Abstract
Despite recent advances in the first-line treatment of multiple myeloma, almost all patients eventually experience relapse with drug-resistant disease. New therapeutic modalities are needed, and to this end, SNS01, a therapeutic nanoparticle, is being investigated for treatment of multiple myeloma. The antitumoral activity of SNS01 is based upon modulation of eukaryotic translation initiation factor 5A (eIF5A), a highly conserved protein that is involved in many cellular processes including proliferation, apoptosis, differentiation and inflammation. eIF5A is regulated by post-translational hypusine modification, and overexpression of hypusination-resistant mutants of eIF5A induces apoptosis in many types of cancer cells. SNS01 is a polyethylenimine (PEI)-based nanoparticle that contains both a B-cell-specific expression plasmid expressing a non-hypusinable mutant of eIF5A and a small interfering RNA (siRNA) which depletes endogenous hypusinated eIF5A. Reducing hypusine-modified eIF5A levels was found to inhibit phosphorylation and activity of ERK MAPK and nuclear factor-κB (NF-κB), and thus sensitize myeloma cells to apoptosis resulting from transfection of a plasmid expressing eIF5AK50R. SNS01 exhibited significant antitumoral activity in both KAS-6/1 (95% inhibition; P < 0.05) and RPMI 8226 (59% inhibition; P < 0.05) multiple myeloma xenograft models following systemic administration. These results highlight the potential of using this approach as a new therapeutic strategy for multiple myeloma.
Introduction
Multiple myeloma is a clonal B-cell neoplasia that causes bone destruction and impairs bone marrow function. Despite advances in chemotherapy and stem cell transplantation, which have improved response rates, multiple myeloma remains an incurable disease, and new therapeutic modalities are needed.
Eukaryotic translation initiation factor 5A (eIF5A) is an abundant protein and the only known protein to undergo post-translational modification of a conserved lysine to hypusine. eIF5A appears to have diverse cellular functions having been implicated in protein translation1,2 and elongation,3,4 mRNA transport,5,6 proliferation,7,8 apoptosis,9,10,11,12 and inflammation.13 The majority of these functions appear to be dependent on the post-translational hypusine modification of eIF5A, with one notable exception—apoptosis. Mutants of eIF5A that cannot be hypusinated induce apoptosis in numerous cancer cell types including colon, cervical, melanoma, and lung9,10,13 and inhibit growth of solid tumors.12 eIF5A is a regulator of p53 expression,9,11,14 but can also initiate apoptosis through the mitochondrial pathway via activation of caspases in p53-deficient cells.10 In addition, eIF5A and deoxyhypusine synthase (DHS), the first of two enzymes mediating eIF5A hypusination, have been identified as markers of neoplastic growth and metastasis, respectively, implicating hypusinated eIF5A in tumor progression.8,15 High expression levels of hypusinated eIF5A have been associated with activation of oncogenes7,16 and with malignant transformation of hematopoietic cells.17 Furthermore, a reduction in the ratio of hypusine-modified to total eIF5A has been observed during apoptosis in response to interferon-α while an increase in the ratio was correlated to protection from apoptosis in response to epidermal growth factor treatment and activation of ERK.18,19 We hypothesized, therefore, that reducing the expression of hypusinated eIF5A using a small interfering RNA (siRNA) might increase apoptosis induced by overexpression of non-hypusinable eIF5A mutants.
Nuclear factor-κB (NF-κB) is a transcription factor involved in the cellular response to cytokines, lipopolysaccharides, free radicals, as well as viral and bacterial pathogens. Activation of NF-κB contributes to the pathology of multiple myeloma in several ways, including the production of cytokines such as interleukin-6, a growth factor for myeloma cells that can inhibit apoptosis and contribute to drug resistance.
In this study, we demonstrate that siRNA-mediated suppression of hypusinated eIF5A increases the antitumoral effect induced by overexpression of non-hypusinable eIF5A mutants in cultured multiple myeloma cells. The potential of this approach for treatment of multiple myeloma was also investigated in vivo using two multiple myeloma xenograft models in which SNS01, a nonviral polyethylenimine (PEI) nanoparticle, was administered intravenously. SNS01 comprises both an RNA interference-resistant DNA plasmid expressing non-hypusinable eIF5AK50R under the control of a B cell-specific promoter/enhancer and an eIF5A siRNA to reduce expression of the abundant hypusinated eIF5A found in myeloma cells. SNS01 inhibited tumor growth in a dose-dependent manner and, at higher dose levels, induced regression of myeloma tumors following both local as well as systemic administration. Inhibition of eIF5A using siRNA or SNS01 was also found to inhibit activation of NF-κB in response to tumor necrosis factor-α (TNF-α), suggesting that NF-κB inhibition may contribute to the antitumoral activity of SNS01 in multiple myeloma models. The effect of SNS01 on the activation of MAPK pathways is also discussed.
Results
siRNA-mediated suppression of eIF5A inhibits activation of NF-κB in myeloma cells
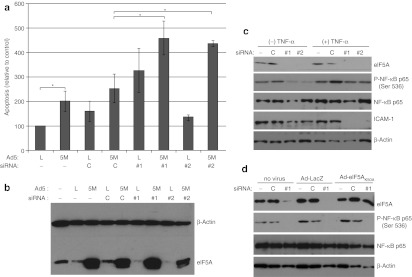
Human multiple myeloma KAS-6/1 cells were transfected with siRNAs targeting eIF5A prior to transduction with adenovirus constructs expressing either β-galactosidase or eIF5AK50A and assessed for apoptosis (Figure 1a). Overexpression of eIF5AK50A increased the incidence of apoptosis in KAS-6/1 cells, and this apoptosis was significantly enhanced by depletion of hypusinated eIF5A using siRNAs targeting eIF5A. The eIF5A siRNAs # 1 and # 2 target the 3′UTR and open-reading frame of eIF5A, respectively. Transfection of eIF5A siRNA significantly depleted endogenous eIF5A protein levels without significantly affecting expression of adenovirus-mediated eIF5AK50A (Figure 1b). Since siRNAs targeting eIF5A have exhibited anti-inflammatory properties in animal models of sepsis and lung injury,13 the effect of depleting eIF5A on the activation of NF-κB was examined. Western blot analysis of KAS-6/1 cells transfected with eIF5A siRNAs and stimulated with TNF-α to induce activation of NF-κB revealed a decrease in phosphorylation of NF-κB p65 on Ser536 (Figure 1c), a site that is associated with transcriptional activity of p65.20,21,22 A reduction in expression of intercellular adhesion molecule-1, a cell surface glycoprotein that is transcriptionally regulated by NF-κB, was also observed following transfection with eIF5A siRNAs (Figure 1c). Overexpression of eIF5AK50A did not restore the phosphorylation of NF-κB following siRNA-mediated suppression of eIF5A (Figure 1d) indicating that it may be an effect of depleting hypusinated eIF5A.
Figure 1.
Pretreatment of multiple myeloma cells with eIF5A small interfering RNA (siRNA) increases apoptosis induced by Ad-eIF5AK50A and inhibits phosphorylation of nuclear factor-κB (NF-κB) p65. KAS-6/1 cells were transfected with either a control siRNA (C) or with one of two siRNAs targeting eIF5A (#1 or #2) and then (a,b,d) transfected with either Ad-LacZ (L) or Ad-eIF5AK50A (5M). Seventy-two hours later the cells were harvested and (a) stained with Annexin/PI and analyzed by flow cytometry or (b,d) analyzed by western blot using antibodies against eIF5A, phospho-NF-κB p65 (Ser 536), NF-κB p65, and actin. Mean values ± SE are shown (*P < 0.05, n = 3). (c) Twenty-four hours after the second transfection, the cells were stimulated with 40 ng/ml tumor necrosis factor-α (TNF-α), and cell lysate was harvested and analyzed by western blot using antibodies against phospho-NF-κB p65 (Ser 536), NF-κB p65, intercellular adhesion molecule-1 (ICAM-1) and actin.
PEI condenses eIF5AK50R expression plasmid and eIF5A siRNA into nanoparticles
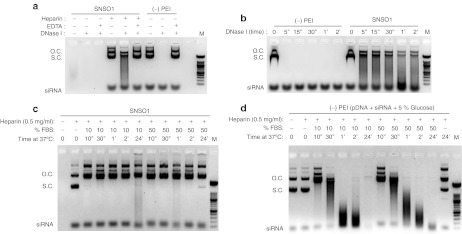
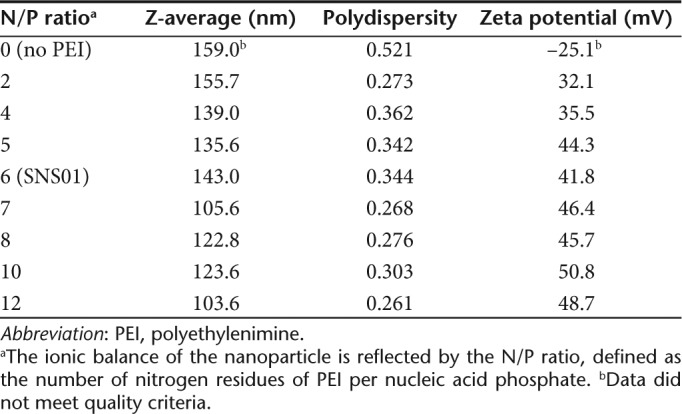
A B cell-specific expression plasmid (pExp5A) was created by subcloning eIF5AK50R into a CpG dinucleotide-reduced expression vector containing a B cell-specific promoter and enhancer (Supplementary Figure S1). The specificity of the B29 promoter and enhancer was assessed in a variety of cell types, and significant reduction of transgene expression was observed in non-B cell lines compared to B cell lines (Supplementary Figure S2). In the presence of nucleic acids, PEI promotes self-assembly of nanoparticles that protect their cargo from serum nucleases. The net charge of the nanoparticle is influenced by the N/P ratio, defined as the number of nitrogen residues of PEI per nucleic acid phosphate. pExp5A plasmid and eIF5A siRNA were combined (2:1 wt/wt) with PEI at an N/P ratio of 6 to produce SNS01 nanoparticles. Interaction with PEI inhibited degradation of both the plasmid DNA and the siRNA in the presence of DNase I and fetal bovine serum (Figure 2). SNS01 nanoparticles have an average zeta diameter of 143 nm, a polydispersity of 0.344 and are positively charged with a zeta potential of +41.8 mV (Table 1).
Figure 2.
Polyethylenimine (PEI) protects nucleic acids from degradation. SNS01 nanoparticles were incubated with either DNase I or fetal bovine serum (FBS) in order to determine whether complexing with PEI protects the nucleic acids from degradation. Nucleic acid solutions in which water was substituted for PEI [(–) PEI] were used as controls. SNS01 or (–) PEI solution was incubated with (a) DNase I at 37 °C for 30 minutes in either the presence or absence of EDTA; (b) DNase I at 37 °C for 5 minutes or up to 2 hours; (c,d) with 10% or 50% FBS. After incubation, the nucleic acids were released from interaction with PEI by addition of 0.5 mg/ml heparin and analyzed by gel electrophoresis. open circle, o.c.; supercoiled, s.c; molecular weight marker, M.
Table 1. Analysis of PEI nanoparticles by dynamic light scattering.
SNS01 inhibits activation of NFκB
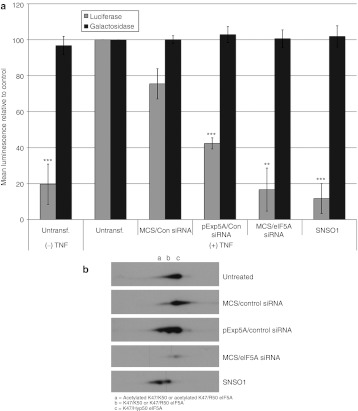
The ability of SNS01, which contains eIF5A siRNA, to inhibit activation of NF-κB was examined using TNF-α-stimulated 293A cells transfected with an NF-κB reporter plasmid. 293A cells were used in these experiments because the B29 promoter is active in 293A cells (Supplementary Figure S2) and transfection is more efficient than in myeloma cell lines. Activation of NF-κB was monitored using the reporter plasmid, pNF-κB-MetLuc2, which contains an NF-κB enhancer element upstream of a secreted metridia luciferase gene. Cells were cotransfected with a β-galactosidase plasmid to confirm that observed inhibitory effects were not due to global inhibition of translation. Transfection of 293A cells with SNS01 or eIF5A siRNA inhibited activation of NF-κB in response to TNF-α stimulation by 88 and 83%, respectively, reducing it to levels equivalent to that observed in unstimulated cells (Figure 3a). Transfection with the pExp5A plasmid also reduced NF-κB activity, although not to the same degree as SNS01 or eIF5A siRNA. The similarity in β-galactosidase activity between SNS01-treated and -untreated cells (Figure 3a) indicates that the reduction in activation of NF-κB in SNS01-treated cells is not due to cell loss or to global inhibition of protein translation.
Figure 3.
SNS01 treatment inhibits nuclear factor-κB (NF-κB) activation in response to tumor necrosis factor-α (TNF-α). Human 293A cells were transfected with control nanoparticles (MCS/Con small interfering RNA (siRNA)), polyethylenimine (PEI) nanoparticles containing only the pExp5A plasmid (pExp5A/Con siRNA) or eIF5A siRNA (MCS/eIF5A siRNA), or SNS01. (a) Twenty-four hours later the cells were cotransfected with the pNF-κB-MetLuc2 and pHM6-LacZ reporter plasmids using Lipofectamine 2000. Four hours later the cells were treated with TNF-α to stimulate NF-κB activity. Luminescence generated by secreted luciferase or β-galactosidase activity was measured in the culture media or cells 24 hours later, respectively. The data shown is the mean + SEM of three independent experiments. The luminescence value for the untransfected positive control [Untransf. (+) TNF] was set to 100 and the remaining samples were expressed relative to the positive control (*P < 0.05, ** P < 0.01, *** P < 0.001). (b) Two-dimensional SDS-PAGE followed by western blotting with an antibody against eIF5A.
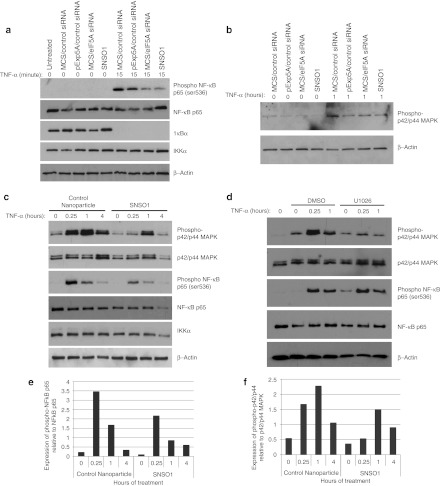
Two-dimensional western blot analysis, which enables resolution of the different post-translationally modified forms of eIF5A, confirmed that SNS01 treatment resulted in reduction of hypusinated eIF5A and a simultaneous accumulation of unhypusinated eIF5AK50R (Figure 3b), including eIF5AK50R that is likely acetylated at K47.23 Moreover, treatment with nanoparticles containing only the pExp5A resulted in a shift in the ratio of hypusinated to unhypusinated eIF5A relative to untreated cells, which may account for the reduction in NF-κB activity observed following transfection of the pExp5A plasmid (Figure 3a,b). Degradation of IκBα was not affected by treatment with SNS01 upon stimulation with TNF-α (Figure 4a) indicating that suppression of NF-κB activation by SNS01 is not attributable to inhibition of its release from IκBα in the cytoplasm. Transcriptional activity of p65 is also regulated by phosphorylation. TNF-α stimulated phosphorylation of p65 and, as was observed for KAS-6/1 cells (Figure 1c), treatment with either eIF5A siRNA or SNS01 inhibited phosphorylation of p65 at Ser536 in 293A cells (Figure 4a,c,e). TNF-α is a well-described activator of the MAPK signaling pathway, a positive regulator of NF-κB activation.24,25 Since eIF5A siRNA has previously been reported to negatively impact activation of MAPK pathways,26 the effect of SNS01 treatment on activation of ERK MAPK in response to TNF-α was investigated. Inhibition of ERK MAPK activation in response to TNF-α was observed following transfection with pExp5A plasmid, eIF5A siRNA, or SNS01 (Figure 4b,c,f). However, inhibition of ERK activation using U1026, an inhibitor of MEK, did not affect the phosphorylation status of NF-κB (Figure 4d), indicating that the effect of SNS01 on NF-κB phosphorylation was not related to its effect on ERK MAPK activation. These data suggest that SNS01 may act in part by sensitizing cells to apoptosis through inhibition of ERK MAPK and NF-κB.
Figure 4.
SNS01 transfection inhibits phosphorylation of nuclear factor-κB (NF-κB) and activation of ERK MAPK. Human 293A cells were transfected with control nanoparticles (MCS/control siRNA), SNS01, or polyethylenimine (PEI) nanoparticles containing only the pExp5A plasmid (pExp5A/control small interfering RNA (siRNA)) or eIF5A siRNA (MCS/eIF5A siRNA). (a–c) Twenty-four hours after transfection the cells were treated with tumor necrosis factor-α (TNF-α) and cell lysate was harvested for western blotting using antibodies against p42/p44 MAPK or proteins involved in NF-κB signaling. (d) 293A cells were treated with either DMSO or U1026 (10 µmol/l) for 2 hours before stimulation with TNF-α. Quantification of expression of phosphorylated p65 and phosphorylated p42/p44 MAPK relative to expression of unphosphorylated total protein from (c) is shown in (e) and (f), respectively.
SNS01 inhibits growth of human multiple myeloma tumors in murine xenograft models
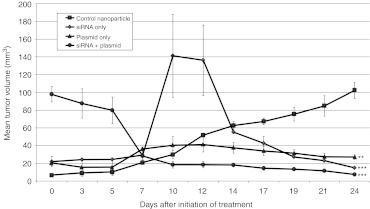
The therapeutic potential of coadministering eIF5A siRNA and an eIF5AK50R expression plasmid using PEI nanoparticles was investigated in SCID mice-bearing KAS-6/1 subcutaneous tumors. Intratumoral injection of eIF5A siRNA inhibited multiple myeloma tumor growth by >80% compared to control animals, whereas eIF5AK50R plasmid inhibited tumor growth by >70% (Figure 5). When both siRNA and plasmid were delivered in combination, the result was significant tumor shrinkage with a 92% inhibition of tumor growth. These results confirm that combining an eIF5AK50R expression plasmid with an siRNA that inhibits expression of hypusinated eIF5A maximizes the antitumoral effect in a multiple myeloma tumor model.
Figure 5.
Coadministration of eIF5AK50R plasmid and eIF5A small interfering RNA (siRNA) delays growth and causes regression of multiple myeloma subcutaneous tumors following intratumoral delivery. SCID mice were injected subcutaneously with KAS-6/1 cells. Treatment was initiated when palpable tumors were observed. Mice were injected intra-tumorally with polyethylenimine (PEI) nanoparticles twice per week at a dose of 1.5 mg/kg. Mice were treated with PEI nanaoparticles containing an eIF5AK50R expression plasmid driven by the human EF1 promoter (pCpG-eIF5AK50R) and either a nontargeting control siRNA (plasmid only) or eIF5A siRNA (siRNA + plasmid). Nanoparticles prepared with a nonexpressing control plasmid and eIF5A siRNA (siRNA only) were also tested. Mice treated with control nanoparticles containing the nonexpressing plasmid pCpG-mcs and control siRNA were used as a control group. All PEI nanoparticles were prepared at an N/P ratio of 8. The data shown is the mean tumor volume + SEM for each group [*P < 0.05; ** P < 0.01, *** P < 0.001 (n = 3)].
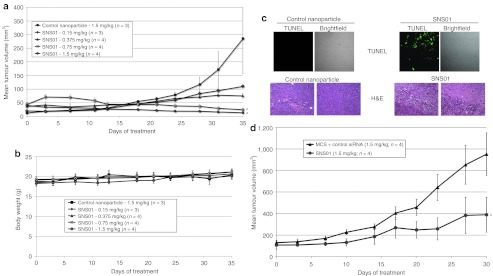
Treatment of cultured KAS-6/1 cells with SNS01 resulted in an 83.5% reduction in expression of eIF5A mRNA and the accumulation of 5.8 × 104 copies of the transgene per nanogram of total RNA (Table 2), indicating that SNS01 is biologically active in myeloma cells. A dose response study using intravenous administration of SNS01 was performed in order to determine the minimum effective dose of SNS01 in the KAS-6/1 tumor model. SNS01 dosed at 1.5 mg/kg resulted in a 95% (P = 0.026) reduction in tumor growth (Figure 6a) with no evidence of tumor burden upon excision. Decreasing the dose of SNS01 by half to 0.75 mg/kg resulted in a 91% (P = 0.03) decrease in tumor volume. The percent change in tumor volume at the end of the study in tumor-bearing mice treated with SNS01 at dose levels of 0.75 mg/kg and 1.5 mg/kg was –244% and –245%, respectively, indicating tumor regression occurred following SNS01 treatment. The tumors of control mice increased in size by more than 2,000% during the same time period. Large regions of necrosis as well as cells undergoing apoptosis were observed in tumors isolated from mice treated with SNS01 (Figure 6c). The efficacy of twice weekly intravenous injections of SNS01 in controlling the growth of RPMI 8226 myeloma subcutaneous tumors was also examined. Mice treated twice weekly with 1.5 mg/kg SNS01 by intravenous administration exhibited a 59% growth inhibition (P < 0.05) compared to mice treated with 1.5 mg/kg of a control nanoparticle (Figure 6d). SNS01 demonstrated significant antitumoral activity in two different models of multiple myeloma and was well tolerated at all tested dosing levels.
Table 2. RT-qPCR analysis of KAS-6/1 cells transfected with SNS01.
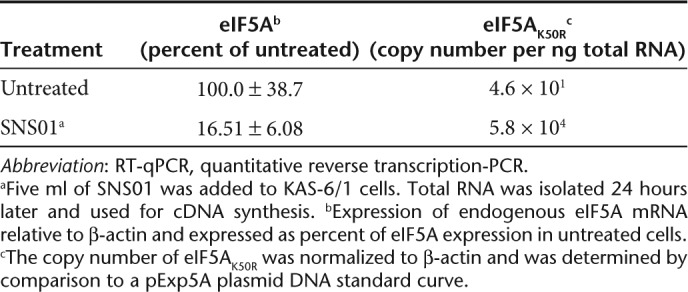
Figure 6.
Intravenous administration of SNS01 delays growth of KAS-6/1 and RPMI 8226 multiple myeloma subcutaneous tumors and induces apoptosis. SCID mice were injected subcutaneously with KAS-6/1 or RPMI 8226 cells. Treatment was initiated when palpable tumors were observed. Mice were injected intravenously twice per week with SNS01 doses ranging from 0.15 to 1.5 mg/kg. Mice treated with 1.5 mg/kg control nanoparticles were used as a control group. (a) KAS-6/1 tumor model mean tumor + SEM (*P < 0.05) and (b) mean body weight ± SEM. (c) Fixed tumors from mice bearing KAS-6/1 tumors treated twice weekly with 1.5 mg/kg of control nanoparticles or SNS01 were embedded and sectioned and either labeled for TUNEL or stained with hematoxylin and eosin (H&E). (d) The data shown is the mean tumor volume ± SEM for RPMI 8226 tumor model (*P < 0.05).
Discussion
eIF5A has been implicated in the regulation of various cellular processes including cell proliferation, inflammation, differentiation, and apoptosis. DHS, an enzyme critical for the conversion of eIF5A to the hypusinated form, is often overexpressed in cancer cells and has been correlated to metastatic disease.15 High levels of hypusinated eIF5A have been associated with aberrant proliferation in neoplasia of the vulva,8 and an eIF5A siRNA was found to increase the cytotoxicity of imatinib in Bcr-Abl-positive cancer cells.17 Inhibitors of DHS inhibit growth of cancer cells both in vitro27 and in vivo,28 indicating that hypusine-eIF5A is a survival factor for some cancer cells. In vitro cell studies and in vivo xenograft studies have demonstrated that overexpression of unhypusinated eIF5A induces apoptosis in multiple cancer cell types9,10 and inhibits tumor growth in vivo.14 Hypusine modification may regulate eIF5A activity in part by controlling its subcellular localization.29
Constitutive activation of NF-κB is a common feature of both primary multiple myeloma cells and multiple myeloma cell lines.30 NF-κB activation is believed to play an important role in plasma cell survival and proliferation and inhibitors of NF-κB increase the sensitivity of myeloma cells to apoptosis.30 The RelA/p65 protein is responsible for most of the transcriptional activity of NF-κB, as p50 and p52 lack transcription-modulating domains. Inhibition of NF-κB activity was observed in TNF-α-treated 293A cells in response to both eIF5A siRNA and SNS01 treatment. Inhibition of NF-κB activity in 293A cells resulting from depletion of eIF5A could not be attributed to inhibition of degradation of the IκBα inhibitory protein since it was phosphorylated and rapidly degraded in response to TNF-α in SNS01-treated cells. Although nuclear translocation of NF-κB is an important aspect of its activity, it is not sufficient for full transcriptional activity of p65. Phosphorylation of p65 at Ser536 has been demonstrated to regulate the transactivation activity of NF-κB.20,21,22 In the present study inhibition of TNF-α-induced NF-κB p65 phosphorylation at Ser536 was observed in response to eIF5A siRNA and SNS01 in KAS-6/1 and 293A cells, respectively. Phosphorylation of p65 at Ser536 appears to regulate the transactivation activity of NF-κB by increasing its association with the transcriptional coactivator, CBP/p300.31 Phosphorylation at this site has been attributed to several kinases including AKT,32 IKKαβ,32 and tyrosine kinase.33 Mutation of Ser536 has been shown to result in loss of p65 transcriptional activity induced by cytokines, including TNF-α34 and lymphotoxin,35 as well as Gram-negative bacteria.32 Furthermore, phosphorylated p65 does not appear to associate with either IκB or p50 in the cytoplasm, and nuclear translocation of phosphorylated p65 occurs even when IκBα degradation has been inhibited by a proteasome inhibitor.22,36 It would appear, therefore, that SNS01 inhibits NF-κB transcriptional activity in an IκBα-independent manner by inhibiting phosphorylation of p65 at Ser536.
The inhibitory effect of eIF5A siRNA on the activity of NF-κB is consistent with reports that eIF5A siRNA can act as an anti-inflammatory in vivo. For example, eIF5A siRNA has been shown to protect mice from a lethal lipopolysaccharides challenge and to reduce the expression of pro-inflammatory cytokines in sepsis and acute lung injury models.13 Inhibitors of the two enzymes that regulate hypusine synthesis, DHS, and deoxyhypusine hydroxylase, have also been reported to have anti-inflammatory properties,37 and DHS haploinsufficiency impairs cytokine signaling.38 It is, therefore, reasonable to suggest that inhibition of NF-κB activity by the eIF5A siRNA may be at least partly responsible for the enhanced apoptosis observed when the siRNA is co-administered to multiple myeloma cells with a proapoptotic eIF5A mutant.
A growing body of evidence suggests that MAPK signaling is involved in the positive regulation of NF-κB activation by TNF-α.24,25 Kinases activated by the MAPK family, including mitogen and stress-activated kinase 1 and ribosomal S6 kinase, can phosphorylate NF-κB p65, thereby linking these two pathways.25,36 Generation of reactive oxygen species (ROS) is a well-described feature of TNF-α signaling, and ROS likely act as signaling molecules leading to activation of both NF-κB and MAPK/SAPK pathways.39 Inhibitors of ROS production interfere with activation of MAPK/SAPK pathways leading to suppression of NF-κB activity.40 Lack of NF-κB activation has been attributed to insufficient p65 phosphorylation on Ser536 as a result of insufficient ERK,40 further supporting a role for MAPK signaling in the regulation of NF-κB activity in response to TNF-α. An siRNA against eIF5A was recently shown to inhibit ROS generation and activation of the p38 and JNK signaling pathways in doxorubicin-treated cardiomyocytes.26 Likewise, a reduction in ERK MAPK activation in response to TNF-α signaling was observed in this study following treatment with SNS01. However, it is unlikely to have contributed to the observed decrease in NF-κB activity since a MEK inhibitor failed to impact phosphorylation of NF-κB. Growth factors such as interleukin-6 and IGF-1 activate the ERK MAPK pathway in multiple myeloma cells, and inhibitors of this pathway are of interest as drug targets in the treatment of multiple myeloma.41 Thus, although the mechanism is unclear, eIF5A may modulate MAPK and NF-κB signaling in stressed cells by regulating ROS production.
A B cell-specific promoter and enhancer were introduced into the pExp5A plasmid in order to minimize expression of the eIF5AK50R transgene in nontarget tissues. The B29 mRNA is expressed throughout all stages of B cell development, including terminally differentiated plasma cells,42 but accumulates to only very low levels in non-B cells,43 indicating that the B29 promoter is suitable for use in gene therapy of multiple myeloma. The B29 DHS4.4 3′ enhancer was included in the pExp5A plasmid due to its ability to increase expression of the B29 promoter in B cells while silencing promoter activity in non-B cells.44 Activity of the B29 promoter was observed in the human embryonic kidney cell line 293 in the present study, indicating that the promoter may be active in certain renal cell lines, although it is inactive in the NRK kidney cell line,44 indicating epigenetic silencing of the B29 promoter may be cell line-dependent, at least in cells of renal origin. The safety and activity of the pExp5A plasmid were further enhanced by use of a CpG-free vector backbone, which has been reported to reduce activation of the vertebrate host immune system45 thereby increasing transgene expression from single and repeat doses.46 Complexing the nucleic acids with in vivo-jetPEI protects plasmid DNA and siRNA from serum nucleases as well as promote entry into cells and facilitates escape from endosomes following cellular uptake. Moreover, systemic injection of DNA or siRNA complexed in vivo-jetPEI does not induce an anti-inflammatory response47 or trigger antibody production.48,49
The efficacy of SNS01 nanoparticles as a means of codelivering an siRNA targeting hypusinated eIF5A and a B-cell specific expression plasmid expressing eIF5AK50R was validated using in vivo murine multiple myeloma models. Intratumoral and systemic administration of SNS01 inhibited growth of subcutaneous multiple myeloma tumors in vivo. Tumor regression and induction of apoptosis were also observed following administration of SNS01. Finally, suppression of endogenous hypusinated eIF5A by SNS01 increased the sensitivity of multiple myeloma cells to apoptotic signals and potentiated the pro-apoptotic activity of eIF5AK50R, likely in part through modulation of NF-κB and MAPK signaling pathways. It is planned to further evaluate this therapeutic approach in multiple myeloma clinical studies.
Materials and Methods
Reagents. Recombinant human interleukin-6 was obtained from R&D Systems (Minneapolis, MN) and recombinant human TNF-α from USBiological (Swampscott, MA). The Beta-Glo Luminescent β-Galactosidase Assay and the DeadEnd Fluoremetric TUNEL Detection System were purchased from Promega (Madison, WI), and the Ready-To-Glow Secreted Luciferase System was purchased from Clontech (Mountain View, CA). The FITC Annexin V Apoptosis Detection Kit II was obtained from BD Pharmingen (San Diego, CA). Antibodies against eIF5A and β-actin were obtained from BD Transduction Laboratories (Franklin Lakes, NJ) and Calbiochem (Rockland, MA), respectively. Antibodies against NF-κB p65, p42/p44 MAPK, IKK, and IκBα were obtained from Cell Signaling Technology (Beverly, MA). The anti-HA-peroxidase (3F10) antibody was purchased from Roche Life Sciences (Indianapolis, IN). In vivo-jetPEI and 10% glucose were from Polyplus Transfection (Illkirch, France).
Cell culture. The KAS-6/1 human multiple myeloma cell line was obtained from Dr John Lust (Mayo Clinic, Rochester, MN). KAS-6/1 cells were maintained in S10 culture media (RPMI-1640 + 10% fetal bovine serum + 4 ng/ml recombinant human interleukin-6) at a cell density between 5 × 105 and 2 × 106 viable cells/ml. The 293A cell line was purchased from Invitrogen (Carlsbad, CA; cat # R705-07) and maintained in culture media containing Dulbecco's modified Eagle's medium high glucose (Invitrogen) with 10% fetal bovine serum and 0.1 mmol/l nonessential amino acids. Cell lines were maintained in a humidified chamber maintained at 37 °C and 5% CO2.
Western blotting. Cell lysate was prepared by incubating cells in lysis buffer [62.5 mmol/l Tris–HCl (pH 6.8), 2% wt/vol SDS, 10% glycerol, and protease inhibitors], and protein concentration was determined using the Bicinchoninic Acid Kit (Sigma, St Louis, MO). Western blot analysis of eIF5A expression was performed as described.9 For analysis of NF-κB pathway proteins, 10 µg of protein was separated by SDS-PAGE and the antibodies were used according to the manufacturer's instructions. Densitometry analysis was performed using ImageQuant software from Molecular Dynamics (Sunnyvale, CA).
Adenovirus constructs, siRNAs, and plasmids. Construction and amplification of the adenoviral constructs and sequences of the eIF5A siRNAs used in this study were described previously.9 The control siRNA used in the present study was a validated nontargeting siRNA (Dharmacon, Lafayette, CO). The CpG dinucleotide-free plasmid, pCpG-mcsG2 (MCS), was obtained from InvivoGen (San Diego, CA). The pCpG-eIF5AK50R plasmid was constructed by subcloning eIF5AK50R into the NcoI and NheI sites of pCpG-mcs. To create pExp5A (refer to Supplementary Figure S1), the pCpG-mcs plasmid was digested with EcoRI to remove the mammalian expression cassette and then ligated to a synthetic linker with a new multiple cloning site. The B29 DHS4.4 3′ enhancer was amplified from KAS-6/1 genomic DNA using the following primers: 5′-GAAGCGGCCGCACCACCCTGGGCCAGGCTGG-3′ and 5′-CCACGCGTAGAGGTGTTAAAAAGTCTTTAGGTAAAG-3′, and ligated into the NotI and MluI sites of the linker (pCpG-DHS4.4). The -167 B29 promoter was amplified from pDrive-hB29 (Invivogen) using primers: 5′ CCAACTAGTGCGACCGCCAAACCTTAGC-3′ and 5′-CAAAAGCTTGACAACGTCCGAGGCTCCTTGG-3′, and ligated into the SpeI and HindIII sites of pCpG-LacZ. The resulting plasmid was used to amplify the B29-eIF5AK50R expression cassette using the primers: 5′-GTTATCGATACTAGTGCGACCGCCAAACC-3′ and 5′-CAAGCGGCCGCCATACCACATTTGTAGAGGTTTTAC-3′, and ligated into the ClaI and NotI sites in the multiple cloning site of pCpG-DHS4.4 to create pExp5A.
KAS-6/1 transfection and apoptosis assays. KAS-6/1 cell suspensions were diluted to 1 × 106 cells/ml in S10 media, seeded at 0.3 ml/well of a 24-well plate and transfected with 52.5 pmol of siRNA combined with 2 µl of Lipofectamine 2000. The transfection was repeated 72 hours later. Four hours later, the transfection media was removed and replaced with RPMI-1640 + 10% fetal bovine serum + 3,000 infectious units of adenovirus. Forty-eight hours later the cells were washed, stained with Annexin V-FITC and propidium iodide, and sorted using a BD Bioscience FACSVantage SE system with an argon lasor. A minimum of 5,000 cells were counted, and the data were analyzed using WinMDI2.8 software. Cells in early apoptosis (Annexin V-positive, PI-negative) were counted and expressed relative to control cells.
SNS01 and nanoparticle preparation and characterization. PEI nanoparticles were prepared by diluting 200 µg of plasmid DNA and 100 µg of siRNA in 5% glucose to a total volume of 500 µl. Invivo-jetPEI sufficient for an N/P ratio of either 8 (48 µl) or 6 (36 µl) was diluted in 5% glucose to a total volume of 500 µl in a separate tube. The diluted PEI was then mixed quickly with the diluted nucleic acids and incubated at room temperature for 30 minutes prior to use. SNS01 nanoparticles contained 0.2 mg/ml pExp5A plasmid and 0.1 mg/ml eIF5A siRNA (eIF5A siRNA # 1 which targets the 3′UTR of human eIF5A) and were prepared at an N/P ratio of 6. Particle size and zeta potential were determined on a Malvern Zetasizer Nano ZS using Dispersion Technology Software 5.10. Both measurements were performed in clear disposable zeta cells (Malvern) on samples diluted in water at 1:10 volumetric ratio.
DNase and serum stability assays. DNase digestion was performed by incubating 2.5 µl SNS01 with 0.5 U of DNase I in 1× DNase digestion buffer [10 mmol/l Tris–HCl (pH 7.5), 2.5 mmol/l MgCl2, 0.1 mmol/l CaCl2] at 37 °C. Digestion was stopped by addition of 83 mmol/l EDTA, and the nucleic acid was released from complexation with PEI by incubation with 0.5 mg/ml heparin for 15 minutes. The samples were analyzed by electrophoresis through a 1.5% agarose gel containing ethidium bromide.
pNF-κB-MetLuc2 reporter assay. 293A cells were transfected in triplicate with 5 µl of SNS01 or PEI nanoparticles containing only the pExp5A plasmid or only the eIF5A siRNA. Twenty-four hours later the cells were cotransfected with 0.5 µg each of the pNF-κB-MetLuc2 (Clontech) and pHM6-LacZ (Roche Life Sciences) reporter plasmids using Lipofectamine 2000. Four hours later the transfection media was removed from the cells and replaced with growth media either with or without 5 ng/ml TNF-α to stimulate NF-κB activity. Twenty-four hours later, duplicate 50 µl volumes of cell culture media were removed from each well, transferred to a 96-well plate and incubated with Ready-to-Glow secreted luciferase reagent according to the manufacturer's instructions. The cells were then trypsinized, transferred to a 96-well plate and incubated with a luminescent β-galactosidase substrate. Luminescence generated by secreted luciferase or β-galactosidase was measured with a luminometer. The experiment described above was performed three times.
Quantitative reverse transcription-PCR. KAS-6/1 cells seeded 300,000 cells/well on a 24-well plate were incubated with 5 µl of SNS01 for 4 hours and then incubated in fresh S10 media for 24 hours. Total RNA was harvested (GenElute Mammalian Total RNA Miniprep Kit; Sigma) and used as a template for cDNA synthesis (Omniscript RT Kit; Qiagen, Hilden, Germany). Human eIF5A was amplified using the primers 5′-AGGCCATGGCAAAATAACTG-3′ and 5′-GGGTGGGGAAAACCAAAATA-3′. The eIF5AK50R transgene was amplified using the primers 5′-GTACAGTAGCTTCCACCATG-3′ and 5′-CATTCTTACGTAATGCTGAGC-3′. The PCR reactions were prepared using SsoFast EvaGreen Supermix (Bio-Rad, Hercules, CA) and quantitative PCR was performed in a Mini-Opticon Thermocycler (Bio-Rad) using β-actin as a reference gene. A plasmid standard curve using 3 × 102 to 3 × 108 copies of the pExp5A plasmid was used to calculate copy number of the expressed transgene.
KAS-6/1 and RPMI 8226 tumor models. All studies involving animals were conducted in accordance with the guidelines set out by the University of Waterloo Animal Care Committee (Waterloo, Ontario, Canada) as established by the Canadian Council on Animal Care and the Province of Ontario Animals for Research Act. The mice were housed in a pathogen-free animal facility at the University of Guelph. Female C.B-17/IcrHsd-Prkdcscid (SCID; Harlan Laboratories, Indianapolis, IN) mice 4–6 weeks of age were implanted subcutaneously with 1.2 × 107 KAS-6/1 cells. To generate the RPMI 8226 tumor model, female CB17-SCID mice (Charles River Laboratories) 4–6 weeks of age were implanted subcutaneously with 1.2 × 107 RPMI 8226 cells. Treatment was initiated when all mice had palpable tumors. The mice were treated by either intratumoral injection or intravenous tail vein injection twice weekly. Animals were dosed with 0.15 mg (nucleic acid)/kg to 1.5 mg/kg of PEI nanoparticles per injection. Tumors were measured twice weekly with digital calipers and tumor volume was calculated using the equation: tumor volume (mm3) = L × W2 × 0.5; where length (L) is the longest diameter of the tumor and W (width) is the smaller diameter. Mice were euthanized by CO2 inhalation at the termination of the study. Tumors were excised and fixed in formalin.
Hematoxylin and eosin and TUNEL. Fixed tissue was embedded and sectioned by the University of Guelph Animal Health Laboratory. Tumor sections were deparaffinized in xylene, rehydrated in ethanol (sequential treatments with 100%, 95%, 85%, 70%, and 50% ethanol), treated with proteinase K, and labeled for TUNEL using the DeadEnd Fluorometric TUNEL System (Promega) according to the manufacturer's instructions. The TUNEL-labeled tissue sections were examined and photographed by fluorescent confocal microscopy. Micrographs of hematoxylin and eosin stained tissue sections were obtained by bright field microscopy.
SUPPLEMENTARY MATERIAL Figure S1. Construction of the pExp5A plasmid. Figure S2. B cell specificity of B29 promoter and enhancer.
Acknowledgments
This work was supported by a research contract from Senesco Technologies Inc. J.E.T. is a Chief Scientific Officer for Senesco Technologies Inc. and holds Senesco shares. R.D. is Vice-President, Research and Development for Senesco. The work presented in manuscript # MT-T-11-834 was supported by a research contract from Senesco Technologies Inc. C.A.T. holds stock options in Senesco Technologies. Senesco Technologies Inc. holds patents pertaining to the use of SNS01 and SNS01-T, a newer formulation of SNS01, as anticancer agents.
Supplementary Material
Construction of the pExp5A plasmid.
B cell specificity of B29 promoter and enhancer.
REFERENCES
- Kang HA., and, Hershey JW. Effect of initiation factor eIF-5A depletion on protein synthesis and proliferation of Saccharomyces cerevisiae. J Biol Chem. 1994;269:3934–3940. [PubMed] [Google Scholar]
- Zanelli CF, Maragno AL, Gregio AP, Komili S, Pandolfi JR, Mestriner CA.et al. (2006eIF5A binds to translational machinery components and affects translation in yeast Biochem Biophys Res Commun 3481358–1366. [DOI] [PubMed] [Google Scholar]
- Gregio AP, Cano VP, Avaca JS, Valentini SR., and, Zanelli CF. eIF5A has a function in the elongation step of translation in yeast. Biochem Biophys Res Commun. 2009;380:785–790. doi: 10.1016/j.bbrc.2009.01.148. [DOI] [PubMed] [Google Scholar]
- Saini P, Eyler DE, Green R., and, Dever TE. Hypusine-containing protein eIF5A promotes translation elongation. Nature. 2009;459:118–121. doi: 10.1038/nature08034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YP, Nemeroff M, Yan YP., and, Chen KY. Interaction of eukaryotic initiation factor 5A with the human immunodeficiency virus type 1 Rev response element RNA and U6 snRNA requires deoxyhypusine or hypusine modification. Biol Signals. 1997;6:166–174. doi: 10.1159/000109123. [DOI] [PubMed] [Google Scholar]
- Maier B, Ogihara T, Trace AP, Tersey SA, Robbins RD, Chakrabarti SK.et al. (2010The unique hypusine modification of eIF5A promotes islet beta cell inflammation and dysfunction in mice J Clin Invest 1202156–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MH, Nishimura K, Zanelli CF., and, Valentini SR. Functional significance of eIF5A and its hypusine modification in eukaryotes. Amino Acids. 2010;38:491–500. doi: 10.1007/s00726-009-0408-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cracchiolo BM, Heller DS, Clement PM, Wolff EC, Park MH., and, Hanauske-Abel HM. Eukaryotic initiation factor 5A-1 (eIF5A-1) as a diagnostic marker for aberrant proliferation in intraepithelial neoplasia of the vulva. Gynecol Oncol. 2004;94:217–222. doi: 10.1016/j.ygyno.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Taylor CA, Sun Z, Cliche DO, Ming H, Eshaque B, Jin S.et al. (2007Eukaryotic translation initiation factor 5A induces apoptosis in colon cancer cells and associates with the nucleus in response to tumour necrosis factor alpha signalling Exp Cell Res 313437–449. [DOI] [PubMed] [Google Scholar]
- Sun Z, Cheng Z, Taylor CA, McConkey BJ., and, Thompson JE. Apoptosis induction by eIF5A1 involves activation of the intrinsic mitochondrial pathway. J Cell Physiol. 2010;223:798–809. doi: 10.1002/jcp.22100. [DOI] [PubMed] [Google Scholar]
- Li AL, Li HY, Jin BF, Ye QN, Zhou T, Yu XD.et al. (2004A novel eIF5A complex functions as a regulator of p53 and p53-dependent apoptosis J Biol Chem 27949251–49258. [DOI] [PubMed] [Google Scholar]
- Caraglia M, Park MH, Wolff EC, Marra M., and, Abbruzzese A. eIF5A isoforms and cancer: two brothers for two functions. Amino Acids. 2011. [DOI] [PMC free article] [PubMed]
- Moore CC, Martin EN, Lee G, Taylor C, Dondero R, Reznikov LL.et al. (2008Eukaryotic translation initiation factor 5A small interference RNA-liposome complexes reduce inflammation and increase survival in murine models of severe sepsis and acute lung injury J Infect Dis 1981407–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, S, Taylor, CA, Liu, Z, Sun, Z, Ye, B., and, Thompson JE. Suppression of primary and disseminated murine tumor growth with eIF5A1 gene therapy. Gene Ther Mol Biol. 2008;12:207–218. [Google Scholar]
- Ramaswamy S, Ross KN, Lander ES., and, Golub TR. A molecular signature of metastasis in primary solid tumors. Nat Genet. 2003;33:49–54. doi: 10.1038/ng1060. [DOI] [PubMed] [Google Scholar]
- Coller HA, Grandori C, Tamayo P, Colbert T, Lander ES, Eisenman RN.et al. (2000Expression analysis with oligonucleotide microarrays reveals that MYC regulates genes involved in growth, cell cycle, signaling, and adhesion Proc Natl Acad Sci USA 973260–3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balabanov S, Gontarewicz A, Ziegler P, Hartmann U, Kammer W, Copland M.et al. (2007Hypusination of eukaryotic initiation factor 5A (eIF5A): a novel therapeutic target in BCR-ABL-positive leukemias identified by a proteomics approach Blood 1091701–1711. [DOI] [PubMed] [Google Scholar]
- Caraglia M, Marra M, Giuberti G, D'Alessandro AM, Baldi A, Tassone P.et al. (2003The eukaryotic initiation factor 5A is involved in the regulation of proliferation and apoptosis induced by interferon-alpha and EGF in human cancer cells J Biochem 133757–765. [DOI] [PubMed] [Google Scholar]
- Caraglia M, Vitale G, Marra M, Del Prete S, Lentini A, Budillon A.et al. (2004Translational and post-translational modifications of proteins as a new mechanism of action of alpha-interferon: review article Amino Acids 26409–417. [DOI] [PubMed] [Google Scholar]
- Schmitz ML, Bacher S., and, Kracht M. I kappa B-independent control of NF-kappa B activity by modulatory phosphorylations. Trends Biochem Sci. 2001;26:186–190. doi: 10.1016/s0968-0004(00)01753-9. [DOI] [PubMed] [Google Scholar]
- Yang F, Tang E, Guan K., and, Wang CY. IKK beta plays an essential role in the phosphorylation of RelA/p65 on serine 536 induced by lipopolysaccharide. J Immunol. 2003;170:5630–5635. doi: 10.4049/jimmunol.170.11.5630. [DOI] [PubMed] [Google Scholar]
- Sasaki CY, Barberi TJ, Ghosh P., and, Longo DL. Phosphorylation of RelA/p65 on serine 536 defines an I{kappa}B{alpha}-independent NF-{kappa}B pathway. J Biol Chem. 2005;280:34538–34547. doi: 10.1074/jbc.M504943200. [DOI] [PubMed] [Google Scholar]
- Klier H, Csonga R, Joäo HC, Eckerskorn C, Auer M, Lottspeich F.et al. (1995Isolation and structural characterization of different isoforms of the hypusine-containing protein eIF-5A from HeLa cells Biochemistry 3414693–14702. [DOI] [PubMed] [Google Scholar]
- Vanden Berghe W, Plaisance S, Boone E, De Bosscher K, Schmitz ML, Fiers W.et al. (1998p38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways are required for nuclear factor-kappaB p65 transactivation mediated by tumor necrosis factor J Biol Chem 2733285–3290. [DOI] [PubMed] [Google Scholar]
- Vermeulen L, De Wilde G, Van Damme P, Vanden Berghe W., and, Haegeman G. Transcriptional activation of the NF-kappaB p65 subunit by mitogen- and stress-activated protein kinase-1 (MSK1) EMBO J. 2003;22:1313–1324. doi: 10.1093/emboj/cdg139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X, Wang DB, Lu X, Wei H, Zhu R, Zhu SS.et al. (2010Doxorubicin induces apoptosis in H9c2 cardiomyocytes: role of overexpressed eukaryotic translation initiation factor 5A Biol Pharm Bull 331666–1672. [DOI] [PubMed] [Google Scholar]
- Shi XP, Yin KC, Ahern J, Davis LJ, Stern AM., and, Waxman L. Effects of N1-guanyl-1,7-diaminoheptane, an inhibitor of deoxyhypusine synthase, on the growth of tumorigenic cell lines in culture. Biochim Biophys Acta. 1996;1310:119–126. doi: 10.1016/0167-4889(95)00165-4. [DOI] [PubMed] [Google Scholar]
- Jasiulionis MG, Luchessi AD, Moreira AG, Souza PP, Suenaga AP, Correa M.et al. (2007Inhibition of eukaryotic translation initiation factor 5A (eIF5A) hypusination impairs melanoma growth Cell Biochem Funct 25109–114. [DOI] [PubMed] [Google Scholar]
- Lee SB, Park JH, Kaevel J, Sramkova M, Weigert R., and, Park MH. The effect of hypusine modification on the intracellular localization of eIF5A. Biochem Biophys Res Commun. 2009;383:497–502. doi: 10.1016/j.bbrc.2009.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni H, Ergin M, Huang Q, Qin JZ, Amin HM, Martinez RL.et al. (2001Analysis of expression of nuclear factor kappa B (NF-kappa B) in multiple myeloma: downregulation of NF-kappa B induces apoptosis Br J Haematol 115279–286. [DOI] [PubMed] [Google Scholar]
- Oh SM, Lee SH, Lee BJ, Pyo CW, Yoo NK, Lee SY.et al. (2007A distinct role of neutrophil lactoferrin in RelA/p65 phosphorylation on Ser536 by recruiting TNF receptor-associated factors to IkappaB kinase signaling complex J Immunol 1795686–5692. [DOI] [PubMed] [Google Scholar]
- Haller D, Russo MP, Sartor RB., and, Jobin C. IKK beta and phosphatidylinositol 3-kinase/Akt participate in non-pathogenic Gram-negative enteric bacteria-induced RelA phosphorylation and NF-kappa B activation in both primary and intestinal epithelial cell lines. J Biol Chem. 2002;277:38168–38178. doi: 10.1074/jbc.M205737200. [DOI] [PubMed] [Google Scholar]
- Doyle SL, Jefferies CA., and, O'Neill LA. Bruton's tyrosine kinase is involved in p65-mediated transactivation and phosphorylation of p65 on serine 536 during NFkappaB activation by lipopolysaccharide. J Biol Chem. 2005;280:23496–23501. doi: 10.1074/jbc.C500053200. [DOI] [PubMed] [Google Scholar]
- O'Mahony AM, Montano M, Van Beneden K, Chen LF., and, Greene WC. Human T-cell lymphotropic virus type 1 tax induction of biologically Active NF-kappaB requires IkappaB kinase-1-mediated phosphorylation of RelA/p65. J Biol Chem. 2004;279:18137–18145. doi: 10.1074/jbc.M401397200. [DOI] [PubMed] [Google Scholar]
- Jiang X, Takahashi N, Matsui N, Tetsuka T., and, Okamoto T. The NF-kappa B activation in lymphotoxin beta receptor signaling depends on the phosphorylation of p65 at serine 536. J Biol Chem. 2003;278:919–926. doi: 10.1074/jbc.M208696200. [DOI] [PubMed] [Google Scholar]
- Bohuslav J, Chen LF, Kwon H, Mu Y., and, Greene WC. p53 induces NF-kappaB activation by an IkappaB kinase-independent mechanism involving phosphorylation of p65 by ribosomal S6 kinase 1. J Biol Chem. 2004;279:26115–26125. doi: 10.1074/jbc.M313509200. [DOI] [PubMed] [Google Scholar]
- Frydas S, Papazahariadou M, Papaioannou N, Hatzistilianou M, Trakatellis M, Merlitti D.et al. (2003Effect of the compound L-mimosine in an in vivo model of chronic granuloma formation induced by potassium permanganate (KMNO4) Int J Immunopathol Pharmacol 1699–104. [DOI] [PubMed] [Google Scholar]
- Templin AT, Maier B, Nishiki Y, Tersey SA., and, Mirmira RG. Deoxyhypusine synthase haploinsufficiency attenuates acute cytokine signaling. Cell Cycle. 2011;10:1043–1049. doi: 10.4161/cc.10.7.15206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., and, Oberley LW. Redox regulation of transcriptional activators. Free Radic Biol Med. 1996;21:335–348. doi: 10.1016/0891-5849(96)00109-8. [DOI] [PubMed] [Google Scholar]
- Hu J, Nakano H, Sakurai H., and, Colburn NH. Insufficient p65 phosphorylation at S536 specifically contributes to the lack of NF-kappaB activation and transformation in resistant JB6 cells. Carcinogenesis. 2004;25:1991–2003. doi: 10.1093/carcin/bgh198. [DOI] [PubMed] [Google Scholar]
- Piazza FA, Gurrieri C, Trentin L., and, Semenzato G. Towards a new age in the treatment of multiple myeloma. Ann Hematol. 2007;86:159–172. doi: 10.1007/s00277-006-0239-5. [DOI] [PubMed] [Google Scholar]
- Hermanson GG, Eisenberg D, Kincade PW., and, Wall R. B29: a member of the immunoglobulin gene superfamily exclusively expressed on beta-lineage cells. Proc Natl Acad Sci USA. 1988;85:6890–6894. doi: 10.1073/pnas.85.18.6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AA, Wood WJ, Jr, Gilly MJ, Damore MA, Omori SA., and, Wall R. The promoter and 5' flanking sequences controlling human B29 gene expression. Blood. 1996;87:666–673. [PubMed] [Google Scholar]
- Malone CS, Kuraishy AI, Fike FM, Loya RG, Mikkili MR, Teitell MA.et al. (2006B29 gene silencing in pituitary cells is regulated by its 3' enhancer J Mol Biol 362173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-Sandoval A., and, Ertl HC. CpG methylation of a plasmid vector results in extended transgene product expression by circumventing induction of immune responses. Mol Ther. 2004;9:249–261. doi: 10.1016/j.ymthe.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Yew NS, Zhao H, Przybylska M, Wu IH, Tousignant JD, Scheule RK.et al. (2002CpG-depleted plasmid DNA vectors with enhanced safety and long-term gene expression in vivo Mol Ther 5731–738. [DOI] [PubMed] [Google Scholar]
- Bonnet ME, Erbacher P., and, Bolcato-Bellemin AL. Systemic delivery of DNA or siRNA mediated by linear polyethylenimine (L-PEI) does not induce an inflammatory response. Pharm Res. 2008;25:2972–2982. doi: 10.1007/s11095-008-9693-1. [DOI] [PubMed] [Google Scholar]
- Lisziewicz J, Trocio J, Whitman L, Varga G, Xu J, Bakare N.et al. (2005DermaVir: a novel topical vaccine for HIV/AIDS J Invest Dermatol 124160–169. [DOI] [PubMed] [Google Scholar]
- Liu H, Liu L, Fletcher BS., and, Visner GA. Sleeping Beauty-based gene therapy with indoleamine 2,3-dioxygenase inhibits lung allograft fibrosis. FASEB J. 2006;20:2384–2386. doi: 10.1096/fj.06-6228fje. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Construction of the pExp5A plasmid.
B cell specificity of B29 promoter and enhancer.