Abstract
Erythropoiesis-stimulating agents are widely used to treat anemia for chronic kidney disease (CKD) and cancer, however, several clinical limitations impede their effectiveness. Nonviral gene therapy systems are a novel solution to these problems as they provide stable and low immunogenic protein expression levels. Here, we show the application of an arginine-grafted bioreducible poly(disulfide amine) (ABP) polymer gene delivery system as a platform for in vivo transfer of human erythropoietin plasmid DNA (phEPO) to produce long-term, therapeutic erythropoiesis. A single systemic injection of phEPO/ABP polyplex led to higher hematocrit levels over a 60-day period accompanied with reticulocytosis and high hEPO protein expression. In addition, we found that the distinct temporal and spatial distribution of phEPO/ABP polyplexes contributed to increased erythropoietic effects compared to those of traditional EPO therapies. Overall, our study suggests that ABP polymer-based gene therapy provides a promising clinical strategy to reach effective therapeutic levels of hEPO gene.
Introduction
Chronic kidney disease (CKD) is widely accepted as a global public health issue.1 Anemia is a major complication of CKD and is primarily due to impaired erythropoietin (EPO) production by the failing kidney, leading to EPO deficiency.2,3 CKD patients with anemia are at a higher risk for adverse medical outcomes, cardiovascular disease, hospitalizations, and mortality.2,4 Today, patients are treated by administration of recombinant human EPO (rHuEPO), which is used to treat anemia caused by CKD and cancer,5,6 and was approved by the US Food and Drug Administration in June 1989. According to a 2009 report of the top ten selling biopharmaceutical products, EPO occupied two spots.7 Despite widespread use of rHuEPO, several clinical limitations remain, including frequent injections, limited routes of administration, high medical expenditures, development of autoimmune pure red cell aplasia, and impacts on hemoglobin variability.2,8,9 To overcome many of these clinical hurdles, gene therapy providing continuous release has been suggested as an attractive alternative to current intermittently administered erythropoiesis-stimulating agents (ESAs).
Over 20 years ago, the first approved gene therapy was performed in humans.10 Gene delivery vectors are classified into viral and nonviral vectors, whose individual advantages and disadvantages have been well documented.11,12,13,14,15 In recent years, nonviral gene therapy has attracted attention due to its ease of modification, and its increased biosafety owing to lower immunogenicity and extrachromosomal maintenance.11,12,13,14,16,17 However, efforts towards using nonviral gene therapy via systemic delivery have been impeded by low levels of transfection and the lack of sustained gene expression.12,14,15,18,19
Recently, we developed an arginine-grafted bioreducible poly(CBA-DAH, disulfide amine) (ABP) polymer for nonviral polymer-based gene delivery.20 Combining the unique properties of bioreducible polymers with the advantages of arginine residues as cell-penetrating peptides, this ABP polymer showed very low cytotoxicity and greatly enhanced in vitro transfection efficiency.20,21,22,23,24 Here, we extended our previous in vitro studies by evaluating the erythropoietic effect of a single systemic ABP polymer-based phEPO delivery system on hematocrit level, reticulocyte count, plasma hEPO protein levels, and organ distribution of hEPO mRNA. Our findings indicate that the ABP polymer may be used as an advanced carrier for hEPO gene delivery, and may provide a potent and attractive clinical approach to enhance erythropoiesis in vivo.
Results
phEPO/ABP sustains higher hematocrit
Human EPO (hEPO) is a 34 kDa acidic glycoprotein hormone that controls erythropoiesis by receptor-mediated regulation of survival, proliferation, and differentiation of erythroid progenitor cells in the bone marrow (BM).25,26,27 The hEPO protein shares 79% amino acid homology with rat and mouse EPO. We selected phEPO rather than a rat EPO plasmid for injection because it enabled us to analyze the quantities of each type of EPO separately, differentiating exogenous from endogenous levels. The particular strength of nonviral polymeric gene delivery systems is their ability to protect genetic material from rapid degradation, improving pharmacokinetic and biodistribution profiles.15 Naked pDNA is not stable in blood and is degraded within minutes after intravenous injection.28 We previously reported phEPO/ABP polyplexes protected pDNA from degradation in vitro for over 6 hours in the presence of fetal bovine serum, which would allow for increased circulation time in vivo.20,24 Design of a hEPO gene therapy strategy using nonviral ABP polymers in the blood circulation may extend serum residence time, resulting in extended in vivo biological potency.
We initially characterized the size and potential changes of polyplexes in several buffer systems and evaluated the polyplex's stability in fresh rat serum, heparin and dithiothreitol by PicoGreen and gel electrophoresis assays (Supplementary Figures S1 and S2).24 The average size of polyplex formed with 100 and 200 µg phEPO/ABP at weight ratio 1/20 in 20 mmol/l HEPES/5% glucose solution was 99.8 ± 0.4 nm and 104.3 ± 0.5 nm, with average zeta-potentials of 25.6 ± 5.5 and 20.2 ± 4.6 mV, respectively. Previously, we investigated the in vitro transfection efficiency and cytotoxicity in various cells as well as the biological functional analysis by colony-forming assay and measurement of antiapoptotic activity.24 We injected a single dose of phEPO delivered by ABP polymer (phEPO/ABP polyplex) to investigate the erythropoietic effects into the tail vein of Sprague-Dawley (SD) rats.
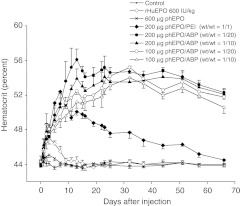
The in vivo effect of phEPO/ABP polyplex delivery was evaluated by hematocrit levels. A single intravenous injection of phEPO/ABP polyplex was able to sustain enhanced hematocrit levels for as long as 60 days after injection with peak levels of 56% (Figure 1). In the phEPO/ABP polyplex group, hematocrit levels were significantly increased at 5 days after injection compared to the control group (P < 0.001) and at 7 days after injection compared to the rHuEPO group (P < 0.001), showing that administration of phEPO/ABP polyplexes produced profound increases in red blood cell levels. The difference in hematocrit levels between the phEPO/ABP polyplex groups and the control group maintained significance through the termination of the study. There were no significant differences in hematocrit levels between the phEPO only group, the GFP pDNA/ABP polyplex group (data not shown) and the control group. Increased hematocrit in the rHuEPO group was lost within the first week (P < 0.05), indicating that constant EPO supply is required for the persistent erythrocytosis.
Figure 1.
Time-dependent increase in hematocrit after polyplex injection. Male Sprague-Dawley (SD) rats received a single intravenous administration of either 600 IU/kg recombinant human erythropoietin (rHuEPO) protein, 600 µg phEPO, phEPO/ABP polyplex, or 200 µg phEPO/PEI polyplex (wt/wt = 1/1). The phEPO/ABP polyplex group was injected with different phEPO amounts (100 and 200 µg) and pDNA/ABP polymer weight ratios (1/10 and 1/20). Data represent means ± SEM with n = 5–6 per group.
Time-dependent hematocrit levels were comparable between all phEPO/ABP polyplex groups, independent of the injected phEPO amounts (100 and 200 µg) and pDNA/ABP polymer weight ratios (1/10 and 1/20). These results imply that in the context of the dose–response relationship, maximal efficacy has been reached. This is supported by our observation that delivery of 50 µg phEPO at both 1/10 and 1/20 weight ratios with ABP increased hematocrit significantly from baseline but did not reach the same level of effect seen with the 100 and 200 µg ABP groups (Supplementary Figure S3). Hematocrit levels of all phEPO/ABP polyplex groups were increased higher and were sustained longer than phEPO/PEI polyplexes from 22 days forward (P < 0.001). This indicates that the ABP polymer-based gene delivery systems are more efficient at achieving long-term, therapeutic expression of hEPO.
Reticulocytosis and hEPO expression reflect the kinetics of phEPO/ABP
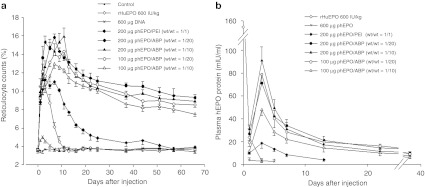
We examined in vivo erythropoietic effects of a single intravenous hEPO gene injection using flow cytometry for reticulocyte counts and ELISA for plasma hEPO levels. The reticulocyte counts in the phEPO/ABP polyplex groups were higher compared with the control group at 1 day postinjection (P < 0.001) and higher than the rHuEPO group at 5 days postinjection (P < 0.001) (Figure 2a). Three weeks after phEPO/PEI polyplex injection, reticulocyte counts had returned to control levels. Reticulocytosis in the rHuEPO group did not last more than 1 week after a single injection, matching the results seen in hematocrit levels. Reticulocytosis of the phEPO/ABP polyplex groups was significant relative to the phEPO/PEI polyplex group at 5 days after injection and remained significantly increased for over a 60-day course (P < 0.001).
Figure 2.
Erythropoietic kinetics of polyplex human erythropoietin (hEPO) gene delivery. (a) Reticulocytosis after injection. Reticulocyte counts as a percent of whole blood were measured by fluorescence-activated cell sorting analysis. (b) Effect of polymer-based hEPO gene delivery on hEPO protein levels. Plasma hEPO protein levels were evaluated by enzyme-linked immunosorbent assay (ELISA). Data represent means ± SEM with n = 5–6 per group.
To sustain erythropoiesis, a picomolar circulating concentration of EPO protein is required to prevent programmed cell death of erythrocyte precursors.9,29,30 The disproportionate relationship between EPO t1/2 and RBC lifespan results in a prolonged erythropoietic effect following a short duration of EPO production.9 In each group, the time-course of reticulocytosis bore a close resemblance to the time-dependent expression levels of plasma hEPO. Consistent with these observations, reticulocytosis and increased expression of hEPO in the phEPO/ABP polyplexes group relative to the control group correlated well with the observed elevated hematocrit levels. Maximum reticulocytosis occurred at 3–7 days after the peak levels in hEPO expression. The expression levels of hEPO in the phEPO/ABP polyplex group peaked 3 days after injection and were still significantly higher than the phEPO/PEI polyplex group through 5 weeks after injection with the exception of the 100 µg phEPO/ABP 1/10 group (Figure 2b). ABP polymer-based gene delivery exhibited markedly higher expression of hEPO than PEI polymer-based gene delivery, even though both polyplexes contained identical amounts of phEPO. The rHuEPO group showed peak levels of hEPO at 30 minutes after injection. Rats injected with 600 µg of phEPO only had nonspecific changes in hematocrit levels and hEPO expression of 3.8 ± 1.5 mIU/ml at 1 day after injection. hEPO expression of untreated control rats was below the sensitivity threshold (<2.5 mIU/ml) of the kit. Taken together these observations provide evidence that ABP polymer-based hEPO gene delivery systems can produce long-term EPO expression and erythropoietic effects in the form of increased reticulocytosis and hematocrit levels.
Temporal and spatial hEPO mRNA expression
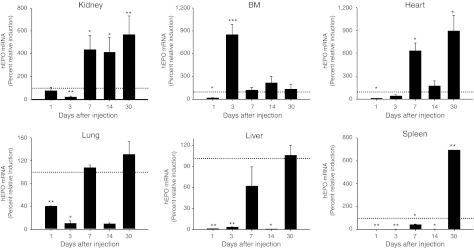
EPO production is primarily controlled through modulation of mRNA and increased mRNA stability by mechanisms that are not completely understood.9,31,32 Because EPO is not stored and is produced de novo primarily by fetal liver, adult kidney, and to some extent by nonrenal tissues, tissue EPO mRNA levels indicate the relative EPO contribution of the individual tissues to the overall pool.9,31,33,34,35 Polyplexes can be cleared from the blood by the reticuloendothelial system and can remain in organs, such as the liver and spleen, for prolonged periods of time.12,36,37,38 In our study, we set out to determine the in vivo mechanisms underlying the sustained long-term effects of phEPO/ABP polyplex gene delivery on erythropoiesis stimulation. To address this question, we focused on the time-dependent quantitative organ distribution of hEPO mRNA between different polyplex groups (Figure 3 and Supplementary Figure S4). We found that expression levels of hEPO mRNA showed different temporal and spatial distribution between the phEPO/ABP polyplex group and the phEPO/PEI polyplex group. Peak expression of hEPO mRNA was observed throughout all tissue types at 1 day following phEPO/PEI polyplex injection, but decreased in all tissues by 3 days, indicating rapid clearance by the reticuloendothelial system. This observation was most apparent in liver and spleen tissue where the drop in expression between 1 and 3 days was several thousand-fold. The expression of hEPO mRNA by both ABP and PEI polymer-based systems were not detectable in brain (data not shown).
Figure 3.
Temporal and spatial distribution of human erythropoietin (hEPO) mRNA expression after polyplex injection. Sprague-Dawley (SD) rats (n ≥ 4) were intravenously injected with either 200 µg phEPO/ABP at wt/wt ratio 1/10 or 200 µg phEPO/PEI at wt/wt ratio 1/1. We evaluated the in vivo expression level of hEPO mRNA using real-time quantitative reverse transcriptase (RT)-PCR at the indicated times in organs after injection of the polyplexes. Error bars represent SEM with n = 5–10 per group. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with phEPO/PEI (dashed line as 100%).
The elevations of hEPO mRNA induced by 200 µg of phEPO complexed with ABP polymer at the pDNA/polymer weight ratio 1/10 caused greater upregulation (P < 0.05) in the BM (3 days after injection), kidney (7, 14, and 30 days after injection), lung (7 days after injection), heart (7 and 30 days after injection) and spleen (30 days after injection) relative to the phEPO/PEI group. In spleen at 30 days after injection, the induction of hEPO mRNA by the phEPO/ABP group was enhanced 673-fold higher than the control group, and sevenfold higher than the phEPO/PEI group (P < 0.01). These data suggest that our ABP polymer-based hEPO gene delivery system is capable of stimulating extramedullary hematopoiesis in the spleen, as late as 30 days after phEPO/ABP polyplex injection and can sustain long-term erythropoietic capacity in vivo. This stands in strong contrast to PEI polymer-based gene delivery which does not exhibit this long-term expression profile.
In vitro cellular uptake supports in vivo erythropoiesis
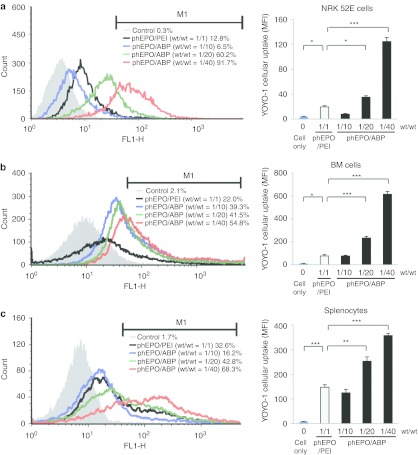
Previously, our group reported that cellular uptake of ABP polyplexes was similar to poly (CBA-DAH) polyplexes but displayed several times higher expression levels. ABP also demonstrated greater transfection efficiency in the presence of serum than poly (CBA-DAH). This lends weight to our hypothesis that the greatly improved transfection efficiency of ABP may be influenced by its arginine moieties, increasing nuclear localization.20 To determine whether in vitro cell-penetrating efficiency can help explain the higher and longer erythropoietic effects of phEPO/ABP in vivo, we measured the cellular uptake of YOYO-1 labeled phEPO in a variety of cell types.
In NRK normal rat kidney cells, the percent gated cellular uptake of phEPO/ABP polyplexes at weight ratio 1/40 was 7.2 times higher than phEPO/PEI polyplexes with 5.7 times higher mean fluorescence intensity (MFI) (Figure 4). This observation also held true in primary rat BM cells (2.5× % gated, and 6.3× MFI phEPO/ABP versus phEPO/PEI, respectively) and in primary rat splenocytes (2.1× % gated, and 2.8× MFI phEPO/ABP versus phEPO/PEI, respectively) (Figure 4). This same data trend was seen albeit to a lesser degree in HEK293 human embryonic kidney cells (1.2× % gated, and 2.4× MFI phEPO/ABP versus phEPO/PEI, respectively) and HepG2 human hepatocellular carcinoma cells (1.2× % gated, and 2.8× MFI phEPO/ABP versus phEPO/PEI, respectively; data not shown). Regarding the fact that cellular uptake is a major barrier which polyplexes must overcome to achieve efficient gene transfection, our in vitro results suggest that the sustained higher erythropoietic effects of our in vivo phEPO/ABP polyplex system can be attributed in part to its superior cell-penetrating transfection abilities.
Figure 4.
In vitro cellular uptake of polymer-based phEPO transfection. Polyplexes were prepared by mixing YOYO-1 iodide-tagged phEPO (2 µg) with ABP polymers at wt/wt ratios of 1/10, 1/20, and 1/40 in 20 mmol/l HEPES/5% glucose solution. The phEPO/PEI (wt/wt 1/1) complex was used as positive control. (a) NRK normal rat kidney cells, (b) primary rat bone marrow (BM) cells, and (c) primary rat splenocytes were analyzed by flow cytometry of M1 gated cellular uptake (%) with mean fluorescence intensity (MFI). Error bars represent SEM with n = 3 per group. *P < 0.05, **P < 0.01, and ***P < 0.001 versus phEPO/PEI polyplex.
Discussion
In the present study, we found that a single systemic injection of ABP-complexed phEPO sustained higher hematocrit levels, increased reticulocytosis, raised expression of plasma hEPO, and time-dependent BM–kidney–spleen distribution of hEPO mRNA compared with PEI-based gene delivery. Our results indicate that the bioreducible ABP polymer is a superior choice for in vivo gene delivery of phEPO and possibly for other genes as well. Thus, our ABP polymer-based gene therapy successfully met the prime requirements to produce therapeutic and sustained levels of functional phEPO transgene products and demonstrated the potential therapeutic value of this approach.
Nondegradable, nonviral carriers are not readily cleared and can accumulate within cells and tissues, eliciting toxicity.11,39 To solve this problem, several biodegradable polycations with lower cytotoxicity and higher transfection efficiencies have been synthesized and investigated as potential gene carriers.11 In our study, in vivo phEPO gene delivery was achieved by administering the transgenes using the nonviral vector, ABP. Arginine-rich sequences (e.g., octa-arginine and Tat sequences) as cell-penetrating peptides have been extensively used to overcome both extracellular penetration and intracellular expression limitations of pDNA, siRNA, proteins, and liposomes.19,40 Our previous in vitro transfection results with endosomal inhibitors chloroquine and nigericin indicate that ABP polyplexes escape from endosomes by direct endosomal membrane penetration of arginine moieties as well as endosome buffering abilities of the polyplexes after cellular uptake.20 ABP was able to condense pDNA into ideally sized (<200 nm), positively charged particles, allowing for prolonged circulation and efficient endosomal uptake.12,24
We observed different temporal and spatial distribution of hEPO mRNA expression between our ABP polymer-transfected and PEI polymer-transfected groups. Several explanations for this potent and long-term erythropoietic effect by phEPO/ABP gene therapy are possible. First, the expression of the EPO gene is regulated in an oxygen-dependent and tissue-specific manner.31,41,42,43 Investigation into tissue-specific regulatory mechanisms of EPO production is significant for designing new strategies to treat human disease. After acute anemia caused by blood loss, there is a major difference between liver and kidney EPO mRNA characteristics.25,41,44 An increasing number of cells with fixed EPO mRNA content are recruited in the kidney, whereas the amount of EPO mRNA per hepatocyte appears to rise under anemic conditions.25,41,44
Second, during periods of hematopoietic stress (including rHuEPO treatment45), pathological conditions, and fetal development,35 hematopoietic stem cells are capable of erythropoiesis in extramedullary organs such as the liver, spleen, brain, and heart.42,46,47 The BM is a major site of erythropoiesis in steady state, whereas the spleen is a reserve erythropoietic organ, which promotes the expansion of a specialized population of stress erythroid progenitors.45,47 The expansion of erythropoiesis that occurs in the murine spleen is due in part to the migration phenomenon of burst-forming unit-erythroid from the BM to the spleen. In addition, the supportive microenvironment more efficiently produces colony-forming unit-erythroid in the spleen than in the BM, which may induce the generation of a hematopoietic niche in spleen.45
Third, transfected organs can serve as depots for the synthesis, controlled release, and secretion of therapeutic protein.37 Due to the potent endocrine/paracrine actions of hormones such as EPO, growth hormone, and parathyroid hormone, the impact of a small amount of DNA is amplified in the body through multiple signaling cascades.37,48,49 Particulate drug delivery systems cause increased accumulation of cargo in the mononuclear phagocyte system cells of the liver, spleen, and BM.15 Our results suggest that the spleen plays a role as a depot organ for phEPO gene expression. Because the phEPO/ABP polyplexes “arrest” in the spleen for extended periods, they are particularly valuable to produce prolonged erythropoiesis in vivo.
Fourth, a direct and disproportionate correlation exists between serum EPO concentrations and RBC production.9 The estimated lifespan of RBCs is between 41 and 60 days in rat, compared to 100–120 days in human, with a daily loss of ~0.8–1.0% of circulating RBCs.9 Because of the long half-life of erythrocytes, once a short duration of high EPO expression triggers prolonged RBC production, it may take months until hematocrit values return to normal.9,50
There are a second set of disorders identified among ESA hyporesponsive patients, such as persistent iron deficiency, inflammatory disease, infection, and hematologic malignancy.2 Upregulation of inflammatory cytokines like interleukin (IL)-6 results in a rapid increase in hepcidin, a key regulatory protein in iron homeostasis. We evaluated the in vivo innate immune response within our experimental groups by measuring plasma IL-6 levels by ELISA (Supplementary Figure S5). The plasma IL-6 levels of 200 µg phEPO/PEI polyplex were significantly increased 6 and 12 hours after injection compared with other groups. No statistically significant increase in IL-6 levels was observed in any of the other treatment groups at any time points.
In the 65-day window within which we monitored therapeutic indicators of EPO effect, hematocrit and reticulocyte levels were decreasing back towards baseline levels but remained higher than at the study onset. We hypothesize that this long-term expression may be due to stimulation of a positive feedback loop by the high levels of transfected EPO after injection.51 Additionally, this study was performed in nondiseased animals with normal EPO levels before treatment. We are currently following up on these results with a CKD animal study to investigate the duration of effect in a diseased animal model.
Systemic nonviral gene therapy has been impeded by the low levels of transfection efficiency and lack of sustained gene expression.14,15,18,19 Overall, our studies demonstrate that a single intravenous administration of phEPO using the nonviral bioreducible ABP polymer-based gene delivery system can be feasible for delivering functional hEPO gene for therapeutically significant systemic erythropoiesis. This bioreducible ABP polymer-based hEPO gene delivery system has the potential to be a convenient, long-lasting, promising, and effective gene-based therapy for the treatment of anemia. ABP polymer-based gene delivery could lead to development of a pipeline of gene therapy products that are suitable for clinical use.
Materials and Methods
Rats. We purchased male SD rats from Charles River Laboratories (Wilmington, MA) at 6–7 weeks of age. All rats were housed in the University of Utah under the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC) guidelines. All experiments were approved by the University of Utah Institutional Animal Care and Use Committee and followed the guidelines provided by the National Institutes of Health in Guide for the Care and Use of Laboratory Animals. All rats had access to food and water ad libitum and were housed in plastic cages on standard 12/12 hours light/dark cycles. The rats were randomly assigned to the one of eight groups: (i) negative control, (ii) rHuEPO protein injection, (iii) hEPO plasmid DNA (phEPO) injection, (iv–vii) phEPO/ABP polyplex injection at varying concentrations, (viii) phEPO/PEI polyplex injection, and (ix) GFP pDNA/ABP polyplex injection.
Preparation of phEPO/polymer polyplexes. We constructed and purified the pCMV-hEPO DNA (phEPO) (4,578 bp) as previously described.24 phEPO and GFP pDNA (gWiz-GFP; Aldevron, Madison, WI) were purified with an endotoxin-free plasmid DNA purification NucleoBond Xtra Maxi plus EF kit (Macherey-Nagel, Bethlehem, PA). The ABP polymer was synthesized as previously described.20 Branched poly(ethylenimine) (bPEI, 25 kDa; Sigma-Aldrich, St Louis, MO) and rHuEPO protein (Aropotin) were used as controls. The phEPO polyplexes were prepared in a 20 mmol/l HEPES/5% glucose buffer. After incubation for 30 minutes at room temperature, the particle size of the polyplex samples was evaluated by dynamic light scattering using a Zetasizer Nano ZS (Malvern Instuments, Malvern, UK). Surface charge was measured by determination of zeta potential using the same instrument.
Single systemic injection of phEPO polyplexes. We administered a single injection for 8–10 minutes into the tail vein of the rats using a 24-gauge intravenous catheter in a final volume of 1 ml. The injected amounts of phEPO were 100 and 200 µg at pDNA/ABP polymer weight ratios of 1/10 and 1/20. The 20 mmol/l HEPES/5% glucose solution was the vehicle for injection. The phEPO alone group received 600 µg of phEPO without polymer. The rHuEPO proteins were injected at 600 IU/kg. The phEPO/PEI polyplex at a 1/1 wt/wt ratio was used as a positive control.
Erythropoietic parameters. Blood samples from the tail vein of SD rats were drawn into both heparinized micro-Hct capillary tubes (Fisher Scientific, Pittsburgh, PA) and K2EDTA tubes (BD Microtainer, Franklin Lakes, NJ). Hematocrit values were measured by the microhematocrit method. For whole blood reticulocyte counts, peripheral blood cells were stained with thiazole orange (BD Retic-Count, Reticulocyte Reagent System; BD Biosciences, Mountain View, CA) for 30 minutes at room temperature in the dark and fluorescence intensity was measured for 50,000 events on a BD FACSCalibur (BD Biosciences). The plasma concentration of hEPO protein was determined with a Quantikine hEPO ELISA kit (R&D Systems, Minneapolis, MN), and the plasma concentration of IL-6 was measured by the Quantikine rat IL-6 ELISA kit (R&D Systems) according to the manufacturer's protocol, respectively.
Real-time quantitative reverse transcriptase-PCR. We intravenously injected SD rats with 200 µg phEPO with ABP at a wt/wt ratio of 1/10 and 200 µg phEPO with PEI at a wt/wt ratio of 1/1. After 1, 3, 7, 14, and 30 days postinjection, rats were sacrificed and tissue from brain, heart, lung, liver, spleen, and kidney were homogenized using a Mini-Beadbeater (Biospec Products, Bartlesville, OK) with TRIzol reagent (Invitrogen, Carlsbad, CA). We isolated total mRNA with the Maxwell 16 instrument and kit (Maxwell 16 tissue LEV total RNA purification kit; Promega, Fitchburg, WI) according to the manufacturer's instructions. Including the no-reverse transcriptase control and no-template control, we performed quantitative real-time PCR analysis of 50 ng of RNA template with Express One-Step SuperScript qRT-PCR kits (Invitrogen) under the StepOnePlus real-time PCR system in a 96-well setup (Applied Biosystems, Carlsbad, CA). We used the FAM Taqman probes and amplified hEPO mRNA with a primer for hEPO (Hs01071097_m1 EPO; Applied Biosystems). Concentrations of hEPO mRNA were normalized to rat β-actin mRNA (Rn00667869_m1 Actb; Applied Biosystems) and the results were expressed as the perecent relative induction between the phEPO/ABP polyplex groups relative to the phEPO/PEI polyplex group.
In vitro cellular uptake assay. Spleens from SD rats were isolated and homogenized by grinding the tissue between the frosted ends of sterilized slides. The homogenate was then passed through a 40-µm nylon cell strainer to produce a single cell suspension. The suspension was centrifuged, erythrocytes were lysed using RBC lysis buffer, and the purified cells were seeded in RPMI-1640 medium. Preparation of BM cell cultures was based on a previously described protocol.42 Briefly, BM cells were isolated from the bilateral femur of 7-week-old SD rats and prepared with Dulbecco's modified Eagle's medium. Also, HepG2, HEK293, and NRK cells were cultured. YOYO-1 iodide- (1 mmol/l solution in DMSO; Molecular Probes, Eugene, OR) tagged phEPO (1 molecule dye per 50 bp nucleotide) was prepared in the dark for 30 minutes. Polyplexes were prepared by mixing YOYO-1 iodide-labeled phEPO (2 µg) with ABP polymer at wt/wt ratios of 1/10, 1/20, and 1/40 in 20 mmol/l HEPES/5% glucose solution, and incubated at room temperature for 30 minutes before transfection. phEPO/PEI (wt/wt 1/1) was used as a positive control. The polyplexes were incubated with cells at 37 °C for 4 hours in serum free media. Samples were analyzed by flow cytometry (FACS Caliber; BD Biosciences, San Jose, CA) at a minimum of 1 × 104 cells using the FL1-height channel for YOYO-1 dye. Untreated cells were used as a negative control for calibration. Cellular uptake (%) was gated on the M1 region and the MFI of each group was recorded. Data were analyzed using Windows Multiple Document Interface Software, version 2.9 (WinMDI; Microsoft, Redmond, WA).
Statistical analysis. We expressed data as mean ± SEM where indicated. Comparisons between multiple groups were performed by analysis of variance followed by Tukey post hoc testing. Comparisons between two samples were analyzed for homogeneity of variance using the Levene test and analyzed by Student t-test or Mann–Whitney rank-sum test as appropriate. Groups with P values <0.05 were considered statistically significant.
SUPPLEMENTARY MATERIAL Figure S1. Average size measurement of ABP polyplexes by DLS. Figure S2. Average zeta potential measurement of ABP polyplex. Figure S3. Hematocrit levels after polyplex injection with 50 µg phEPO/ABP (at wt/wt ratio 1/10 and 1/20), 200 µg phEPO/ABP (at wt/wt ratio 1/20), 100 µg phEPO/PEI (at wt/wt ratio 1/1), and 200 µg phEPO/PEI (at wt/wt ratio 1/1). Figure S4. Percent induction of hEPO mRNA relative to the 1-day phEPO/PEI mRNA levels as 100%. Figure S5. In vivo time-dependent plasma concentration of IL-6.
Acknowledgments
This work was supported by NIH grant HL065477. We thank Arlo N. McGinn (University of Utah) for technical support. We extend thanks to Jerry Spangrude (School of Medicine, University of Utah) for general. Recombinant human erythropoietin (Aropotin) was a generous gift from the TS Corporation (Seoul, Republic of Korea).
Supplementary Material
Average size measurement of ABP polyplexes by DLS.
Average zeta potential measurement of ABP polyplex.
Hematocrit levels after polyplex injection with 50 µg phEPO/ABP (at wt/wt ratio 1/10 and 1/20), 200 µg phEPO/ABP (at wt/wt ratio 1/20), 100 µg phEPO/PEI (at wt/wt ratio 1/1), and 200 µg phEPO/PEI (at wt/wt ratio 1/1).
Percent induction of hEPO mRNA relative to the 1-day phEPO/PEI mRNA levels as 100%.
In vivo time-dependent plasma concentration of IL-6.
REFERENCES
- KDOQI and NKF 2002K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification Am J Kidney Dis 392 Suppl 1): S1–S266. [PubMed] [Google Scholar]
- KDOQI and NKF KDOQI clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease. Am J Kidney Dis. 2006;47 5 Suppl 3:S11–S145. doi: 10.1053/j.ajkd.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Eschbach JW., and, Adamson JW. Anemia of end-stage renal disease (ESRD) Kidney Int. 1985;28:1–5. doi: 10.1038/ki.1985.109. [DOI] [PubMed] [Google Scholar]
- Locatelli F, Pisoni RL, Combe C, Bommer J, Andreucci VE, Piera L.et al. (2004Anaemia in haemodialysis patients of five European countries: association with morbidity and mortality in the Dialysis Outcomes and Practice Patterns Study (DOPPS) Nephrol Dial Transplant 19121–132. [DOI] [PubMed] [Google Scholar]
- Eschbach JW, Egrie JC, Downing MR, Browne JK., and, Adamson JW. Correction of the anemia of end-stage renal disease with recombinant human erythropoietin. Results of a combined phase I and II clinical trial. N Engl J Med. 1987;316:73–78. doi: 10.1056/NEJM198701083160203. [DOI] [PubMed] [Google Scholar]
- Rizzo JD, Brouwers M, Hurley P, Seidenfeld J, Arcasoy MO, Spivak JL, American Society of Clinical Oncology; American Society of Hematology et al. American Society of Clinical Oncology/American Society of Hematology clinical practice guideline update on the use of epoetin and darbepoetin in adult patients with cancer. J Clin Oncol. 2010;28:4996–5010. doi: 10.1200/JCO.2010.29.2201. [DOI] [PubMed] [Google Scholar]
- Walsh G. Biopharmaceutical benchmarks 2010. Nat Biotechnol. 2010;28:917–924. doi: 10.1038/nbt0910-917. [DOI] [PubMed] [Google Scholar]
- Levin NW, Fishbane S, Cañedo FV, Zeig S, Nassar GM, Moran JE, MAXIMA study investigators et al. Intravenous methoxy polyethylene glycol-epoetin beta for haemoglobin control in patients with chronic kidney disease who are on dialysis: a randomised non-inferiority trial (MAXIMA) Lancet. 2007;370:1415–1421. doi: 10.1016/S0140-6736(07)61599-2. [DOI] [PubMed] [Google Scholar]
- Elliott S, Pham E., and, Macdougall IC. Erythropoietins: a common mechanism of action. Exp Hematol. 2008;36:1573–1584. doi: 10.1016/j.exphem.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Gershon D. Human gene therapy. First experiment approved. Nature. 1990;346:402. doi: 10.1038/346402a0. [DOI] [PubMed] [Google Scholar]
- Park TG, Jeong JH., and, Kim SW. Current status of polymeric gene delivery systems. Adv Drug Deliv Rev. 2006;58:467–486. doi: 10.1016/j.addr.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Petros RA., and, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov. 2010;9:615–627. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- Papapetrou EP, Zoumbos NC., and, Athanassiadou A. Genetic modification of hematopoietic stem cells with nonviral systems: past progress and future prospects. Gene Ther. 2005;12 Suppl 1:S118–S130. doi: 10.1038/sj.gt.3302626. [DOI] [PubMed] [Google Scholar]
- Duncan R. The dawning era of polymer therapeutics. Nat Rev Drug Discov. 2003;2:347–360. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- Allen TM., and, Cullis PR. Drug delivery systems: entering the mainstream. Science. 2004;303:1818–1822. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- Miller HI. Gene therapy on trial. Science. 2000;287:591–592. doi: 10.1126/science.287.5453.591c. [DOI] [PubMed] [Google Scholar]
- Wadman M. NIH under fire over gene-therapy trials. Nature. 2000;403:237. doi: 10.1038/35002176. [DOI] [PubMed] [Google Scholar]
- Mastrobattista E, van der Aa MA, Hennink WE., and, Crommelin DJ. Artificial viruses: a nanotechnological approach to gene delivery. Nat Rev Drug Discov. 2006;5:115–121. doi: 10.1038/nrd1960. [DOI] [PubMed] [Google Scholar]
- Heitz F, Morris MC., and, Divita G. Twenty years of cell-penetrating peptides: from molecular mechanisms to therapeutics. Br J Pharmacol. 2009;157:195–206. doi: 10.1111/j.1476-5381.2009.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TI, Ou M, Lee M., and, Kim SW. Arginine-grafted bioreducible poly(disulfide amine) for gene delivery systems. Biomaterials. 2009;30:658–664. doi: 10.1016/j.biomaterials.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M., and, Weissleder R. Intracellular cargo delivery using tat peptide and derivatives. Med Res Rev. 2004;24:1–12. doi: 10.1002/med.10056. [DOI] [PubMed] [Google Scholar]
- Brooks H, Lebleu B., and, Vivès E. Tat peptide-mediated cellular delivery: back to basics. Adv Drug Deliv Rev. 2005;57:559–577. doi: 10.1016/j.addr.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Tung CH., and, Weissleder R. Arginine containing peptides as delivery vectors. Adv Drug Deliv Rev. 2003;55:281–294. doi: 10.1016/s0169-409x(02)00183-7. [DOI] [PubMed] [Google Scholar]
- Nam HY, Lee Y, Lee M, Shin SK, Kim TI, Kim SW.et al. (2012Erythropoietin gene delivery using an arginine-grafted bioreducible polymer system J Control Release 157437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koury ST, Bondurant MC., and, Koury MJ. Localization of erythropoietin synthesizing cells in murine kidneys by in situ hybridization. Blood. 1988;71:524–527. [PubMed] [Google Scholar]
- Krantz SB. Erythropoietin. Blood. 1991;77:419–434. [PubMed] [Google Scholar]
- Dessypris E, Graber SE, Krantz SB., and, Stone WJ. Effects of recombinant erythropoietin on the concentration and cycling status of human marrow hematopoietic progenitor cells in vivo. Blood. 1988;72:2060–2062. [PubMed] [Google Scholar]
- Lechardeur D, Sohn KJ, Haardt M, Joshi PB, Monck M, Graham RW.et al. (1999Metabolic instability of plasmid DNA in the cytosol: a potential barrier to gene transfer Gene Ther 6482–497. [DOI] [PubMed] [Google Scholar]
- Brines M. The therapeutic potential of erythropoiesis-stimulating agents for tissue protection: a tale of two receptors. Blood Purif. 2010;29:86–92. doi: 10.1159/000245630. [DOI] [PubMed] [Google Scholar]
- Brines M., and, Cerami A. Erythropoietin-mediated tissue protection: reducing collateral damage from the primary injury response. J Intern Med. 2008;264:405–432. doi: 10.1111/j.1365-2796.2008.02024.x. [DOI] [PubMed] [Google Scholar]
- Weidemann A., and, Johnson RS. Nonrenal regulation of EPO synthesis. Kidney Int. 2009;75:682–688. doi: 10.1038/ki.2008.687. [DOI] [PubMed] [Google Scholar]
- Goldberg MA, Gaut CC., and, Bunn HF. Erythropoietin mRNA levels are governed by both the rate of gene transcription and posttranscriptional events. Blood. 1991;77:271–277. [PubMed] [Google Scholar]
- Jacobson LO, Goldwasser E, Fried W., and, Plzak L. Role of the kidney in erythropoiesis. Nature. 1957;179:633–634. doi: 10.1038/179633a0. [DOI] [PubMed] [Google Scholar]
- Zanjani ED, Poster J, Burlington H, Mann LI., and, Wasserman LR. Liver as the primary site of erythropoietin formation in the fetus. J Lab Clin Med. 1977;89:640–644. [PubMed] [Google Scholar]
- Dame C, Fahnenstich H, Freitag P, Hofmann D, Abdul-Nour T, Bartmann P.et al. (1998Erythropoietin mRNA expression in human fetal and neonatal tissue Blood 923218–3225. [PubMed] [Google Scholar]
- Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A.et al. (1990Direct gene transfer into mouse muscle in vivo Science 2474949 Pt 11465–1468. [DOI] [PubMed] [Google Scholar]
- Felgner PL., and, Rhodes G. Gene therapeutics. Nature. 1991;349:351–352. doi: 10.1038/349351a0. [DOI] [PubMed] [Google Scholar]
- Lammers T, Kiessling F, Hennink WE., and, Storm G. Nanotheranostics and image-guided drug delivery: current concepts and future directions. Mol Pharm. 2010;7:1899–1912. doi: 10.1021/mp100228v. [DOI] [PubMed] [Google Scholar]
- He CX, Tabata Y., and, Gao JQ. Non-viral gene delivery carrier and its three-dimensional transfection system. Int J Pharm. 2010;386:232–242. doi: 10.1016/j.ijpharm.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Nakase I, Takeuchi T, Tanaka G., and, Futaki S. Methodological and cellular aspects that govern the internalization mechanisms of arginine-rich cell-penetrating peptides. Adv Drug Deliv Rev. 2008;60:598–607. doi: 10.1016/j.addr.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Stockmann C., and, Fandrey J. Hypoxia-induced erythropoietin production: a paradigm for oxygen-regulated gene expression. Clin Exp Pharmacol Physiol. 2006;33:968–979. doi: 10.1111/j.1440-1681.2006.04474.x. [DOI] [PubMed] [Google Scholar]
- Tan CC, Eckardt KU., and, Ratcliffe PJ. Organ distribution of erythropoietin messenger RNA in normal and uremic rats. Kidney Int. 1991;40:69–76. doi: 10.1038/ki.1991.181. [DOI] [PubMed] [Google Scholar]
- Chikuma M, Masuda S, Kobayashi T, Nagao M., and, Sasaki R. Tissue-specific regulation of erythropoietin production in the murine kidney, brain, and uterus. Am J Physiol Endocrinol Metab. 2000;279:E1242–E1248. doi: 10.1152/ajpendo.2000.279.6.E1242. [DOI] [PubMed] [Google Scholar]
- Koury ST, Bondurant MC, Koury MJ., and, Semenza GL. Localization of cells producing erythropoietin in murine liver by in situ hybridization. Blood. 1991;77:2497–2503. [PubMed] [Google Scholar]
- Nijhof W, Goris H, Dontje B, Dresz J., and, Loeffler M. Optimal erythroid cell production during erythropoietin treatment of mice occurs by exploiting the splenic microenvironment. Exp Hematol. 1993;21:496–501. [PubMed] [Google Scholar]
- Digicaylioglu M., and, Lipton SA. Erythropoietin-mediated neuroprotection involves cross-talk between Jak2 and NF-kappaB signalling cascades. Nature. 2001;412:641–647. doi: 10.1038/35088074. [DOI] [PubMed] [Google Scholar]
- Kiel MJ., and, Morrison SJ. Uncertainty in the niches that maintain haematopoietic stem cells. Nat Rev Immunol. 2008;8:290–301. doi: 10.1038/nri2279. [DOI] [PubMed] [Google Scholar]
- Hojman P, Gissel H., and, Gehl J. Sensitive and precise regulation of haemoglobin after gene transfer of erythropoietin to muscle tissue using electroporation. Gene Ther. 2007;14:950–959. doi: 10.1038/sj.gt.3302951. [DOI] [PubMed] [Google Scholar]
- Murua A, Orive G, Hernández RM., and, Pedraz JL. Emerging technologies in the delivery of erythropoietin for therapeutics. Med Res Rev. 2011;31:284–309. doi: 10.1002/med.20184. [DOI] [PubMed] [Google Scholar]
- Sebestyén MG, Hegge JO, Noble MA, Lewis DL, Herweijer H., and, Wolff JA. Progress toward a nonviral gene therapy protocol for the treatment of anemia. Hum Gene Ther. 2007;18:269–285. doi: 10.1089/hum.2006.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palani S., and, Sarkar CA. Positive receptor feedback during lineage commitment can generate ultrasensitivity to ligand and confer robustness to a bistable switch. Biophys J. 2008;95:1575–1589. doi: 10.1529/biophysj.107.120600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Average size measurement of ABP polyplexes by DLS.
Average zeta potential measurement of ABP polyplex.
Hematocrit levels after polyplex injection with 50 µg phEPO/ABP (at wt/wt ratio 1/10 and 1/20), 200 µg phEPO/ABP (at wt/wt ratio 1/20), 100 µg phEPO/PEI (at wt/wt ratio 1/1), and 200 µg phEPO/PEI (at wt/wt ratio 1/1).
Percent induction of hEPO mRNA relative to the 1-day phEPO/PEI mRNA levels as 100%.
In vivo time-dependent plasma concentration of IL-6.