Abstract
The interleukin-17 (IL-17) cytokine family is crucial to the progression of experimental autoimmune encephalomyelitis (EAE), an animal model of multiple sclerosis (MS). It has been shown in a neuroectoderm-specific knockout study that astrocyte-restricted ablation of Act1, a key and common transcription factor for signals mediated by IL-17 family members (IL-17A, IL-17F, and IL-17C), ameliorates EAE. However, the effect of Act1 deficiency in astrocytes on ongoing disease, which is of clinical relevance for MS therapy, has not been investigated. Here we report that intracerebroventricular (i.c.v.) injection of a novel lentiviral vector (shAct1) to knockdown Act1 expression in astrocytes effectively inhibited disease progression at EAE induction, clinical onset, and peak of disease (ongoing phases), with significantly reduced numbers of infiltrating inflammatory cells and percentage of Th17 cells in the central nervous system (CNS). This was mainly due to the suppressed expression of Th17-related chemokines in astrocytes, while neurotrophic factors in the CNS and immune responses in the periphery were not affected. These results demonstrate that blocking the IL-17 pathways in astrocytes is a promising therapeutic approach for MS in a CNS-specific manner, which does not interfere with systemic immune responses, a major concern in conventional MS therapy.
Introduction
Multiple sclerosis (MS) and its animal model, experimental autoimmune encephalomyelitis (EAE), are autoimmune demyelinating diseases of the central nervous system (CNS).1 MS is thought to begin when peripherally activated myelin-reactive T cells infiltrate into the CNS, followed closely by other cells, including T cells, macrophages, dendritic cells, B cells, and neutrophils.2,3 Once in the CNS, myelin-reactive T cells undergo reactivation and produce proinflammatory cytokines, thereby triggering an inflammatory cascade that results in myelin damage.2,3,4 In spite of extensive research to develop pharmacotherapeutic agents to reduce myelin damage, only a few therapies are available, all with systemic side effects and only mild to moderate efficacy.5 A therapeutic strategy that specifically targets inflammation in the CNS is highly desirable.
Although EAE and MS had been considered a typical Th1 cell-mediated diseases,6,7 growing evidence indicate that Th17 cells play an important role in the effector mechanisms of these and other autoimmune diseases.8,9,10,11,12 Thus, any factor that directly impairs development of Th17 cells, or a deficiency of factors that promote this lineage (IL-1, IL-6, IL-23), consistently abrogates EAE.13,14 Interleukin-17A (IL-17A) is the hallmark cytokine of Th17 lineage.15 IL-17A, as a homodimer or a heterodimer with its homologue IL-17F, binds to the heteromeric transmembrane receptor (IL-17RA and IL-17RC), which results in recruitment of Act1 and formation of a signaling complex that facilitates inflammatory responses.16 Both IL-17A and IL-17F have proinflammatory effects via induction of proinflammatory cytokines, chemokines, and metalloproteinases in a broad spectrum of cells.17 Mice that lack the IL-17 or IL-17 receptor are less susceptible to EAE induction, and IL-17–specific inhibition attenuates inflammation, suggesting that IL-17 signaling plays a critical role in the effector stage of EAE.18 Importantly, increased IL-17 production and messenger RNA expression were reported in MS patients with active disease.19,20,21
Astrocytes are the most abundant cell type in the mammalian CNS constituting ~90% of human brain tissue, and have been considered both an ally and enemy in the fight against CNS immune infiltration and restoration of neuronal function.22 Astrocytes support neural transmission, maintain survival of neurons and other glia, and sustain the integrity of the blood-brain-barrier; on the other hand, astrocytes are now considered important non-professional antigen presenting cells that play complex roles in CNS immunopathogenesis.22 During CNS inflammation, astrocytes secrete proinflammatory cytokines, activate autoreactive T cells23 and are the major producers of chemokines, thus promoting secondary waves of immune cell infiltration into the CNS.23,24 Further, astrocytes proliferate and upregulate their expression of glial fibrillary acidic protein (GFAP) upon activation in a process termed astrogliosis, a main cause of the formation of MS plaque.25 Thus, targeting inflammatory pathways in astrocytes, while not interfering with their protective properties, could be a highly effective CNS-specific approach for MS treatment.
A recent study using neuroectoderm-specific knockout mice showed that blockade of IL-17 signaling, through ablation of Act1 in astrocytes, inhibited the induction of EAE.26 This result demonstrated an important role of IL-17 signaling in astrocytes during disease development; however, it is not known whether inhibition of IL-17 signaling in astrocytes after disease onset would have a therapeutic effect. To test this possibility, in the current study we generated a novel lentiviral vector to specifically knockdown Act1 expression and block the IL-17-IL-17R pathway in astrocytes. We found that this approach not only prevented EAE development, but also effectively suppressed ongoing EAE, without affecting secretion of neurotrophic factors by astrocytes and the peripheral immune system. This study provides a unique CNS-specific treatment for EAE/MS that avoids systemic side effects caused by conventional MS therapies.
Results
Lentiviral vector for expression of Act1-specific shRNA in astrocytes
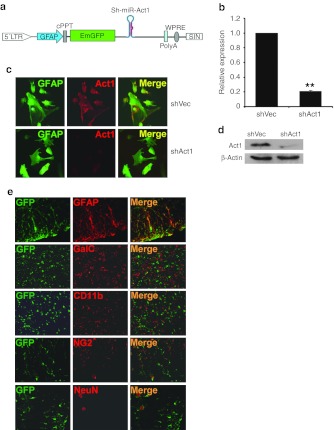
To test the therapeutic effects and mechanisms of astrocyte-restricted interference with IL-17 signaling in EAE, we generated a novel lentiviral vector that expresses Act1-specific short hairpin RNA (shRNA) in astrocytes. A schematic map of pLenti-GFAPpro-EGFP-shAct1 vector is shown in Figure 1a; the vector map is shown in Supplementary Figure S1. To obtain an astrocyte-specific transcription, CMV and EF1 promoters were substituted with a 682-bp long GFAP promoter. Enhanced green fluorescent protein (EGFP) was used as a reporter gene; miR-30 based shAct1 cassette was inserted downstream of the EGFP stop codon, which was thus transcribed simultaneously with EGFP.
Figure 1.
Efficacy and astrocyte specificity of shAct1 lentiviral vector. (a) Schematic map of the astrocyte-specific shAct1 lentiviral vector. (b) Primary astrocytes were infected with shAct1- and control lentivirus. Three days later, Act1 mRNA expression was assayed by real-time RT-PCR. **P < 0.01 (n = 3 each group). (c) Act1 protein expression (red) in astrocytes (green) was determined by immunohistochemistry. (d) Act1 protein expression in astrocytes was determined by western blot. (e) Immunofluorescence of GFP (green) and neural specific markers (red) in spinal cord tissue sections 3 days after i.c.v. injection. GFAP, astrocytes; NeuN, neurons; GalC, oligodendrocytes; CD11b, microglia; NG2, oligodendrocyte progenitor cells (OPCs). GFAP, glial fibrillary acidic protein; i.c.v., intracerebroventricular; LTR, long terminal repeat; mRNA, messenger RNA; RT-PCR, reverse transcription-PCR.
In vitro cultured primary astrocytes were infected with shAct1 or control lentivirus (shVec) and the expression of Act1 was examined 3 days later. shAct1 lentivirus markedly reduced messenger RNA and protein levels of Act1, as determined by real-time reverse transcription-PCR (Figure 1b), immunohistochemistry (Figure 1c), and western blot (Figure 1d). We then intracerebroventricularly (i.c.v.) injected lentivirus containing shAct1 vectors to mice, and killed the mice 3 days later to test cell-specific expression of EGFP. As shown in Figure 1e, only astrocytes (GFAP+) were co-stained with EGFP. More than 70% of astrocytes from white and gray matter of brain and spinal cord expressed EGFP (data not shown), while other CNS cell types, including neurons, oligodendrocytes, microglia, and oligodendrocyte precursor cells, did not express EGFP (Figure 1e), thus confirming astrocyte-specific expression of shAct1.
Act1 knockdown in astrocytes suppresses EAE
To determine whether interfering with IL-17 signaling in astrocytes affects EAE induction, shAct1 lentivirus and control lentivirus (1 × 107 infectious units (IU) in 20 µl phosphate-buffered saline (PBS)/mouse) were given to mice by i.c.v. administration 3 days before immunization. A substantial reduction in Act1 expression was observed 36 hours after shAct1 lentivirus injection, and the effect lasted until the end of the experiment (data not shown). The shVec control lentivirus-treated group developed typical EAE, while shAct1 treatment resulted in significantly decreased disease severity (P < 0.01; Figure 2a).
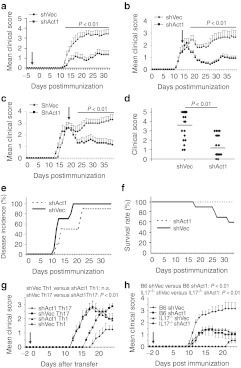
Figure 2.
i.c.v. injection of shAct1 lentivirus ameliorates disease severity. C57BL/6 mice were injected i.c.v. with 1 × 107 IU shAct1 lentivirus (a) 3 days before EAE induction (n = 10 each group), (b) 14 days p.i. (disease onset) (n = 8 each group), or (c) 20 days p.i. (peak disease) (n = 8 each group). Control mice were given the same amount of control lentivirus. Clinical scores were recorded daily. Results are shown as mean ± SEM (n = 10 or 8 in each group). (d) Disease distribution at the end points of experiment, (e) disease incidence, and (f) survival rate of shAct1 lentivirus- and control virus-treated groups are shown. (g) C57BL/6 mice were injected i.c.v. with 1 × 107 IU shAct1 or control lentivirus a day before cell adoptive transfer; clinical scores of Th1 cell- and Th17 cell-induced EAE are shown as mean ± SEM (n = 5 each group). (h) IL-17A deficient and WT C57BL/6 mice were i.c.v. injected with 1 × 107 IU shAct1 or control lentivirus a day before immunization; clinical scores were shown as mean ± SEM (n = 5 each group). One representative of three independent experiments is shown in a,b,c,g, and h. The data in d,e, and f come from three independent experiments when virus was injected 3 days before immunization. EAE, experimental autoimmune encephalomyelitis; i.c.v., intracerebroventricular; IL, interleukin; IU, infectious units; n.s., not significant; p.i., postimmunization; Th1, T-helper 1; WT, wild-type.
We then tested the therapeutic effect of shAct1 on ongoing EAE. Mice were i.c.v. injected with shAct1 lentivirus at disease onset (day 14 postimmunization (p.i.)) or its peak (day 20 p.i.). shAct1 treatment at both time points significantly suppressed EAE progression (Figure 2b,c). Consistent with these observations, treated groups of mice showed reduced disease scores (Figure 2d), delayed disease onset (Figure 2e), and improved survival rate (Figure 2f) compared to control groups. The improvement in clinical symptoms was observed 3–4 days after virus injection, and the effect persisted until the end of the experiment. These data show that knocking down Act1 in astrocytes by lentiviral shRNA expression has a significant therapeutic effect in EAE, which may translate in a novel approach for treatment of MS.
Given that Act1 participates in signaling of IL-17, which is produced by Th17 but not Th1 cells, we tested our hypothesis that shAct1 would suppress only Th17 cell-induced, but not Th1 cell-induced adoptive EAE. Polarized myelin oligodendrocyte glycoprotein (MOG)-specific Th1 and Th17 cells were transferred into shAct1 and shVec lentivirus-injected recipient mice for EAE induction. EAE severity in mice receiving Th17 cells was effectively reduced by shAct1; by contrast, shAct1 injection in mice receiving Th1 cells did not protect them from EAE (Figure 2g). These data confirm the specific effect of astrocyte-specific shAct1 lentivirus on Th17 cell-, but not Th1 cell-induced EAE pathogenesis.
To further examine the relationship between shAct1 and IL-17 in suppression of EAE we used IL-17A−/− mice. While these mice developed milder EAE compared to wild-type mice, strikingly, astrocyte-specific Act1 knockdown significantly delayed disease onset and reduced EAE severity in IL-17A−/− mice as well (Figure 2h). These results are consistent with known functions of Act1 as a common adaptor protein for several cytokines of IL-17 cytokine family that also play a pathogenic role in EAE.
Astrocyte-restricted blocking of IL-17 signaling does not alter the peripheral immune system
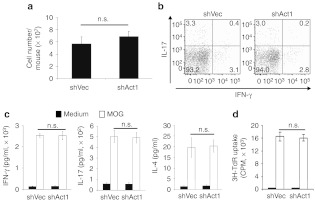
We then tested whether Act1 knockdown in astrocytes impacts peripheral immune response in EAE mice. There was no significant difference in spleen cell numbers between treatment and control groups (Figure 3a), and the numbers of Th1 and Th17 cells in spleens of the shAct1-treated group were not changed in comparison with those treated with control virus, as determined by intracellular cytokine staining of interferon-γ (IFN-γ) and IL-17 (Figure 3b). Splenocytes from the shAct1- and shVec-treated groups produced similar amounts of IFN-γ, IL-17, and IL-4 upon MOG-stimulation (Figure 3c). Also, there was no significant difference in spleen cell proliferation between the two groups after restimulation with MOG (Figure 3d). Together, these results suggest that interference with IL-17 signaling through astrocyte-restrict knockdown of Act1 does not affect peripheral immune response during EAE.
Figure 3.
Peripheral immune system was not impacted by i.c.v. injection of shAct1 lentivirus. Splenocytes were harvested at the end point of the experiment shown in Figure 2b. (a) Total cell numbers were counted and (b) frequencies of IFN-γ+ and IL-17+ cells in gated CD4+ T cells were measured by flow cytometry. (c) After 3 days' culture, cytokine production in culture supernatants was determined by ELISA and (d) T cell proliferation determined by 3H-incorporation. Results are shown as mean ± SD (n = 5 each gro up). One representative of three experiments is shown. cpm, counts per minute; ELISA, enzyme-linked immunosorbent assay; i.c.v., intracerebroventricular; IFN, interferon; IL, interleukin, n.s., not significant.
Astrocyte-restricted blocking of IL-17 signaling inhibits inflammatory cell infiltration into the CNS
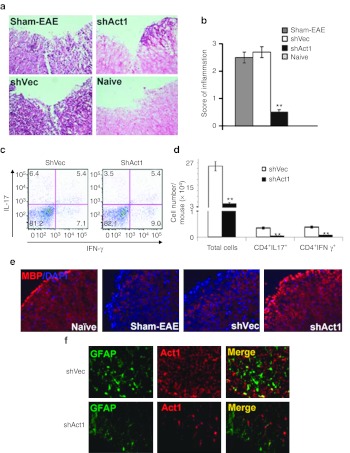
Consistent with clinical signs, typical mononuclear cell (MNC) infiltration foci were observed in the spinal cord white matter of control virus-treated mice, with an inflammation score of 2.7 ± 0.3, which was similar to sham-treated EAE mice (Figure 4a,b). In contrast, the score for inflammatory infiltration in shAct1-treated mice was decreased to 0.5 ± 0.1 (P < 0.01). The average numbers of MNCs per spinal cord were 26 × 104 in control virus-treated mice versus 5.0 × 104 in shAct1-treated mice (Figure 4d; P < 0.01). While shAct1- and shVec-treated mice showed similar percentages of CD4+ T cells that produced IL-17 and IFN-γ in flow cytometric analysis (Figure 4c), the absolute numbers of these cells were significantly reduced in shAct1-treated mice (3,320 ± 118 versus 427 ± 25 for IL-17 and 3,530 ± 220 versus 689 ± 45 for IFN-γ Figure 4d). Staining for myelin basic protein expression showed that knockdown of Act1 in astrocytes substantially inhibited demyelination compared to control treatments (Figure 4e).
Figure 4.
Knocking down Act1 expression in astrocytes suppressed inflammatory infiltration in the CNS. Spinal cords of mice in Figure 2b were harvested at day 34 p.i. and inflammatory infiltration scores were assessed by H&E staining at L3 level. (a) Quantification of CNS inflammation and demyelination was performed on six sections per mouse (n = 5 each group). (b) **P < 0.01 compared to Sham-EAE group. (c) Similar amounts of the remaining spinal cord tissues were processed for cell suspension, and frequencies of Th1 and Th17 cells were assessed by flow cytometry. (d) Total mononuclear cell (MNC) numbers in spinal cord suspensions were counted, and absolute numbers of Th1 and Th17 cells were calculated by multiplying the percentages of these cells in c and total MNC numbers in each spinal cord; **P < 0.01. (e) Demyelination in each group was assessed by MBP staining. (f) Act1 expression in spinal cord after treatment was determined by immunohistochemistry. One representative of three experiments is shown. CNS, central nervous system; DAPI, 4′,6-diamidino-2-phenylindole; EAE, experimental autoimmune encephalomyelitis; H&E, hematoxylin and eosin; IFN, interferon; IL, interleukin; MBP, myelin basic protein; p.i., postimmunization; Th1, T-helper 1.
Given that numbers of Act1+ astrocytes in the CNS were greatly decreased in the shAct1-treated mice compared to shVec-treated mice (Figure 4f), we tested whether this reduction in Act1+ cells resulted from a possible cytotoxicity of shAct1. shAct1 lentivirus infection did not affect lactate dehydrogenase levels in cultured primary astrocytes (Supplementary Figure S2), indicating that the infection had no cytotoxic effect. Staining for activated caspase-3 showed that shAct1 lentivirus infection did not promote astrocytes death compared to the control virus (data not shown). These results indicate that the reduction in Act1+ cell numbers was indeed due to knocking down its expression, but not due to the death of infected cells.
To analyze which types of inflammatory infiltration were inhibited, MNCs were isolated from spinal cords and analyzed by flow cytometry. As shown in Figure 5a, percentages of CD4+ T cells, CD11c+ dendritic cells and Gr-1+ neutrophils were reduced in the shAct1-treated mice compared to the control group. Although percentages of CD19+ B cells and F4/80+ macrophage/microglia were slightly increased in these mice, due to the dramatically reduced total numbers of MNCs in the CNS after shAct1 treatment (Figure 4d), the absolute numbers of all infiltrating cell types were significantly reduced in shAct1-treated mice (all P < 0.05–0.01; Figure 5b).
Figure 5.
Subtypes of reduced inflammatory cells in the CNS after shAct1 treatment. Cell suspensions from spinal cord tissues were prepared as described in Figure 4c and (a) percentages of CD4+ T cells, B cells (CD19+), DCs (CD11c+), macrophages/microglia (F4/80+), and neutrophils (Gr-1+) cells in inflammatory infiltrates were determined by flow cytometry. (b) Quantification of CNS-infiltrating inflammatory cells. Absolute numbers of different subtypes of CNS i nfiltrates were calculated by multiplying the percentages of these cells in a and the total number of MNCs in each spinal cord is shown in Figure 4d. *P < 0.05, **P < 0.01, comparison between shVec and shAct1 treated groups (n = 5 each group). One representative of two experiments is shown. CNS, central nervous system; DCs, dendritic cells; MNC, mononuclear cell.
Blocking IL-17 signaling in astrocytes impairs chemokine expression in vivo
To determine why interfering with IL-17 signaling in astrocytes inhibits inflammatory cell infiltration, we explored an array of cytokine/chemokine gene expression in the spinal cords of shAct1- and control-treated EAE mice. As shown in Figure 6, expression of both Act1 and receptors of IL-17 family members, including IL-17RA and IL-17RE, in the spinal cords of EAE mice was reduced by shAct1 treatment (Figure 6a,b), and expression of inflammatory cytokines, including granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-17A, IL-17F, IL-23, insulin-like growth factor-1 (IGF-1), and fibroblast growth factor-2 (FGF-2) was substantially reduced as well (Figure 6c). Expression of certain chemokines including CXCL1, CXCL2, CCL20, and matrix metalloproteinase MMP3, was greatly reduced, while expression of chemokines induced by IFN-γ signaling (CXCL-9, CXCL-10, and CXCL11)26 did not change (Figure 6d). When levels of several Th1- and Th17-related chemokines and cytokines in the spinal cord tissue lysate were tested by western blot, we found that expression of CXCL1, CXCL2, CCL20, and IL-17A was reduced after shAct1 treatment, and expression of CXCL9, CXCL10, CXCL11, and IFN-γ remained unchanged (Figure 6e), consistent with real-time PCR results. While inducible nitric oxide synthase has been reported to play various roles in the progression of EAE27, there was no difference in the expression level of inducible nitric oxide synthase in the spinal cord of EAE mice treated with shAct1 or control lentivirus (Figure 6f). These results suggest that the impact of astrocyte-restricted blocking of IL-17 signaling on the induction of EAE was probably due to a defect in IL-17-induced inflammatory molecules.
Figure 6.
Expression of chemokines, cytokines, and neurotrophic factors in the CNS after shAct1 treatment. Total RNA was prepared at the end of experiment from a fraction of spinal cords of shAct1-treated or control lentivirus-treated EAE mice described in Figure 2b. RT-PCR was performed to determine mRNA levels of (a) Act1, (b) receptors for IL-17 family cytokines, (c) cytokines, and (d) chemokines. (e) Expression of several Th1- and Th17-related chemokines and cytokines was confirmed by western blot. (f) Expression of iNOS, and (g) neurotrophic factors BDNF and NT-3 was determined by real-time PCR. Relative expression was calculated by −ΔΔCt values from triplicate PCR. *P < 0.05, **P < 0.01 (n = 5 each group). One representative of two experiments is shown. BDNF, brain-derived neurotrophic factor; CNS, central nervous system; CNTF, ciliary neurotrophic factor; EAE, ex perimental autoimmune encephalomyelitis; FGF2, fibroblast growth factor-2; GM-CSF, granulocyte-macrophage colony-stimulating factor; IGF1, insulin-like growth factor-1; IL, interleukin; iNOS, inducible nitric oxide synthase; mRNA, messenger RNA; n.s., not significant; NT-3, neurotrophin-3; RT-PCR, reverse transcription-PCR; TGF-β, transforming growth factor-β Th1, T-helper 1.
Blocking IL-17 signaling in astrocytes does not change the production of neurotrophic factors
Astrocytes favor neuron/oligodendrocyte survival by secreting various neurotrophic factors such as neurotrophin-3 (NT-3) and brain-derived neurotrophic factor (BDNF).22 To determine whether interfering with IL-17 signaling in astrocytes affects the secretion of neurotrophic factors, the transcription levels of BDNF and NT-3 in spinal cords of EAE mice were analyzed by real-time PCR. There was no significant difference in expression of these two neurotrophic factors between the shAct1-treated and control groups (Figure 6g), suggesting that shAct1 treatment influences only the inflammatory response activity of astrocytes, but does not affect their capacity for neurotrophic factor secretion.
Blocking IL-17 signaling in astrocytes impairs their chemokine expression in vitro
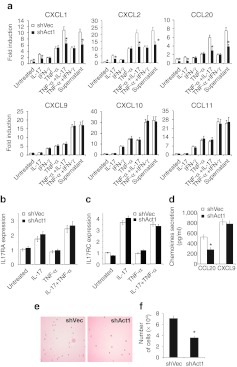
To further confirm the in vivo finding that blocking IL-17 signaling in astrocytes inhibited their chemokine production, we determined the profile of chemokine expression of astrocytes under different culturing conditions. The expression of CXCL-1, CXCL-2, and CCL-20, which are inducible by IL-17,26 was reduced in shAct1-treated astrocytes, either with or without IL-17, IL-17 plus tumor necrosis factor-α (TNF-α) treatment, or treatment with supernatant of MOG-stimulated spleen cells from 2D2 mice (Figure 7a). The expression of these three chemokines was not changed by TNF-α, IFN-γ or TNF-α plus IFN-γ treatment, but IL-17 and TNF-α in combination enhanced the expression of these chemokines. In contrast, CXCL-9, CXCL-10, and CXCL-11 were only induced by IFN-γ or IFN-γ combined with TNF-α, but not by IL-17 or IL-17 plus TNF-α, regardless of infection with shAct1 or shVec (Figure 7a). We also measured expression of IL-17 receptors IL-17RA and IL-17RC by real-time PCR. Treatment with IL-17 or IL-17 with TNF-α induced the expression of these IL-17 receptors, but there was no significant difference between shAct1 and control lentivirus treatment (Figure 7b,c). This suggests that Act1 interference did not affect the signaling pathway upstream of IL-17. The chemokine secretion capacity of astrocytes treated with shAct1 and control lentivirus was compared by enzyme-linked immunosorbent assay. Consistent with real-time PCR data, CCL20 content was significantly reduced after shAct1 treatment in the culture medium of astrocytes stimulated with IL-17 plus TNF-α, while CXCL9 secretion remained unchanged (Figure 7d).
Figure 7.
Expression and chemotaxis function of shAct1-treated astrocytes in vitro. (a) Primary astrocytes generated from neonatal mice were infected with shAct1 or control lentivirus and stimulated by different cytokines or culture supernatants of MOG35-55-stimulated spleen cells from 2D2 mice. Twenty-four hours later, chemokine expression was determined by RT-PCR. (b,c) Expression of IL-17RA and IL-17RC was determined by RT-PCR after 24 hours of different treatments. (d) Concentrations of CCL20 and CXCL9 in culture supernatants were determined by ELISA. They were selected as the representative chemokines responsive to IL-17 signaling or IFN-γ signaling, respectively. (e) Primary astrocytes were infected with shAct1 or control lentivirus for 72 hours, then stimulated with IFN-γ, IL-17, and/or TNF-α for 16 hours. MOG35-55-stimulated 2D2 splenic cells were loaded onto the luminal side of transwell inserts with a pore size of 3 µm and incubated for 24 hours. (f) Numbers of cells in the abluminal side were quantified. Data in this figure represent mean ± SEM of three independent experiments. *P < 0.05. ELISA, enzyme-linked immunosorbent assay; IFN, interferon; IL, interleukin; MOG, myelin oligodendrocyte glycoprotein; RT-PCR, reverse transcription-PCR; TNF, tumor necrosis factor.
Furthermore, a transmigration assay was performed to test the chemoattractive capability of astrocytes whose IL-17 signaling had been blocked. Compared to the control lentivirus-treated astrocytes, knocking down Act1 expression in astrocytes significantly inhibited the transmigration of MOG-stimulated splenocytes of 2D2 mice (Figure 7e,f).
Discussion
In the present study, we introduced a novel lentiviral vector to specifically knockdown Act1 expression and block the IL-17-IL-17R pathway in astrocytes; we then tested its therapeutic effects and mechanisms of action in EAE. This approach effectively suppressed clinical EAE when treatment was started at induction phase or at peak of disease, and strongly reduced numbers of infiltrating cells in the CNS, including Th1 and Th17 cells. The therapeutic effect was mainly attributable to the suppressed expression of chemokines in astrocytes, such as CXCL1, CXCL2, CCL20, and matrix metalloproteinase MMP3, which are essential for the recruitment of peripheral leukocytes into the CNS. Importantly, this approach did not impair the immune system in the periphery or the production of neurotrophic factors in the CNS. Thus, this study, for the first time, provides a promising therapeutic approach for MS in a CNS-specific manner, without affecting systemic immune responses.
IL-17 is a potent proinflammatory cytokine and a hallmark of Th17 cells, which play a crucial role in EAE.11,12 Mice deficient in IL-17A developed less severe EAE,18 and anti-IL-17A treatment of relapsing–remitting EAE delayed relapse and reduced relapse incidence and severity.28 Recently, it has been shown that neuroectoderm-restricted knocking out of Act1, the key adaptor molecule in IL-17–mediated signaling, delayed disease onset and reduced disease severity, suggesting that IL-17 signaling in astrocytes may be a promising target for MS treatment.16 However, it is hard to translate this approach into therapy because: (i) Act1 expression may cause severe side effects due to the important functions of IL-17 in immune responses, especially in infectious disease and cancer;29,30,31 and (ii) it is impossible to define the effect of a knockout approach on ongoing disease, which represents the real clinical situation. Our novel lentiviral vector can bridge this gap by specifically targeting Act1 in astrocytes.
The GFAP gene promoter has been found to be astrocyte-specific and the ABC1D part of this promoter has enhanced activity and astrocyte specificity.32 We thus cloned different parts of the GFAP promoter region from mouse genomic DNA and combined them with a new GFAP promoter made up of ABC1D to drive shRNA expression. As GFAP protein expression will be largely induced in EAE and MS,25 it is an ideal promoter to strongly drive gene or shRNA expression in astrocytes of EAE mice, while the emergence of microRNAs and their transcription characteristics makes it possible to use cell-specific RNA polymerase II promoter to transcribe microRNA-based shRNAs for cell-targeted gene knocking down. Several reports have shown that incorporating sequences encoding small interfering RNAs targeting different genes into a human miR-30 pre-microRNA (pre-microRNA) backbone can express small interfering RNAs in cells,33,34 and this method can achieve a more efficient knockdown than small interfering RNA expressed as conventional shRNA. We therefore introduced a miR-30 based shAct1 cassette directly downstream of the terminal codon of marker gene EGFP driven by astrocyte-specific promoter GFAP, such that the EGFP marker gene and shAct1 would be transcribed as one cassette, and EGFP fluorescence could be used to monitor the amount and location of shRNA. Our in vitro and in vivo data have shown that this novel vector can specifically deliver an exogenous gene and shRNA into astrocytes, whose Act1 pathway can be dramatically reduced.
It has been suggested that IL-17A exerts its proinflammatory function via inducing chemokine production, such as CXCL1, CXCL2, CCL20,35 which in turn leads to an accumulation of immune cells, particularly neutrophils and monocytes, at the site of inflammation.16 In EAE, IL-17A, primarily produced by infiltrating Th17 cells, activates astrocytes to produce large amounts of these chemokines, thus attracting the secondary wave of inflammatory infiltration, and causing myelin damage.26 Inflammatory infiltrations result in an environment containing elements that are intrinsically hostile to the oligodendrocyte lineage, thus blocking possible spontaneous remyelination.36,37 Reducing autoimmune responses in the CNS would convert the hostile environment into one supportive of the remyelination process. Inhibition of CNS inflammation could also reverse neural stem cell niche dysfunction, and promote neural repair.38 Our study showed that knocking down Act1 signaling in astrocytes effectively reduced expression of IL-17-induced chemokines CXCL1, 2, and CCL20. As a result, treated mice exhibited significantly decreased CNS infiltrations of neutrophils, macrophages/microglia, CD4+ T cells, B cells, dendritic cells, and decreased expression of proinflammatory molecules such as IL-17A, IL-17F, IL-23, IGF-1, and FGF-2. Interestingly, blocking IL-17 signaling in astrocytes did not influence their expression of neurotrophic factors (e.g., BDNF and NT-3), which play an important role in remyelination and neuroregeneration.36,39 Together, this approach succeeds in retaining the protective properties of astrocytes while blocking their pathogenic properties.
While astrocyte specificity and Act1 knockdown efficiency have been confirmed in vitro and in naive mice in vivo, we found that the numbers of non-astrocyte Act1+ cells in CNS lesions were also reduced in EAE mice after shAct1 virus injection. The reasons for this phenomenon could be that: (i) numbers of Act1+ infiltrating cells were significantly reduced; (ii) IL-17 signaling is required to trigger Act1 expression. After infection with shAct1, IL-17 secretion in the CNS decreased because of the reduced absolute number of IL-17-secreting cells in the CNS, and thus Act1 expression by CNS cells was not triggered. The reduced numbers of Act1+ cells in the CNS after shAct1 treatment were not due to cell death of Act1+ astrocytes, given that both in vivo activated caspase-3 staining and in vitro cytotoxicity assay showed that shAct1 lentivirus treatment was not toxic to astrocytes. Unchanged expression of NT-3 and BDNF after shAct1 lentivirus treatment also indicates a comparable number of astrocytes, the major producer of neurotrophic factors.22 Together, these results suggest that astrocyte-restricted Act1 knockdown also has some indirect effects on CNS environment that may favor disease treatment.
It has been found that expression IL-17 receptors were upregulated in the CNS of EAE mice in vivo and in activated astrocytes in vitro.40 In the current study, expression of both IL-17RA and IL-17RE (responsive to IL-17C) in the CNS of shAct1-treated mice was significantly lower than in control mice. However, in vitro studies showed no difference in expression of any of the IL-17 receptors tested in astrocytes infected by shAct1 or shVec lentivirus. These results suggest that shAct1 does not directly influence expression of IL-17 receptors; instead, reduced expression in the CNS after shAct1 treatment may be an indirect effect of the reduction of inflammatory cell infiltration and of proinflammatory mediators in the CNS, which fail to upregulate the IL-17 receptors as typically observed in inflammation.
Interestingly, astrocyte-specific shAct1 knocking down also delayed disease onset and reduced the severity of disease in IL-17A deficient mice. This suggests that, in addition to the IL-17A-IL-17RA/IL-17RC-Act1 pathway, other Act1-related pathway(s) in astrocytes are involved in EAE pathogenesis. This hypothesis is supported by a recent finding that IL-17C-IL-17RE-Act1 pathway also plays an important role in EAE development.16 These authors did not study whether this pathway is also functional in astrocytes. However, our results showed that IL-17RE expression greatly increased during EAE (Supplementary Figure S3a). In addition, the supernatant of MOG-stimulated spleen cells from 2D2 mice can induce expression of IL-17RE in astrocytes, similarly to IL-17RA and IL-17RC (Supplementary Figure S3b). These data indicate that an IL-17C-IL-17RE-Act1 pathway may play a role similar to that of the IL-17A-IL-17-RA/IL-17RC-Act1 pathway in astrocytes during EAE pathogenesis. More importantly, the observation that Act1 knockdown blocks all rather than only one of such IL-17–related pathways is a promising aspect of this approach for potential clinical use in MS.
All of the currently available MS therapies target immune molecules that are neither antigen-specific, nor organ-specific, and carry the inherent risk of infection and systemic side effects.5 An approach to reach this goal may be CNS-specific manipulation, i.e., therapeutic agents that target only CNS tissue without affecting systemic immune responses. Indeed, Mi et al. found that clinical EAE was dramatically suppressed in mice lacking LINGO-1, a key negative regulator of oligodendrocyte differentiation and remyelination, while MOG-induced T cell proliferation and Th1/Th2/Th17 cytokine production in the periphery remained unchanged.41 Croxford et al. found that, while systemic administration of IL-10 failed to suppress EAE, delivery of cells that expressed IL-10 into the CNS had a significant effect.42 We, in the current study, provide evidence that specifically blocking IL-17 signaling in astrocytes significantly protected CNS tissue from inflammatory infiltration of systemic immune cells, while the peripheral immune system remained unaffected. These results indicate that systemic immunomodulation, as with all current MS therapies, may not be necessary. Instead, CNS-targeted manipulation could be a novel and sufficient MS therapy, with the great advantage of not disturbing the global immune system.
In summary, our data demonstrate that blocking IL-17 signaling in astrocytes by knocking down Act1 expression can effectively treat ongoing EAE without affecting the peripheral immune system. The lentiviral vector, which has been widely used in clinical trails,43,44,45 has been confirmed as a highly safe tool for gene therapy. I.c.v. injection, by which the therapeutic reagents are rapidly diffused in the CNS via cerbrospinal fluid circulation, closely mimics intrathecal injection (subarachnoid space injection) in humans and is a simple and common practice in treating a broad range of CNS diseases.46,47 Further, an inducible regulation system (e.g., Tet-on) can be added in the vector to turn on/off Act1 signal in astrocytes, as desired, for future clinical use. Taken together, the approach tested in the present study represents a novel CNS-specific strategy for EAE/MS therapy.
Materials and Methods
Mice. Female C57BL/6 mice, 8–10 weeks of age, were purchased from the Jackson Laboratory (Bar Harbor, ME). 2D2 (TCRMOG) transgenic mice (B6 background; purchased from the Jackson Laboratory) and IL-17A deficient mice (B6 background; purchased from the Amgen, Thousand Oaks, CA), were bred in the animal facility of Thomas Jefferson University. All animal procedures were performed in accordance with the guidelines of the Institutional Animal Care and Committee of Thomas Jefferson University.
Construction of pLenti-GFAP-EGFP-mi-shAct1 and control lentiviral vectors. Lentiviral vector backbone plasmid pCDH-CMV-MCS-EF1-copGFP was digested with SpeI and SalI to remove CMV promoter, multiple cloning site, EF1 promoter and copGFP, were then replaced with a fragment containing ABC1D region of human gfa2 promoter.32 The ABC1D region of human gfa2 promoter was separately amplified with primer pairs of gfaaF, gfaaR and gfabF, gfabR (Supplementary Table S1). The two purified PCR products were digested with SpeI, SacI (for gfaa fragment) and SacI, SalI (for gfab fragment). The digested fragments were ligated with digested lentiviral backbone. The EGFP ORF fragment was amplified by primer pair EGFPF and EGFPR (Supplementary Table S1) and cloned into downstream of GFAP promoter, after which a fragment containing miR-30 based shAct1 cassette (Open Biosystems, Lafayette, CO; Cat No. V2LMM2517) was amplified by primer pair shMiAct1F and shMiAct1R, and then cloned into multiple cloning sites downstream of the EGFP gene to form a pLenti-GFAPpro-EGFP-mi-shAct1 lentiviral vector. The constructed vector sequence was verified by sequencing. The vector without the insertion of mi-shAct1 was used as a vector control.
Virus packaging, concentrating, and titering. The newly generated pLenti-GFAppro-EGFP-mi-shAct1 and three other helper plasmids pLP1, pLP2, pLP/VSV-G (Invitrogen, Carlsbad, CA) were amplified and their concentration was adjusted to 1 µg/µl. Ninety percent of confluent 293T cells in 100 mm dishes were transfected with plasmid DNA containing 15 µg pLenti-GFAppro-EGFP-mi-shAct1 or 15 µg pLenti-GFAppro-EGFP-MCS (negative control vector), 6.5 µg pLP1, 2.5 µg pLP2, and 3.5 µg pLP/VSV-G through CaCl2 method.48 After overnight incubation, the medium with plasmids was replaced by 10 ml fresh Dulbecco's modified Eagle's medium medium supplemented with 10% fetal bovine serum, 2 mmol/l L-Glutamine, 100 IU/ml penicillin, and 100 µg/ml Streptomycin (Mediatech, Herndon, VA). Supernatants were harvested after 30 hours' culture and filtered through a 0.45 µm membrane. The filtered supernatant was mixed with 10% PEG-10000 and incubated at 4 °C overnight, then was centrifuged for 1 hour at 3,500 rpm. The pellet containing lentivirus was resuspended in PBS, and aliquots were stored at −80 °C. Viral titers were assayed by infection of 293T cells at different dilutions; titers were adjusted to 5 × 108 IU/ml before infection and injection.
Viral infection of purified astrocytes and in vivo injection. For in vitro virus infection, purified astrocytes were rooted in poly-lysine coated 6-well plates at a concentration of 5 × 105 cells/well. Two days later, the culture medium was replaced by fresh complete Dulbecco's modified Eagle's medium supplemented with 1 × 106 IU/well different lentivirus and 8 µg/ml polybrene, and then incubated for 16 hours at 37 °C. After incubation, the medium with virus soap was replaced by fresh medium, and cultured for further use.
For in vivo injection, mice were anaesthetized and fitted with i.c.v. cannula for virus microinjection. A microsyringe was inserted into 2.0-mm lateral, 1.0-mm caudal to bregma, and 2.5 mm below the skull surface; 1 × 107 IU/mouse shAct or control virus (in 20 µl volume) was given to the mice. Injection speed was maintained at 1 µl/minute to prevent leaking.
Induction and assessment of EAE. For active EAE, mice were immunized subcutaneously on the back with 200 µg of MOG35-55 (MEVGWYRSPFSRVVHLYRNGK) emulsified in CFA (Difco Lab, Detroit, MI) containing 4 mg/ml Mycobacterium tuberculosis H37Ra (Difco). Two hundred nanogram of pertussis toxin (List Biological Lab, Epsom, England) was given intraperitoneally on days 0 and 2 (p.i.). For passive EAE, shAct1 or shVec lentivirus-injected mice were transferred with 3.0 × 107 polarized MOG35-55-specific Th1 or Th17 cells/mouse 4 hours after sublethal irradiation (550 Rad). To prepare MOG-specific polarized T cell populations, draining lymph nodes and spleen cells were prepared from mice immunized as described above at day 9 p.i. Cells were cultured for 4 days with MOG35-55 at a concentration of 25 µg/ml under Th1- (20 ng/ml rmIL-12 (PeproTech, Rocky Hill, NJ), 2 µg/ml anti-IL23p19 (eBioscience, San Diego, CA)) or Th17- (20 ng/ml rmIL-23 (PeproTech)) polarizing conditions.26 Mice were scored daily for appearance of clinical signs of EAE on a scale from 0 to 5 as described previously49: 0, no clinical sign; 1, fully limp tail; 2, paralysis of one hind limb; 3, paralysis of both hind limbs; 4, paralysis of trunk; 5, moribund or death.
Isolation of primary astrocytes. Primary astrocytes were isolated and cultured as previously described.40 Briefly, newborn mice (P0) were killed, and the whole brain was harvested and dissociated with Neural Tissue Dissociation Kit (Miltenyi Biotech, Auburn, CA) following the manufacturer's instructions. The dissociated cells were centrifuged at 300g for 10 minutes, then resuspended with Dulbecco's modified Eagle's medium/10% fetal bovine serum, and seeded on poly-lysine-coated 60-mm dishes at a density of 1 × 106/dish. After 7 days, cultures were trypsinized and replated in petri dishes. Cells from cultures that had been passaged twice were used as astrocytes. Purity of astrocytes was >95% as determined by immunostaining with anti-GFAP antibodies.
Astrocyte treatment in vitro. Primary astrocytes were seeded on poly-lysine-coated 60-mm dishes at a density of 1 × 106/dish and cultured in Dulbecco's modified Eagle's medium/10% fetal bovine serum medium except when specified differently in figure legends. Cells were treated with IFN-γ (10 ng/ml), IL-17A (50 ng/ml), and/or TNF-α (10 ng/ml). For the treatment with supernatant of MOG35-55-stimulated 2D2 spleen cells, 1 million cells/ml of 2D2 splenocytes were stimulated with 20 µg/ml MOG35-55 for 3 days, then the astrocyte culture medium was replaced by the supernatant from the MOG35-55-stimulated 2D2 spleen cells. Cells were harvested at 24 hours for RNA purification experiments, and at 48–60 hours for western blot.
Flow cytometry analysis. For intracellular staining, cells isolated from spleen and spinal cord were stimulated with PMA (50 ng/ml) and ionomycin (500 ng/ml) (Sigma-Aldrich, St Louis, MN) and GolgiStop (1 µg/106 cells) (BD PharMingen, San Jose, CA) for 4 hours at a density of 1 × 106/ml in RPMI1640 complete medium. Cells were harvested, washed in staining buffer (PBS) containing 1% fetal calf serum, 0.1% NaN3 and, in some cases, blocked with anti-CD16/CD32 antibodies. After washes, cells were first stained with fluorescent antibodies to surface markers, followed by intracellular staining. For cell surface staining, MNCs isolated from spinal cord were first counted, after which 1 × 106 cells/tube were washed with staining buffer and then stained with different fluorescent antibodies to surface marker for 30 minutes, then washed and resuspended in staining buffer for flow cytometry analysis.
Migration assay. Astrocytes were infected by shAct1 and control lentivirus for 48 hours, then stimulated with IL-17 plus TNF-α for 24 hours. Migration assays were performed as previously described, with some modifications.50 Briefly, prestimulated astrocytes, either infected by shAct1 or control lentivirus were cultured in 24-well plates. Then, 2D2 spleen cells that had been stimulated with 20 µg/ml MOG35-55 for 3 days were loaded onto the luminal side of Transwell inserts (3 µm pore size; CORNING, Corning, NY) and incubated at 37 °C/5% CO2. Twenty-four hours later, 50 µl of 0.5 mol/l ethylenediaminetetraacetic acid was added to the abluminal side and the plates were placed on a shaker for 15 minutes to dislodge cells. Cells in the abluminal side were then harvested and counted under light microscopy.
Immunohistochemical staining. Mice treated with shAct1 and control lentivirus were, extensively perfused, and the brain and spinal cords were harvested. Immunohistochemical staining was performed as previously described, with some modifications.37,51 Briefly, spinal cords were carefully excised from the brain stem to the lumbar region and cryoprotected with 30% sucrose in PBS. The lumbar enlargement was identified and then transected at the exact midpoint of the lumbar enlargement to standardize a site along the longitudinal axis of the cord, ensuring that the same lumbar spinal cord regions were analyzed for all conditions. Transverse sections of brain and spinal cord were cut, and immunohistochemistry was performed using different antibodies. Immunofluorescence controls were routinely generated with irrelevant IgGs as first antibody. Finally, slides were covered with Vectashield Mounting Medium (Vector Laboratories, Burlingame, CA), containing 1 µmol/l 4′,6-diamidino-2-phenylindole (DAPI). Results were visualized by fluorescent microscopy (Eclipse 800; Nikon, Melville, NY).
Reagents, antibodies, and detailed methods for cell preparation, proliferation assay, western blot, ELISA, LDH cytotoxicity, real-time quantitative RT-PCR, and histopathology can be found in Supplementary Materials and Methods. Primers used for real-time quantitative RT-PCR analysis were listed in Supplementary Table S2.
Statistical analysis. Differences for clinical scores of EAE were analyzed by using the two-way analysis of variance test. Inflammation scores were analyzed using the Mann–Whitney test. Differences for other parameters were analyzed by unpaired, two-tailed Student's t-test. A value of P < 0.05 was considered statistically significant.
SUPPLEMENTARY MATERIAL Figure S1. Vector map for astrocytes-specific expression. Figure S2. Cytotoxicity of shAct1 lentivirus on astrocytes in vitro. Figure S3. Expression of IL-17 family receptors. Table S1. Primers used for pLenti-GFAP-EGFP-mi-shAct1 vector construction. Table S2. Primers used for real-time quantitative RT-PCR analysis. Materials and Methods.
Acknowledgments
This study was supported by the National Multiple Sclerosis Society, the National Institute of Health, and the Groff Foundation. Y.Y. is partially supported by Chinese Natural Science Foundation (81100888). We thank Katherine Regan for editorial assistance. The authors declared no conflict of interest.
Supplementary Material
Vector map for astrocytes-specific expression.
Cytotoxicity of shAct1 lentivirus on astrocytes in vitro.
Expression of IL-17 family receptors.
Primers used for pLenti-GFAP-EGFP-mi-shAct1 vector construction.
Primers used for real-time quantitative RT-PCR analysis.
REFERENCES
- Baxter AG. The origin and application of experimental autoimmune encephalomyelitis. Nat Rev Immunol. 2007;7:904–912. doi: 10.1038/nri2190. [DOI] [PubMed] [Google Scholar]
- Hohlfeld R. Biotechnological agents for the immunotherapy of multiple sclerosis. Principles, problems and perspectives. Brain. 1997;120 (Pt 5):865–916. doi: 10.1093/brain/120.5.865. [DOI] [PubMed] [Google Scholar]
- Peterson LK., and, Fujinami RS. Inflammation, demyelination, neurodegeneration and neuroprotection in the pathogenesis of multiple sclerosis. J Neuroimmunol. 2007;184:37–44. doi: 10.1016/j.jneuroim.2006.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakowski ML., and, Owens T. Naive T lymphocytes traffic to inflamed central nervous system, but require antigen recognition for activation. Eur J Immunol. 2000;30:1002–1009. doi: 10.1002/(SICI)1521-4141(200004)30:4<1002::AID-IMMU1002>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Greenberg BM., and, Calabresi PA. Future research directions in multiple sclerosis therapies. Semin Neurol. 2008;28:121–127. doi: 10.1055/s-2007-1019133. [DOI] [PubMed] [Google Scholar]
- Segal BM. Experimental autoimmune encephalomyelitis: cytokines, effector T cells, and antigen-presenting cells in a prototypical Th1-mediated autoimmune disease. Curr Allergy Asthma Rep. 2003;3:86–93. doi: 10.1007/s11882-003-0017-6. [DOI] [PubMed] [Google Scholar]
- Shevach EM, Chang JT., and, Segal BM. The critical role of IL-12 and the IL-12R beta 2 subunit in the generation of pathogenic autoreactive Th1 cells. Springer Semin Immunopathol. 1999;21:249–262. doi: 10.1007/BF00812256. [DOI] [PubMed] [Google Scholar]
- Lovett-Racke AE, Yang Y., and, Racke MK. Th1 versus Th17: are T cell cytokines relevant in multiple sclerosis. Biochim Biophys Acta. 2011;1812:246–251. doi: 10.1016/j.bbadis.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gran B, Zhang GX, Yu S, Li J, Chen XH, Ventura ES.et al. (2002IL-12p35-deficient mice are susceptible to experimental autoimmune encephalomyelitis: evidence for redundancy in the IL-12 system in the induction of central nervous system autoimmune demyelination J Immunol 1697104–7110. [DOI] [PubMed] [Google Scholar]
- Zhang GX, Gran B, Yu S, Li J, Siglienti I, Chen X.et al. (2003Induction of experimental autoimmune encephalomyelitis in IL-12 receptor-beta 2-deficient mice: IL-12 responsiveness is not required in the pathogenesis of inflammatory demyelination in the central nervous system J Immunol 1702153–2160. [DOI] [PubMed] [Google Scholar]
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM.et al. (2005Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages Nat Immunol 61123–1132. [DOI] [PubMed] [Google Scholar]
- Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH.et al. (2005A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17 Nat Immunol 61133–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B.et al. (2003Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain Nature 421744–748. [DOI] [PubMed] [Google Scholar]
- El-Behi M, Ciric B, Dai H, Yan Y, Cullimore M, Safavi F.et al. (2011The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF Nat Immunol 12568–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal S., and, Gurney AL. IL-17: prototype member of an emerging cytokine family. J Leukoc Biol. 2002;71:1–8. [PubMed] [Google Scholar]
- Chang SH., and, Dong C. Signaling of interleukin-17 family cytokines in immunity and inflammation. Cell Signal. 2011;23:1069–1075. doi: 10.1016/j.cellsig.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolls JK., and, Lindén A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S.et al. (2006IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis J Immunol 177566–573. [DOI] [PubMed] [Google Scholar]
- Matusevicius D, Kivisäkk P, He B, Kostulas N, Ozenci V, Fredrikson S.et al. (1999Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis Mult Scler 5101–104. [DOI] [PubMed] [Google Scholar]
- McFarland HF., and, Martin R. Multiple sclerosis: a complicated picture of autoimmunity. Nat Immunol. 2007;8:913–919. doi: 10.1038/ni1507. [DOI] [PubMed] [Google Scholar]
- Tzartos JS, Friese MA, Craner MJ, Palace J, Newcombe J, Esiri MM.et al. (2008Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis Am J Pathol 172146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A, Frederick TJ., and, Miller SD. Astrocytes in multiple sclerosis: a product of their environment. Cell Mol Life Sci. 2008;65:2702–2720. doi: 10.1007/s00018-008-8059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y., and, Benveniste EN. Immune function of astrocytes. Glia. 2001;36:180–190. doi: 10.1002/glia.1107. [DOI] [PubMed] [Google Scholar]
- Calderon TM, Eugenin EA, Lopez L, Kumar SS, Hesselgesser J, Raine CS.et al. (2006A role for CXCL12 (SDF-1alpha) in the pathogenesis of multiple sclerosis: regulation of CXCL12 expression in astrocytes by soluble myelin basic protein J Neuroimmunol 17727–39. [DOI] [PubMed] [Google Scholar]
- Oksenberg JR, Seboun E., and, Hauser SL. Genetics of demyelinating diseases. Brain Pathol. 1996;6:289–302. doi: 10.1111/j.1750-3639.1996.tb00856.x. [DOI] [PubMed] [Google Scholar]
- Kang Z, Altuntas CZ, Gulen MF, Liu C, Giltiay N, Qin H.et al. (2010Astrocyte-restricted ablation of interleukin-17-induced Act1-mediated signaling ameliorates autoimmune encephalomyelitis Immunity 32414–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias AS, de la Hoz C, Castro FR, Oliveira EC, Ribeiro dos Reis JR, Silva JS.et al. (2007Nitric oxide and TNFalpha effects in experimental autoimmune encephalomyelitis demyelination Neuroimmunomodulation 1432–38. [DOI] [PubMed] [Google Scholar]
- Chen Y, Langrish CL, McKenzie B, Joyce-Shaikh B, Stumhofer JS, McClanahan T.et al. (2006Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis J Clin Invest 1161317–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou W., and, Restifo NP. T(H)17 cells in tumour immunity and immunotherapy. Nat Rev Immunol. 2010;10:248–256. doi: 10.1038/nri2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison PJ, Ballantyne SJ., and, Kullberg MC. Interleukin-23 and T helper 17-type responses in intestinal inflammation: from cytokines to T-cell plasticity. Immunology. 2011;133:397–408. doi: 10.1111/j.1365-2567.2011.03454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Veerdonk FL, Gresnigt MS, Kullberg BJ, van der Meer JW, Joosten LA., and, Netea MG. Th17 responses and host defense against microorganisms: an overview. BMB Rep. 2009;42:776–787. doi: 10.5483/bmbrep.2009.42.12.776. [DOI] [PubMed] [Google Scholar]
- Lee Y, Messing A, Su M., and, Brenner M. GFAP promoter elements required for region-specific and astrocyte-specific expression. Glia. 2008;56:481–493. doi: 10.1002/glia.20622. [DOI] [PubMed] [Google Scholar]
- Bauer M, Kinkl N, Meixner A, Kremmer E, Riemenschneider M, Förstl H.et al. (2009Prevention of interferon-stimulated gene expression using microRNA-designed hairpins Gene Ther 16142–147. [DOI] [PubMed] [Google Scholar]
- Boden D, Pusch O, Silbermann R, Lee F, Tucker L., and, Ramratnam B. Enhanced gene silencing of HIV-1 specific siRNA using microRNA designed hairpins. Nucleic Acids Res. 2004;32:1154–1158. doi: 10.1093/nar/gkh278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laan M, Cui ZH, Hoshino H, Lötvall J, Sjöstrand M, Gruenert DC.et al. (1999Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways J Immunol 1622347–2352. [PubMed] [Google Scholar]
- Franklin RJ., and, Ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci. 2008;9:839–855. doi: 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- Yang J, Jiang Z, Fitzgerald DC, Ma C, Yu S, Li H.et al. (2009Adult neural stem cells expressing IL-10 confer potent immunomodulation and remyelination in experimental autoimmune encephalitis J Clin Invest 1193678–3691. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Rasmussen S, Imitola J, Ayuso-Sacido A, Wang Y, Starossom SC, Kivisäkk P.et al. (2011Reversible neural stem cell niche dysfunction in a model of multiple sclerosis Ann Neurol 69878–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama T, Momoki-Soga T., and, Inoue N. Astrocyte-derived factors instruct differentiation of embryonic stem cells into neurons. Neurosci Res. 2003;46:241–249. doi: 10.1016/s0168-0102(03)00063-4. [DOI] [PubMed] [Google Scholar]
- Das Sarma J, Ciric B, Marek R, Sadhukhan S, Caruso ML, Shafagh J.et al. (2009Functional interleukin-17 receptor A is expressed in central nervous system glia and upregulated in experimental autoimmune encephalomyelitis J Neuroinflammation 614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi S, Hu B, Hahm K, Luo Y, Kam Hui ES, Yuan Q.et al. (2007LINGO-1 antagonist promotes spinal cord remyelination and axonal integrity in MOG-induced experimental autoimmune encephalomyelitis Nat Med 131228–1233. [DOI] [PubMed] [Google Scholar]
- Croxford JL, Feldmann M, Chernajovsky Y., and, Baker D. Different therapeutic outcomes in experimental allergic encephalomyelitis dependent upon the mode of delivery of IL-10: a comparison of the effects of protein, adenoviral or retroviral IL-10 delivery into the central nervous system. J Immunol. 2001;166:4124–4130. doi: 10.4049/jimmunol.166.6.4124. [DOI] [PubMed] [Google Scholar]
- Fiandaca MS., and, Bankiewicz KS. Gene therapy for Parkinson's disease: from non-human primates to humans. Curr Opin Mol Ther. 2010;12:519–529. [PubMed] [Google Scholar]
- Escors D., and, Breckpot K. Lentiviral vectors in gene therapy: their current status and future potential. Arch Immunol Ther Exp (Warsz) 2010;58:107–119. doi: 10.1007/s00005-010-0063-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bank A, Dorazio R., and, Leboulch P. A phase I/II clinical trial of beta-globin gene therapy for beta-thalassemia. Ann N Y Acad Sci. 2005;1054:308–316. doi: 10.1196/annals.1345.007. [DOI] [PubMed] [Google Scholar]
- Di Cianni S, Rossi M, Casati A, Cocco C., and, Fanelli G. Spinal anesthesia: an evergreen technique. Acta Biomed. 2008;79:9–17. [PubMed] [Google Scholar]
- Winn SR., and, Emerich DF. Managing chronic pain with encapsulated cell implants releasing catecholamines and endogenous opiods. Front Biosci. 2005;10:367–378. doi: 10.2741/1534. [DOI] [PubMed] [Google Scholar]
- Jin D, Liu HX, Hirai H, Torashima T, Nagai T, Lopatina O.et al. (2007CD38 is critical for social behaviour by regulating oxytocin secretion Nature 44641–45. [DOI] [PubMed] [Google Scholar]
- Yan Y, Zhang GX, Gran B, Fallarino F, Yu S, Li H.et al. (2010IDO upregulates regulatory T cells via tryptophan catabolite and suppresses encephalitogenic T cell responses in experimental autoimmune encephalomyelitis J Immunol 1855953–5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Sonobe Y, Akahori T, Jin S, Kawanokuchi J, Noda M.et al. (2011IL-9 promotes Th17 cell migration into the central nervous system via CC chemokine ligand-20 produced by astrocytes J Immunol 1864415–4421. [DOI] [PubMed] [Google Scholar]
- Yang J, Yan Y, Ciric B, Yu S, Guan Y, Xu H.et al. (2010Evaluation of bone marrow- and brain-derived neural stem cells in therapy of central nervous system autoimmunity Am J Pathol 1771989–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Vector map for astrocytes-specific expression.
Cytotoxicity of shAct1 lentivirus on astrocytes in vitro.
Expression of IL-17 family receptors.
Primers used for pLenti-GFAP-EGFP-mi-shAct1 vector construction.
Primers used for real-time quantitative RT-PCR analysis.