOI] [PubMed] [Google Scholar]
- Giannakakou P, Sackett DL, Kang YK, Zhan Z, Buters JT, Fojo T.et al. (1997Paclitaxel-resistant human ovarian cancer cells have mutant beta-tubulins that exhibit impaired paclitaxel-driven polymerization J Biol Chem 27217118–17125. [DOI] [PubMed] [Google Scholar]
- Kavallaris M, Tait AS, Walsh BJ, He L, Horwitz SB, Norris MD.et al. (2001Multiple microtubule alterations are associated with Vinca alkaloid resistance in human leukemia cells Cancer Res 615803–5809. [PubMed] [Google Scholar]
- Holland JF, Scharlau C, Gailani S, Krant MJ, Olson KB, Horton J.et al. (1973Vincristine treatment of advanced cancer: a cooperative study of 392 cases Cancer Res 331258–1264. [PubMed] [Google Scholar]
- Hussain M, Wozniak AJ., and, Edelstein MB. Neurotoxicity of antineoplastic agents. Crit Rev Oncol Hematol. 1993;14:61–75. doi: 10.1016/1040-8428(93)90006-p. [DOI] [PubMed] [Google Scholar]
- Martin V. Overview of paclitaxel (TAXOL) Semin Oncol Nurs. 1993;9 4 Suppl 2:2–5. doi: 10.1016/s0749-2081(16)30035-3. [DOI] [PubMed] [Google Scholar]
- Cavaletti G, Cavalletti E, Montaguti P, Oggioni N, De Negri O., and, Tredici G. Effect on the peripheral nervous system of the short-term intravenous administration of paclitaxel in the rat. Neurotoxicology. 1997;18:137–145. [PubMed] [Google Scholar]
- Windebank AJ. Chemotherapeutic neuropathy. Curr Opin Neurol. 1999;12:565–571. doi: 10.1097/00019052-199910000-00010. [DOI] [PubMed] [Google Scholar]
- Bocquet A, Berges R, Frank R, Robert P, Peterson AC., and, Eyer J. Neurofilaments bind tubulin and modulate its polymerization. J Neurosci. 2009;29:11043–11054. doi: 10.1523/JNEUROSCI.1924-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard JP, Melikov K, Vives E, Ramos C, Verbeure B, Gait MJ.et al. (2003Cell-penetrating peptides. A reevaluation of the mechanism of cellular uptake J Biol Chem 278585–590. [DOI] [PubMed] [Google Scholar]
- Stojiljkovic M, Piperski V, Dacevic M, Rakic L, Ruzdijic S., and, Kanazir S. Characterization of 9L glioma model of the Wistar rat. J Neurooncol. 2003;63:1–7. doi: 10.1023/a:1023732619651. [DOI] [PubMed] [Google Scholar]
- Van Meir EG. CNS cancer: models, markers, prognostic factors, targets, and therapeutic approaches 2009.
- Els T, Eis M, Hoehn-Berlage M., and, Hossmann KA. Diffusion-weighted MR imaging of experimental brain tumors in rats. MAGMA. 1995;3:13–20. doi: 10.1007/BF02426396. [DOI] [PubMed] [Google Scholar]
- Wang TH, Wang HS., and, Soong YK. Paclitaxel-induced cell death: where the cell cycle and apoptosis come together. Cancer. 2000;88:2619–2628. doi: 10.1002/1097-0142(20000601)88:11<2619::aid-cncr26>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Terzis A-J, Thorsen F, Heesel O, Visted T, Bjerkvig R, Dahl O.et al. (1997Proliferation, migration and invasion of human glioma cells exposed to paclitaxel (Taxol) in vitro Br J Cancer 751744–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panopoulos A, Howell M, Fotedar R., and, Margolis RL. Glioblastoma motility occurs in the absence of actin polymer. Mol Biol Cell. 2011;22:2212–2220. doi: 10.1091/mbc.E10-10-0849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzeau J, Peterson A., and, Eyer J. The vimentin-tubulin binding site peptide (Vim-TBS.58-81) crosses the plasma membrane and enters the nuclei of human glioma cells. Int J Pharm. 2012;423:77–83. doi: 10.1016/j.ijpharm.2011.04.067. [DOI] [PubMed] [Google Scholar]
- Shadidi M., and, Sioud M. Selective targeting of cancer cells using synthetic peptides. Drug Resist Updat. 2003;6:363–371. doi: 10.1016/j.drup.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Shadidi M., and, Sioud M. Identification of novel carrier peptides for the specific delivery of therapeutics into cancer cells. FASEB J. 2003;17:256–258. doi: 10.1096/fj.02-0280fje. [DOI] [PubMed] [Google Scholar]
- Ho IA, Lam PY., and, Hui KM. Identification and characterization of novel human glioma-specific peptides to potentiate tumor-specific gene delivery. Hum Gene Ther. 2004;15:719–732. doi: 10.1089/1043034041648372. [DOI] [PubMed] [Google Scholar]
- Wu C, Lo SL, Boulaire J, Hong ML, Beh HM, Leung DS.et al. (2008A peptide-based carrier for intracellular delivery of proteins into malignant glial cells in vitro J Control Release 130140–145. [DOI] [PubMed] [Google Scholar]
- McCarthy KD., and, de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray J, Peterson DA, Schinstine M., and, Gage FH. Proliferation, differentiation, and long-term culture of primary hippocampal neurons. Proc Natl Acad Sci USA. 1993;90:3602–3606. doi: 10.1073/pnas.90.8.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech S., and, Banker G. Culturing hippocampal neurons. Nat Protoc. 2006;1:2406–2415. doi: 10.1038/nprot.2006.356. [DOI] [PubMed] [Google Scholar]
- Terzis AJ, Thorsen F, Heese O, Visted T, Bjerkvig R, Dahl O.et al. (1997Proliferation, migration and invasion of human glioma cells exposed to paclitaxel (Taxol) in vitro Br J Cancer 751744–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redgate ES, Deutsch M., and, Boggs SS. Time of death of CNS tumor-bearing rats can be reliably predicted by body weight-loss patterns. Lab Anim Sci. 1991;41:269–273. [PubMed] [Google Scholar]
- Jing R, Pizzolato G, Robson RM, Gabbiani G., and, Skalli O. Intermediate filament protein synemin is present in human reactive and malignant astrocytes and associates with ruffled membranes in astrocytoma cells. Glia. 2005;50:107–120. doi: 10.1002/glia.20158. [DOI] [PubMed] [Google Scholar]
- Mathieu D, Lecomte R, Tsanaclis AM, Larouche A., and, Fortin D. Standardization and detailed characterization of the syngeneic Fischer/F98 glioma model. Can J Neurol Sci. 2007;34:296–306. doi: 10.1017/s0317167100006715. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
NFL-TBS.40-63 peptide internalization and microtubule network disruption in glioma and primary cells from human or mouse.
In vitro effects of NFL-TBS.40-63 peptide on viability, proliferation and migration of human and mouse glioma cells and astrocytes.
The NFL-TBS.40-63 peptide has no effect on normal brain.
Internalization of the NFL-TBS.40-63 peptide by LNCaP, Hela and NIH-3T3 cell lines, effects on their microtubule networks and proliferation.