Abstract
Tolerance induction, and thus prevention of autoimmunity, is linked with the amount of self-antigen presented on thymic stroma. We describe that intrathymic (i.t.) delivery of the autoantigen, myelin oligodendrocyte glycoprotein (MOG), via a lentiviral vector (LV), led to tolerance induction and prevented mice from developing fulminant experimental autoimmune encephalomyelitis (EAE). This protective effect was associated with the long-term expression of antigen in transduced stromal cells, which resulted in the negative selection of MOG-specific T cells and the generation of regulatory T cells (Tregs). These selection events were effective at decreasing T-cell proliferative responses and reduced Th1 and Th17 cytokines. In vivo, this translated to a reduction in inflammation and demyelination with minimal, or no axonal loss in the spinal cords of treated animals. Significantly intrathymic delivery of MOG to mice during the priming phase of the disease failed to suppress clinical symptoms despite mice being previously treated with a clearing anti-CD4 antibody. These results indicate that targeting autoantigens to the thymic stroma might offer an alternative means to induce the de novo production of tolerant, antigen-specific T cells; however, methods that control the number and or the activation of residual autoreactive cells in the periphery are required to successfully treat autoimmune neuroinflammation.
Introduction
Multiple sclerosis, the most common nontraumatic neurologic disease in young adults, is a chronic inflammatory disease of the central nervous system, associated with the destruction of the myelin sheath and axonal loss.1 Several lines of evidence suggest that the disease is autoimmune in nature, promoted by myelin-specific CD4+ T cells, at least in the initial phases of the disease.2
It is accepted that immunological self-tolerance is managed at several checkpoints. Recessive mechanisms, determined in a cell intrinsic manner, constitute the purging of autoreactive T cells either by deletion or by receptor editing.3 In addition, dominant cell-extrinsic mechanisms, which constitute the generation of regulatory T cells (Tregs), are essential at maintaining immune tolerance in peripheral tissues.4
The selection of self-tolerant T cells is predicated on the binding efficiency of the T-cell receptor (TCR) with major histocompatibility complex (MHC) molecules in association with self-peptides (MHC/pep).5 Thymocytes that do not bind MHC/pep complexes die by neglect whereas interactions with one or two MHC/pep complexes with high affinity are sufficient to induce apoptosis associated with negative selection. In contrast, thymocytes with a high number of low TCR interactions receive a survival signal and are positively selected.6,7 Compared to conventional T cells, selection of thymic Tregs requires avidity interactions that are proposed to be higher than those for positively selected T cells, but lower than cells that are negatively selected.8 Accruing evidence now supports the concept that tissue-specific antigens, representing most, if not all organs in the body,9 including those for myelin10 are expressed on defined thymic stromal cell populations. More importantly, it is the absolute concentration of these self-antigens that influences the efficacy of tolerance mechanisms. For example, perturbations in antigen expression can increase the susceptibility to autoimmune diseases11,12,13 including experimental autoimmune encephalomyelitis (EAE).14,15,16 Conversely, intrathymic (i.t.) administration of organ fragments,17 cells,18 proteins, peptides19 and the administration of antigen-encoding viral vectors20,21 have been shown to establish specific and robust tolerance induction in various disease models.
Current strategies to treat autoimmune diseases including multiple sclerosis often rely on generalized immunosuppression that can reduce clinical symptoms but simultaneously predisposes patients to opportunistic infection. Gene therapy applications have the potential to provide a precise, antigen-specific approach to selectively target autoaggressive T cells during thymocyte ontogeny, install immune tolerance, and treat autoimmunity in the absence of chronic immunosuppression. We demonstrate herein that manipulation of thymic central tolerance pathways by lentiviral-mediated overexpression of myelin oligodendrocyte glycoprotein (MOG), a principal target autoantigen in multiple sclerosis,22 established antigen-specific tolerance and prevented chronic EAE. However, control of neuroinflammation was compromised when mice, previously preconditioned with a clearing anti-CD4 antibody, were treated after disease had been established. This lack of an effect was evident despite the fact that CD4+ T-cell numbers, which are the principal drivers of disease in this model,2 were significantly reduced in peripheral organs. Collectively these findings suggest that the immune system can be resynchronized to instil tolerance mechanisms; however, effective control of tissue inflammation will most likely require adjunct immune suppressive approaches.
Results
Analysis of transduced thymic cells
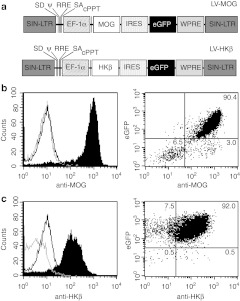
To specifically target tolerance induction against a major autoantigen involved in immune-mediated demyelination, we constructed a self-inactivating bicistronic lentiviral vector (LV) encoding a full-length cDNA for mouse MOG (LV-MOG-IRES-eGFP). For comparison, we constructed a vector encoding the β subunit of the hydrogen-potassium ATPase (HKβ) (LV-HKβ-IRES-eGFP), an autoantigen associated with pernicious anemia.23 Both vectors transcriptionally regulated MOG and HKβ, respectively, using the elongation factor (EF)-1α promoter and incorporated an expression cassette for enhanced green fluorescent protein (eGFP) under the translational control of an internal ribosomal entry site (IRES) sequence (Figure 1a). As a prelude to in vivo experiments, we transduced the mouse thymic cortical epithelial cell line, 427.1, with LV-MOG or LV-HKβ. Flow cytometric analysis of transduced cells revealed that both vectors efficiently transduced 427.1 cells with the majority of cells expressing MOG or HKβ antigens (Figure 1b,c). More importantly, coordinated expression of MOG or HKβ with eGFP in >90% of cells was demonstrated. Given these findings, we used eGFP expression in subsequent experiments as a surrogate marker to estimate the proportion of cells expressing MOG or HKβ peptides in the absence of antigen-specific tetramers.
Figure 1.
Schematic representation of recombinant lentiviral vectors and in vitro expression of engineered transgenes. (a) The proviral, self-inactivating (SIN) forms of LV-MOG or LV-HKβ lentiviral vectors encoding mouse MOG or the β subunit of the hydrogen-potassium cDNA, respectively, under the control of the EF-1α promoter. Both vectors incorporate an eGFP tag under the translational control of an internal ribosomal entry site (IRES) sequence. (b) Expression of MOG or (c) HKβ in transduced 427.1 cells as determined by flow cytometry. Left hand panel shows expression profile of cells transduced with LV-MOG or LV-HKβ and stained with anti-MOG or anti-HKβ antibodies, respectively (black shaded curve). Isotype staining (grey curve) and staining of transduced 427.1 cells expressing an irrelevant antigen (black curve) served as controls. Dot plot analysis of trasduced 427.1 cells co-expressing eGFP and MOG or HKβ (right hand panel). Proportions of MOG:eGFP and HKβ:eGFP subsets are numerically indicated in each quandrant. Data are representative of two independent experiments. EF, elongation factor; eGFP, enhanced green fluorescent protein; LTR, long-term repeat; LV, lentiviral vector; MOG, myelin oligodendrocyte glycoprotein; ψ, packaging signal; RRE, rev response element; SA, splice acceptor site; SD, splice donor site; WPRE, woodchuck hepatitis virus post-transcriptional regulatory element.
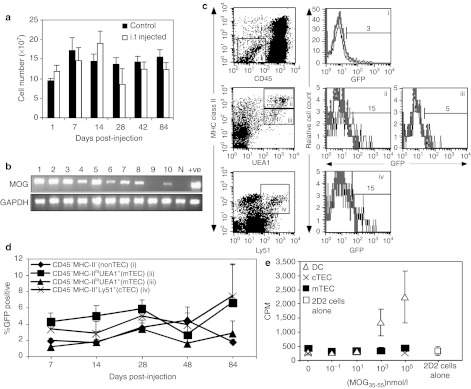
Next, we undertook time course experiments to phenotypically characterize the proportion of marked cells in hematopoietic and stromal compartments. We introduced into both thymic lobes, 1–2 × 107 LV-MOG particles, and subsequently analyzed thymii from individual mice on days 7, 14, 28, 42, and 84 post-injection.
Total thymocyte numbers did not significantly differ between injected and noninjected controls, indicating that neither the i.t. injection procedure, nor the vectors, caused any adverse effects (Figure 2a). In a separate cohort, reverse transcriptase PCR analysis of vector-mediated MOG or HKβ transcripts in i.t.-treated animals revealed that the majority of animals maintained transgene expression for at least 4 months (Figure 2b, Supplementary Figure S1). Notably, mRNA transcripts were predominantly confined to the thymus, although transgene expression was found in peripheral organs in a small proportion of animals (Supplementary Figure S2). Next, phenotypic analyses of cells from i.t.-injected animals were performed using a panel of well-characterized antibodies, which delineate hematopoietic and nonhematopoietic stromal cell subsets. Low proportions of eGFP+ thymic stromal cells encompassing thymic nonepithelial cells (non-TECs) (CD45−MHC-II−), and thymic epithelial cells (TECs) constituting cortical (cTEC) (CD45−MHC-II+Ly51+) and medullary TECs (mTECs) (CD45−MHC-II+UEA-1+) were observed (Figure 2c). Notably, the expression of eGFP in CD45−MHC-IIhiUEA-1+ mTECs was consistently higher in comparison to CD45−MHC-IIloUEA-1+ mTECs, which probably pertains to the higher proliferative potential of the former cell type,24 and to the fact that LVs preferentially transduce cells that have actively transitioned into the G1b phase of the cell cycle.25 Temporal analysis revealed that while transgene expression was low, it persisted in TEC and non-TEC subsets throughout the entire 84-day time course (Figure 2d). Transgene expression in thymic endothelial cells (CD45−CD31+) was undetectable. Moreover, the expression of eGFP in all hematopoietic-derived CD45+ cells, including T cells (CD45+TCRβ+), dendritic cells (DCs) (CD45+CD11c+), and MHC class II+ and class II− subsets from the thymus or from peripheral lymphoid organs was not detected in any of the time points assessed (data not shown). Thus, biased expression of vesicular stomatitis virus-glycoprotein (VSV-G) pseudotyped lentiviral particles was demonstrated in i.t.-injected mice, with cTEC, mTEC, and non-TEC stromal cells being preferentially transduced over thymic-derived hematopoietic and endothelial subpopulations.
Figure 2.
In vivo transgene expression and functional assessment of thymic stromal cells. (a) Graph of the total thymic cellularity in mice following intrathymic injection with LV-MOG. Each bar represents the mean ± SEM of three mice per group per time point. (b) RT-PCR analysis of thymic MOG mRNA transgene expression in individual animals i.t. injected with LV-MOG 4 months prior to analysis. (c) Representative dot plot profiles and gating of defined thymic stromal subsets are shown in the left hand panel. Non-TECs (CD45−/MHC-II−) and TECs subpopulations including mTECs (CD45−MHC-II+UEA-1+) and cTECs (CD45−MHC-II+Ly51+). Both medullary and cortical epithelial dot plots were generated from CD45−-gated populations. Representative histograms of eGFP expression in corresponding stromal subsets from normal C57Bl/6 mice (grey curve) and i.t.-injected mice with LV-MOG (black curve) shown in right hand panel. Percent eGFP-positive cells were analyzed after 84 days post-i.t. injection with LV-MOG. (d) Expression of eGFP in phenotypically defined stromal subsets over time. Data shown are the mean ± SEM from three i.t. LV-MOG-injected mice per time point. (e) Proliferative responses of 2D2 transgenic T-cells cocultured with FACS sorted thymic stromal cells that were previously pulsed with serially diluted pMOG35–55. Limiting numbers of cTECs allowed for only two peptide concentrations to be tested. Each data point is the mean ± SEM of three observations. Data are representative of two independent experiments. CPM, counts per minute; cTEC, cortical thymic epithelial cells; DC, dendritic cell; eGFP; enhanced green fluorescent protein; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; i.t., intrathymic; LV, lentiviral vector; MHC, major histocompatibility complex; MOG, myelin oligodendrocyte glycoprotein; mTEC, medullary TEC; N, naive (noninjected) animal; RT-PCR, reverse transcription PCR; +ve = positive control constituted 427.1 cells transduced with LV-MOG.
Positive and negative selection of thymocytes are predicated on TCR interactions with MHC–peptide complexes, akin to what occurs with the activation of mature T cells in peripheral tissues.26 With this caveat, we investigated the functional capacity of purified TEC subsets (DCs, mTEC, and cTECs) pulsed with MOG peptide encompassing amino acids 35–55 (pMOG35–55) to stimulate the in vitro proliferation of MOG35–55-specific, 2D2 transgenic T cells (Figure 2e). Peptide-pulsed thymic DCs induced a modest proliferative response, while both mTEC and cTEC subsets did not elicit a discernable response.
Intrathymic administration of LV-MOG suppresses the neurological signs of EAE with decreased T-cell proliferative responses to pMOG35–55
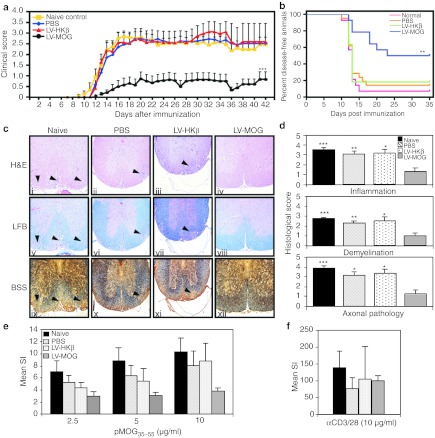
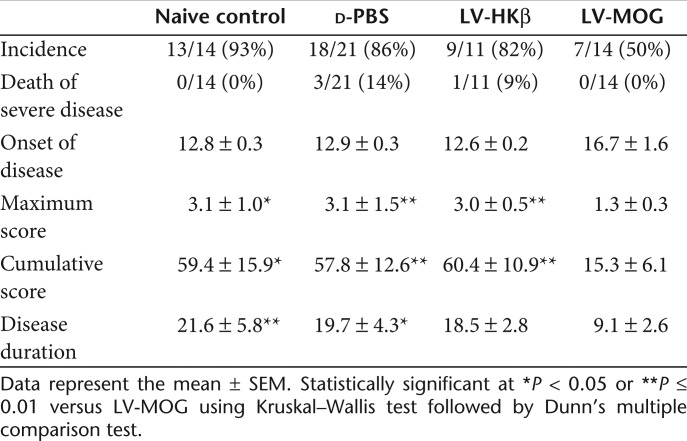
Having shown that i.t. administration of a LV co-expressing MOG and eGFP targeted cortical and medullary epithelium, two important cell types involved in tolerance induction,27 we next assessed whether this administration protocol could protect animals from EAE induced by immunization with pMOG35–55. C57Bl/6 mice were i.t. injected with LV-MOG or LV-HKβ at a final dose of 1–2 × 107 functional particles per thymic lobe. A third group was injected with phosphate-buffered saline (PBS) as a vehicle control, while a fourth, naive group of mice, did not undergo any treatment. Following a 12-week period to allow for transgene stabilization, all animal groups were immunized with pMOG35–55 to induce EAE and monitored for 6 weeks. In comparison to controls (naive, PBS, and LV-HKβ), mice that received LV-MOG demonstrated significant reductions in the incidence of EAE, a finding that is comparable to protein-based intrathymic approaches. 19 The mean neurological, maximal clinical, and cumulative disease scores as well as the disease duration were also decreased (Figure 3a, Table 1). Furthermore, while LV-MOG administration did not alter the onset of the disease, the disease-free status of injected mice was significantly enhanced (Figure 3b).
Figure 3.
Intrathymic (i.t.) injection of LV-MOG prevents EAE and induces a state of antigen-specific tolerance in C57Bl/6 mice. (a) Clinical score of mice i.t. injected with LV-MOG (n = 14), LV-HKβ (n = 11), or PBS (n = 21) 12 weeks post-EAE induction. Naive untouched animals (n = 14) served as a further control. Data (mean ± SEM) are pooled from two independent experiments. Six weeks after immunization, organs were removed and processed for histology or cultured with pMOG35–55 or anti-CD3 and anti-CD28. (b) Kaplan–Meier plot of the proportion of disease-free animals after EAE induction in LV-MOG versus LV-HKβ, PBS, and normal animal cohorts. P = 0.003, logrank test with Bonferroni correction. (c) Representative stained histological sections of spinal cords from immunized animals from untreated naive controls or animals i.t. treated with LV-MOG, LV-HKβ, or PBS. Serial sections were stained with hematoxylin and eosin (H&E) to determine the degree of inflammatory cell infiltrates, luxol fast blue (LFB) to establish myelin integrity and Bielschowski silver stain (BSS) to ascertain for axonal loss and damage. Arrows indicate areas of inflammatory cell accumulation, which corresponded to areas of demyelination and axon damage. Original magnification ×100. (d) Histological scores for inflammation, demyelination, and axonal damage. (e) Splenocytes isolated from mice i.t. injected with LV-MOG, LV-HKβ, PBS or naive mice where stimulated with varying doses of pMOG35–55 and their proliferative responses measured. (f) As a comparison, proliferative responses of splenocytes from all animal groups stimulated with the polyclonal T-cell activators anti-CD3 and anti-CD28 are shown. Data represents the mean ± SEM. *P < 0.05, **P ≤ 0.01 or ***P ≤ 0.001. EAE, experimental autoimmune encephalomyelitis; LV, lentiviral vector; MOG, myelin oligodendrocyte glycoprotein; PBS, phosphate-buffered saline; SI, stimulation index.
Table 1. Clinical features of EAE in animals administered intrathymically with recombinant lentiviral vectors.
Histopathological evaluation of stained spinal cord sections revealed that LV-MOG-treated animals had fewer inflammatory cell infiltrates with an associated preservation of the myelin architecture and significantly less axonal damage. In contrast, LV-HKβ, PBS, and naive animals manifested typical EAE histopathological hallmarks encompassing extensive mononuclear cell lesions primarily around the meninges and blood vessels with concomitant myelin loss and axonal injury (Figure 3c,d).
We then determined whether LV-MOG administration into the thymus had any effects on the immune response using an in vitro recall proliferation assay. Spleen cells from naive, PBS, and LV-HKβ mice all responded vigorously to pMOG35–55 stimulation in a dose-dependent manner. In contrast, mean stimulation indices from LV-MOG-derived splenocytes were reduced in the order of 63–33% in comparison to naive, PBS, and LV-HKβ mice (Figure 3e). Notably, splenocytes from all animal groups proliferated robustly in response to anti-CD3/28 stimulation, indicating that LV-MOG-treated splenocytes were specifically tolerized against pMOG35–55 (Figure 3f). Interestingly, no correlation between the severity of clinical disease and the production of anti-MOG antibodies was observed in mice tolerized with LV-MOG, as high serum anti-MOG35–55 antibody titres were determined in all animal cohorts (Supplementary Figure S3). Importantly, low antibody titers were determined in overtly diseased EAE control animals, indicating that the antibody response to pMOG35–55 may not play a major role in mediating EAE in the current model setting.
Ectopic overexpression of MOG induces the deletion of autoreactive thymocytes while promoting the generation of Tregs
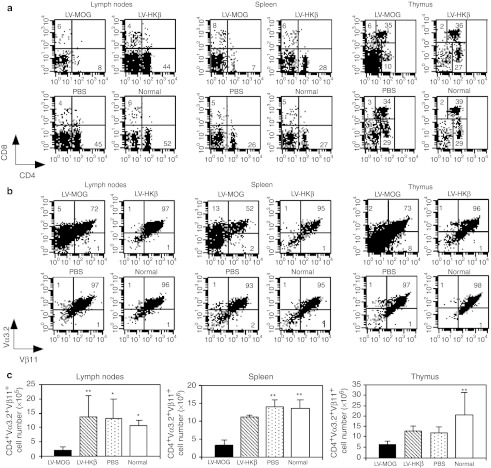
To further clarify the mechanism(s) by which tolerance was induced in LV-MOG-injected mice, we utilized the 2D2 TCR transgenic mouse model. The majority (>95%) of CD4+ T cells in this mouse line express the Vα3.2 and Vβ11 TCR combination, which recognize pMOG35–55 in the context of H-2 IAb.28 As such, this animal model has the advantage of allowing the tracking and quantification of autoreactive T cells throughout development or during immune responses utilizing MOG-specific tetramers or anti-Vα3.2- and anti-Vβ11-specific antibodies. Animals were i.t. injected with LV-MOG, LV-HKβ, PBS or left untouched and allowed to recover for 12 weeks. Flow cytometric analysis revealed that while there was no change in the percentage of CD4+CD8+ double positive subset in all animal cohorts, a 1.5- to twofold reduction in the proportion of CD4+CD8− thymocytes was observed in LV-MOG-treated animals versus all other animal groups. In addition, a three- to fourfold decrease in the percentage of mature CD4+ single positive cells in the spleen and lymph nodes, respectively, was also determined (Figure 4a). Analysis of clonotypic CD4+Vα3.2+Vβ11+ cells revealed a significant reduction in the proportion and cell number of this population in the thymus, spleen, and lymph nodes in LV-MOG-treated animals in comparison to control animal cohorts (Figure 4b,c). Collectively these observations suggest that extensive negative selection of autoreactive MOG-specific thymocytes had occurred following i.t. injection of LV-MOG.
Figure 4.
Ectopic expression of MOG leads to the deletion of MOG35–55 antigen-specific autoreactive T cells. Leucocytes from spleen, lymph nodes, and thymus, isolated from normal 2D2 mice or 2D2 mice previously injected i.t. with LV-MOG, LV-HKβ, or PBS were stained with antibodies specific for (a) CD4 and CD8 or (b) Vα3.2 and Vβ11. Expression of Vα3.2 and Vβ11 was analyzed through a CD4+ gate. Markers were set according to isotype controls. Numbers represent the frequencies of stained populations within defined quadrants. (c) Absolute numbers of clonotypic CD4+Vα3.2+Vβ11+ cells from spleen, lymph nodes and thymus. Data represents the mean ± SEM. *P < 0.05 or **P ≤ 0.01. LV, lentiviral vector; MOG, myelin oligodendrocyte glycoprotein; PBS, phosphate-buffered saline.
Characterization and therapeutic evaluation of regulatory T cells in LV-MOG-injected mice
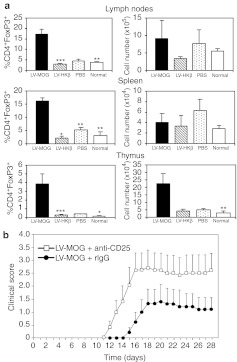
Alongside the deletion of self-reactive T cells, central immune tolerance is supplemented by the positive selection of Tregs. Accumulating evidence suggests that agonist self-ligands presented in the thymus are essential for the generation of Tregs,29,30 which characteristically express the forkhead/winged helix transcription factor FoxP3.4 To determine whether i.t. transfer of LV-MOG enhanced the production of Tregs, we analyzed the proportions and cell numbers of antigen-specific CD4+FoxP3+ Tregs in the spleen, lymph nodes, and thymus in injected 2D2 animals. As controls, normal untouched 2D2 mice or animals i.t. injected with PBS or LV-HKβ were also analyzed. The percentage of CD4+FoxP3+ Tregs in the thymus of LV-MOG-treated mice increased ~ten- to 28-fold with a concomitant four- to eightfold increase in cell number over control animal groups (Figure 5a). While the frequency of CD4+FoxP3+ Tregs in LV-MOG-treated animals had significantly increased in lymph nodes and spleen in comparison to controls, total CD4+FoxP3+ Treg numbers did not differ, because of the massive depletion of CD4+Vα3.2+Vβ11+ conventional T cells (Figure 4). These results suggest that CD4+FoxP3+ Treg numbers expanded in the thymus while being preserved in peripheral lymphoid organs.
Figure 5.
MOG35–55-specific Tregs significantly impact the disease course in intrathymically injected LV-MOG mice. (a) Proportions and cells numbers of CD4+FoxP3+ Tregs from spleen, lymph nodes, and thymus in normal 2D2 mice or 2D2 mice previously injected i.t. with LV-MOG, LV-HKβ, or PBS. All data (means ± SEM) showing proportions and absolute numbers are representative of seven to nine animals per group that were pooled from two independent experiments. *P < 0.05 or **P ≤ 0.01. (b) Treatment of recipients with anti-CD25 Ab abrogates the protective effects of LV-MOG. C57BL/6 mice were i.t. injected with LV-MOG. Twelve weeks later, mice were injected with 750 µg anti-CD25 (n = 5) or isotype control (n = 5) and subsequently immunized with pMOG35–55. Mice were monitored daily for clinical signs of EAE. Statistical differences between anti-CD25 and isotype-treated animals were significant (P = 0.03) between days 12 and 16. EAE, experimental autoimmune encephalomyelitis; LV, lentiviral vector; MOG, myelin oligodendrocyte glycoprotein; PBS, phosphate-buffered saline; Tregs, regulatory T cells.
A number of compelling reports have shown that Tregs impact the course of autoimmunity including EAE.31 To address the question of whether LV-MOG-mediated generation of Tregs contributed to tolerance mechanisms, C57BL/6 mice were i.t. injected with LV-MOG with one cohort receiving an anti-CD25 depleting antibody (CD4+FoxP3+ Tregs express high levels of CD25),4 while the other received an IgG isotype control. Three days after antibody treatment, animals were immunized with pMOG35–55 and monitored daily for clinical signs of disease. Animals that received the anti-CD25 depleting antibody presented with clinical signs earlier and displayed a statistical increase in clinical and cumulative scores between days 12 and 19 in comparison to isotype-treated animals, in accordance to previously published results32 (Figure 5b). These results suggest that deletion of CD25+ Tregs abrogated the suppressive responses induced by ectopic expression of MOG. It further consolidates the concept that the protective effect manifest in mice administered i.t. with LV-MOG was, in part, mediated by the generation of Tregs.
Reduction in proinflammatory cytokine responses following i.t. administration of LV-MOG
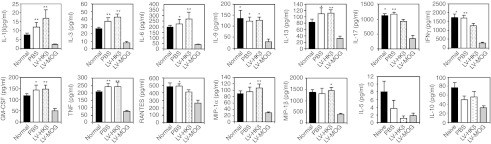
We next assessed whether any cytokine perturbations correlated with tolerance induction in LV-MOG-treated animals. 2D2 mice were i.t. injected with LV-MOG, LV-HKβ, or PBS. Twelve weeks after i.t. injection, all animal cohorts, including a naive untreated control group, were immunized with pMOG35–55 to induce EAE and humanely killed after 23 days. Splenocyte conditioned medium from pMOG35–55-stimulated spleen cell cultures from LV-MOG, LV-HKβ, PBS, and naive animals were assessed in cytokine bead array assays or by ELISA (Figure 6). Of the 21 cytokines that were analyzed, 12, Th1/Th17 cytokines, were significantly reduced in LV-MOG splenocyte cultures in comparison to controls. These include IL-1β, IL-3, IL-6, IL-9, IL-13, IL-17, GM-CSF, IFNγ, TNF and the chemokines RANTES, macrophage inflammatory protein (MIP)-1α, and MIP-1β. No changes in Th2-type cytokines such as IL-5 and IL-10 were found.
Figure 6.
Reduction in proinflammatory cytokines in LV-MOG-treated mice. Normal 2D2 or i.t.-injected 2D2 mice were immunized with pMOG35–55 12 weeks after i.t. administration of LV-MOG, LV-HKβ, or PBS. Spleens were removed 23 days after immunization and spleens stimulated in vitro with pMOG35–55 for 3 days with supernatant collected and cytokine content analyzed either by cytokine bead array or ELISA. Data were generated from 9 to 13 animals per group that were pooled from two independent experiments and are expressed as the mean ± SEM. Statistical differences (*P <0.05 or **P ≤ 0.01) between LV-MOG and normal, PBS, or LV-HKβ animals were calculated using a Kruskal–Wallis test followed by Dunn's multiple comparison test. IFN, interferon; IL, interleukin; LV, lentiviral vector; MIP, macrophage inflammatory protein; MOG, myelin oligodendrocyte glycoprotein; PBS, phosphate-buffered saline; TNF, tumor necrosis factor.
Intrathymic injection of LV-MOG does not attenuate EAE progression
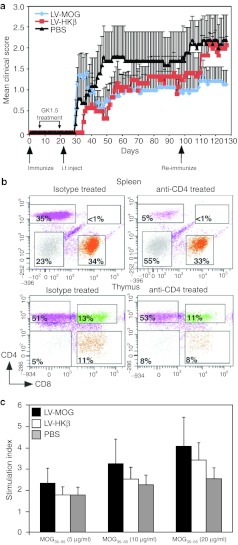
Having established that intrathymic administration of LV-MOG prevented the development of EAE we wanted to assess whether this protocol could ameliorate disease symptoms in mice with pre-established disease. To test this, animals were immunized with pMOG35–55 and 6 days later, were preconditioned with an anti-CD4 clearing antibody to deplete peripheral CD4+ T cells with the aim of transiently suppressing encephalitogenic immune responses. Following anti-CD4 treatment, animals were intrathymically injected with LV-MOG, LV-HKβ, or PBS and monitored for 10 weeks. Following this period, mice that had a clinical score of less than two were rechallenged with pMOG35–55 and further assessed. In contrast to the preventative model, intrathymic administration of LV-MOG during the priming phase of the disease did not alter the mean clinical score in comparison to mice i.t. injected with LV-HKβ or PBS (Figure 7a). Moreover, there were no significant changes in the mean neurological, maximal clinical, and cumulative disease scores (data not shown). One possible reason for a lack of efficacy in this setting may have been because of the impaired clearance of CD4+ T cells following antibody treatment. Alternatively, anti-CD4+ antibody may have breached the thymus–blood barrier thus perturbing thymic lymphopoiesis. To address these issues, a separate cohort of mice were injected with anti-CD4 or rat IgG isotype control with splenocytes and thymocytes analyzed by fluorescent activated cell sorting (FACS) 7 days after the cessation of antibody treatment—a time point (~day 27) which corresponds to the onset of disease in LV-MOG-treated animals (Figure 7a). Predicably, greater than 85% of the starting CD3+CD4+ T-cell population in the spleen was specifically depleted in anti-CD4-injected animals indicating that CD4+ populations were efficiently cleared. Moreover, there were no changes in the percentages of mature CD3:CD4 or CD3:CD8 thymic subsets suggesting that thymopoiesis was not affected (Figure 7b). Although intrathymic treatment with LV-MOG did not promote disease remission in the current model, we asked whether LV-MOG treatment could still promote the production of tolerant, antigen-specific T cells. To this end, spleens from treated mice where removed at the completion of the study on day 127 with splenocytes stimulated with graded concentrations of pMOG35–55 and their in vitro proliferative responses assessed. There was no significant difference in the proliferative response to pMOG35–55 or anti-CD3/CD28 stimulation between all animal cohorts (Figure 7c and data not shown) suggesting that the selection of a tolerant T-cell repertoire in LV-MOG-treated mice after disease onset, while probable, was overshadowed by the expansion of autoreactive T effectors in the periphery.
Figure 7.
Intrathymic (i.t.) delivery of MOG during disease onset does not ameliorate EAE progression. (a) Normal C57Bl/6 mice were immunized with pMOG35–55 and 6 days later received 1 mg anti-CD4 antibody (clone GK1.5) in the peritoneum as described in materials and methods. Mice were subsequently randomized and i.t. injected with LV-MOG (n = 7), LV-HKβ (n = 6), or PBS (n = 5), 1 day after the cessation of antibody treatment. Mice were monitored daily for clinical signs of EAE for 70 days, subsequently re-immunized with pMOG35–55 and monitored for a further 28 days. Data denotes the mean ± SEM and is representative of two independent experiments. (b) Efficacy of CD4+ T-cells clearance was performed in mice injected with 1 mg of anti-CD4 antibody or rIgG isotype control as described in materials and methods. FACS analysis of thymocytes and splenocytes stained with CD3, CD4, and CD8 was performed 7 days after the cessation of antibody treatment. Shown are the proportions of mature CD4:CD8 subsets gated on the CD3+ cells. Data are representative of five mice per antibody treatment. (c) Splenocytes isolated from mice i.t. injected with LV-MOG, LV-HKβ, or PBS where stimulated with varying doses of pMOG35–55 and their proliferative responses measured. Data represent the mean ± SEM. EAE, experimental autoimmune encephalomyelitis; LV, lentiviral vector; MOG, myelin oligodendrocyte glycoprotein; PBS, phosphate-buffered saline; pMOG35–55, MOG peptide encompassing amino acids 35–55.
Discussion
In this study, we postulated that overexpression of MOG in the thymus could be used as a therapeutic tool to modulate central tolerance mechanisms and ameliorate damage to neural tissue in EAE. We show that (i) in vivo lentiviral-mediated transduction of MOG in thymic stromal cells promoted deletion of autoantigen-specific T cells as well as the generation of Tregs, (ii) these mechanisms ameliorated EAE symptoms before disease has been established, however, (iii) induction of central tolerance mechanisms after disease onset was not curative as it failed to control tissue inflammation and neurodegeneration.
In extension to previous findings,21 we have demonstrated that in situ delivery of LVs could transduce cTEC, mTEC, and non-TEC stroma. Both mTECs and cTECs play defined roles in central tolerance mechanisms.33,34 Importantly, LV-MOG transgene expression in these stromal subsets coincided with tolerance induction. Intriguingly, DCs, which are known to be major purveyors of thymocyte negative selection,30 were not targeted by LV-mediated transduction. Yet, results generated from in vitro coculture experiments clearly showed that this cell type had a significantly higher propensity to stimulate transgenic T-cell proliferation over mTECs or cTECs. Collectively, these data support the model where mTECs act as an antigen reservoir and influence tolerance induction by either cross-presenting tolerogenic peptides (in this scenario, MOG) or MHC-II-loaded complexes to DCs, thus facilitating the elimination of autoreactive T cells.35,36,37 The lack of detectable T-cell stimulation by TECs in vitro, however, does not unequivocally indicate a lack of direct presentation by TECs in vivo and furthermore may be confounded by the relatively poor proliferative response observed in MHC-II-restricted coculture systems (D. Gray, personal communications, Walter and Eliza Hall Institute, 17 July 2010) compared with MHC-I-restricted transgenic models.26
Having confirmed that the protective effect imparted by intrathymic over expression of MOG was, in part, mediated by the deletion of MOG-specific autoreactive T cells, we also showed that intrathymic administration of LV-MOG led to the expansion of CD4+FoxP3+ Tregs in the thymus while Treg number was preserved in secondary immune organs. Although the relative contributions of clonal deletion versus Treg production in maintaining a state of tolerance was not directly ascertained, we did demonstrate that antibody-mediated abolition of Tregs eliminated the protective effects of LV-MOG treatment, thus validating a primary role of Tregs in the maintenance of tolerance mechanisms and the suppression of fulminant signs of EAE. Current theories on the underlying mechanisms on the generation of Tregs by intrathymic overexpression of MOG are not clearly defined. However, our studies, in agreement with other published reports,38 indicate that the generation of Tregs were highly sensitive to the relative amounts of MOG expressed. This was exemplified by the observations that expansion of the proportions and numbers of Tregs were confined to sites, in this case, the thymus, where virus-generated mRNA MOG transcripts were predominantly expressed. Based on these observations, and data from several published reports,29,30,38 it is hypothesized that antigen plays an instructive role in the positive selection of antigen-specific Tregs upon encountering MOG on cortical epithelial cells. An alternative hypothesis suggests that Tregs are not positively selected, rather, they are enriched during selection processes owing to immature thymocytes being preferentially predisposed to differentiate along the Treg cell lineage. Conversely, developing Tregs might be more recalicitrant to negative selection because of an inherent resistance to cell death-mediated signals than conventional T cells.39 It is possible that a combination of mechanisms could contribute to the observed increase in numbers of antigen-specific Tregs in the current model setting.
While we could not evaluate the absolute concentration of expressed MOG in recipient thymii, we demonstrated that <5% of eGFP expression in thymic stromal subsets was sufficient to induce tolerance and limit autoimmunity. The relatively low levels of thymic cellular chimerism is consistent with the notion that transduction of a high proportion of target cells may not be required to promote antigen-specific tolerance, as attested in several transplant and autoimmune disease models.40,41,42 The chronicity of transgene expression in the thymus is also an important factor in maintaining tolerance mechanisms.42 In tracking experiments, we demonstrated that transgene expression in medullary and cortical epithelium persisted for at least 3 months. Given that the turnover of TECs in young adolescent mice occurs every 10–14 days,26 preservation of tolerance mechanisms described herein, may have been regulated by putative epithelial stem/progenitor cell, akin to what has previously been described in the embryonic thymus43 and other epithelized organs.44
We have shown that ectopic transfer of MOG into the thymus ameliorated the signs of disease before disease onset; however, control of disease symptoms was negated when mice were treated 6 days after immunization—a time which corresponds to the priming phase of the disease. We also report that this effect ensued despite the depletion of ~85% peripheral CD4+ T cells prior to the onset of disease symptoms. Collectively, these results highlight the fact that activation of a limited number of highly pathogenic T cells can overcome peripheral regulatory mechanisms, establish autoimmunity, and propagate neurodegeneration. The mechanisms as to why intrathymic overexpression of MOG was effective in a prophylactic rather than a therapeutic setting remain to be elucidated. One plausible hypothesis is that resistance might be linked with the hyperactivity of certain signaling cascades in residual effector T cells, as recently demonstrated for protein kinase B/c-akt45 or p38 MAP kinase,46 which have been shown to modulate Treg-mediated suppression. As a further consequence of this activation process, establishment of a proinflammatory milleu in the central nervous system, as exemplified by the secretion of tumor necrosis factor (TNF)α and IL-647 and a plethora of other events, further exacerbates the disease process.
Successful treatment of multiple sclerosis will require several approaches including current therapies that globally suppress immune function, as well as therapies that regenerate damaged and lost neural tissue and induction of tolerance mechanisms. Our results provide proof-of-principle that a direct and relatively simple method of genetically modifying TECs can lead to the installation of antigen-specific tolerance and further adds to the armamentarium of approaches that specifically target autoreactive T cells. While various safety concerns of using integrating vectors need to be addressed, it is envisioned that this approach, together with other strategies that purge or suppress residual autoreactive T cells, could be adopted as a potentially synergistic approach to protect against the development of untoward autoimmune responses.
Materials and Methods
Induction and assessment of EAE. C57Bl/6 mice were purchased from Monash University Animal Services. MOG-specific TCR (2D2) transgenic mice where bred at the Monash University Animal Services facility. EAE was induced in female mice, aged 8–12 weeks, as previously described.48 Neurological signs were determined using an arbitrary clinical score as previously described.48 All breeding and animal experiments were performed in accordance with the Australian code of practice for the care and use of animals for scientific purposes (2004, 7th edition), after approval by the Monash Medical Centre and Monash University School of Biomedical Sciences animal ethics committees.
LV construction, production, and intrathymic administration of viral vectors. The second generation, self-inactivating bicistronic lentiviral transfer vector, pWPI, was used to engineer transgene expression under the control of an elongation factor 1α promoter. Using standard molecular biology techniques, the open reading frames for mouse MOG and HKβ were subcloned upstream of an IRES-eGFP cassette by blunt-end ligation to generate the vectors pWPI-MOG-IRES-eGFP and pWPI-HKβ-IRES-eGFP, respectively. Viral stocks were generated by triple transfection of the recombinant vectors together with accessory plasmids, pSPAX2 and pMD2.G using Fugene6 (Roche) into 293T cells. Supernatants were collected, passed through a 0.22-µm filter, concentrated by ultracentrifugation and titres calculated after flow cytometric determination of eGFP in transduced HeLa cells. Typically, 0.5–1 × 109 transducing units/ml concentrated vector was generated in this manner. We injected 10 µl of concentrated vector in each thymic lobe in anaesthetized mice after a median sternotomy.
In vivo clearance of CD4+ and CD25+ cells. CD4+ T cells were depleted from EAE mice as a preconditioning regimen using a modified protocol originally described by Walder et al.49 In brief, 6 days after immunization, mice were injected intraperitoneally with 100 µg purified anti-CD4 antibody (clone GK1.5) or rat IgG isotype (Sigma-Aldrich, St. Louis, MO) every day for 6 consecutive days and subsequently every second day for a further four treatments. To examine the functional contribution of CD25+ cells, mice, previously i.t. injected with LV-MOG, were injected intraperitoneally with 750 µg of purified anti-CD25 Ab (clone PC61), or rat IgG 3 days prior to immunization.
Histological assessment of inflammation, demyelination, and axonal damage. Brains and spinal cords were removed, prior to immersion in 4% paraformaldehyde, 0.1 mol/l phosphate buffer solution. Segments of brain, cerebellum, and spinal cord were embedded in paraffin. Sections (5 µm) were prepared and stained with hematoxylin-eosin (H&E), luxol fast blue, and Bielschowsky silver stain for evidence of inflammation, demyelination, and axonal damage, respectively. All histological stained sections were semiquantitatively scored in a blinded manner as previously described.48 Microscopy was performed using an Olympus BX41 microscope interfaced with an Olympus D12 digital camera (Olympus, Tokyo, Japan) and processed for luminosity and contrast in Adobe Photoshop CS and subsequently imported to Canvas for minimizing and figure collation.
Flow cytometry. A complete list of all antibodies used in this study is shown in Supplementary Table S1. Staining of cell surface antigens was executed in the following manner. Mononuclear cells (1–5 × 106) from thymus, spleen lymph nodes, or central nervous system were isolated as previously described50 and stained with primary antibodies for 20 minutes on ice. Transduced 427.1 cells expressing MOG and eGFP were stained with an anti-MOG monoclonal antibody, washed in FACS buffer, then stained with a goat anti-mouse Alexa 647 secondary conjugate for 20 minutes on ice. Staining of thymic stromal subsets was performed as previously described.26 Cells were washed and finally resuspended in 200 µl FACS buffer. Sample data were acquired using a FACSCalibur or FACS Canto flow cytometer (BD Bioscience, San Jose, CA) and analyzed using CellQuest Pro or FACSDiva software (both from BD Bioscience). For analysis of eGFP and HKβ in transduced 427.1 cells, 1 × 106 cells were fixed and permeabilized using fixation/permeabilization solution (BD Bioscience), washed in permeabilization buffer (BD Bioscience) and stained using a two-step procedure starting with 25 µl of anti-HKβ for 20 minutes on ice, followed by a wash in permeabilization buffer and finally staining with goat anti-mouse Alexa 647. Cells were washed in permeabilization buffer and resuspended in 200 µl FACS buffer. Electronic events were acquired and analyzed as above. For analysis of FoxP3, cells were stained according to the manufacturer's protocol (eBioscience, San Diego, CA).
Preparation, isolation, and functional analysis of thymic stromal cells. Digestion of thymii was performed as previously described.26 DCs (CD11c+, MHC-II+), mTECs (CD45−, Ly51−, EpCAM+), and cTECs (CD45−, Ly51+, EpCAM+) were sterile sorted using an Influx 2 cell sorter (BD Bioscience) to greater than 90%. Purified thymic stromal cell subpopulations were incubated with pMOG35–55 at 37 °C for 1 hour and then thoroughly washed. Stromal cells (10,000) were then plated in 96-well, round-bottom plates and cocultured with 50,000, spleen-derived T cells from 2D2 mice using a pan-T-cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany). As controls, T cells were cultured alone. Cocultures were maintained in a humidified incubator at 37 °C 5% CO2 for 72 hours with proliferation of T cells assessed as described below.
T-cell proliferation and cytokine production. Both procedures were performed as previously described.50 Proliferation data are presented as a stimulation index which was calculated according the formula: mean proliferation counts of cells cultured with pMOG35–55 or anti-CD3ε and anti-CD28 divided by the mean proliferation counts of cells cultured in medium alone.
Anti-MOG antibody detection. Anti-MOG antibody detection in serum samples were determined by ELISA. Sera were collected at the experimental end point and diluted 1:50, 1:200, and 1:2000 in PBS. Ninety-six well microtitre plates (Nunc-Immunoplate, Roskilde, Denmark) were coated with 100 µl of pMOG35–55 (5 µg/ml in carbonate buffer, pH 9.6) for 12 hours at 4 °C. Control wells were also coated with a scrambled pMOG35–55 sequence. Wells were washed twice with PBS and were subsequently blocked with Protein-Free block medium (Thermo-Fischer, Rockford, IL) supplemented with 5% goat serum (Sigma-Aldrich) for 2 hours at room temperature. Following incubation, wells were washed three times with PBS containing 0.05% Tween 20 (PBS/Tween20) and incubated with 100 µl diluted mouse sera for 1 hour at room temperature. As a positive control, wells were incubated with the anti-MOG antibody at 5 µg/ml. After washing four times with PBS/Tween20, wells were incubated for 1 hour at room temperature with the following horse radish peroxidase-conjugated goat anti-mouse antibodies (diluted 1:2000); IgGAM (Sigma-Aldrich), IgG, IgG1, IgG2a, IgG2b, IgG3, IgM, and IgA (all from Caltag, Burlingame, CA). Following incubation, wells were washed five times with PBS/Tween20 and reaction products visualized using the chromogen 1,2-Diaminobenzene, 1,2-Phenylenediamine (Sigma-Aldrich), and absorbances read at 492 nm using a microplate reader.
Cytokine detection and analysis. Quantitation of mouse cytokines and chemokines including, interferon (IFN)γ, interleukin (IL)-1α, IL-1β IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12p70, IL-13, G-CSF, GM-CSF, KC, MCP-1, MIG, MIP-1α, MIP-1β, RANTES, and TNF were simultaneously determined using a multiplexed bead assay (Cytometric Bead Array Flex sets (CBA)) according to the manufacturer's recommended protocol (BD Bioscience). Acquisition of events was performed using a FACS Canto II flow cytometer and Diva software and data analyzed and fitted to a 4-parameter logistic equation using the FCAP array software (Soft Flow, Pecs, Hungary). Mouse IL-17 was quantified by ELISA (eBioscience).
Reverse transcription PCR. Tissue was removed and stored in RNAlater (Qiagen, Hilden, Germany). Total RNA was extracted and purified using an RNeasy mini kit (Qiagen). RNA was reversed transcribed using Superscript III first-strand synthesis system (Invitrogen, Carlsbad, CA) and analyzed by semiquantitative PCR. To distinguish between endogenous and lentiviral-mediated transgene mRNA expression of MOG and HKβ, the following primer pairs were used: MOG-F 5′-GGCAGGACAGTTTCTTGAAG-3′ and eGFP-R 5′-CTGAACTTGTGGCCGTTTAC-3′ HKβ-F 5′-ACCACGTGACCTTC AACAAC-3′ and eGFP-R 5′-CTGAACTTGTGGCCGTTTAC-3′. For housekeeping control expression, mouse GAPDH was analyzed using the following primer pair; GAPDH-F 5′-CATGACAACTTTGGCATTGTGG-3′ and GAPDH-R 5′-CAGATCCACAACGGATACATTGGG-3′. PCR conditions for the amplification of viral-encoded MOG was 94 °C for 2 minutes, 32 cycles of 94 °C for 30 seconds, 55 °C for 30 seconds, 72 °C for 1 minute, and a final elongation step for 7 minutes. PCR conditions for amplification of viral-encoded HKβ and endogenous GAPDH was 94 °C for 2 minutes, 32 cycles of 94 °C for 30 seconds, 53 °C for 30 seconds, 72 °C for 1 minute, and a final elongation step for 7 minutes.
Statistics. Data are presented as the mean ± the standard error of the mean (SEM). All statistical analyses were performed using Instat 3.0b or Prism 5.0a (Graphpad software, San Diego, CA). The significances of differences between two groups were determined using an unpaired Student's t-test. Statistical analysis of three or more groups was performed using Kruskal–Wallis with Dunn's post-hoc test. P values of less than 0.05 were considered to be significant. Comparison of the proportion of animals remaining disease free over time between all treatment groups was assessed using the Kaplan–Meier method and compared with the log-rank test. Multiple comparisons of curves were performed incorporating a Bonferroni correction with P values below 0.016 considered significant.
SUPPLEMENTARY MATERIAL Figure S1. RT-PCR analysis of thymic HKβ mRNA transgene expression. Figure S2. RT-PCR analyses of MOG and HKβ mRNA transgene expression in CNS, lymph node, and spleen tissues. Figure S3. Antibody responses in i.t.-injected animals. Table S1. Antibodies used in study.
Acknowledgments
The authors thank Didier Trono for the lentiviral transfer vector and accessory plasmids, Shunhe Wang for help with histology, and Jade Homann for her assistance with the i.t. procedures. This work is supported by grants from the Baker Foundation, the National Health and Medical Research Council of Australia, Cure MS Inc. Ltd, Diane Asmar funds, Bethlehem Griffiths Research Foundation, Multiple Sclerosis Society of New York and Multiple Sclerosis Research Australia. R.L.B. is Chief Scientific Officer of Norwood Immunology. The authors declared no conflict of interest.
Supplementary Material
RT-PCR analysis of thymic HKβ mRNA transgene expression.
RT-PCR analyses of MOG and HKβ mRNA transgene expression in CNS, lymph node, and spleen tissues.
Antibody responses in i.t.-injected animals.
Antibodies used in study.
REFERENCES
- Hauser SL., and, Oksenberg JR. The neurobiology of multiple sclerosis: genes, inflammation, and neurodegeneration. Neuron. 2006;52:61–76. doi: 10.1016/j.neuron.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Zamvil SS., and, Steinman L. Diverse targets for intervention during inflammatory and neurodegenerative phases of multiple sclerosis. Neuron. 2003;38:685–688. doi: 10.1016/s0896-6273(03)00326-x. [DOI] [PubMed] [Google Scholar]
- von Boehmer H., and, Melchers F. Checkpoints in lymphocyte development and autoimmune disease. Nat Immunol. 2010;11:14–20. doi: 10.1038/ni.1794. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Yamaguchi T, Nomura T., and, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Goldrath AW., and, Bevan MJ. Selecting and maintaining a diverse T-cell repertoire. Nature. 1999;402:255–262. doi: 10.1038/46218. [DOI] [PubMed] [Google Scholar]
- Klein L, Hinterberger M, Wirnsberger G., and, Kyewski B. Antigen presentation in the thymus for positive selection and central tolerance induction. Nat Rev Immunol. 2009;9:833–844. doi: 10.1038/nri2669. [DOI] [PubMed] [Google Scholar]
- Palmer E., and, Naeher D. Affinity threshold for thymic selection through a T-cell receptor-co-receptor zipper. Nat Rev Immunol. 2009;9:207–213. doi: 10.1038/nri2469. [DOI] [PubMed] [Google Scholar]
- Schwartz RH. Natural regulatory T cells and self-tolerance. Nat Immunol. 2005;6:327–330. doi: 10.1038/ni1184. [DOI] [PubMed] [Google Scholar]
- Derbinski J, Schulte A, Kyewski B., and, Klein L. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat Immunol. 2001;2:1032–1039. doi: 10.1038/ni723. [DOI] [PubMed] [Google Scholar]
- Kyewski B., and, Derbinski J. Self-representation in the thymus: an extended view. Nat Rev Immunol. 2004;4:688–698. doi: 10.1038/nri1436. [DOI] [PubMed] [Google Scholar]
- Egwuagu CE, Charukamnoetkanok P., and, Gery I. Thymic expression of autoantigens correlates with resistance to autoimmune disease. J Immunol. 1997;159:3109–3112. [PubMed] [Google Scholar]
- Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ.et al. (2002Projection of an immunological self shadow within the thymus by the aire protein Science 2981395–1401. [DOI] [PubMed] [Google Scholar]
- Liston A, Gray DH, Lesage S, Fletcher AL, Wilson J, Webster KE.et al. (2004Gene dosage–limiting role of Aire in thymic expression, clonal deletion, and organ-specific autoimmunity J Exp Med 2001015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AC, Nicholson LB, Legge KL, Turchin V, Zaghouani H., and, Kuchroo VK. High frequency of autoreactive myelin proteolipid protein-specific T cells in the periphery of naive mice: mechanisms of selection of the self-reactive repertoire. J Exp Med. 2000;191:761–770. doi: 10.1084/jem.191.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein L, Klugmann M, Nave KA, Tuohy VK., and, Kyewski B. Shaping of the autoreactive T-cell repertoire by a splice variant of self protein expressed in thymic epithelial cells. Nat Med. 2000;6:56–61. doi: 10.1038/71540. [DOI] [PubMed] [Google Scholar]
- Huseby ES, Sather B, Huseby PG., and, Goverman J. Age-dependent T cell tolerance and autoimmunity to myelin basic protein. Immunity. 2001;14:471–481. doi: 10.1016/s1074-7613(01)00127-3. [DOI] [PubMed] [Google Scholar]
- Ellison GW., and, Waksman BH. Role of the thymus in tolerance. IX. Inhibition of experimental autoallergic encephalomyelitis by intrathymic injection of encephalitogen. J Immunol. 1970;105:322–326. [PubMed] [Google Scholar]
- Murakami K, Maruyama H, Nishio A, Kuribayashi K, Inaba M, Inaba K.et al. (1993Effects of intrathymic injection of organ-specific autoantigens, parietal cells, at the neonatal stage on autoreactive effector and suppressor T cell precursors Eur J Immunol 23809–814. [DOI] [PubMed] [Google Scholar]
- Khoury SJ, Sayegh MH, Hancock WW, Gallon L, Carpenter CB., and, Weiner HL. Acquired tolerance to experimental autoimmune encephalomyelitis by intrathymic injection of myelin basic protein or its major encephalitogenic peptide. J Exp Med. 1993;178:559–566. doi: 10.1084/jem.178.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilan Y, Attavar P, Takahashi M, Davidson A, Horwitz MS, Guida J.et al. (1996Induction of central tolerance by intrathymic inoculation of adenoviral antigens into the host thymus permits long-term gene therapy in Gunn rats J Clin Invest 982640–2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marodon G, Fisson S, Levacher B, Fabre M, Salomon BL., and, Klatzmann D. Induction of antigen-specific tolerance by intrathymic injection of lentiviral vectors. Blood. 2006;108:2972–2978. doi: 10.1182/blood-2006-03-010900. [DOI] [PubMed] [Google Scholar]
- Bernard CC, Johns TG, Slavin A, Ichikawa M, Ewing C, Liu J.et al. (1997Myelin oligodendrocyte glycoprotein: a novel candidate autoantigen in multiple sclerosis J Mol Med 7577–88. [DOI] [PubMed] [Google Scholar]
- Toh BH, Gleeson PA, Simpson RJ, Moritz RL, Callaghan JM, Goldkorn I.et al. (1990The 60- to 90-kDa parietal cell autoantigen associated with autoimmune gastritis is a beta subunit of the gastric H+/K(+)-ATPase (proton pump) Proc Natl Acad Sci USA 876418–6422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SJ, Ahn S, Park CS, Holmes KL, Westrup J, Chang CH.et al. (2006The quantitative assessment of MHC II on thymic epithelium: implications in cortical thymocyte development Int Immunol 18729–739. [DOI] [PubMed] [Google Scholar]
- Korin YD., and, Zack JA. Progression to the G1b phase of the cell cycle is required for completion of human immunodeficiency virus type 1 reverse transcription in T cells. J Virol. 1998;72:3161–3168. doi: 10.1128/jvi.72.4.3161-3168.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray DH, Seach N, Ueno T, Milton MK, Liston A, Lew AM.et al. (2006Developmental kinetics, turnover, and stimulatory capacity of thymic epithelial cells Blood 1083777–3785. [DOI] [PubMed] [Google Scholar]
- Klein L., and, Kyewski B. Self-antigen presentation by thymic stromal cells: a subtle division of labor. Curr Opin Immunol. 2000;12:179–186. doi: 10.1016/s0952-7915(99)00069-2. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Pagany M, Weiner HL, Linington C, Sobel RA., and, Kuchroo VK. Myelin oligodendrocyte glycoprotein-specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. J Exp Med. 2003;197:1073–1081. doi: 10.1084/jem.20021603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA.et al. (2001Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide Nat Immunol 2301–306. [DOI] [PubMed] [Google Scholar]
- Apostolou I, Sarukhan A, Klein L., and, von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nat Immunol. 2002;3:756–763. doi: 10.1038/ni816. [DOI] [PubMed] [Google Scholar]
- Vandenbark AA., and, Offner H. Critical evaluation of regulatory T cells in autoimmunity: are the most potent regulatory specificities being ignored. Immunology. 2008;125:1–13. doi: 10.1111/j.1365-2567.2008.02900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill A, Spittle E., and, Bäckström BT. Partial depletion of CD69low-expressing natural regulatory T cells with the anti-CD25 monoclonal antibody PC61. Scand J Immunol. 2007;65:63–69. doi: 10.1111/j.1365-3083.2006.01870.x. [DOI] [PubMed] [Google Scholar]
- Klein L., and, Kyewski B. Self-antigen presentation by thymic stromal cells: a subtle division of labor. Curr Opin Immunol. 2000;12:179–186. doi: 10.1016/s0952-7915(99)00069-2. [DOI] [PubMed] [Google Scholar]
- McCaughtry TM, Baldwin TA, Wilken MS., and, Hogquist KA. Clonal deletion of thymocytes can occur in the cortex with no involvement of the medulla. J Exp Med. 2008;205:2575–2584. doi: 10.1084/jem.20080866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallegos AM., and, Bevan MJ. Central tolerance to tissue-specific antigens mediated by direct and indirect antigen presentation. J Exp Med. 2004;200:1039–1049. doi: 10.1084/jem.20041457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet V, Naquet P., and, Guinamard RR. Intercellular MHC transfer between thymic epithelial and dendritic cells. Eur J Immunol. 2008;38:1257–1263. doi: 10.1002/eji.200737982. [DOI] [PubMed] [Google Scholar]
- Perchellet A, Brabb T., and, Goverman JM. Crosspresentation by nonhematopoietic and direct presentation by hematopoietic cells induce central tolerance to myelin basic protein. Proc Natl Acad Sci USA. 2008;105:14040–14045. doi: 10.1073/pnas.0804970105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman MA, Larkin J 3rd, Cozzo C, Jordan MS., and, Caton AJ. CD4+ CD25+ regulatory T cell repertoire formation in response to varying expression of a neo- self-antigen. J Immunol. 2004;173:236–244. doi: 10.4049/jimmunol.173.1.236. [DOI] [PubMed] [Google Scholar]
- van Santen HM, Benoist C., and, Mathis D. Number of T reg cells that differentiate does not increase upon encounter of agonist ligand on thymic epithelial cells. J Exp Med. 2004;200:1221–1230. doi: 10.1084/jem.20041022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman D, Tian C., and, Iacomini J. Induction of donor-specific tolerance in sublethally irradiated recipients by gene therapy. Mol Ther. 2005;12:353–359. doi: 10.1016/j.ymthe.2005.03.035. [DOI] [PubMed] [Google Scholar]
- Tian C, Bagley J, Cretin N, Seth N, Wucherpfennig KW., and, Iacomini J. Prevention of type 1 diabetes by gene therapy. J Clin Invest. 2004;114:969–978. doi: 10.1172/JCI22103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian C, Bagley J., and, Iacomini J. Persistence of antigen is required to maintain transplantation tolerance induced by genetic modification of bone marrow stem cells. Am J Transplant. 2006;6:2202–2207. doi: 10.1111/j.1600-6143.2006.01455.x. [DOI] [PubMed] [Google Scholar]
- Rossi SW, Jenkinson WE, Anderson G., and, Jenkinson EJ. Clonal analysis reveals a common progenitor for thymic cortical and medullary epithelium. Nature. 2006;441:988–991. doi: 10.1038/nature04813. [DOI] [PubMed] [Google Scholar]
- Blanpain C., and, Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol. 2009;10:207–217. doi: 10.1038/nrm2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrens EJ, Mijnheer G, Duurland CL, Klein M, Meerding J, van Loosdregt J.et al. (2011Functional human regulatory T cells fail to control autoimmune inflammation due to PKB/c-akt hyperactivation in effector cells Blood 1183538–3548. [DOI] [PubMed] [Google Scholar]
- Noubade R, Krementsov DN, Del Rio R, Thornton T, Nagaleekar V, Saligrama N.et al. (2011Activation of p38 MAPK in CD4 T cells controls IL-17 production and autoimmune encephalomyelitis Blood 1183290–3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, Petersen TR.et al. (2007Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation Nat Med 13423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnezis T, Mandemakers W, McQualter JL, Zheng B, Ho PP, Jordan KA.et al. (2004The neurite outgrowth inhibitor Nogo A is involved in autoimmune-mediated demyelination Nat Neurosci 7736–744. [DOI] [PubMed] [Google Scholar]
- Waldor MK, Sriram S, Hardy R, Herzenberg LA, Herzenberg LA, Lanier L.et al. (1985Reversal of experimental allergic encephalomyelitis with monoclonal antibody to a T-cell subset marker Science 227415–417. [DOI] [PubMed] [Google Scholar]
- Barnard AL, Chidgey AP, Bernard CC., and, Boyd RL. Androgen depletion increases the efficacy of bone marrow transplantation in ameliorating experimental autoimmune encephalomyelitis. Blood. 2009;113:204–213. doi: 10.1182/blood-2008-05-156042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
RT-PCR analysis of thymic HKβ mRNA transgene expression.
RT-PCR analyses of MOG and HKβ mRNA transgene expression in CNS, lymph node, and spleen tissues.
Antibody responses in i.t.-injected animals.
Antibodies used in study.