Abstract
Liver gene transfer for hemophilia B has shown very promising results in recent clinical studies. A potential complication of gene-based treatments for hemophilia and other inherited disorders, however, is the development of neutralizing antibodies (NAb) against the therapeutic transgene. The risk of developing NAb to the coagulation factor IX (F.IX) transgene product following adeno-associated virus (AAV)-mediated hepatic gene transfer for hemophilia is small but not absent, as formation of inhibitory antibodies to F.IX is observed in experimental animals following liver gene transfer. Thus, strategies to modulate antitransgene NAb responses are needed. Here, we used the anti-B cell monoclonal antibody rituximab (rtx) in combination with cyclosporine A (CsA) to eradicate anti-human F.IX NAb in rhesus macaques previously injected intravenously with AAV8 vectors expressing human F.IX. A short course of immunosuppression (IS) resulted in eradication of anti-F.IX NAb with restoration of plasma F.IX transgene product detection. In one animal, following IS anti-AAV6 antibodies also dropped below detection, allowing for successful AAV vector readministration and resulting in high levels (60% or normal) of F.IX transgene product in plasma. Though the number of animals is small, this study supports for the safety and efficacy of B cell-targeting therapies to eradicate NAb developed following AAV-mediated gene transfer.
Introduction
Adeno-associated virus (AAV) vectors are among the most efficient tools for in vivo gene transfer.1 Successful correction of hemophilia B has been demonstrated in small and large animal models of the disease2,3,4,5 using AAV vectors to express coagulation factor IX (F.IX) in the host liver.6,7,8,9 These findings were clinically translated in two clinical studies utilizing AAV vectors to transfer the F.IX transgene to the liver of severe hemophilia B subjects,10,11 both resulting in therapeutic levels of transgene expression.
One of the most important complications of hemophilia treatment is the formation of inhibitory antibodies directed against the therapeutic protein, commonly referred to as “inhibitors”. Inhibitor formation following conventional, protein replacement therapy for hemophilia B occurs in ~3% of patients.12 Several studies suggest that both genetic and environmental factors impact the risk of mounting an immune response to the infused F.IX protein.13 In preclinical studies of gene transfer for hemophilia B, the risk of inhibitor formation also seems to be a function of the underlying mutation within the F.IX gene, as a higher incidence of anti-F.IX antibody formation is observed in animals carrying null mutations.14,15 In addition, the gene transfer target tissue in part determines the overall risk of inhibitor formation, with gene transfer to muscle being more immunogenic than the same transgene delivered to liver.16 Liver gene transfer, in particular, is more likely to induce tolerance to the expressed transgene via the expansion of antigen-specific CD4+CD25+FoxP3+ regulatory T cells (Tregs).17,18,19,20 No inhibitor formation has been documented in nearly 50 hemophilia A and B subjects who have been enrolled in in vivo or ex vivo clinical gene transfer protocols thus far,10,11,21,22,23 confirming the safety of the approach. However, in all human gene transfer studies for hemophilia conducted to date, only patients at low risk of inhibitor formation (patients with repeated exposures to clotting factor with no history of inhibitor) were enrolled. To move gene therapy for hemophilia forward and make it clinically relevant, it will be important to be able to treat a broader spectrum of patients, including those at higher risk of inhibitor formation.
Here, we describe the pharmacological eradication of anti-human F.IX inhibitory antibodies in a nonhuman primate (NHP) model of AAV vector-mediated gene transfer to liver. Two animals developing long-lasting inhibitors following AAV gene transfer of F.IX to the liver were treated with a course of the calcineurin inhibitor cyclosporine A (CsA) combined with the B cell-depleting monoclonal antibody rituximab (rtx). This approach resulted in complete eradication of inhibitor in both animals and, in one animal, the additional benefit of reducing the anti-AAV antibody titer to levels that allowed for successful vector readministration.
This study provides evidence that relatively non-toxic short-term immunosuppression (IS) can result in eradication of inhibitors and the reduction of overall B-cell immunity in the setting of AAV-mediated gene transfer to the liver.
Results
Administration of a course of CsA and rtx results in the eradication of inhibitory antibodies to the human F.IX transgene product
NHP have been used extensively to study the safety of gene transfer approaches in a variety of settings; because of the high level of conservation of sequence between human and NHP, human transgenes can often be used without triggering an immune response. Development of neutralizing antibodies (NAb) to human coagulation factors, however, has been documented in NHP,7,24,25 and these can serve as a model for the study of NAb (inhibitors) that may arise in the course of novel gene therapy approaches. In the current study, following the intravenous administration of 2 × 1013 vector genomes (vg)/kg of an AAV8 vector encoding human F.IX under the control of a liver-specific promoter (AAV8-hAAT-hF.IX) to two animals (RQ6871 and RQ6889), both animals developed an anti-human F.IX inhibitory antibody. Human F.IX transgene expression was detected for a period of few weeks following gene transfer, but was then lost (Figure 1a). Concomitant with the disappearance of detectable hF.IX from plasma, anti-human F.IX inhibitory antibodies, measured with a modified Bethesda assay, became detectable, reaching a peak of about 25 Bethesda units (B.U.) in both animals (Figure 1b). Inhibitor titer declined over time to levels between 1.5 and 10 B.U., and persisted for at least 50 weeks following gene transfer; hF.IX transgene product was undetectable in plasma during this entire period.
Figure 1.
Human F.IX transgene plasma levels and anti-hF.IX antibody titers. (a) Human F.IX transgene expression levels in plasma measured by an ELISA assay specific for human F.IX, which did not cross-react with rhesus F.IX. (b) Anti-hF.IX inhibitory antibody titer measured with a modified Bethesda assay. Results are expressed in Bethesda units (B.U.), one B.U. corresponds to a residual activity of F.IX in plasma of 50%. (c) Anti-human F.IX IgG titer measured with a capture assay. Arrow (⇑): AAV8-hAAT-hF.IX vector administration at a dose of 2 × 1013 vg/kg. Asterisk (*): AAV6-hAAT-hF.IX vector readministration at 1 × 1013 vg/kg. Open circles: animal RQ6871; filled squares: animal RQ6889. Gray area: CsA administration; filled diamonds: rtx intravenous infusions. CsA, cyclosporine A; F.IX, coagulation factor IX; rtx, rituximab; vg, vector genomes.
Both animals retained normal clotting time while the inhibitor was detectable (not shown), indicating that the anti-human F.IX antibodies did not cross-react with endogenous rhesus F.IX.
In an attempt to eradicate the anti-hF.IX inhibitor, an immunosuppressive (IS) regimen based on oral CsA and intravenous infusion of rtx was administered to the animals. CsA was given orally for 9 weeks (week 58–66), whereas rtx was given as three intravenous doses at week 62, 65, and 69. A bolus of an intravenous antihistamine was also given to the animals at the time of rtx administration to prevent allergic reactions to the infused monoclonal antibody. Drug regimens based on combinations of rtx and other IS drugs have been previously used in patients affected by acquired hemophilia, showing a good efficacy and tolerability profile.26,27 No acute toxicity and no infections were associated with IS in either animal. In both animals, the Bethesda titer rapidly declined when IS was started, returning to baseline levels, without reappearing following cessation of therapy (Figure 1b). Eradication of inhibitor was associated with the detection of hF.IX transgene product in peripheral blood of both animals (Figure 1a); in animal RQ6889, hF.IX plasma levels returned to those measured before inhibitor development (~500 ng/ml). In animal RQ6871, immediately after IS hF.IX were considerably lower than the peak levels measured immediately after vector administration; in this animal, both neutralizing (Bethesda titer, Figure 1b) and total (IgG, Figure 2c) anti-hF.IX antibodies decreased to baseline levels over a longer period of time compared to RQ6889. After week 80, when eventually anti-hF.IX antibodies became undetectable also in animal RQ6871, hF.IX transgene levels in plasma increased up to ~200 ng/ml, probably reflecting the original plateau levels of hF.IX transgene expression for this animal.
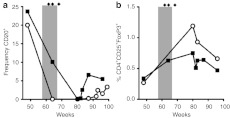
Figure 2.
Effect of IS on B and Treg cells. (a) Frequency of CD20+ B cells in peripheral blood in function of time. Results represented as % of CD20+ cell in total PBMC. (b) Frequency of CD4+CD25+FoxP3+ T cells in PBMC. Results represented as % CD4+CD25+FoxP3+ cells in total PBMC. Open circles: animal RQ6871; filled squares: animal RQ6889. Gray area: CsA administration; filled diamonds: rtx intravenous infusions. CsA, cyclosporine A; IS, immunosuppression; PBMC, peripheral blood mononuclear cells; rtx, rituximab.
Anti-human F.IX IgG capture assay performed on plasma from the two animals confirmed the development of anti-hF.IX antibodies following AAV8-hAAT-hF.IX, which declined to baseline levels following IS (Figure 1c).
IS results in profound depletion of B cells in peripheral blood with no change in frequency of circulating CD4+CD25+FoxP3+ T cells
Administration of CsA in combination with rtx resulted in disappearance of CD20+ B cells from peripheral blood of both animals treated (Figure 2a). B-cell depletion lasted for several weeks after IS was discontinued, with B cells again detectable after week 80 of observation, although at frequencies lower than baseline. Prolonged B-cell depletion following rtx administration at doses similar to the current study has been documented in humans and was not associated with adverse events such as an increased risk of infections.27,28 Furthermore, studies in humans receiving rtx also showed that the drug does not completely deplete B cells in lymphoid organs like the spleen,29 a finding which may explain why the kinetics of reduction in the frequency of CD20+ B cells in peripheral blood (Figure 2a) did not precisely overlap with that of eradication of anti-hF.IX antibodies (Figure 1a,b).
We and others previously showed that CD4+CD25+FoxP3+ regulatory T cells (Tregs) are important to establish tolerance to a transgene product following liver gene transfer,20 and that pharmacological interference with Tregs induction results in antitransgene product inhibitory antibody formation in NHP.24 To assess the impact of IS with CsA and rtx on Tregs, peripheral blood mononuclear cells (PBMC) from the treated animals were analyzed and no drop in frequency of CD4+CD25+FoxP3+ Tregs was documented (Figure 2b), suggesting that the IS regimen used did not result in Tregs depletion. This result is consistent with studies in humans showing that rtx does not affect the frequency of T cells.29,30 An increase in the frequency of Tregs was measured at week 80 in animal RQ6889, however, the significance of this finding is unclear (Figure 2b).
Influence of IS on anti-AAV vector NAb titer
To test the influence of combined CsA and rtx administration on anti-AAV antibodies, we used an in vitro neutralization assay.
After AAV8-hAAT-hF.IX vector administration, anti-AAV8 NAb titer rose from undetectable to >1:1,000; IS did not lower the anti-AAV8 NAb titer below the upper limit of detection of the NAb assay used in this study (1:1,000, data not shown).
An effect of IS was observed on anti-AAV6 NAb titer in at least one of the animals treated. In animal RQ6889, following AAV8-hAAT-hF.IX vector administration anti-AAV6 NAb titer increased from 1:1 (naive) to 1:31.6, a titer sufficient to block AAV vector transduction,10,31 and IS resulted in a drop of the titer back to the baseline level (Figure 3a). In this animal, anti-AAV6 IgG levels, which increased slightly after AAV8 vector administration, dropped further below the detection limit of the IgG capture assay (Figure 3b). The second animal tested, RQ6871, displayed higher baseline titer anti-AAV6 NAb, which increased further to 1:1,000 after AAV8 vector administration. IS did not result in a significant drop in anti-AAV6 NAb titer (Figure 3a), and anti-AAV6 IgG, despite dropping markedly, did not disappear completely (Figure 3b).
Figure 3.
Anti-AAV6 antibody titers. (a) Anti-AAV6 NAb titer over time. Titers are reported as the plasma dilution at which greater than 50% inhibition of luciferase signal was observed (log scale). (b) Anti-AAV6 IgG titer measured with a capture assay. Arrow (⇑): AAV8-hAAT-hF.IX vector administration at a dose of 2 × 1013 vg/kg. Asterisk (*): AAV6-hAAT-hF.IX vector readministration at 1 × 1013 vg/kg. Open circles: animal RQ6871; filled squares: animal RQ6889. Gray area: CsA administration; diamonds: rtx intravenous infusions. CsA, cyclosporine A; NAb, neutralizing antibody; rtx, rituximab; vg, vector genomes.
Vector readministration in the absence of NAb results in increased hF.IX transgene expression levels
The drop in anti-AAV6 antibody titer observed following IS administration provided a rationale to attempt vector readministration. For this purpose, an AAV6-hAAT-hF.IX vector, expressing the hF.IX transgene from the same transgene expression cassette used in the AAV8 vector initially administered, but packaged into an AAV serotype 6 capsid, was delivered intravenously at a dose of 1 × 1013 vg/kg, 3 and 8 months after IS was discontinued in animals RQ6889 and RQ6871, respectively. In animal RQ6889, the one with the lowest anti-AAV6 NAb titer after IS, hF.IX levels increased significantly (P = 0.0001) after vector administration from 644 ± 180 ng/ml (average ± SD weeks 60–83, ~12% of normal) to up to 2,258 ± 521 ng/ml (weeks 84–98, ~50% of normal, Figure 1a). Increase in hF.IX transgene expression levels was not associated with the development of inhibitory or noninhibitory antibody responses against the hF.IX transgene product (Figure 1b,c). Accordingly, no hF.IX-specific T-cell responses were detectable by IFN-γ ELISpot in PBMC (data not shown).
Vector readministration was also attempted in animal RQ6871, in which a drop of anti-AAV6 IgG antibody titer was observed following IS (Figure 3b). This animal, however, still retained an elevated anti-AAV NAb titer after IS (Figure 3a), and showed a modest increase in circulating hF.IX transgene product following vector readminstration (Figure 1a) (from 22 ± 9 ng/ml, weeks 60–83, to 81 ± 124 ng/ml, weeks 81–62, P = 0.0297).
Results of the vector readministration experiments are summarized in Table 1.
Table 1. Summary of study design and findings.
In both animals, following vector readministration anti-AAV6 IgG and NAb increased sharply, showing that both animals at this time had normal B-cell reactivity against the AAV capsid. No T-cell responses directed against AAV2, AAV6, or AAV8 capsids were detected following vector readministration (data not shown).
Discussion
AAV-mediated gene transfer for hemophilia B has recently been reported to produce promising results in human trials.10,11 Although the clinical experience with gene transfer for both hemophilia A and B suggests that the approach is safe, the risk of developing an inhibitor against the therapeutic transgene product remains a major concern. In all clinical studies of gene therapy for hemophilia to date,10,11,21,22,23 this risk was minimized by enrolling into trials only those subjects who had a history of repeated exposures to infused clotting factors with no evidence of inhibitor formation and, at least in one study (of muscle-directed gene transfer for hemophilia B),21 by enrolling only subjects carrying missense mutations within the F.IX gene, allowing production of a non-functional protein but tolerance to F.IX epitopes. Studies in rhesus macaques using human F.IX (which differs from the endogenous rhesus F.IX by 11 amino acid residues),32 however, showed the development of both non-neutralizing and NAb following plasma-derived protein infusion32 or liver expression of the human F.IX transgene,7,24,25 respectively. These findings suggest that the rhesus model could be utilized to study manipulation of inhibitor formation following human F.IX gene transfer.
It has been shown that liver expression of a transgene is likely to induce immune tolerance to the expressed antigen, rather than immunity. Following the initial description of tolerance induction to the human F.IX transgene product in mice with AAV hepatic gene transfer,20 several studies showed that hepatic gene transfer can trigger the expansion of a population of antigen-specific Tregs,17,18,19 which in turn modulate immune responses directed against the transgene itself.
Studies in mice and NHP reported that blockade of the IL-2 receptor CD25 (expressed at high levels in Tregs) prevented Treg expansion and led to inhibitor formation following AAV vector-mediated hepatic gene transfer.17,24 This is in agreement with recently published studies showing that IL-2 plays a fundamental role in the induction of Tregs in vivo.33,34 Thus, any interference with the induction of Tregs, such as the proposed use of IS drugs to modulate AAV capsid-specific T-cell responses,35 may increase the risk of inhibitor formation in the setting of AAV liver gene transfer for hemophilia.24,36
Another factor that may increase the overall immunogenicity of AAV-mediated gene transfer for hemophilia is the activation of the host innate immune system.37,38,39,40 Innate immune responses have been implicated in inhibitor development in hemophilia patients receiving protein replacement therapy41; in the setting of gene transfer with AAV vectors, recent data show that the structure of the viral genome and its interaction with toll-like receptors may increase the likelihood of developing responses against the transgene product.39
Given that inhibitor may develop, an important risk mitigation strategy is the development of a regimen to eradicate neutralizing antibodies to F.IX should they arise in the context of gene therapy. Here, we describe the successful pharmacological eradication of inhibitory antibodies directed against human F.IX in two rhesus macaques. Anti-B cell therapy with the monoclonal antibody rtx is used to treat acquired hemophilia; similarly, isolated reports showed that the combination of rtx administration with immune tolerance induction, is effective in eradicating inhibitory antibodies to F.IX.42,43 These results are in agreement with a previous report25 in which the successful eradication of an inhibitory antibody directed against the human F.IX transgene was achieved in one rhesus macaque using the same IS regimen used here; this report did not look at the effects of IS on B and T cells or the effect of this intervention on anti-AAV NAb. Rtx in combination with CsA has been used in the clinic to eradicate inhibitor in acquired hemophilia A,44 to treat lymphoproliferative disorders,45 and to treat autoimmune hemolytic anemia in infants.46
In the current study, animals developed persistent inhibitors following gene transfer, despite the continuous expression of the human F.IX transgene in hepatocytes. Finn and colleagues showed that AAV-mediated hepatic gene transfer for coagulation factor VIII was sufficient to eradicate inhibitors in hemophilia A dogs.36 The fact that in the current study, the pro-tolerogenic expression of the human F.IX transgene in liver failed to clear inhibitors without IS may simply reflect the experience with hemophilia B patients with inhibitor, where inhibitor eradication with immune tolerance induction has generally had a lower success rate than in hemophilia A patients with inhibitor.12
Finally, we showed that depletion of B cells with rtx in conjunction with administration of CsA resulted in a drop in anti-AAV NAb. This result is potentially important, as it may represent a relatively safe and simple intervention that would allow for vector administration in subjects otherwise ineligible for systemic vector delivery. Our results, however, also suggest that this approach is ineffective at lowering high-titer NAb, restricting the use of the IS regimen described here to subjects with low NAb titer or requiring additional measures to lower high-titer NAb to AAV vectors, such as plasmapheresis,47 to enhance the antibody-lowering effect of anti-B cell therapies. These results are in agreement with results in human subjects receiving rtx to manage nonmalignant autoimmune disease,48 in which a decrease in the anti-AAV NAb titer was observed only in those subjects with a low NAb titer before rtx administration. The fact that rtx administration does not invariably result in a drop of circulating antibodies is likely due to the fact that this anti-CD20–depleting antibody does not target CD20neg plasma cells and the fact that the administration of the drug does not result in complete depletion of B cells in lymphoid organs like the spleen.29 In the current study, coadministration of CsA, an IS drug targeting T cells, with rtx may have enhanced the overall anti-B cell effect of the IS regimen through the inhibition of cytokine production by T-helper cells.
In conclusion, we showed that a short course of IS with CsA and rtx resulted in inhibitor eradication in a NHP model of AAV-mediated liver gene transfer for hemophilia B. IS regimens targeting B cells may also facilitate systemic AAV gene transfer by further lowering low-titer NAb to vector capsid to levels that would allow for successful vector administration. These results provide a rationale for pharmacological intervention to eradicate inhibitors in AAV gene transfer to liver for hemophilia B and other inherited protein deficiencies, and raise the question as to whether these maneuvers could also be revisited in the setting of NAb to F.IX in protein replacement therapy.
Materials and Methods
Animals and animal procedures. Rhesus macaques (Macaca mulatta) were housed and handled in accordance with the guidelines set by the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council (DHHS publication no. NIH 85-23). Animals were screened for anti-AAV8 NAb before inclusion in the study and only naive (seronegative) animals were selected.
AAV vectors, rtx, and diphenhydramine were given intravenously as a bolus. CsA was given mixed with food. All experiments performed were approved by the Animal Care and Use Committees of the Children's Hospital of Philadelphia and the National Heart, Lung, and Blood Institute.
Vectors. Empty capsid-free AAV vectors preparations were obtained by triple transfection followed by cesium chloride gradient-based gradient purification as previously described.49 Vectors were resuspended in phosphate-buffered saline supplemented with F68. Vectors were titered by gel electrophoresis followed by silver staining and by quantitative real-time PCR. The expression cassette driving the expression of coagulation human F.IX under the control of the hepatocyte-specific promoter human α-1 antitrypsin (hAAT) has been previously described.10 For the purpose of the study, the same expression cassette was packaged into AAV serotype 6 and 8 vectors and delivered intravenously in saline solution.
IS agents. CsA (Neoral; Novartis Pharmaceuticals, East Hanover, NJ) was given orally at doses up to 25 mg/kg twice daily for about 9 weeks as previously described.25 Rituximab (Genentech-Roche, South San Francisco, CA) was given intravenously at a dose of 20 mg/kg (equivalent to ~375 mg/m2).25 Regimens based on a similar dose of rtx in addition to other immunosuppressants have been previously tested in patients with acquired hemophilia, showing good efficacy and tolerability profile.26,27 Animals received three doses of rtx at intervals of 3 and 4 weeks between the first and the second dose, and the second and the third dose, respectively. Rtx was coadministered with the antihistamine drug diphenhydramine given intravenously at a dose of 4 mg/kg to prevent allergic reactions against the infused monoclonal antibody rtx.
Flow cytometry. Frequency of CD20+ B cells in peripheral blood was determined by staining PBMC with an anti-CD20 antibody (Biolegend, San Diego, CA) followed by flow cytometry. Frequency of CD4+CD25+FoxP3+ Treg cells was determined with an antibody set from eBioscience (San Diego, CA) following the manufacturer's protocol. Samples were acquired on a BD Canto II flow cytometer (BD Bioscience, San Jose, CA) and analyzed with FlowJo version 8.8.3 software (Tree Star, Ashland, OR).
Factor IX antigen and anti-F.IX antibody determination. Human F.IX antigen levels in plasma were determined as previously described24 using an enzyme-linked immunosorbent assay specific for the human protein, which did not cross-react with the endogenous rhesus F.IX. Anti-hF.IX antibodies were measured as described24 with a capture assay in which human F.IX was coated onto enzyme-linked immunosorbent assay plates, test sera were added, and IgG specific for human F.IX were detected with a goat anti-rhesus IgG (H+L) antibody (Southern Biotech, Birmingham, AL).
Inhibitory antibodies to the human F.IX transgene product were determined with a modified Bethesda assay24 in which test plasma was heat-inactivated at 56 °C for 1 hour before testing to eliminate the activity of the endogenous rhesus F.IX.
Anti-AAV antibody determination. Anti-AAV IgG levels were determined with a capture assay as described by Mingozzi and colleagues.24 Anti-AAV NAb titers were determined using a modified version of a previously described in vitro assay.10 Briefly, an AAV vector carrying a self-complementary genome for the expression of the Renilla luciferase transgene under the control of the cytomegalovirus promoter/enhancer was incubated with semi-log dilutions of test sera. Luciferase activity on cell lysate was measured with a commercially available kit from Promega (Madison, WI). NAb titers were expressed as the reciprocal dilution at which 50% inhibition of luciferase activity was measured compared to the maximum activity (no inhibition).
ELISpot assay. Blood was collected from animals by venipuncture and PBMC were isolated from animals using heparinized DB Vacutainer CTP tubes (BD, Franklin Lakes, NJ). PBMC were cryopreserved until tested.
T-cell reactivity to either the human F.IX transgene or to alternate AAV serotypes was measured with an IFN-γ ELISpot assay as previously described,50 using an antibody set for human IFN-γ which showed high levels of cross-reactivity with the monkey protein (MabTech, Mariemont, OH).
As test antigens, recombinant human F.IX (Benefix; Pfizer, New York, NY) and purified AAV2, AAV6, and AAV8 empty capsids were used at a concentration of 25 µg/ml. Results were expressed as spot forming units per million PBMC plated in the assay; a positive response to an antigen was defined as a spot count of at least 50 spot forming units/million PBMC and threefold the count of the medium-only negative control.
Statistical analysis. Statistical analysis was performed with the Prism Version 5.0b (GraphPad Software, La Jolla, CA). Two-tailed unpaired t-test was used to compare means, P values <0.05 were considered significant.
Acknowledgments
This work was supported by the Howard Hughes Medical Institute (to K.A.H.) and by the National Heart, Lung, and Blood Institute (grant P01 HL078810 to K.A.H. and intramural funding to M.E.M., S.D.P., R.E.D., and C.E.D.). We thank the staff at 5 Research Court for their excellent care and handling of the rhesus macaques. F.M., J.F.W., and K.A.H. are inventors on patents related to AAV gene therapy and have consulted for companies developing gene therapeutics. The other authors declared no conflict of interest.
REFERENCES
- Mingozzi F., and, High KA. Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nat Rev Genet. 2011;12:341–355. doi: 10.1038/nrg2988. [DOI] [PubMed] [Google Scholar]
- Lin HF, Maeda N, Smithies O, Straight DL., and, Stafford DW. A coagulation factor IX-deficient mouse model for human hemophilia B. Blood. 1997;90:3962–3966. [PubMed] [Google Scholar]
- Evans JP, Brinkhous KM, Brayer GD, Reisner HM., and, High KA. Canine hemophilia B resulting from a point mutation with unusual consequences. Proc Natl Acad Sci USA. 1989;86:10095–10099. doi: 10.1073/pnas.86.24.10095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauser AE, Whitlark J, Whitney KM., and, Lothrop CD., Jr A deletion mutation causes hemophilia B in Lhasa Apso dogs. Blood. 1996;88:3451–3455. [PubMed] [Google Scholar]
- Wang L, Zoppè M, Hackeng TM, Griffin JH, Lee KF., and, Verma IM. A factor IX-deficient mouse model for hemophilia B gene therapy. Proc Natl Acad Sci USA. 1997;94:11563–11566. doi: 10.1073/pnas.94.21.11563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount JD, Herzog RW, Tillson DM, Goodman SA, Robinson N, McCleland ML.et al. (2002Sustained phenotypic correction of hemophilia B dogs with a factor IX null mutation by liver-directed gene therapy Blood 992670–2676. [DOI] [PubMed] [Google Scholar]
- Nathwani AC, Rosales C, McIntosh J, Rastegarlari G, Nathwani D, Raj D.et al. (2011Long-term safety and efficacy following systemic administration of a self-complementary AAV vector encoding human FIX pseudotyped with serotype 5 and 8 capsid proteins Mol Ther 19876–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemeyer GP, Herzog RW, Mount J, Arruda VR, Tillson DM, Hathcock J.et al. (2009Long-term correction of inhibitor-prone hemophilia B dogs treated with liver-directed AAV2-mediated factor IX gene therapy Blood 113797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder RO, Miao C, Meuse L, Tubb J, Donahue BA, Lin HF.et al. (1999Correction of hemophilia B in canine and murine models using recombinant adeno-associated viral vectors Nat Med 564–70. [DOI] [PubMed] [Google Scholar]
- Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ.et al. (2006Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response Nat Med 12342–347. [DOI] [PubMed] [Google Scholar]
- Nathwani AC, Tuddenham EG, Rangarajan S, Rosales C, McIntosh J, Linch DC.et al. (2011Adenovirus-associated virus vector-mediated gene transfer in hemophilia B N Engl J Med 3652357–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMichele D. Inhibitor development in haemophilia B: an orphan disease in need of attention. Br J Haematol. 2007;138:305–315. doi: 10.1111/j.1365-2141.2007.06657.x. [DOI] [PubMed] [Google Scholar]
- Chambost H. Assessing risk factors: prevention of inhibitors in haemophilia. Haemophilia. 2010;16 suppl. 2:10–15. doi: 10.1111/j.1365-2516.2009.02197.x. [DOI] [PubMed] [Google Scholar]
- Cao O, Hoffman BE, Moghimi B, Nayak S, Cooper M, Zhou S.et al. (2009Impact of the underlying mutation and the route of vector administration on immune responses to factor IX in gene therapy for hemophilia B Mol Ther 171733–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields PA, Arruda VR, Armstrong E, Chu K, Mingozzi F, Hagstrom JN.et al. (2001Risk and prevention of anti-factor IX formation in AAV-mediated gene transfer in the context of a large deletion of F9 Mol Ther 4201–210. [DOI] [PubMed] [Google Scholar]
- Herzog RW, Fields PA, Arruda VR, Brubaker JO, Armstrong E, McClintock D.et al. (2002Influence of vector dose on factor IX-specific T and B cell responses in muscle-directed gene therapy Hum Gene Ther 131281–1291. [DOI] [PubMed] [Google Scholar]
- Cao O, Dobrzynski E, Wang L, Nayak S, Mingle B, Terhorst C.et al. (2007Induction and role of regulatory CD4+CD25+ T cells in tolerance to the transgene product following hepatic in vivo gene transfer Blood 1101132–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrzynski E, Mingozzi F, Liu YL, Bendo E, Cao O, Wang L.et al. (2004Induction of antigen-specific CD4+ T-cell anergy and deletion by in vivo viral gene transfer Blood 104969–977. [DOI] [PubMed] [Google Scholar]
- Mátrai J, Cantore A, Bartholomae CC, Annoni A, Wang W, Acosta-Sanchez A.et al. (2011Hepatocyte-targeted expression by integrase-defective lentiviral vectors induces antigen-specific tolerance in mice with low genotoxic risk Hepatology 531696–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingozzi F, Liu YL, Dobrzynski E, Kaufhold A, Liu JH, Wang Y.et al. (2003Induction of immune tolerance to coagulation factor IX antigen by in vivo hepatic gene transfer J Clin Invest 1111347–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manno CS, Chew AJ, Hutchison S, Larson PJ, Herzog RW, Arruda VR.et al. (2003AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B Blood 1012963–2972. [DOI] [PubMed] [Google Scholar]
- Powell JS, Ragni MV, White GC 2nd, Lusher JM, Hillman-Wiseman C, Moon TE.et al. (2003Phase 1 trial of FVIII gene transfer for severe hemophilia A using a retroviral construct administered by peripheral intravenous infusion Blood 1022038–2045. [DOI] [PubMed] [Google Scholar]
- Roth DA, Tawa NE, Jr, O'Brien JM, Treco DA., and, Selden RF. Nonviral transfer of the gene encoding coagulation factor VIII in patients with severe hemophilia A. N Engl J Med. 2001;344:1735–1742. doi: 10.1056/NEJM200106073442301. [DOI] [PubMed] [Google Scholar]
- Mingozzi F, Hasbrouck NC, Basner-Tschakarjan E, Edmonson SA, Hui DJ, Sabatino DE.et al. (2007Modulation of tolerance to the transgene product in a nonhuman primate model of AAV-mediated gene transfer to liver Blood 1102334–2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathwani AC, Gray JT, Ng CY, Zhou J, Spence Y, Waddington SN.et al. (2006Self-complementary adeno-associated virus vectors containing a novel liver-specific human factor IX expression cassette enable highly efficient transduction of murine and nonhuman primate liver Blood 1072653–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field JJ, Fenske TS., and, Blinder MA. Rituximab for the treatment of patients with very high-titre acquired factor VIII inhibitors refractory to conventional chemotherapy. Haemophilia. 2007;13:46–50. doi: 10.1111/j.1365-2516.2006.01342.x. [DOI] [PubMed] [Google Scholar]
- Stasi R, Brunetti M, Stipa E., and, Amadori S. Selective B-cell depletion with rituximab for the treatment of patients with acquired hemophilia. Blood. 2004;103:4424–4428. doi: 10.1182/blood-2003-11-4075. [DOI] [PubMed] [Google Scholar]
- Sidner RA, Book BK, Agarwal A, Bearden CM, Vieira CA., and, Pescovitz MD. In vivo human B-cell subset recovery after in vivo depletion with rituximab, anti-human CD20 monoclonal antibody. Hum Antibodies. 2004;13:55–62. [PubMed] [Google Scholar]
- Audia S, Samson M, Guy J, Janikashvili N, Fraszczak J, Trad M.et al. (2011Immunologic effects of rituximab on the human spleen in immune thrombocytopenia Blood 1184394–4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arad U, Tzadok S, Amir S, Mandelboim M, Mendelson E, Wigler I.et al. (2011The cellular immune response to influenza vaccination is preserved in rheumatoid arthritis patients treated with rituximab Vaccine 291643–1648. [DOI] [PubMed] [Google Scholar]
- Jiang H, Couto LB, Patarroyo-White S, Liu T, Nagy D, Vargas JA.et al. (2006Effects of transient immunosuppression on adenoassociated, virus-mediated, liver-directed gene transfer in rhesus macaques and implications for human gene therapy Blood 1083321–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozier JN, Metzger ME, Donahue RE., and, Morgan RA. The rhesus macaque as an animal model for hemophilia B gene therapy. Blood. 1999;93:1875–1881. [PubMed] [Google Scholar]
- Grinberg-Bleyer Y, Baeyens A, You S, Elhage R, Fourcade G, Gregoire S.et al. (2010IL-2 reverses established type 1 diabetes in NOD mice by a local effect on pancreatic regulatory T cells J Exp Med 2071871–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadoun D, Rosenzwajg M, Joly F, Six A, Carrat F, Thibault V.et al. (2011Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis N Engl J Med 3652067–2077. [DOI] [PubMed] [Google Scholar]
- Mingozzi F., and, High KA. Immune responses to AAV in clinical trials. Curr Gene Ther. 2011;11:321–330. doi: 10.2174/156652311796150354. [DOI] [PubMed] [Google Scholar]
- Finn JD, Favaro P, Wright JF, Mingozzi F, High KA., and, Arruda VR. Rabbit anti-thymocyte globulin (rATG) administrated concomitantly with liver delivery of AAV2-hFIX can promote inhibitor formation in rhesus macaques. Blood. 2010;116:3765. [Google Scholar]
- Hösel M, Broxtermann M, Janicki H, Esser K, Arzberger S, Hartmann P.et al. (2012Toll-like receptor 2-mediated innate immune response in human nonparenchymal liver cells toward adeno-associated viral vectors Hepatology 55287–297. [DOI] [PubMed] [Google Scholar]
- Jayandharan GR, Aslanidi G, Martino AT, Jahn SC, Perrin GQ, Herzog RW.et al. (2011Activation of the NF-kappaB pathway by adeno-associated virus (AAV) vectors and its implications in immune response and gene therapy Proc Natl Acad Sci USA 1083743–3748. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Martino AT, Suzuki M, Markusic DM, Zolotukhin I, Ryals RC, Moghimi B.et al. (2011The genome of self-complementary adeno-associated viral vectors increases Toll-like receptor 9-dependent innate immune responses in the liver Blood 1176459–6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers GL, Martino AT, Aslanidi GV, Jayandharan GR, Srivastava A., and, Herzog RW. Innate Immune Responses to AAV Vectors. Front Microbiol. 2011;2:194. doi: 10.3389/fmicb.2011.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allacher P, Baumgartner CK, Pordes AG, Ahmad RU, Schwarz HP., and, Reipert BM. Stimulation and inhibition of FVIII-specific memory B-cell responses by CpG-B (ODN 1826), a ligand for Toll-like receptor 9. Blood. 2011;117:259–267. doi: 10.1182/blood-2010-06-289009. [DOI] [PubMed] [Google Scholar]
- Barnes C, Davis A, Furmedge J, Egan B, Donnan L., and, Monagle P. Induction of immune tolerance using rituximab in a child with severe haemophilia B with inhibitors and anaphylaxis to factor IX. Haemophilia. 2010;16:840–841. doi: 10.1111/j.1365-2516.2007.01446.x. [DOI] [PubMed] [Google Scholar]
- Fox RA, Neufeld EJ., and, Bennett CM. Rituximab for adolescents with haemophilia and high titre inhibitors. Haemophilia. 2006;12:218–222. doi: 10.1111/j.1365-2516.2006.01215.x. [DOI] [PubMed] [Google Scholar]
- Kam G, Lee YS, Tan TT, Chow P., and, Ng HJ. Surgery-associated acquired haemophilia and response to combined rituximab and cyclosporine treatment. Haemophilia. 2011;17:715–716. doi: 10.1111/j.1365-2516.2010.02458.x. [DOI] [PubMed] [Google Scholar]
- Ogimi C, Tanaka R, Arai T, Kikuchi A, Hanada R., and, Oh-Ishi T. Rituximab and cyclosporine therapy for accelerated phase Chediak-Higashi syndrome. Pediatr Blood Cancer. 2011;57:677–680. doi: 10.1002/pbc.23231. [DOI] [PubMed] [Google Scholar]
- Svahn J, Fioredda F, Calvillo M, Molinari AC, Micalizzi C, Banov L.et al. (2009Rituximab-based immunosuppression for autoimmune haemolytic anaemia in infants Br J Haematol 14596–100. [DOI] [PubMed] [Google Scholar]
- Monteilhet V, Saheb S, Boutin S, Leborgne C, Veron P, Montus MF.et al. (2011A 10 patient case report on the impact of plasmapheresis upon neutralizing factors against adeno-associated virus (AAV) types 1, 2, 6, and 8 Mol Ther 192084–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vervoordeldonk M, Mingozzi F, Edmonson S, Thurlings R, High K., and, Tak P. Pre-existing immunity to different adeno-associated virus serotypes in serum and synovial fluid of RA patients: implications for vector efficacy. Mol Ther. 2009;17:S316. [Google Scholar]
- Ayuso E, Mingozzi F, Montane J, Leon X, Anguela XM, Haurigot V.et al. (2010High AAV vector purity results in serotype- and tissue-independent enhancement of transduction efficiency Gene Ther 17503–510. [DOI] [PubMed] [Google Scholar]
- Mingozzi F, Maus MV, Hui DJ, Sabatino DE, Murphy SL, Rasko JE.et al. (2007CD8(+) T-cell responses to adeno-associated virus capsid in humans Nat Med 13419–422. [DOI] [PubMed] [Google Scholar]