Abstract
Recent modest successes in ex vivo dendritic cell (DC) immunotherapy have motivated continued innovation in the area of DC manipulation and activation. Although ex vivo vaccine approaches continue to be proving grounds for new DC manipulation techniques, the intrinsic limits of ex vivo therapy, including high cost, minimal standardization, cumbersome delivery, and poor accessibility, incentivizes the development of vaccines compatible with in vivo DC targeting. We describe here a method to co-deliver both tumor-specific antigen (TSA) and an iMyD88/CD40 adjuvant (iMC), to DCs that combines toll-like receptor (TLR) and CD40 signaling. In this study, we demonstrate that simple TSA delivery via adenoviral vectors results in strong antitumor immunity. Addition of iMC delivered in a separate vector is insufficient to enhance this effect. However, when delivered simultaneously with TSA in a single bicistronic vector (BV), iMC is able to significantly enhance antigen-specific cytotoxic T-cell (CTL) responses and inhibit established tumor growth. This study demonstrates the spatial-temporal importance of concurrent DC activation and TSA presentation. Further, it demonstrates the feasibility of in vivo molecular enhancement of DCs necessary for effective antitumor immune responses.
Introduction
Widely accepted as the most potent antigen-presenting cells of the immune system, dendritic cells (DCs) serve to capture, process, and present antigens to naive T-cells. T-cell receptor/antigen/major histocompatibility complex (MHC) interactions lead to antigen-specific T-cell priming and expansion, provided that appropriate secondary signals are present on the DCs.1,2 For this reason, tumor antigen-specific DC vaccines have been developed for a variety of different neoplasias. Despite some modest successes, including recent FDA approval of Sipuleucel-T, a DC-based prostate cancer vaccine, most DC tumor immunotherapies have not led to robust clinical outcomes that warrant their use as first-line cancer-fighting agents.3,4,5,6
One likely explanation for this shortcoming is the suboptimal activation profile of DCs produced by many ex vivo maturation protocols, which can result in DCs expressing low levels of costimulatory molecules as well as TH1-polarizing cytokines, such as interleukin (IL)-12.7,8 To overcome these DC activation deficits, we recently developed a synthetic molecule, termed iMyD88/CD40 (iMC), capable of simultaneous toll-like receptor (TLR) and CD40 activation in the presence of a small molecule chemical inducer of dimerization (CID).9 This technology has enabled us to strongly activate DCs in vitro without the need for exogenous proinflammatory cytokines or potentially harmful TLR agonists such as lipopolysaccharide (LPS), or its derivatives.5,8 When delivered to DCs in vitro using first-generation Ad5 adenoviral (Adv) vectors, iMC induced upregulation of DC activation markers as well as high expression of TH1-associated cytokines such as IL-12, leading to cytotoxic T-cell (CTL) responses capable of killing established tumors in preclinical models.9
Although the iMC-DC vaccine has been shown to be highly efficacious, the cumbersome requirements of an autologous DC therapy create a short-term obstacle for its broadest application.10 Current ex vivo DC vaccine protocols involve a highly specialized, labor-intensive process in a quality-controlled good manufacturing practice environment, leading to high costs, suboptimal standardization, and low accessibility that limits the application of these therapies to highly insured cancer patients with access to specialized medical centers.5,11
To overcome these challenges, investigators have begun developing strategies to target the delivery of antigens and activation factors (“adjuvants”) directly to DCs in vivo.10,11,12 Several delivery vehicle choices for such an “off-the-shelf” therapy have already been tested including viruses, plasmids, and naked RNA.12,13,14,15 In our current work, we chose to utilize first generation, replication-deficient ΔE1, ΔE3 Ad5 adenoviruses. Their relative ease of production, excellent transduction efficiency, high “downstream” immunogenicity, especially in the context of CD8+ T-cell induction, strong CD11c+ DC tropism, and general safety make them a logical, well-established choice for this proof-of-principle study.12,16,17,18,19,20
We first confirmed that direct subcutaneous (s.c.) injection of tumor-specific antigen (TSA)-encoding Ad5 vectors (Ad-TSA) induces an antigen-specific CD8+ response, capable of significantly decreasing preexisting tumor growth in mice. Despite the reported potency of the iMC adjuvant, co-injection of Ad-TSA with an iMC-encoding adenovector was ineffective in augmenting the antitumor effect. Upon further investigation, this result was consistent with inefficient (~40%) uptake of both vectors within the same cell. To ensure coexpression of both antigen and inducible adjuvant, we developed a bicistronic adenovector, Ad5-iMC-β-Gal (BAdv), encoding iMC and β-galactosidase (as the TSA). A picornavirus-derived “F2A” sequence was used to coexpress both TSA and iMC.21 Assay of BAdv signaling in vitro revealed that moderate to high BAdv levels strongly induced nuclear factor-κB (NF-κB) signaling and robust IL-12 secretion even without CID addition, thereby eliminating the need for CID administration in subsequent in vivo experiments. When injected into mouse footpads, BAdv induced significantly (P < 0.05) higher levels of antigen-specific IFN-γ–secreting CD8+ T-cells compared to Ad-TSA alone. In addition, more of these antigen-specific, functional CD8+ T-cells localized within the tumors of BAdv-treated mice than Ad-TSA–treated mice. Finally, this enhanced immunogenicity by BAdv translated to significantly (P < 0.05) improved control of preestablished tumors in mice, compared to treatment with Ad-TSA alone. Our results indicate that in vivo delivery of the iMC-based DC-enhancing system is possible and can significantly improve on an already efficacious Ad-TSA treatment of preexisting tumors in preclinical models. Overall, this proof-of-principle study lays the groundwork for the development of an in vivo immunotherapy capable of genetically enhancing DCs and inducing potent, effective antitumor immune responses without the need for prolonged leukapheresis and ex vivo DC manipulations.
Results
TSA-encoding Ad5 (Ad-TSA) inhibits tumor growth in mice via expansion of antigen-specific CD8+ T-cells
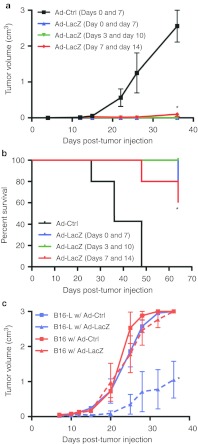
Previous reports by others showed that direct in vivo injection of adenoviral vectors encoding TSAs can induce antigen-specific CD8+ T-cell responses capable of prophylactic as well as therapeutic treatment of tumors in mice.16,22 However, the therapeutic efficacy was limited to the first few days after tumor challenge. To investigate whether we could further boost efficacy, we initially evaluated an Ad5 vector expressing β-galactosidase (Ad-LacZ) antigen in the treatment of a B16 melanoma subline expressing β-galactosidase (B16-βgal). Mice were injected s.c. with tumor cells and subsequently treated via footpad injections of Ad-LacZ at 0, 3, or 7 days post-tumor injection with a second viral treatment 7 days later. Day 0 treated mice showed no tumor growth. Similarly, mice receiving treatment 3 days post-tumor inoculation displayed little to no tumor growth. Even when treatment was delayed for 7 days, the now palpable preestablished tumors underwent a significant decrease in volume or growth rate compared to control (Ad-Ctrl)-injected animals (Figure 1a). Correspondingly, all Ad-LacZ–treated mice experienced a significantly higher survival advantage compared to Ad-Ctrl (Figure 1b). Mice inoculated with parental B16 cells exhibited similar tumor growth curves whether treated with Ad-LacZ or Ad-Ctrl, attributing Ad-LacZ–induced tumor growth reduction to the induction of antigen-specific CD8+ T-cells as previously reported (Figure 1c).16 Flow cytometric analysis of peripheral blood lymphocytes from Ad-LacZ or Ad-Ctrl–treated mice using anti-CD8 antibody and MHC class I (MHC-I) tetramers loaded with the β-gal, Kb-restricted, immunodominant peptide, ICPMYARV also revealed that Ad-LacZ treatment led to significant proliferation of β-gal–specific CD8+ T-cells, undetectable in Ad-Ctrl treated mice (Figure 2a).
Figure 1.
Ad-LacZ vaccination can eliminate preestablished B16-lacZ tumors. (a) Mice (n = 5) were injected with adenovirus (1 × 1010 vp/mouse) in the footpad as indicated at either 0, 3, or 7 days after subcutaneous injection of 10,000 B16-LacZ tumor cells and boosted again 7 days later with another adenoviral injection. Tumor growth was monitored by measuring the length and width and volume calculated as 0.52 × length × width2. (b) Survival curves of mice treated as described in a. (c) Mice (n = 5) were subcutaneously inoculated with either 50,000 B16 or B16-LacZ tumor cells and injected with the indicated adenovirus in the footpad, 7 days later. Results in a and b are representative of five experiments. *P < 0.05. vp, virus particles.
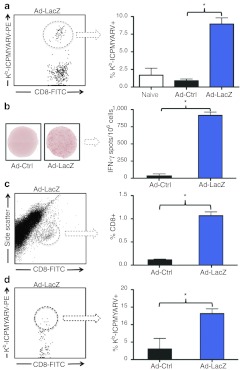
Figure 2.
Ad-LacZ induces a potent antigen-specific CD8+ T-cell response. (a) H2-Kb-ICPMYARV tetramer (β-gal-tet) and CD8 staining of PBLs extracted from mice 8 days after footpad injection with Ad-LacZ (1 × 1010 vp/mouse). Panel indicates a representative dot plot of flow cytometry data from the Ad-LacZ group and the average % tetramer+ peripheral blood CD8+ cells of 15 mice per group (two mice in naive group). (b) IFN-γ ELISpot of splenocytes extracted from mice 9 days after treatment as in a. Panel shows one representative well of each treatment group and the average number of spots of four mice per group. (c) Intratumoral lymphocyte analysis shows an increased number of CD8+ T-cells resident within the tumors of Ad-LacZ treated mice (1.6 × 108 pfu per mouse). Panel shows one Ad-LacZ representative dot plot of flow cytometry data and the overall combined percentage of CD8+ cells in the tumors for three mice per group. Tumors were disaggregated by digestion and strained to make single-cell suspensions before staining with anti-CD8 antibodies and MHCI tetramers. (d) β-gal-tet staining of CD8+ cells from c indicate the presence of tumor antigen-specific T-cells in the tumors of Ad-LacZ treated mice (1 × 1010 vp/mouse). Panel shows one Ad-LacZ representative dot plot of flow cytometry data and the average+ % intratumoral CD8+ cells (n = 3). All results are representative of two or more independent experiments. *P < 0.05. FITC, fluorescein isothiocyanate; IFN-γ, interferon-γ MHCI, major histocompatibility complex class I; PBL, peripheral blood lymphocytes; pfu, plaque-forming unit; vp, virus particles.
To elucidate functionality of antigen-specific CD8+ T-cells, splenocytes were isolated from immunized animals, and IFN-γ secretion, following ICPMYARV exposure, was examined by Enzyme-linked immunosorbent spot (ELISpot) assays. IFN-γ–secreting β-gal–specific CD8+ T-cells were detected in the spleens of Ad-LacZ–treated mice, whereas control-treated mice yielded significantly fewer antigen responsive T-cells (Figure 2b). To demonstrate that Ad-LacZ–induced CD8+ cells are able to home to and kill tumor cells, we analyzed the tumor-localized, antigen-specific CD8+ population. Tumors were harvested from mice 11 days after vaccination with Ad-Ctrl or Ad-LacZ, following an initial tumor growth period of 8 days. Tumor cells were disaggregated, and infiltrating CD8+ lymphocytes were analyzed by flow cytometry (Figure 2c,d). Significantly more CD8+ cells were present in the tumors of Ad-LacZ treated mice (Figure 2c), and a significantly higher percentage of these CD8+ cells were β-gal–specific compared to Ad-Ctrl treated mice (Figure 2d). Taken together, these data demonstrate the ability of Ad-LacZ to induce a functional, antigen-specific CD8+ T-cell pool capable of homing to the target tumor.
Development of a bicistronic vector encoding both functional iMC and TSA
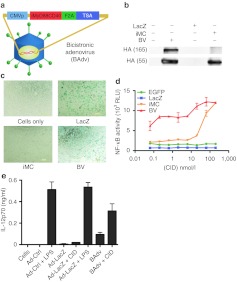
In an effort to enhance the antitumor immune responses induced by Ad-LacZ and to harness the powerful DC activation potential of previously described iMC, we coadministered Ad-LacZ and Ad-iMC as two distinct vectors.9 However, tumor growth experiments reflected no significant difference in tumor reduction or survival between Ad-LacZ or Ad-LacZ + Ad-iMC-treated mice (Supplementary Figure S1a,b). Further exploration into this lack of augmentation revealed that coadministration of two adenoviruses encoding distinct transgenes in vivo results in less than 40% coexpression of transgenes within draining lymph node CD11c+ DCs (Supplementary Figure S2). To overcome this logistical obstacle and to better achieve simultaneous, dual-transgene expression, we exploited the ability of a picornavirus-derived, peptide-bond-skipping “F2A” sequence to generate two separate gene products from a single open reading frame.21,23,24 Specifically, iMC and β-galactosidase were subcloned in-frame upstream and downstream, respectively, of the Foot and Mouth Disease Virus F2A sequence, under cytomegalovirus promoter transcriptional control. The resulting expression vector was termed the “bicistronic vector (BV)” Supplementary Materials and Methods (Figure 3a). Dual-gene expression was tested in vitro via western blot analysis, which demonstrated the production of both iMC and an iMC-β-galactosidase fusion protein, a minor side-product of 2A systems (Figure 3b).24 In addition, X-Gal staining of BV-transfected cells revealed the production of functional β-galactosidase (Figure 3c). Next, using an NF-κB–responsive secreted alkaline phosphatase (SEAP) reporter assay, we verified the ability of the BV-produced iMC to induce NF-κB.9,25 Addition of CID enhanced NF-κB activation, while the BV also led to detectable CID-independent NF-κB activity (Figure 3d). Thus, the BV enabled us to coexpress both a functional DC-enhancing molecule, iMC, and a TSA, and thereby determine whether the inclusion of iMC to Ad5-TSA delivery in vivo could further augment antitumor immune responses.
Figure 3.
Development of iMC/LacZ bicistronic vector. (a) Illustration of bicistronic vector (BV) showing iMC and TSA (LacZ) separated by the picornavirus F2A sequence. (b) Western blot of 293-HEK cells transfected with BV shows the production of a 55-kDa HA-tagged protein identified as iMC. The uncleaved 165-kDa iMC-LacZ fusion protein is also apparent. (c) X-gal staining of HEK-293 cells transfected as in b. (d) NF-κB-SEAP reporter assay indicating BV-mediated NF-κB induction. HEK-293 cells were co-transfected with BV (or other constructs as indicated) along with NF-κB-SEAP reporter plasmid. After 24 hours, AP20187 was added as indicated. (e) IL-12p70 ELISA of primary mBMDCs transduced with bicistronic adenovirus (BAdv) (10,000 vp/cell) (or other viruses as indicated) for 48 hours with or without CID treatment. Results are representative of 2 (b and c) or ≥ 3 (as shown in d and e) experiments. BAdv, bicistronic adenovirus; CID, chemical inducer of dimerization; IL, interleukin; LPS, lipopolysaccharide; mBMDC, mouse bone marrow-derived dendritic cell; NF-κB, nuclear factor-κB; TSA, tumor-specific antigen; vp, virus particles.
Bicistronic Ad5-iMC/βGal induces IL-12 secretion in primary bone marrow DCs
To compare Ad-LacZ to BV, we further developed a corresponding bicistronic adenovirus (BAdv), encoding iMC-F2A-β-Gal, and verified expression by western blotting and X-Gal staining, as above Supplementary Materials and Methods (data not shown). To assess the ability of the BAdv to activate primary DCs, we transduced mouse bone marrow-derived DCs (mBMDCs) and measured secretion of IL-12p70, a pro-Th1 cytokine previously shown to be highly induced by iMC signaling together with other proinflammatory cytokines such as IL-1α, IL-1β, IFN-γ, and IP-10.9 Using an IL-12–specific enzyme-linked immunosorbent assays (ELISA), we observed that the BAdv was capable of inducing high-level IL-12 secretion in BMDCs, which was further enhanced by CID addition. Conversely, IL-12 secretion was not detected in Ad-LacZ–treated control cells, without LPS supplementation (Figure 3e). Thus, the BAdv showed a similar DC activation profile to that previously described for iMC but with increased CID-independent activity, at least with this variant of “2A” sequence, thereby obviating the need for CID treatment in subsequent in vivo DC-delivery experiments.9
The BAdv reduces tumor growth more effectively than Ad-LacZ
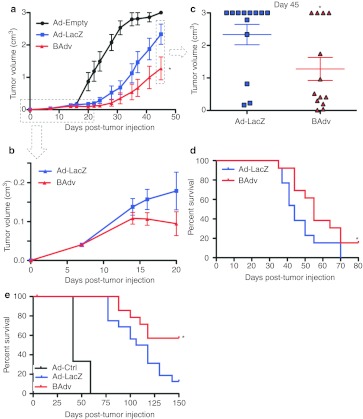
To test whether iMC-TSA coexpression could enhance antitumor response over that conferred by adenoviral TSA delivery, we compared the growth of B16-βgal melanoma tumors following vaccination with Ad-LacZ or BAdv. To optimize the tumor growth assay, we utilized a more stringent model with delayed vaccination for 8 days post-tumor inoculation along with a tenfold increase in tumor cell challenge. As expected, Ad-LacZ treatment led to tumor growth inhibition, which was further inhibited by BAdv treatment (Figure 4a). Notably, this divergence in tumor growth rate occurred 6–7 days after treatment, suggesting a more potent induction of the adaptive immune response by the BAdv compared to that observed for the monocistronic vector containing TSA alone (Figure 4b). Growth rates continued to diverge with time, reaching a maximum difference in tumor size by day 45 post-tumor injection (Figure 4c). In addition, the disparity in growth between Ad-LacZ and BAdv-treated tumors led to a significant survival advantage for BAdv-treated mice (Figure 4d). Finally, similar tumor growth rate reductions were observed in B16 tumor-laden mice treated with an adenovector expressing the endogenous B16 antigen protein TRP2 (Ad-TRP2) or a bicistronic vector expressing both iMC and TRP2 (BAdv-TRP2) (Supplementary Figure S3a,b). Thus, concurrent expression of iMC with TSA leads to better outcomes both in tumor growth and overall survival compared to TSA treatment alone.
Figure 4.
Co-delivery of iMC and LacZ (TSA) in one adenovector significantly improves tumor growth reduction and survival compared to Ad-LacZ alone. (a) Tumor growth curve indicating a significant decrease in tumor growth of mice treated with BAdv when compared to the already highly effective Ad-LacZ. Mice (n = 13) were injected subcutaneously with 100,000 B16-LacZ cells. Tumors were allowed to grow for 8 days before footpad treatment with the indicated adenovectors (1 × 1010 vp/mouse) and tumor growth measured over time. (b) The first 20 days of the tumor experiment described in a. (c) Plot shows tumor size of individual mice 45 days after tumor cell injection. (d) Survival curve of experiment in a shows a significant survival advantage in mice treated with BAdv compared to Ad-LacZ–treated mice. Results in a–d are representative of five experiments. (e) Survival graph of mice pretreated with indicated adenovector and challenged subcutaneously with 50,000 B16-LacZ tumor cells 2 months after treatment. Results for each treatment type (i.e., adenovector type) are a compilation of data from several groups treated with varying amount of adenovector. Ad-Ctrl data represents one group of three mice treated with 1 × 1010 vps. Four groups of four mice each were injected with one of the following amount of Ad-LacZ vps: 1 × 1010, 3.16 × 109, 1 × 109, and 3.16 × 108. Four groups of four mice each were injected with one of the following amount of BAdv vps: 1 × 1010, 3.16 × 109, 1 × 109, and 3.16 × 108. *P < 0.05. BAdv, bicistronic adenovirus; TSA, tumor-specific antigen; vp, virus particle.
Although therapeutic tumor treatment would likely be the first clinical application of iMC/TSA technology, we wanted to test whether a BAdv could also provide long-lasting, prophylactic efficacy against TSA+ tumors. Mice were injected with tumors cells s.c. 2 months after BAdv or Adv treatment. Consistent with reports that IL-12 enhances CD8+ T-cell memory development, mice given prophylactic BAdv treatment exhibited a significant survival advantage compared to those given Ad-TSA alone (Figure 4e).26 More than 50% of the BAdv-treated mice were living 150 days after tumor cell injection compared to 12.5% given Ad-TSA.
The BAdv elicits an enhanced antigen-specific immune response compared to Ad-LacZ
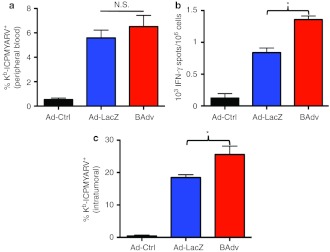
To better understand the immunological basis for BAdv's enhanced therapeutic efficacy, we focused on its effect on CTL responses, implicated as the main target of action involved in Ad-TSA therapy.16,18 Surprisingly, tetramer+ CD8+ T-cells were not significantly increased in the peripheral blood of BAdv compared to Ad-LacZ–treated mice (BAdv) (Figure 5a). Similar MHC-I:SVYDFFVWL (TRP2 immunodominant peptide) tetramer+ CD8+ T-cells were observed in Ad-TRP2 and BAdv-TRP2 treated mice, albeit at much lower levels (Supplementary Figure S4). Therefore, we hypothesized that BAdv's significant improvement in antitumor therapy likely results from the induction of a functionally augmented immune response rather than a mere increase in antigen-specific T-cell numbers.
Figure 5.
BAdv significantly improves upon the already potent antigen-specific CD8+ T-cell response of Ad-LacZ. (a) β-gal-tetramer+ and CD8 staining of PBLs of mice (n = 13) treated with the indicated adenovectors (1 × 1010 vp/mouse). (b) IFN-γ ELISpot of splenocytes extracted from mice 9 days after treatment with indicated adenovectors. Results reflect the combining of two experiments with four mice per group. In one experiment, vp was held constant, and mice were injected with 1 × 1010 vp of Ad-Ctrl (Ad-Empty, 1.5 × 109 pfu), Ad-LacZ (1.6 × 109 pfu), and BAdv (1.2 × 109 pfu) per mouse. In the second experiment, pfu was held constant, and mice were injected with 1.6 × 108 pfu of Ad-Ctrl (Ad-Empty, 1.1 × 109 vp), Ad-LacZ (1 × 109 vp), and BAdv (1.4 × 109 vp) per mouse. The average number of ELISpots counted for a given treatment was comparable across the two experiments. (c) β-gal-tet+ and CD8 staining of intratumoral lymphocytes shows significantly more antigen-specific, CD8+ T-cells in tumors of mice treated with BAdv compared to Ad-LacZ. Mice (n = 4) were inoculated with 50,000 tumor cells and footpad-injected with the indicated adenovectors (1.6 × 108 pfu/mouse) 8 days later. Tumors were allowed to grow for 11 more days before they were removed and processed in single cell suspensions. Results are representative of two (b and d) or five (a) experiments. *P < 0.05. BAdv, bicistronic adenovirus; IFN-γ, interferon-γ N.S., not significant; PBL, peripheral blood lymphocytes; pfu, plaque-forming unit; vp, virus particle.
We previously reported that mBMDC expression of iMC resulted in a significant upregulation of surface markers CD86, CD40, CCR7 and MHC-II (I-Ab), as well as secretion of proinflammatory cytokines, IL-1α, IL-1β, IL-12, IFN-γ, and IP-10.9 To show that iMC retains its ability to activate DCs when expressed within a BV system, mBMDCs were transduced with BAdv-TRP2, resulting in significant upregulation of surface markers CD86, CD40, CCR7, MHC-I (H2-Kb), and MHC-II ((I-Ab) in a CID-dependent fashion (Supplementary Figure S5a–e). In addition, multiplex analysis of BAdv-TRP2–transduced mBMDC supernatants showed CID-dependent release of proinflammatory cytokines, IL-1α, IL-1β, IL-6, IL-12, IFN-γ, and IP-10 (Supplementary Figure S6a–f). Our previous characterization of the effects of iMC signaling on DCs led us to conclude that high IL-12 secretion correlated with improved ex vivo DC vaccine efficacy.9 Notably, the presence of IL-12 during DC:T-cell interaction has been shown to increase both T-cell IFN-γ production and functional avidity.5,27 As mentioned above, primary DCs transduced in vitro with BAdv produced high levels of IL-12, whereas no IL-12 secretion was detectable by cells transduced with Ad-LacZ. Therefore, to determine if IL-12 contributed to the enhanced CD8+ T-cell activity seen after BAdv treatment, we compared IFN-γ production of Ad-LacZ–induced antigen-specific CD8+ T-cells to BAdv-induced cells. IFN-γ ELISpot analysis revealed that BAdv induced a significantly higher amount of functional IFN-γ–secreting CD8+ T-cells compared to Ad-LacZ (Figure 5b). Furthermore, size analysis of the spots revealed significantly higher IFN-γ–secretion by BAdv-induced versus Ad-LacZ–induced cells (Supplementary Figure S7).
It has also been shown that the presence of IL-12 during DC:T-cell interaction, enhances the ability of CTLs to home to tumors.5,28,29 Therefore, we analyzed the presence of antigen-specific CD8+ T-cells within preestablished tumors in mice treated with either Ad-LacZ or BAdv 8 days after tumor inoculation. Ten days following vaccination, tumors were removed and processed into single-cell suspensions, stained with anti-CD8α and ICPMYARV MHC-I tetramer reagents, and analyzed by flow cytometry. Tumors of BAdv-treated mice were smaller at the time of harvest (data not shown) and contained a significantly higher number of β-gal–specific CD8+ T-cells, indicating that BAdv-induced T-cells had an enhanced ability to home to the tumor site (Figure 5c). Collectively, the comparisons between Ad-LacZ and BAdv treatment demonstrate that Adv-mediated TSA expression in conjunction with coexpressed iMC induces more potent CD8+ T-cell responses, which can lead to a significant reduction in the growth rate of even preestablished aggressive tumors.
Discussion
Over the past 15 years, ex vivo DC-based vaccines have continued to evolve, resulting in the first ever FDA approval of this class of therapeutics in 2010, Sipuleucel-T.3,30 Although we have created several novel methods to enhance DC function to address specific shortcomings of current ex vivo DC protocols,9,25,31,32 several limitations remain. Procurement, culturing, manipulation, and DC reinjection are inherently time-consuming and technically challenging procedures requiring the use of specialized equipment and infrastructure, and contributing to the high costs, difficult standardization, and limited accessibility of autologous DC therapy.11 Therefore, in vivo targeting of DC adjuvants and antigens are ultimately required to circumvent the need for ex vivo DC manipulation and to expand the administration of DC therapies to entry-level practitioners in broad settings.
Stability, relative ease of production and administration, and potential mechanistic advantages of in vivo DC therapy, make it ideally suited for overcoming the challenges faced by current ex vivo protocols.11 Nevertheless, in vivo DC therapies also face challenges that must be overcome for broad acceptance as a first-line treatment modality. Most current in vivo DC techniques lack adequate DC maturation and activation control.11 Initial in vivo DC vaccines consisted of simple co-injection of protein antigen with TLR-based adjuvants, known for their ability to activate DCs and induce strong CTL responses.33,34,35 However, to avoid antigen-independent DC activation and subsequent generation of misdirected immune responses, newer protein-based vaccines co-deliver various adjuvants with protein antigens either via simple conjugation or novel nanoparticle technology.36,37,38 Nevertheless, protein vaccines are limited in the amount and duration of antigen they can deliver. To overcome this limitation, researchers have utilized antigen-encoding vectors, both viral and nonviral, to deliver antigen to DCs in vivo resulting in continuous cytosolic antigen production and MHC-I presentation without the need for cell uptake and cross-presentation, resulting in longer-lived peptide/MHC complexes.33,39 In addition, viral vectors can partially activate DCs in the absence of additional exogenous adjuvants. This phenomenon is especially true of adenovectors, which have been shown to activate DCs via pattern-recognition receptors.40 Furthermore, Adv is readily taken up by CD11c+ DCs in vivo, diminishing the need for DC-specific targeting required by protein vaccines to augment antigen loading and to reduce off-target effects.19 Finally, previous studies, as well as results presented here, show that Adv is capable of inducing potent, transgene-specific CD8+ T-cell responses, capable of significantly decreasing antigen-specific tumor growth.16 Therefore, Adv presents an excellent starting platform for evaluating further improvements to in vivo DC activation technology.
Many strategies to augment viral-induced transgene immunity have been developed.33,40,41 Recently, a phase II clinical trial involving PROSTVAC, a poxviral-based vaccine, encoding prostate-specific antigen and three DC costimulatory molecules, has enjoyed good success, reporting a significant improvement in overall survival of 8.5 months.42 Although this result is quite promising, PROSTVAC did not prolong progression-free survival, emphasizing the need for additional improvement.42 Therefore, we sought to augment Adv-based, transgene-specific antitumor immunity by enhancing DC activation, utilizing our recently described inducible iMC composite system. The iMC activates DCs by combining TLR with CD40 signaling, resulting in highly synergistic signaling that generates strong TH1-polarizing conditions and consequentially potent CTL responses.9,31,43,44
Herein, we demonstrate that the addition of iMC signaling to Adv-mediated in vivo TSA delivery substantially augments antigen-specific, functional CD8+ T-cell responses, leading to significant tumor growth reductions and prolonged survival. Furthermore, it is important to note that simple co-injection of iMC with TSA, in separate Adv vectors, failed to enhance the antitumor effect of Ad-TSA treatment. This lack of improvement is likely due to poor cotransduction of adenovectors in vivo, as demonstrated using surrogate Adv encoding RFP or GFP. In that case, less than half of target DCs coexpressed both proteins, as detected by flow cytometry. Therefore, we posited that the lack of sufficient iMC and TSA coexpression resulted in inadequate activation of TSA-expressing DCs and thereby no improvement in subsequent antitumor immunity. In addition, it is possible that iMC-activated, non-TSA–expressing DCs present irrelevant antigens (especially Adv-derived), leading to undesired off-target side effects. As a result, we proposed that an iMC/TSA-encoding, coexpression vector would fully benefit from the potent DC adjuvant properties of iMC and therefore augment Ad-TSA in vivo DC therapy.
Initial functional tests of the dual-expressing vector revealed that the CID-regulated characteristics of iMC had been largely eliminated at the relatively high viral titers used. We hypothesize that CID-independent activity derives in part from excessive protein aggregation caused by large, uncleaved iMC:TSA fusion proteins, which is much less pronounced when iMC is expressed by itself. As a result, this unanticipated side effect has simplified our system by removing the need for dimerizer treatment whereas retaining the overall immune enhancement characteristics of iMC. However, since the initially inducible characteristic of iMC may be desirable in some cases, if restoration of iMC inducibility in the context of a BAdv was needed, one could utilize more efficient 2A systems such as “P2A,” replace the 2A with an internal ribosome entry sequence, or develop a dicistronic vector.45 In addition, it is possible that CID-inducibility may depend on the properties of the TSA being expressed by the bicistronic system, such as size or immunogenicity, as the BAdv-TRP2 seems to retain more of iMC's inducibility while expressing a much less immunogenic antigen that is half the size of β-galactosidase.
Immune-function data suggest that BAdv's enhanced antitumor efficacy is directly related to a potent boost of the antigen-specific CD8+ T-cell response. Further analysis of the BAdv-induced T-cell responses, together with previous investigations into the nature of enhanced iMC-DC immunogenicity, as well as BAdv-induced IL-12 secretion, presented here, leads us to infer that iMC-mediated, high-level IL-12 secretion is one of the major mechanisms responsible for BAdv's immune enhancement.9 Specifically, the BAdv does not induce more antigen-specific, circulating T-cells, but instead confers augmented function as is evident by higher IFN-γ production and the increased tumor-homing abilities of BAdv-DC–activated T-cells. Such a functional boost directly correlates with the effect of IL-12 on T-cells, as previously described in the literature.5,27,28,29 However, our results do not exclude additional mechanisms of immune enhancement. For example, we previously reported that natural killer cell induction is another sequelae of iMC-mediated immune augmentation.9 Also, iMC-induced surface marker upregulation and broad proinflammatory cytokine secretion, reported previously and shown here to be retained in the bicistronic system, present other potential mechanisms of iMC immune enhancement to be elucidated in detail in future studies. Indeed, recent clinical data suggests that IL-12-mediated vaccine augmentation is enhanced by and possibly requires additional immunological adjuvants to elicit useful clinical outcomes.46
Finally, preexisting antivector immunity can severely decrease the efficacy of Adv-based in vivo vaccines.33,40 Nevertheless, we show that neutralizing antibodies can be partially overcome by iMC, presumably due to low-level iMC transgene expression that can overcome limited antigen delivery (Supplementary Figure S8a–c). Further enhancements to transgene delivery should be achievable via vectors that are more immunologically “invisible”, such as pseudotyped adenovectors, or that are less susceptible to neutralizing humoral immunity, such as plasmids or nonviral vectors.12,13,47,48 In fact, nonimmunogenic delivery systems would be the most optimal, as the anti-TSA immune response could be boosted indefinitely. The efficacy of these nonviral as well as viral vectors could be further enhanced by specifically targeting iMC expression to DCs using either DC-specific promoters or by tethering vectors to the DC surface using DC-tropic ligands and antibodies targeting DC receptors, such as the mannose receptor, DEC205/CD205, DC-SIGN/CD209, or CLEC9A/DNGR-1.11,49
Materials and Methods
Mice, cell lines, peptides, and dimerizer drug. Four- to six-week-old female C57BL/6 mice were purchased from the Center for Comparative Medicine at Baylor College of Medicine and maintained under pathogen-free conditions in the Transgenic Mouse Facility at Baylor College of Medicine. The Institutional Animal Care and Use Committee of Baylor College of Medicine approved animal procedures. B16.F10 and HEK293 cells were purchased from ATCC (Manassas, VA) and cultured as recommended by vendor. B16.D5-LacZ cells were a generous gift from Dr Alison McCormick, Touro University, CA, and cultured in complete RPMI supplemented with L-glutamine (Mediatech, Manassas, VA), 0.1 mmol/l nonessential amino acids (Gibco by Invitrogen, (Carlsbad, CA), 1 mmol/l Sodium Pyruvate (Gibco by Invitrogen, Carlsbad, CA), and 50 µmol/l β-mercaptoethanol (Bio-Rad, Hercules, CA). Peptides were produced at 90–95% purity by Genemed Synthesis (San Antonio, TX). Dimerizer drug AP20187 was provided by ARIAD Pharmaceuticals (Cambridge, MA).
Preparation of mouse DCs. Murine BMDCs were prepared as previously described.25,32
Mouse IL-12p70 ELISAs. BMDCs were transduced with Ad5 vectors (10,000 virus particles (vp)/cell) and treated with 100 nmol/l AP20187 or 500 ng/ml LPS (Sigma-Aldrich, St Louis, MO). Supernatants were collected 2 days post-transduction and assessed for presence of IL-12 (p70) using a standard mouse IL-12 (p70) ELISA test (BD Biosciences, San Diego, CA) as per manufacturers recommendations. Briefly, flat-bottom immunosorbent 96-well plates (Thermo Scientific, Rochester, NY) were coated overnight with anti-mouse IL-12 (p70) monoclonal capture antibody (BD Biosciences). Supernatants were then incubated in coated plates and biotinylated anti-mouse IL-12 (p70) monoclonal detection antibody (BD Biosciences) was used to bind to captured IL-12 (p70) from supernatants. Finally, streptavidin-horseradish peroxidase conjugate (BD Biosciences) was used to quantify presence of IL-12 (p70)-bound detection antibody in wells.
Tumor experiments. Groups (n = 5–16) of C57BL/6 female mice were inoculated s.c. with 10,000–100,000 B16.F10 or B16.D5 LacZ tumor cells. Tumors were allowed to grow for 7–8 days (until visible and palpable) and measured by length and width. Tumor volume was calculated as 0.52 × length × width2. Mice were allocated to treatment groups so that the average tumor volumes were approximately equal. In LacZ tumor experiments, mice were footpad-injected with 1 × 1010 vp of Ad-Ctrl (Ad-Empty, 1.5 × 109 pfu (plaque-forming unit)), Ad-LacZ (1.6 × 109 pfu), and BAdv (1.2 × 109 pfu). Viruses were diluted with phosphate-buffered saline (PBS) to 1 × 1010 vp/100 µl PBS, and 50 µl were injected into each footpad. In TRP2 tumor experiments, mice were footpad-injected with 1.6 × 109 pfu of Ad-Ctrl (Ad-Empty, 1.05 × 1010 vp), Ad-TRP2 (6.7 × 1010 vp), BAdv-TRP2 (5.3 × 1010 vp). Viruses were diluted with PBS to 1.6 × 109 pfu/100 µl PBS, and 50 µl were injected into each footpad. Tumor growth was assessed by caliper measurement and tumor volume calculation, as described above, every 2–3 days. Mice with tumors of greater than 10% of body weight or exceeding 3 cm3 were euthanized and recorded as a tumor volume of 3 cm3.
Memory tumor experiments were carried out as stated above with the following modifications. On day 0, mice were footpad-injected with adenoviral treatment of 1 × 1010, 3.16 × 109, 1 × 109, and 3.16 × 108 vp/mouse (four mice per treatment amount for a total of 16 mice per treatment type) for Ad-LacZ and BAdv. Control mice (n = 3) were injected with 1 × 1010 vp of Ad-Ctrl per mouse. Two months postadenoviral injection, mice were injected with 50,000 tumor cells as stated above, and tumors measured and calculated as above.
Tetramer analysis. Peripheral blood was obtained via retro-orbital bleed from mice 8–10 days after adenoviral treatment. Lymphocytes were extracted using Histopaque (Sigma-Aldrich) gradient. Cells were then stained using anti-CD8-FITC (BD Pharmingen, San Diego, CA) and ICPMYARV-H2Kb-PE tetramer for β-galactosidase immunizations or SYDFFVWL-H2Kb-PE tetramer for TRP2 immunizations (Baylor College of Medicine, Tetramer Core, Houston, TX). Stained cells were analyzed using an LSRII flow cytometer (BD Biosciences).
IFN-γ ELISpot. Spleens were dissected from mice 8–10 days after Adv treatment. In one experiment, vp was held constant, and mice were injected with 1 × 1010 vp of Ad-Ctrl (Ad-Empty, 1.5 × 109 pfu), Ad-LacZ (1.6 × 109 pfu), and BAdv (1.2 × 109 pfu) per mouse. In another experiment, PFU was held constant, and mice were injected with 1.6 × 108 pfu of Ad-Ctrl (Ad-Empty, 1.1 × 109 vp), Ad-LacZ (1 × 109 vp), and BAdv (1.4 × 109 vp) per mouse. Splenocytes were purified and seeded into 96-well nitrocellulose-base plates (Millipore, Billerica, MA) that had been coated overnight with 10 µg/ml anti-mouse IFN-γ mAb (AN18; Mabtech, Mariemont, OH). Splenocytes were stimulated with 1 µg/ml β-gal497-504 (ICPMYARV) H2-Kb-restricted peptide or irrelevant TRP2180-188 (SVYDFFVWL) peptide (negative control) overnight at 37 °C in 5% CO2. Cells were removed and plates washed with PBS containing 0.5% Tween-20 (Bio-Rad). Plates were incubated with 1 µg/ml biotinylated anti-mouse IFN-γ mAb (R4-6A2; Mabtech) at 37 °C in 5% CO2 for 2 hours. Plates were then washed and incubated with avidin/biotinylated enzyme complex (Vector Laboratories, Burlingame, CA) for 1 hour and IFN-γ spots detected using 3-amino-9-ethylcarbazole substrate (Sigma-Aldrich). Spots were counted and analyzed by ZellNet Consulting (Fort Lee, NJ) using KS ELISPOT 4.3 software.
Intratumoral lymphocytes. Groups (n = 4) of mice were injected s.c. with 50,000 B16.D5 LacZ tumor cells, allowed to grow for 8 days and then allocated according to tumor volume as described above. Adenovirus (1.6 × 108 pfu/mouse) was injected after 8 days as described above. Twelve days after adenoviral treatment, tumors were removed and digested using 2 µg/ml collagenase A (Roche, Mannheim, Germany). Single-cell suspensions were made using a cell strainer. Cells were then stained with anti-CD8-FITC (BD Pharmingen) and ICPMYARV-MHCI-PE tetramer (Baylor College of Medicine, Tetramer Core) and analyzed using an LSRII flow cytometer.
Double expression. Three mice were injected with 1 × 1010 vp of Ad-EGFP and Ad-dsRed per mouse. Viruses were footpad-injected as described above. Popliteal lymph nodes were dissected 48 hours after virus injection. Single-cell suspensions were made and stained with anti-CD11c-PE-Cy7 (BD Pharmingen). Cells were analyzed by flow cytometry.
Western blotting. HEK-293 cells were transfected via CaPO4 transfection with 0.5 µg of plasmid, expressing EGFP, LacZ, iMC, or iMC-F2A-LacZ. Cells were harvested 48 hours post-transfection, lysed using Laemmli Buffer (Bio-Rad) with 5% β-mercaptoethanol (Bio-Rad), and incubated at 100 °C for 10 minutes. Samples were then run on a polyacrylamide gel and transferred to a 0.45-µm nitrocellulose membrane (Bio-Rad). Membranes were blocked and subsequently incubated with anti-HA primary antibody (Covance, Princeton, NJ). After washing, HRP-conjugated goat anti-mouse antibody (Santa Cruz Biotechnology, Santa Cruz, CA) was added to membrane and visualized using Super Signal West Dura (Thermo Scientific) and a Kodak Image Station 4000MM (Kodak, Rochester, NY).
β-Galactosidase assay. HEK-293 cells were transfected with 0.5 µg of plasmid, expressing either EGFP, LacZ, iMC, or iMC-F2A-LacZ via CaPO4 transfection. After 48 hours, cells were washed and stained for β-galactosidase activity using the β-gal Staining Kit (Invitrogen) as per manufacturers instructions.
NF-κB SEAP reporter assay. HEK-293 cells were transfected with 0.5 µg of plasmid expressing EGFP, LacZ, iMC, or iMC-F2A-LacZ via CaPO4 transfection. All cells were also transfected with 1 µg of NF-κB SEAP reporter via CaPO4 transfection. After 24 hours incubation, cells were harvested, plated into 96-well plates (Greiner Bio-One, Monroe, NC), and treated with increasing amounts of AP20187. After 24 hours, supernatants were harvested and analyzed for SEAP activity, as previously described.25
In vitro DC activation and cytokine secretion studies. mBMDCs were transduced in triplicate with indicated Ad5 vectors (20,000 vp/cell) and treated with 100 nmol/l AP20187 where indicated. Cells were stained with anti-CD11c-APC (eBioscience, San Diego, CA) and either with anti-CD40-PE (BD Pharmingen), anti-CD86-FITC (BD Pharmingen), and anti-CCR7-PE-Cy7 (eBioscience) or with anti-H2-Kb-FITC (BD Pharmingen) and anti-I-Ab-PE (BD Pharmingen). Cells were then analyzed by flow cytometry.
Supernatants of similarly treated mBMDCs were harvested and analyzed in duplicate (supernatants from two separate wells treated with the same condition) using Milliplex MAP technology (Millipore) as per manufacturer's protocol. Briefly, supernatants were mixed with fluorescently color-coded beads linked to specific antianalyte antibodies. Biotinylated secondary antibodies were added to the beads. Finally PE-labeled streptavidin was added to detect the secondary antibody. Beads were analyzed using a Bio-Plex multiplex reader (Bio-Rad).
Statistics. All data are presented as the mean ± SEM. Data were analyzed using Prism 5.0 software (GraphPad Software, La Jolla, CA). An unpaired Student's t-test was used to calculate two-tailed P values in order to determine statistical significance between two groups. One-way analysis of variance with Bonferroni's post-test correction was used to determine statistical significance between three or more groups. Survival statistics was analyzed using Gehan-Breslow-Wilcoxon test.
SUPPLEMENTARY MATERIAL Figure S1. Co-injection of iMC-encoding adenovirus does not enhance Ad-LacZ–mediated antitumor efficacy. Figure S2. Co-injection of two adenoviruses expressing distinct fluorescent proteins results in suboptimal coexpression. Figure S3. Co-delivery of iMC and the endogenous antigen TRP2 in one adenovector (BAdv-TRP2) improves B16 tumor growth reduction compared to Ad-TRP2 alone. Figure S4. Mouse footpad injection of Ad-TRP2 and BAdv-TRP2 induces TRP2-tetramer+ CD8+ T-cells. Figure S5. BAdv-TRP2 induces surface marker upregulation of in vitro cultured mBMDCs in a CID-dependent manner. Figure S6. BAdv-TRP2 induces multiple proinflammatory cytokine release by in vitro cultured mBMDCs in a CID-dependent manner. Figure S7. BAdv induced CD8+ T-cells secrete more IFN-γ than those induced by Ad-LacZ. Figure S8. BAdv partially overcomes adenoviral pretreatment and significantly decreases tumor growth compared to Ad-Ctrl. Materials and Methods.
Acknowledgments
We are grateful to Natalia Lapteva, Shin-Heng Chiou, Payam Shahi, Matthew Collinson-Pautz, and Christopher Sundby for technical assistance and William Decker and Matthew Halpert for a critical reading of the manuscript. In addition, we thank Franco DeMayo, Mary Estes, Martha Mims, and Kevin M Slawin for their generous insights along the way. B16.D5-LacZ tumor cells were generously provided by Suyu Shu (Cleveland Clinic, OH) and AP20187 was generously provided by ARIAD Pharmaceuticals (Cambridge, MA). This work was supported by CPRIT grant (RP100728), the Vanguard Urologic Institute Fund (Houston, TX) and Department of Defense prostate cancer training grant DAMD W81-XWH-09-1-0231. D.M.S. is acting CSO of Bellicum Pharmaceuticals (Houston, TX). The others authors declared no conflict of interest.
Supplementary Material
Co-injection of iMC-encoding adenovirus does not enhance Ad-LacZ–mediated antitumor efficacy.
Co-injection of two adenoviruses expressing distinct fluorescent proteins results in suboptimal coexpression.
Co-delivery of iMC and the endogenous antigen TRP2 in one adenovector (BAdv-TRP2) improves B16 tumor growth reduction compared to Ad-TRP2 alone.
Mouse footpad injection of Ad-TRP2 and BAdv-TRP2 induces TRP2-tetramer+ CD8+ T-cells.
BAdv-TRP2 induces surface marker upregulation of in vitro cultured mBMDCs in a CID-dependent manner.
BAdv-TRP2 induces multiple proinflammatory cytokine release by in vitro cultured mBMDCs in a CID-dependent manner.
BAdv induced CD8+ T-cells secrete more IFN-γ than those induced by Ad-LacZ.
BAdv partially overcomes adenoviral pretreatment and significantly decreases tumor growth compared to Ad-Ctrl.
REFERENCES
- Koski GK, Cohen PA, Roses RE, Xu S., and, Czerniecki BJ. Reengineering dendritic cell-based anti-cancer vaccines. Immunol Rev. 2008;222:256–276. doi: 10.1111/j.1600-065X.2008.00617.x. [DOI] [PubMed] [Google Scholar]
- Steinman RM., and, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF.et al. (2010Sipuleucel-T immunotherapy for castration-resistant prostate cancer N Engl J Med 363411–422. [DOI] [PubMed] [Google Scholar]
- Eubel J., and, Enk AH. Dendritic cell vaccination as a treatment modality for melanoma. Expert Rev Anticancer Ther. 2009;9:1631–1642. doi: 10.1586/era.09.139. [DOI] [PubMed] [Google Scholar]
- Czerniecki BJ, Koski GK, Koldovsky U, Xu S, Cohen PA, Mick R.et al. (2007Targeting HER-2/neu in early breast cancer development using dendritic cells with staged interleukin-12 burst secretion Cancer Res 671842–1852. [DOI] [PubMed] [Google Scholar]
- Smits EL, Anguille S, Cools N, Berneman ZN., and, Van Tendeloo VF. Dendritic cell-based cancer gene therapy. Hum Gene Ther. 2009;20:1106–1118. doi: 10.1089/hum.2009.145. [DOI] [PubMed] [Google Scholar]
- Gabrilovich DI. Dendritic cell vaccines for cancer treatment. Curr Opin Mol Ther. 2002;4:452–458. [PubMed] [Google Scholar]
- Gilboa E. DC-based cancer vaccines. J Clin Invest. 2007;117:1195–1203. doi: 10.1172/JCI31205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan P, Lapteva N, Seethammagari M, Levitt JM, Slawin KM., and, Spencer DM. A composite MyD88/CD40 switch synergistically activates mouse and human dendritic cells for enhanced antitumor efficacy. J Clin Invest. 2011;121:1524–1534. doi: 10.1172/JCI44327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacken PJ., and, Figdor CG. Targeted antigen delivery and activation of dendritic cells in vivo: steps towards cost effective vaccines. Semin Immunol. 2011;23:12–20. doi: 10.1016/j.smim.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Tacken PJ, de Vries IJ, Torensma R., and, Figdor CG. Dendritic-cell immunotherapy: from ex vivo loading to in vivo targeting. Nat Rev Immunol. 2007;7:790–802. doi: 10.1038/nri2173. [DOI] [PubMed] [Google Scholar]
- Liu MA. Immunologic basis of vaccine vectors. Immunity. 2010;33:504–515. doi: 10.1016/j.immuni.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Sardesai NY., and, Weiner DB. Electroporation delivery of DNA vaccines: prospects for success. Curr Opin Immunol. 2011;23:421–429. doi: 10.1016/j.coi.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang DC, DeVit M., and, Johnston SA. Genetic immunization is a simple method for eliciting an immune response. Nature. 1992;356:152–154. doi: 10.1038/356152a0. [DOI] [PubMed] [Google Scholar]
- Diken M, Kreiter S, Selmi A, Britten CM, Huber C, Türeci ö.et al. (2011Selective uptake of naked vaccine RNA by dendritic cells is driven by macropinocytosis and abrogated upon DC maturation Gene Ther 18702–708. [DOI] [PubMed] [Google Scholar]
- Suleman M, Galea S, Gavard F, Merillon N, Klonjkowski B, Tartour E.et al. (2011Antigen encoded by vaccine vectors derived from human adenovirus serotype 5 is preferentially presented to CD8+ T lymphocytes by the CD8a+ dendritic cell subset Vaccine 295892–5903. [DOI] [PubMed] [Google Scholar]
- Liniger M, Zuniga A., and, Naim HY. Use of viral vectors for the development of vaccines. Expert Rev Vaccines. 2007;6:255–266. doi: 10.1586/14760584.6.2.255. [DOI] [PubMed] [Google Scholar]
- Millar J, Dissanayake D, Yang TC, Grinshtein N, Evelegh C, Wan Y.et al. (2007The magnitude of the CD8+ T cell response produced by recombinant virus vectors is a function of both the antigen and the vector Cell Immunol 25055–67. [DOI] [PubMed] [Google Scholar]
- Lindsay RW, Darrah PA, Quinn KM, Wille-Reece U, Mattei LM, Iwasaki A.et al. (2010CD8+ T cell responses following replication-defective adenovirus serotype 5 immunization are dependent on CD11c+ dendritic cells but show redundancy in their requirement of TLR and nucleotide-binding oligomerization domain-like receptor signaling J Immunol 1851513–1521. [DOI] [PubMed] [Google Scholar]
- Rollier CS, Reyes-Sandoval A, Cottingham MG, Ewer K., and, Hill AV. Viral vectors as vaccine platforms: deployment in sight. Curr Opin Immunol. 2011;23:377–382. doi: 10.1016/j.coi.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Szymczak AL, Workman CJ, Wang Y, Vignali KM, Dilioglou S, Vanin EF.et al. (2004Correction of multi-gene deficiency in vivo using a single 'self-cleaving' 2A peptide-based retroviral vector Nat Biotechnol 22589–594. [DOI] [PubMed] [Google Scholar]
- Zhai Y, Yang JC, Kawakami Y, Spiess P, Wadsworth SC, Cardoza LM.et al. (1996Antigen-specific tumor vaccines. Development and characterization of recombinant adenoviruses encoding MART1 or gp100 for cancer therapy J Immunol 156700–710. [PubMed] [Google Scholar]
- Funston GM, Kallioinen SE, de Felipe P, Ryan MD., and, Iggo RD. Expression of heterologous genes in oncolytic adenoviruses using picornaviral 2A sequences that trigger ribosome skipping. J Gen Virol. 2008;89 Pt 2:389–396. doi: 10.1099/vir.0.83444-0. [DOI] [PubMed] [Google Scholar]
- Donnelly ML, Hughes LE, Luke G, Mendoza H, ten Dam E, Gani D.et al. (2001The 'cleavage' activities of foot-and-mouth disease virus 2A site-directed mutants and naturally occurring '2A-like' sequences J Gen Virol 82Pt 51027–1041. [DOI] [PubMed] [Google Scholar]
- Hanks BA, Jiang J, Singh RA, Song W, Barry M, Huls MH.et al. (2005Re- engineered CD40 receptor enables potent pharmacological activation of dendritic-cell cancer vaccines in vivo Nat Med 11130–137. [DOI] [PubMed] [Google Scholar]
- Xiao Z, Casey KA, Jameson SC, Curtsinger JM., and, Mescher MF. Programming for CD8 T cell memory development requires IL-12 or type I IFN. J Immunol. 2009;182:2786–2794. doi: 10.4049/jimmunol.0803484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Koski GK, Faries M, Bedrosian I, Mick R, Maeurer M.et al. (2003Rapid high efficiency sensitization of CD8+ T cells to tumor antigens by dendritic cells leads to enhanced functional avidity and direct tumor recognition through an IL-12-dependent mechanism J Immunol 1712251–2261. [DOI] [PubMed] [Google Scholar]
- Ogawa M, Tsutsui T, Zou JP, Mu J, Wijesuriya R, Yu WG.et al. (1997Enhanced induction of very late antigen 4/lymphocyte function-associated antigen 1-dependent T-cell migration to tumor sites following administration of interleukin 12 Cancer Res 572216–2222. [PubMed] [Google Scholar]
- Fujiwara H., and, Hamaoka T. Coordination of chemokine and adhesion systems in intratumoral T cell migration responsible for the induction of tumor regression. Int Immunopharmacol. 2001;1:613–623. doi: 10.1016/s1567-5769(00)00049-7. [DOI] [PubMed] [Google Scholar]
- Ridgway D. The first 1000 dendritic cell vaccinees. Cancer Invest. 2003;21:873–886. doi: 10.1081/cnv-120025091. [DOI] [PubMed] [Google Scholar]
- Lapteva N, Seethammagari MR, Hanks BA, Jiang J, Levitt JM, Slawin KM.et al. (2007Enhanced activation of human dendritic cells by inducible CD40 and Toll-like receptor-4 ligation Cancer Res 6710528–10537. [DOI] [PubMed] [Google Scholar]
- Park D, Lapteva N, Seethammagari M, Slawin KM., and, Spencer DM. An essential role for Akt1 in dendritic cell function and tumor immunotherapy. Nat Biotechnol. 2006;24:1581–1590. doi: 10.1038/nbt1262. [DOI] [PubMed] [Google Scholar]
- Robson NC, Hoves S, Maraskovsky E., and, Schnurr M. Presentation of tumour antigens by dendritic cells and challenges faced. Curr Opin Immunol. 2010;22:137–144. doi: 10.1016/j.coi.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Merad M, Sugie T, Engleman EG., and, Fong L. In vivo manipulation of dendritic cells to induce therapeutic immunity. Blood. 2002;99:1676–1682. doi: 10.1182/blood.v99.5.1676. [DOI] [PubMed] [Google Scholar]
- Okano F, Merad M, Furumoto K., and, Engleman EG. In vivo manipulation of dendritic cells overcomes tolerance to unmodified tumor-associated self antigens and induces potent antitumor immunity. J Immunol. 2005;174:2645–2652. doi: 10.4049/jimmunol.174.5.2645. [DOI] [PubMed] [Google Scholar]
- Wagner H. The immunogenicity of CpG-antigen conjugates. Adv Drug Deliv Rev. 2009;61:243–247. doi: 10.1016/j.addr.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Maraskovsky E, Schnurr M, Wilson NS, Robson NC, Boyle J., and, Drane D. Development of prophylactic and therapeutic vaccines using the ISCOMATRIX adjuvant. Immunol Cell Biol. 2009;87:371–376. doi: 10.1038/icb.2009.21. [DOI] [PubMed] [Google Scholar]
- Tacken PJ, Zeelenberg IS, Cruz LJ, van Hout-Kuijer MA, van de Glind G, Fokkink RG.et al. (2011Targeted delivery of TLR ligands to human and mouse dendritic cells strongly enhances adjuvanticity Blood 1186836–6844. [DOI] [PubMed] [Google Scholar]
- Zhang X, Gordon JR., and, Xiang J. Advances in dendritic cell-based vaccine of cancer. Cancer Biother Radiopharm. 2002;17:601–619. doi: 10.1089/108497802320970217. [DOI] [PubMed] [Google Scholar]
- Nayak S., and, Herzog RW. Progress and prospects: immune responses to viral vectors. Gene Ther. 2010;17:295–304. doi: 10.1038/gt.2009.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JL, Gulley JL, Arlen PM, Beetham PK, Tsang KY, Slack R.et al. (2005Phase I study of sequential vaccinations with fowlpox-CEA(6D)-TRICOM alone and sequentially with vaccinia-CEA(6D)-TRICOM, with and without granulocyte-macrophage colony-stimulating factor, in patients with carcinoembryonic antigen-expressing carcinomas J Clin Oncol 23720–731. [DOI] [PubMed] [Google Scholar]
- Kantoff PW, Schuetz TJ, Blumenstein BA, Glode LM, Bilhartz DL, Wyand M.et al. (2010Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer J Clin Oncol 281099–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napolitani G, Rinaldi A, Bertoni F, Sallusto F., and, Lanzavecchia A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat Immunol. 2005;6:769–776. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells JW, Cowled CJ, Farzaneh F., and, Noble A. Combined triggering of dendritic cell receptors results in synergistic activation and potent cytotoxic immunity. J Immunol. 2008;181:3422–3431. doi: 10.4049/jimmunol.181.5.3422. [DOI] [PubMed] [Google Scholar]
- Kim JH, Lee SR, Li LH, Park HJ, Park JH, Lee KY.et al. (2011High cleavage efficiency of a 2A peptide derived from porcine teschovirus-1 in human cell lines, zebrafish and mice PLoS ONE 6e18556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker WK., and, Safdar A. Cytokine adjuvants for vaccine therapy of neoplastic and infectious disease. Cytokine Growth Factor Rev. 2011;22:177–187. doi: 10.1016/j.cytogfr.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Davis ME. Non-viral gene delivery systems. Curr Opin Biotechnol. 2002;13:128–131. doi: 10.1016/s0958-1669(02)00294-x. [DOI] [PubMed] [Google Scholar]
- Lungwitz U, Breunig M, Blunk T., and, Göpferich A. Polyethylenimine-based non-viral gene delivery systems. Eur J Pharm Biopharm. 2005;60:247–266. doi: 10.1016/j.ejpb.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Sancho D, Joffre OP, Keller AM, Rogers NC, Martínez D, Hernanz-Falcón P.et al. (2009Identification of a dendritic cell receptor that couples sensing of necrosis to immunity Nature 458899–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Co-injection of iMC-encoding adenovirus does not enhance Ad-LacZ–mediated antitumor efficacy.
Co-injection of two adenoviruses expressing distinct fluorescent proteins results in suboptimal coexpression.
Co-delivery of iMC and the endogenous antigen TRP2 in one adenovector (BAdv-TRP2) improves B16 tumor growth reduction compared to Ad-TRP2 alone.
Mouse footpad injection of Ad-TRP2 and BAdv-TRP2 induces TRP2-tetramer+ CD8+ T-cells.
BAdv-TRP2 induces surface marker upregulation of in vitro cultured mBMDCs in a CID-dependent manner.
BAdv-TRP2 induces multiple proinflammatory cytokine release by in vitro cultured mBMDCs in a CID-dependent manner.
BAdv induced CD8+ T-cells secrete more IFN-γ than those induced by Ad-LacZ.
BAdv partially overcomes adenoviral pretreatment and significantly decreases tumor growth compared to Ad-Ctrl.