Abstract
Prostate cancer is a common cancer in men and continues to be a major health problem. Imaging plays an important role in the clinical management of patients with prostate cancer. An important goal for prostate cancer imaging is more accurate disease characterization through the synthesis of anatomic, functional, and molecular imaging information. Positron emission tomography (PET)/computed tomography (CT) in oncology is emerging as an important imaging tool. The most common radiotracer for PET/CT in oncology, 18F- fluorodeoxyglucose (FDG), is not very useful in prostate cancer. However, in recent years other PET tracers have improved the accuracy of PET/CT imaging of prostate cancer. Among these, choline, labelled with 18F or 11C, 11C-acetate and 18F- fluoride have demonstrated promising results, and other new radiopharmaceuticals are currently under development and evaluation in pre-clinical and clinical studies. Large prospective clinical PET/CT trials are needed to establish the role of PET/CT in prostate cancer patients. Because there are only limited available therapeutic options for advanced metastatic prostate cancer, there is an urgent need for the development of more effective treatment modalities that could improve outcome. Prostate cancer represents an attractive target for radioimmunotherapy (RIT) for several reasons, including pattern of metastatic spread (lymph nodes and bone marrow, sites with good access to circulating antibodies), and small volume disease (ideal for antigen access and antibody delivery). Furthermore, prostate cancer is also radiation sensitive. Prostate-specific membrane antigen (PSMA) is expressed by virtually all prostate cancers, and represents an attractive target for RIT. Anti PSMA RIT demonstrates antitumor activity and is well tolerated. Clinical trials are underway to further improve upon treatment efficacy and patient selection. This review focuses on the recent advances of clinical PET/CT imaging and RIT of prostate cancer.
Keywords: positron-emission tomography, PET, PET/CT, radioimmunotherapy, RIT, prostate cancer
Introduction
Prostate cancer is the most common cancer in men in United States and Europe1,2. Despite early detection of prostate cancer through screening, about 27 000 deaths per year are attributable to prostate cancer in the United States2. In several countries, screening programmes have been introduced, although no randomised controlled trials have been completed with mature follow up, to prove or disprove the effectiveness of such an approach3. As a result of screening, the proportion of men diagnosed before the age of 70 has increased, as has the proportion of well and moderately differentiated tumors. Apart from age and ethnic origin, a positive family history is probably the strongest known risk factor.
Prostate cancer is most commonly diagnosed, when it is still localized. At present, diagnosis is based on histological examination of tissue specimens from the prostate gland usually obtained by systematic transrectal core biopsies, with transrectal ultrasound guidance. The most commonly used system for grading adenocarcinoma of the prostate is the Gleason score. The system describes a score between 2 and 10, with 2 being the least aggressive and 10 the most aggressive. This score is the sum of the two most common patterns (grades 1–5) of tumor growth.
The choice of treatment for localized prostate cancer, i.e. active surveillance, radical prostatectomy, or any type of radiotherapy, depends on tumor characteristics, Gleason score, PSA value and the patient’s life expectancy. Treatment with intent to cure is not used in all patients with prostate cancer since many cases of well to moderately differentiated prostate cancers have a very indolent history and are not lethal. Men in these low risk groups, especially if they have a short life expectancy, may be actively monitored however, active surveillance is being offered more commonly to patients with normal life expectancy1,4 The basic concept of active surveillance is that most men diagnosed with low grade, small-volume disease are not destined to have any clinical manifestations of prostate cancer during their lifetime4. However, in the case of poorly differentiated tumors in patients with an otherwise long life expectancy, treatment with curative intent is offered. Radical prostatectomy can be performed as an open operation or by conventional or robotic laparoscopy. In the recent years, the methods for delivery of external-beam radiation therapy (RT) have improved. The addition of image guidance has resulted in more accurate radiation treatment plans using newer conformal therapy methods such as three-dimensional conformal RT, intensity-modulated RT, and proton beam RT5. Radiotherapy may also be delivered with high-dose brachytherapy, combined with external-beam radiotherapy, or by permanently implanting radioactive seeds, either as monotherapy or in combination with external-beam radiation1. Other treatment options for patients with localised prostate cancer may include high-intensity focused ultrasound and cryosurgery.
The first sign of failure after primary treatment with curative intent is generally a rising serum PSA concentration, occurring months to years before clinical symptoms or radiographic signs of recurrent disease. In general, local recurrence is characterized by a late PSA increase, a long PSA-doubling time, and a less aggressive disease at diagnosis with low Gleason score, and no invasion of the seminal vesicles or lymph nodes. However, in patients who demonstrate early PSA recurrence accompanied by rapid PSA doubling times, metastases are more likely to be the cause. Local salvage therapy is available and may be effective in individual cases, but most men will ultimately suffer from progressive disease because of subclinical sites of disease outside of the prostate area that are not evident on standard imaging modalities.
For several decades, chemical androgen-ablation has been the mainstay of the clinical management of advanced prostate cancer due to the dependence of prostate cancer cells on androgen stimulation. About 70–80% of treated patients with advanced metastatic disease will have symptomatic relief after androgen ablation. After progression on hormonal therapy, metastatic castration-resistant prostate cancer is the final stage of this disease. Cytotoxic chemotherapy has been demonstrated to improve symptoms and length of life in this setting, but is not curative6-9 More recently, autologous cellular immunotherapy with sipuleucel-T has been demonstrated to have a survival benefit and has been approved for clinical use by the U.S. FDA and a new chemotherpeutic agent (cabazitaxel) has also demonstrated a survival benefit leading to FDA approval. However, with all available treatment strategies, responses are transient and lead to only incremental benefits. Novel therapeutic approaches are in development, including new cytotoxic agents, hormonal agents, biologic agents, antiproliferative therapies, immunotherapies (vaccine-based approaches, immune-regulating agents), and antiangiogenic agents1,10,11. Other new treatment strategies in advanced prostate cancer may involve targeted radionuclide therapy (TRT)12-15.
The current treatment options for advanced metastatic prostate cancer demonstrate limited efficacy and severe side effects. Therefore, there is a need for new diagnostic imaging agents and therapeutic strategies in the clinical management of prostate cancer patients. In this review, we summarize recent developments in clinical positron emission tomography (PET)/computed tomography (CT) imaging for detection and monitoring of prostate cancer and advances in the radioimmunotherapy (RIT) of prostate cancer.
PET/CT and prostate cancer
The successful management of prostate cancer requires early detection, appropriate risk assessment, and optimum treatment. Imaging has become more important in the diagnosis, local staging, and treatment follow-up of prostate cancer, and recent developments in imaging technologies, particularly magnetic resonance imaging (MRI) and PET/CT, may lead to significant improvements in lesion detection and staging16,17. Imaging is a powerful tool because most imaging techniques are non- or minimal invasive, and can provide dynamic real-time data, and repeated observations. However, no consensus exists regarding the use of imaging for evaluating primary prostate cancers.
Ultrasonography (US) is mainly used for biopsy guidance and brachytherapy seed placement. Endorectal MRI including MR spectroscopy (MRS) is helpful for evaluating tumor location and extent. MRI with superparamagnetic nanoparticles has high sensitivity and specificity in depicting lymph node metastases, but guidelines have not yet been developed for its use, which remains restricted to the research setting17. CT is mainly reserved for the evaluation of advanced disease.
Functional imaging techniques, such as PET, detect pathologic processes using specific molecular probes labelled with radionuclides, and particularly PET/CT imaging plays an increasingly important role in oncology. The advantage of PET/CT include high sensitivity and spatial resolution and the ability to quantify uptake. The most commonly used PET tracer in oncology is 18F-FDG. However, the results of 18F-FDG PET in detecting prostate cancer have been disappointing18-21. To improve the usefulness of PET in prostate cancer detection, molecular probes with higher sensitivity and specificity are currently being developed.
PET tracers for prostate cancer
FDG
18F-FDG uptake in the cell is mediated by several glucose transporters in the cell membrane, which allow active 18F-FDG passage across the membrane to the cytoplasm and trapping without further metabolism. Most malignant cells are characterized by an enhanced rate of glucose metabolism due to increased numbers of cell surface glucose transporter proteins and by increased intracellular enzyme levels of hexokinase and phosphofructokinase, which promote glycolysis22. The most common glucose transport protein overexpressed on the tumor cell membranes is Glut-1, which is insulin independent. Once inside the cell, FDG is phosphorylated by hexokinase into FDG-6-phosphate. FDG-6-phosphate is not metabolized and accumulates intracellularly. FDG is not very useful in prostate cancer mainly because of the low metabolism of prostate cancer cells but also because of the urinary excretion of 18F-FDG that masks uptake in the prostate gland and loco-regional lymph nodes23. A large fraction of prostate cancer possess a relatively slow metabolic rate and expresses fewer Glut-1 binding sites, leading to lower 18F-FDG uptake compared with other cancers22. Table 1 summarizes clinical reports on 18F-FDG in prostate cancer24-42 and indicates that uptake is mainly seen in more advanced disease. Figure 1 illustrates 18F-FDG uptake in a patient with aggressive prostate cancer.
Table 1.
| Tracer(s) | Patient number |
Disease stage | Study objective |
Sensitivity | Specificity | Year | Group |
|---|---|---|---|---|---|---|---|
| 18F-FDG | 48 | Early | Staging | 81% | NA | 1996 | Effert PJ, et al. |
| 18F-FDG | 13 | Advanced | Staging | NA | NA | 1996 | Yeh SD, et al. |
| 18F-FDG | 34 | Advanced | Staging | 65% | NA | 1996 | Shreve PD, et al |
| 18F-FDG | 18 | Mixed | Restaging | NA | NA | 1999 | Hofer C, et al |
| 18F-FDG | 44 | Early | Staging | 64% | NA | 1999 | Oyama N, et al. |
| 18F-FDG | 24 | Early | Staging | 4% | NA | 2001 | Liu IJ, et al. |
| 18F-FDG | 10 | Advanced | Restaging | NA | NA | 2001 | Oyama N, et al. |
| 18F-FDG | 42 | Early | Staging | NA | NA | 2002 | Oyama N, et al. |
| 18F-FDG | 17 | Advanced | Staging | NA | NA | 2002 | Morris MJ, et al. |
| 18F-FDG | 24 | Advanced | Re-staging | 75% | 100% | 2003 | Chang CH, et al. |
| 18F-FDG | 91 | Mixed | Restaging | 31% | NA | 2005 | Schoder H, et al |
| 18F-FDG, 11C-methionine | 10 | Advanced | Staging | NA | NA | 1999 | Mascapinlac HA, et al |
| 18F-FDG, 11C-methionine | 12 | Advanced | Staging | 48% (72%) | NA | 2002 | Nunez R, et al. |
| 18F-FDG, 11C-acetate | 15 (25) | Mixed | Staging | NA | NA | 2003 | Fricke E, et al |
| 18F-FDG, 11C-acetate | 18(22) | Early | Staging | NA | NA | 2002 | Oyama N, et al. |
| 18F-FDG, 11C-acetate | 46 | Advanced | Restaging | NA | NA | 2003 | Oyama N, et al. |
| 18F-FDG, 11C-choline | 100 | Advanced | Restaging | 27% (47%) | NA | 2003 | Picchio M, et |
| 18F-FDG, 18F-FDHT | 7 | Advanced | Staging | 97% (78%) | NA | 2004 | Larson SM, et al. |
| 18F-FDG, 11C-choline, | 26 | Early | Staging | 73% | 59% | 2010 | Watanabe H, et al. |
Figure 1.
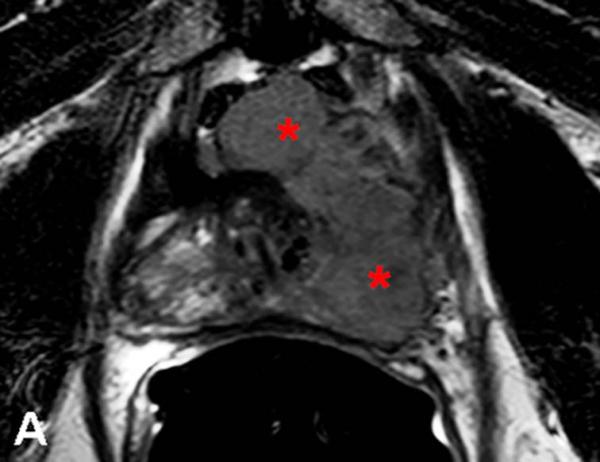
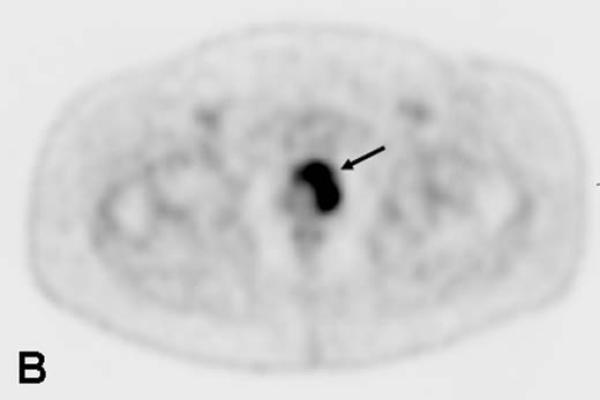
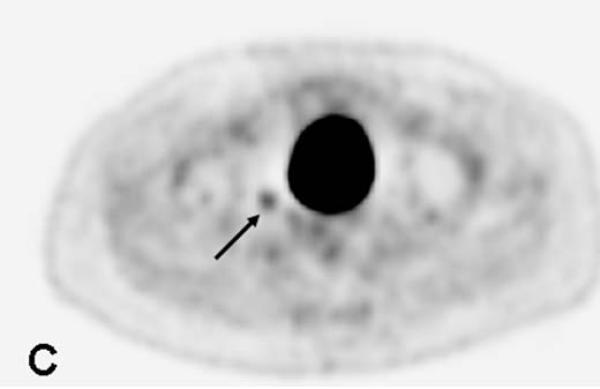
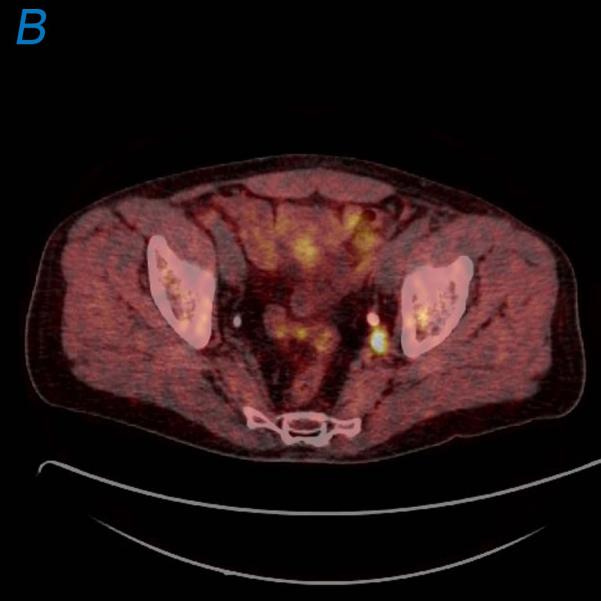
67-year-old male with a PSA<0.6 ng/ml (under hormone therapy). Axial T2W MR image demonstrates a large multi-lobular mass of Gleason 9 in the left hemi-prostate (asterix) (a); 18F-FDG PET image demonstrates significant tracer uptake by the large mass (arrow) (b), a metastatic lymph node within right pelvis also shows increased uptake (arrow) (c).
Choline
The most commonly used PET tracer in prostate cancer is choline radiolabelled with 11C (11C-choline) or 18F as in 18F-fluoroethylecholine and 18F- fluoromethyldimethyl- 2-hydroxyethylammonium (18F-FCH)43. Choline is incorporated into malignant cells by conversion into phosphorylcholine, which is trapped inside the cell. This is followed by synthesis of phosphatidylcholine, which constitutes a main component of cell membranes. Increased choline uptake in prostate cancer cells may be explained by increased cell proliferation in tumors and by upregulation of choline kinase in cancer cells; overexpression of choline kinase has been found in cancer cell lines, including human derived prostate cancer. Thus, the uptake of choline labelled with 11C or 18F in malignant tumors represents the rate of tumor cell proliferation. 18F-FCH has the advantage of a longer half-life of 18F (110 min), compared with 11C (20 min). Thus, an onsite cyclotron is not necessary for 18F-based agents as it is for 11C-based agents. However, urinary excretion of 18F-FCH is higher than 11C-choline. Table 2 summarizes clinical trials with 11C-choline40,42,44-66 and 18F-choline67-81. Figure 2 illustrates 18F-FCH PET/CT scan in a prostate cancer patient.
Table 2.
| Tracer(s) | Patient number |
Disease stage | Study objective |
Sensitivity | Specificity | Year | Group |
|---|---|---|---|---|---|---|---|
| 11C-choline | 10 | Mixed | Staging | NA | NA | 1998 | Hara T, et al. |
| 11C-choline | 23 | Advanced | Staging | NA | NA | 2000 | Kotzerke J, et al. |
| 11C-choline | 25 | Early | Staging | NA | NA | 2002 | De Jong IJ, et al. |
| 11C-choline | 67 | Mixed | Staging | 80% | 96% | 2003 | De Jong IJ, et al. |
| 11C-choline | 36 | Mixed | Restaging | 38% | NA | 2003 | De Jong IJ, et al. |
| 11C-choline | 14 | Early | Staging | NA | NA | 2004 | Sutinen E, et al. |
| 11C-choline | 36 | Early | Staging | 66% | 81% | 2005 | Farsad M, et al. |
| 11C-choline | 20 | Early | Staging | 100% | NA | 2005 | Yamaguchi T, et al. |
| 11C-choline | 13 | Mixed | Staging | 56.3% | 12.5% | 2005 | Yoshida S, et al. |
| 11C-choline | 43 | Early | Staging | 66% | 84% | 2006 | Martorana G, et al. |
| 11C-choline | 26 | Early | Staging | NA | NA | 2006 | Reske SN, et al. |
| 11C-choline | 50 | Advanced | Restaging | 91% | 50% | 2007 | Rinnab J, et al. |
| 11C-choline | 55 | Early | Staging | 36% | NA | 2007 | Rinnab J, et al |
| 11C-choline | 58 | Early | Staging | 86.5% | 62% | 2007 | Scher B, et al. |
| 11C-choline | 26 | Early | Staging | 55% | 86% | 2007 | Testa C, et al. |
| 11C-choline | 15 | Recurrence | Restaging | NA | NA | 2008 | Rinnab J, et al. |
| 11C-choline | 57 | Mixed | Staging | 60% | 97.6% | 2008 | Schiavina R, et al. |
| 11C-choline | 49 | Early | Staging | 90.5% | 85.7% | 2008 | Li X, et al. |
| 11C-choline | 41 | Advanced | Restaging | 75-89% | 40% | 2009 | Rinnab J, et al. |
| 11C-choline | 190 | Advanced | Restaging | 73% | 69% | 2009 | Castellucci P, et al |
| 11C-choline | 6 | Advanced | Restaging | NA | NA | 2010 | Winter A, et al |
| 11C-choline | 25 | Recurrence | Restaging | 86% | 100% | 2010 | Fucchio C, et al. |
| 11C-choline, 18F-FDG | 26 | Early | Staging | 73% (31%) | NA | 2010 | Watanabe H, et al. |
| 11C-choline, 18F-FDG | 100 | Advanced | Restaging | 47% (27%) | NA | 2003 | Picchio M, et |
| 11C-choline, 11C-acetate, | 12 | Advanced | Staging | NA | NA | 2003 | Kotzerke J, et al. |
| 18F-FCH | 17 | Mixed | Staging | 93% | 48% | 2005 | Kwee SA, et al. |
| 18F-FCH | 19 | Early | Restaging | 100% | NA | 2005 | Schmid DT, et al. |
| 18F-FCH | 26 | Mixed | Staging | 60% | 90% | 2006 | Kwee SA, et al. |
| 18F-FCH | 100 | Advanced | Restaging | 98% | 100% | 2006 | Cimitan M, et al. |
| 18F-FCH | 20 | Advanced | Staging | 10% | 80% | 2006 | Hacker A, et al. |
| 18F-FCH | 34 | Advanced | Restaging | NA | NA | 2006 | Heinisch M, et al. |
| 18F-FCH | 111 | Mixed | Staging | 86% | NA | 2008 | Husarik DB, et al. |
| 18F-FCH | 20 | Early | Staging | NA | NA | 2008 | Igerc I, et al |
| 18F-FCH | 15 | Early | Staging | NA | NA | 2008 | Kwee SA, et al. |
| 18F-FCH | 70 | Advanced | Staging | 79% | 97% | 2009 | Beheshti M, et al. |
| 18F-FCH | 20 | Early | Staging | NA | NA | 2010 | Steuber T, et al |
| 18F-FCH | 111 | Mixed | Staging | 45-66% | 96% | 2010 | Beheshti M, et al. |
| 18F-FCH | 25 | Early | Staging | 100% | 95% | 2010 | Poulsen MH, et al |
| 18F-FCH, 1C-acetate, | 11 (11) | Advanced | Restaging | NA | NA | 2007 | Vees H, et al. |
| 18F-FCH, 18F-Fluoride | 38 | Mixed | Staging | 74% (81%) | 99% (93%) | 2008 | Beheshti M, et al. |
Figure 2.
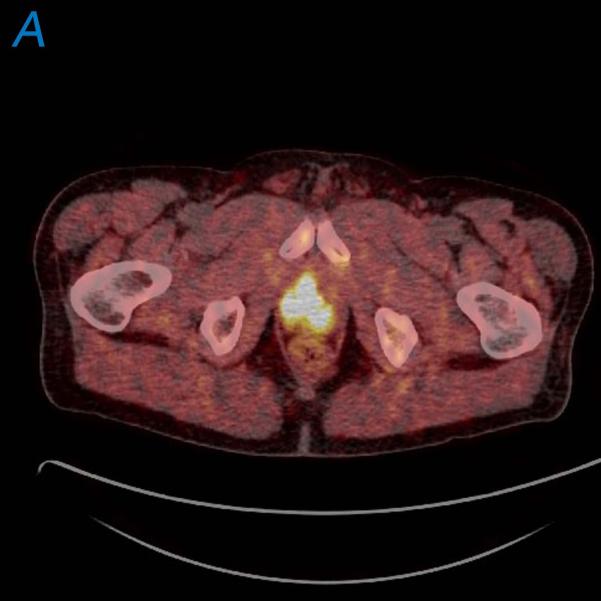
59-year-old male with newly diagnosed high risk prostate cancer. Fused 18F-FCH PET/CT images demonstrate significant increased tracer uptake in the prostate gland (a) and in a lymph node within left pelvis (b).
Acetate
Another commonly used tracer for PET imaging in prostate cancer is 11C-acetate. The mechanism of tumor uptake appears to be incorporation into cell membrane lipids. The uptake of acetate in malignant cells is proportional to lipid synthesis and fatty acid metabolism. Prostate cancer is associated with an increase in fatty acid synthesis and with overexpression of fatty acid synthase82. Acetate is metabolized and incorporated into the cellular lipid pool, and finally into the cell membrane. There is little excretion of this agent into the urine and relatively rapid clearance of the tracer from most other tissues because of its oxidative metabolism to 11C-CO2. Because 11C has a very short half-life, an on-site cyclotron is necessary to use this tracer for clinical studies. Recently, acetate has also been labelled with the longer lived positron emitter 18F for PET imaging of prostate cancer83. Table 3 summarizes the current clinical experience with 11C-acetate in prostate cancer37-39,66,80,84-89, and figure 3 illustrates an 11C-acetate PET/CT scan.
Table 3.
| Tracer(s) | Patient number |
Disease stage | Study objective |
Sensitivity | Specificity | Year | Group |
|---|---|---|---|---|---|---|---|
| 11C-acetate | 30 | Early | Staging | NA | NA | 2002 | Kato T, et al. |
| 11C-acetate | 31 | Advanced | Re-staging | 83% | NA | 2002 | Kotzerke J, et al. |
| 11C-acetate | 20 | Advanced | Restaging | 75% | NA | 2006 | Sanblom G, et al. |
| 11C-acetate | 50 | Advanced | Re-staging | NA | NA | 2006 | Wachter S, et al. |
| 11C-acetate | 32 | Advanced | Restaging | 82% | NA | 2007 | Albrecht S, et al. |
| 11C-acetate | 12 | Advanced | Staging | NA | NA | 2009 | Seppala J, et al. |
| 11C-acetate, 11C-choline | 12 | Advanced | Staging | NA | NA | 2003 | Kotzerke J, et al. |
| 11C-acetate, 18F-FDG | 25 (15) | Mixed | Staging | NA | NA | 2003 | Fricke E, et al |
| 11C-acetate, 18F-FDG | 22(18) | Early | Staging | NA | NA | 2002 | Oyama N, et al. |
| 11C-acetate, 18F-FDG | 46 | Advanced | Restaging | NA | NA | 2003 | Oyama N, et al. |
| 11C-acetate, 18F-FCH | 11 (11) | Advanced | Restaging | NA | NA | 2007 | Vees H, et al. |
Figure 3.
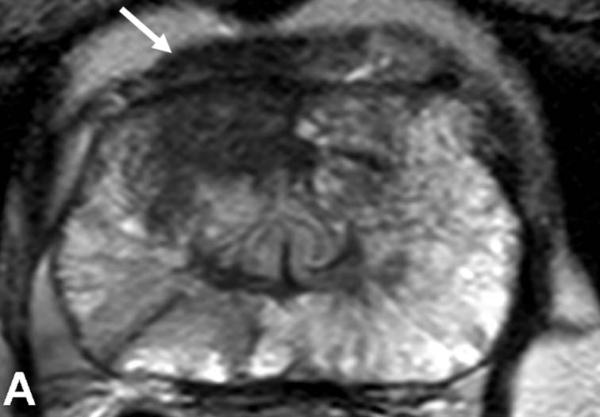
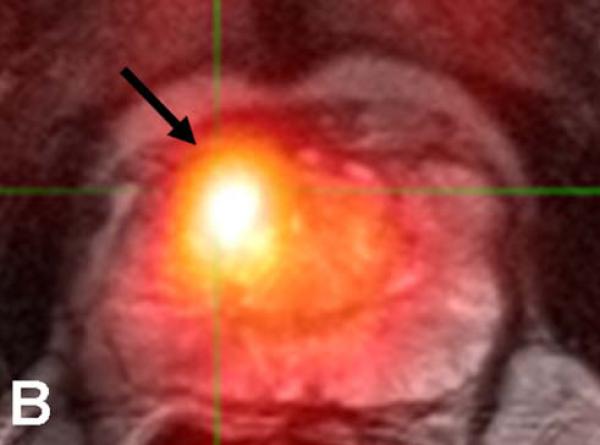
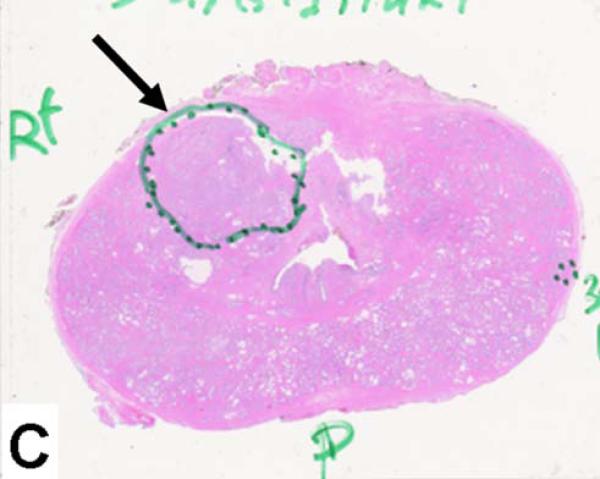
64-year-old male with a PSA of 7.5 ng/dl. Axial T2W MR Image demonstrates a low signal intensity focus in the right anterior PZ (arrow) (a). Fused 11C-acetate PET/MRI image localizes the tumor (arrow) (b). Histopathology confirms presence of a Gleason 3+4 tumor (arrow and inked in green) (c).
Amino acids
Uptake of 11C-methionine is proportional to the amino acid cellular transport and, by implication, protein synthesis. In cancer, methionine uptake has been correlated with the amount of viable tumor tissue and with active tumor proliferation. 11C-methionine is rapidly cleared from the blood and is metabolized in both the liver and the pancreas without renal excretion82. Two studies have demonstrated 11C-methionine to be superior to FDG35,36. 11C-methionine has also only been used in small studies, and larger clinical trails are needed to evaluate the role of this tracer in prostate cancer patients. Anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid (anti-18F-FACBC) is a synthetic l-leucine analogue with delayed bladder excretion that has been shown to be taken up by prostate tumors90. Initial experience with anti-18F-FACBC, a synthetic amino acid analogue, has been promising91. Currently, a tracer similar to anti-18F-FACBC is being developed commercially and tested in Phase I/II clinical trials. Table 4 includes clinical reports on 11C-methionine35,36,92and anti-18F-FACBC91.
Table 4.
| Tracer(s) | Patient number |
Disease stage | Study objective |
Sensitivity | Specificity | Year | Group |
|---|---|---|---|---|---|---|---|
| 11C-methionine, 18F-FDG | 10 | Advanced | Staging | NA | NA | 1999 | Mascapinlac HA, et al |
| 11C-methionine, 18F-FDG | 12 | Advanced | Staging | 72% (48%) | NA | 2002 | Nunez R, et al. |
| 11C-methionine | 20 | Early | Staging | 35% | 47% | 2005 | Tooth G, et al |
| 18F-FACBC | 15 | Advanced | Staging, restaging |
NA | NA | 2007 | Schuster DM, et al. |
| 18F-FDHT, 18F-FDG | 7 | Advanced | Staging | 78% (97%) | NA | 2004 | Larson SM, et al. |
| 18F-FDHT | 20 | Advanced | Staging | 63% | NA | 2005 | Dehdasthi F, et al. |
| 18F-fluoride | 44 | Bone metastases | Staging | 100% | 100% | 2006 | Even-Sapir E, et al |
| 18F-fluoride, 18F-FCH | 38 | Bone metastases | Staging | 81% (74%) | 93% (99%) | 2008 | Beheshti M, et al |
FDHT
The androgen receptor plays an important role in prostate cancer, and anti-androgen treatment is widely used in the treatment of prostate cancer. 18F-fluoro-5α-dihydrotestosterone (FDHT) is a radiolabelled analogue of dihydrotestosterone, the main ligand of androgen receptor. Only few small studies have used 18F-FDHT in progressive metastatic castration-resistant prostate cancer patients41,93. 18F-FDHT PET/CT may have a role in monitoring viable, androgen-sensitive, advanced prostate cancer, and in the assessment of therapeutic response to anti-androgen treatment. However, the experience with 18F-FDHT PET is limited and it has not yet entered large multicenter clinical trials. Clinical experience with 18F-FDHT41,93 is included in table 4.
Fluoride
18F-fluoride PET is highly sensitive for detection of malignant bone metastases, and uptake in malignant bone lesions reflects the increase in regional blood flow and bone turnover. The uptake mechanism resembles that of 99mTc-MDP94. Furthermore, the plasma clearance is more rapid than that of 99mTc-MDP, the extraction is higher because of its smaller molecular weight, and the protein binding is negligible whereas binding of 99mTc-MDP to plasma proteins varies from 25% to 70%. The fast blood clearance of 18F-fluoride results in a better target- to background ratio as compared to 99mTc-MDP. In the bone, 18F-fluoride ions exchange with hydroxyl groups in the hydroxyapatite at the surface of bone crystals resulting in fluoroapatite, mainly at sites of bone remodelling with high turnover. 18F-fluoride bone scans benefit from the intrinsically better spatial resolution of PET scans compared to planar gamma camera images and because of the ability to co-localize uptake in registered CT scans. Clinical studies with 18F-fluoride PET in prostate cancer is summarized in table 481,95
Antibodies
Because prostate specific membrane antigen (PSMA) a transmembrane protein, is expressed by virtually all prostate cancers, and its expression is further increased in poorly differentiated, metastatic, and castrate-resistant carcinomas, it is a very attractive target. Molecules targeting PSMA can be labelled with radionuclides to become both diagnostic and/or therapeutic agents. A number of PSMA antibodies have been developed that target the external domain of the antigen and these are demonstrating promising results in imaging and RIT of prostate cancer14,15. There is increasing evidence that HER2 also plays a role in advanced prostate cancer13. Monoclonal antibodies such as trastuzumab and pertuzumab, or the small scaffold Affibody molecule are used as HER2-targeting agents. A HER2-binding Affibody molecule has been labelled with 18F for in vivo monitoring of the HER2 expression by PET, and the same tracer has been used to assess the changes of HER2 expression following therapeutic intervention96,97. Recently, 111In- and 68Ga-labeled Affibody molecules were used for clinical imaging of HER2 positive tumors in 3 breast cancer patients98.
Primary diagnosis
The goal of current prostate cancer care is to administer risk-adjusted, patient-specific treatment, planned to maximize cancer control while minimizing the risk of complications. Therefore, accurate characterization of the tumor and staging of disease is of great importance in choosing the appropriate therapeutic strategy, i.e., observation, active surveillance, androgen ablation, radical surgery, external radiation, etc. Prostate cancer is diagnosed by pathologic examination of needle biopsy specimens, most often prompted by abnormal clinical findings on digital rectal examination and by elevated serum PSA. Since approximately 85% of prostate cancers are multifocal in origin, the current 12-18 spatially distributed prostate core biopsies under TRUS guidance may not provide accurate information about the extent and grade of disease17. Even when the number of core biopsies is increased underdiagnosing and undergrading of biopsy specimens (compared to radical prostatectomy specimens) is common. Biopsy and radical prostatectomy Gleason score categories correlate in only about 69% of the patients99. Thus, using the current diagnostic procedures both underdiagnosis and overdiagnosis exists and a new and more accurate approach that identifies clinically significant disease and differentiates it from indolent disease is urgently needed.
18F-FDG PET/CT is not effective in the diagnosis of localized prostate cancer because of low glucose metabolism in these tumors. However, some localized prostate cancers are highly glucose dependent and will be positive on 18F-FDG PET/CT scans performed for other reasons than investigation of prostate cancer, although this PET positive finding is the exception rather than the normal finding. Several PET studies have used choline and acetate for detection of malignancy in the prostate gland. However, careful interpretation of the PET images of prostate cancer is necessary because the tracer uptake for the normal prostate and for BPH may overlap with those for prostate cancer 68,74,84,100. It is possible that combining PET with MRI may improve the detection rate of malignancy in the prostate gland in the future. Furthermore, the utility of PET to detect locally confined prostate cancer will be improved by molecular probes with higher sensitivity and specificity. Thus, new PET tracers are being developed for prostate cancer.
Initial staging
Accurate detection of lymph-node metastases in prostate cancer is an essential component of the approach to treatment. Pelvic lymph node metastases are considered the strongest predictor of disease recurrence and progression, and the presence of metastases often means the difference between local and systemic therapy. CT and MRI are the main imaging techniques for N-staging of prostate cancer101. For the assessment of lymph node metastases, MR imaging, like CT, has relative low sensitivity102. The low sensitivity of MR imaging and CT is mainly due to the inability of cross-sectional imaging to detect metastases in normal-sized nodes. Both CT and MRI mainly rely on size criteria to detect malignant lymph node involvement, and small metastases or micrometastases cannot be detected using conventional size criteria. The use of lymphotropic ultrasmall supermagnetic particles of iron oxide (USPIO) as a contrast agent for MRI enables reliable detection of metastases in pelvis lymph nodes smaller than 0.5 mm in patients with prostate cancer. Thus, this new promising technique is able to detect malignant involvement of normal sized lymph nodes. USPIO particles are consumed by macrophages in normal lymph nodes resulting in decrease in signal on T2/T2*-weighted MRI sequences. In a study of 80 patients with prostate cancer MRI with lymphotropic superparamagnetic nanoparticles (Sinerem, Guerbet, Paris, France) correctly identified all patients with nodal metastases, and a node-by-node analysis had a significantly higher sensitivity than conventional MRI (90.5 percent vs. 35.4 percent, P<0.001) or nomograms103. The new technique also had a sensitivity of 100% and a specificity of 95.7 in detecting nodal metastasis on a per patient basis. Unfortunately, this agent is very unlikely to become commercially available. A second generation USPIO, ferumoxytal, has been developed and approved for iron replacement therapy in chronic renal failure. This agent may also have efficacy as a lymph node imaging agent.
18F-FDG has been used for N-staging but, because prostate cancer has variable accumulation of 18F-FDG, 18F-FDG PET/CT is not widely used23,104. In recent years, eports have focused on the potential role of PET performed with radiotracers such as 11C-acetate, 11C- and 18F- FCH in the assessment of patients with prostate cancer. However, the value of PET with choline and acetate in lymph node staging of prostate cancer has been a subject of controversy, and varying results have been reported in the primary assessment of malignant lymph node involvement48,60,73. Recently, Behesthi et al. assessed the value of FCH PET/CT imaging in the preoperative staging of intermediate- and high-risk patients with prostate cancer77. In this large prospective study, 132 patients with prostate cancer with intermediate or high risk of extra capsular disease were enrolled. Overall, 912 lymph nodes were histopathologically examined, and a per-patient analysis revealed the sensitivity, specificity, and positive and negative predictive values of 18F-FCH PET/CT in the detection of malignant lymph nodes were 45%, 96%, 82%, and 83%, respectively. In lymph nodes 5 mm in diameter or larger, the sensitivity, specificity, and positive and negative predictive values were 66%, 96%, 82%, and 92%, respectively. In clinical staging, 18F-FCH PET/CT led to a change in the therapeutic care of 15% of the patient population (19/130 patients). When considering the entire high-risk group, 20% of the patient population (17/83 patients) had findings that were upstaged after 18F-FCH PET/CT. At least two other large prospective clinical trials of 18F-FCH PET/CT are underway76,79. The role of 11C-methionine, 18F-FACBC, and 18F-FDHT for detection of nodal involvement remains to be evaluated further in large prospective trials.
Recurrence
Biochemical recurrence, a rise in PSA, occurs in 20%-40% of patients within 10 years of “definitive” treatment, usually proceeding clinically detectable disease. After definitive radical prostatectomy or radiation therapy, biochemical recurrence is usually the first sign of prostate cancer recurrence. After radical prostatectomy, PSA should fall to undetectable values within 3-4 weeks, while the PSA level decrease slowly and may never reach undetectable values after radiation therapy. The time from biochemical recurrence to metastases depends on preoperative pathologic stage, Gleason score, and PSA doubling time. A shorter PSA doubling time (< 10 months) after radical prostatectomy is a strong indicator for malignant disease progression, while defining biochemical recurrence after irradiation is more complex. When a biochemical recurrence is observed in patients, accurate delineation of local versus metastatic disease is crucial for selection of appropriate therapy. Imaging plays an important role in distinguishing local recurrence from distant malignant disease.
Because of low metabolism of most prostate cancers, FDG PET/CT is not very useful for this purpose. In a study of 91 patients, 18F-FDG-PET detected local or systemic disease in 31% of patients with PSA relapse34. However, several studies have reported both choline and acetate to be useful for detecting recurrence in patients with PSA relapse39,40,87,105. Rinnab et al. evaluated the detection of biochemical recurrence of prostate cancer after radical prostatectomy with 11C-choline PET/CT in 41 patients, and reported a sensitivity value of 89% for patients with a PSA<2.5ng/ml62. Recently, Winter et al. reported the initial results of 11C-choline PET/CT-guided secondary lymph node surgery in 6 patients with biochemical failure after radical prostatectomy and, after resection of lymph nodes, in all patients the oncologic criteria of a remission were fulfilled64. In a large prospective study Cimitan et al. identified prostate cancer recurrence with 18F-FCH PET/CT in 53 of 100 patients with PSA relapse; however, 89% of patients with presumably false-negative scans had a serum PSA level < 4 ng/dL resulting in a lower sensitivity for 18F-FCH for detecting recurrent prostate cancer if the PSA was low70. The authors concluded that 18F-FCH PET/CT is not likely to have a significant impact on the care of prostate cancer patients with biochemical recurrence until PSA increases to above 4 ng/ml. Recently, Castellucci et al. investigated the effect of total PSA at the time of 11C-choline PET/CT (trigger PSA), PSA velocity (PSAvel), and PSA doubling time (PSAdt) on 11C-choline PET/CT detection rate in patients (n=190) treated with radical prostatectomy who showed biochemical failure during follow-up63. The study demonstrated that the 11C-choline PET/CT detection rate is influenced by trigger PSA, PSAdt, and PSAvel. Trigger PSA and PSAvel were found to be independent predictive factors for a PET-positive result (P = 0.002; P = 0.04), while PSAdt was found to be an independent factor only in patients with trigger PSA less than 2 ng/mL (P = 0.05) using multivariate analysis. The results from this study may be used to improve the selection of patients for PET/CT scanning by reducing the number of false-negative scans and increasing the detection rate of disease in patients with early relapse and potentially curative disease.
The role of 11C-acetate for detecting prostate cancer recurrence was examined by Sandblom et al. in 20 patients with increasing PSA after radical prostatectomy. In this study recurrence was detected in 75% of the patients while 15 % of the cases showed false positive uptake86. Kotzerke et al. studied the potential utility of 11C-acetate in the detection of local recurrence in 31 patients and positively identified local recurrence in 15 of 18 patients with 11C-acetate PET66. Friecke et al. compared 11C-acetate and 18F-FDG in patients with rising PSA after radical prostatectomy and radiation therapy. The results showed that 11C-acetate detected relapse in 20 of 24 patients whereas 18F-FDG was positive in 10 of 15106. Seppala et al. demonstrated the feasibility of 11C-acetate PET/CT in prospectively delineating prostate cancer lesions in 12 patients who had received external beam radiation therapy107. No large prospective clinical trial has directly compared choline and acetate PET/CT for detection of prostate cancer. 18F-FACBC PET/CT may also be used for detection of recurrence91. However, the study is small and it has to be confirmed in larger clinical trial.
Bone metastases
A typical feature of prostate cancer is its ability to metastasize to bone. It has been estimated that >80% of men who die from prostate cancer develop bone metastases108. It is mainly osteoblastic, and is caused by a relative excess of osteoblast activity induced by adjacent cancer cells, leading to abnormal bone formation. Bone metastases are the result of a complex series of steps that depend on dynamic crosstalk between metastatic cancer cells, cellular components of the bone marrow microenvironment, and bone matrix (osteoblasts and osteoclasts). Bone scintigraphy using 99mTc-labelled diphosphonates has long been the mainstay investigation for bony metastasis. However, planar bone scans have a relative poor specificity. It can be difficult to distinguish between metastases and other pathological conditions such as degenerative disease, which often coexist in prostate cancer patients. Single photon emission computed tomography (SPECT) has been used in such situations to clarify the location of focal hotspots. Recently, Helyar et al. investigated the additional value of SPECT/CT over whole-body planar bone scintigraphy and SPECT in prostate cancer patients109. The addition of SPECT/CT improved the diagnostic confidence compared to SPECT alone and planar imaging in prostate cancer patients with suspected bone metastases. SPECT/CT resulted in a significant reduction of equivocal reports, and a definitive diagnosis was given in the majority of the patients as compared to planar or SPECT imaging alone.
FDG
A few 18F-FDG PET studies have looked specifically at the skeleton, and these studies indicate that FDG is less sensitive than bone scintigraphy in the identification of osseous metastases. Thirty four patients were evaluated in a study by Shreve et al., in which PET was compared with 99mTc bone scan, CT, and clinical follow up for the presence of skeletal metastases26. In 202 untreated osseous metastases in 22 patients, the sensitivity of 18F-FDG PET was 65% (131 of 202 metastases), with a positive predictive value of 98% (131 of 133 positive findings). In that study there were also 6 patients receiving hormonal treatment and 1 studied after orchiectomy, with 131 metastases identified on the bone scan but only 4 seen on 18F However, in study by Morris et al. 18F-FDG PET demonstrated a sensitivity of 77% -FDG PET. of for detection bone metastases patients with advanced metastatic prostate cancer, but FDG was effective in detecting soft-tissue metastases32
Fluoride
18F-fluoride PET/CT is a promising modality for the evaluation of bone metastases with higher sensitivity for lesion detection, when compared with the routine conventional bone scan. Schirrmeister et al. compared the diagnostic accuracy of 18F-fluoride PET scanning of the skeletal trunk with the diagnostic accuracy of conventional bone scintigraphy110. Sensitivities in the detection of benign and malignant lesions were compared in different regions of the skeleton. It was clearly demonstrated that bone imaging with 18F-fluoride PET is more sensitive than planar bone scan in the detection of benign and malignant osseous lesions. The sensitivity in detecting benign and malignant bone lesions with bone scan is highly dependent on their anatomic localization. Recently, it was demonstrated that 18F-fluoride PET is more accurate than 99mTc-diphosphonate SPECT for identifying both malignant and benign lesions of the skeleton111,112. In a prospective study by Even-Sapir et al., planar and SPECT 99mTc-MDP bone scans, 18F-fluoride PET, and 18F-fluoride PET/CT were performed on 44 patients with high-risk prostate cancer95. Among these 23 patients were characterized as having metastatic disease. As was the case in prior reports, 18F-fluoride PET was more sensitive in detecting skeletal metastases than was planar 99mTc-MDP scintigraphy, either alone or in combination with 99mTc-MDP SPECT. 18F-fluoride PET detected skeletal metastases in all 23 patients, whereas 99mTc-MDP imaging detected malignant lesions in only 18 patients.
In another prospective study, Behesthi et al. compared the potential value of 18F-FCH and 18F-fluoride PET/CT for the detection of bony metastases from prostate cancer81. Thirty-eight patients with prostate cancer underwent both imaging modalities within 2 weeks. Overall, 321 lesions were evaluated in this study. The sensitivity, specificity and accuracy of PET/CT in the detection of bone metastases in prostate cancer was 81%, 93% and 86% for 18F-fluoride, and 74% (p=0.12), 99% (p=0.01) and 85% for 18F-FCH, respectively. Thus, 18F-fluoride PET/CT demonstrated higher raw sensitivity than 18F-FCH PET/CT for detection of bone metastases; however, upon analysis this difference was not statistically significant. Furthermore, 18F-FCH PET/CT proved to be more specific than 18F-fluoride PET/CT. For evaluation of bone metastases in prostate cancer patients, 18F-FCH and 18F-fluoride PET/CT were concordant in 80% of lesions. The remaining “discordant group” (constituting 20% of the study) could be classified into two categories. In the group with 18F-FCH positive/18F-fluoride negative results, the findings may be due to bone marrow metastases without significant bone reaction and remodelling, which suggests that 18F-FCH PET/CT has an advantage in the early detection of bone metastases. In the other group, demonstrating 18F-FCH negative/18F-fluoride positive results, this pattern was mainly seen in densely sclerotic malignant lesions. Most of these lesions were positive in previous 18F-FCH PET/CT studies. Thus, with increasing density of sclerotic lesions, the intensity of 18F-FCH uptake was reduced so that no 18F-FCH uptake was detected in densely sclerotic malignant lesions. Almost all of these lesions were detected in patients who were under hormone therapy, which supports the theory that 18F-FCH-negative sclerotic lesions may no longer be as metabolically active.
Radioimmunotherapy and prostate cancer
Radioimmunotherapy refers to the use of a radiolabeled antibody to deliver a therapeutic radiation dose, most frequently to tumor. This “targeted” form of radiotherapy allows radiation delivery to tumors while sparing normal organs. Targeted radionuclide therapy utilizes a charged particle since this form of ionizing radiation is absorbed locally with efficient transfer of energy to the biochemical system of the targeted cells, disrupting the processes necessary for cell survival. Beta particles, Auger electrons, and Alpha particles have been used for these purposes but most current clinical applications utilize beta particle emission.
General aspects of RIT
Currently, the antibodies used for RIT are IgG proteins derived from murine hybridoma cells [mouse lymphocyte fused with a mouse malignant plasma (myeloma) cell]. These giant cells are capable of producing large amounts of the specific immunoglobulin for which the lymphocyte had been encoded by prior immunization of an intact animal. Each hybridoma cell colony produces a monoclonal antibody that can be assessed for binding affinity and epitope specificity to select a preferred reagent for radiolabeling and further evaluation. The immuno-recognition portion of the large immunoglobulin resides in the terminal portion of heavy and light chains known as the hypervariable region. The remainder of the molecule is involved in complement binding and evoking a macrophage response – properties that are important in terms of the antitumor effect of the antibody. However, murine immunoglobulins are recognized as foreign proteins in humans leading to development of anti-murine antibodies [Human Anti-Murine Antibodies or HAMA]. The hypervariable region can be split off from the intact molecule and either evaluated as is or fused with a portion of a human immunoglobulin. These immunoglobulin constructs are identified as either “chimeric” or “humanized” depending upon the amount of murine component retained. A standard nomenclature has been developed. All generic names for monoclonal antibodies end with the suffix “mab”. Mouse monoclonal antibodies are “momabs”; the chimeric molecules are “ximabs” and the humanized molecules are “zumabs”. Antibodies directed against tumor antigens often include the syllable “tu”; hence “…tumomab”, a murine monoclonal antibody to a tumor antigen; “…tuximab”, a chimeric monoclonal antibody to a tumor antigen and “…tuzumab”, a humanized monoclonal antibody.
In order to prevent non-specific binding of the radiolabeled antibody or even specific binding to similar epitopes expressed on tissue other than the tumor target, it is necessary to administer unlabeled antibody prior to or at the time of administration of the labeled antibody. In the instance of anti-CD20 radioimmunotherapy of low-grade B cell non-Hodgkin’s lymphoma, several hundred milligrams of an unlabeled immunoglobulin are administered prior to the labeled antibody to saturate the abundant CD20 expression on normal B cells. By contrast, since the prostate is the only normal tissue in males expressing significant amounts of PSMA, only a relatively small quantity of “carrier” antibody is necessary as there is little competition for the radiolabeled antibody. PSMA is a large molecule with an extra-cellular, transmembrane and intracellular portion. Antibodies have been developed to each of these components. One of the antibodies, J591 with affinity for the extra-cellular portion of the PSMA epitope has been evaluated extensively as a vehicle to deliver targeted radiation.
Radioimmunotherapy can be delivered in a single dose or in multiple fractions. The degree of anti-tumor response following the administration of radiolabeled mAbs depends on several variables, especially total (cumulative) radiation dose to the tumor, dose-rate, and tumor radiosensitivity. As with conventional external beam ionizing radiotherapy, dose fractionation may result in the ability to deliver a higher tumor dose with less toxicity. Fractionated dose RIT may decrease the dose to bone marrow while increasing the cumulative radiation dose to the tumor at an optimal dose-rate113-115. Preclinical data have shown that dose fractionation or multiple low dose treatments can decrease toxicity while increasing the efficacy116-118. Early clinical studies have supported the ability to increase the cumulative maximum tolerated dose by dose fractionation119-121.
It is clear that external beam radiotherapy can be combined with cytotoxic chemotherapy. Though there may be increased toxicity, efficacy of concurrent chemoradiotherapy may be superior to sequential use. This may be especially true when utilizing chemotherapeutic agents with radiosensitizing effects. Combining RIT with cytotoxic chemotherapy has also been investigated122-124. These combinations have the possibility of increasing the therapeutic yield of RIT, particularly in the face of bulky, metastatic solid tumors.
With “targeted” therapy in general, patient selection can be important. While all our ability to pre-select optimal patients based upon expression of a target may be limited, in other cases, it can be quite helpful either in selecting patients more likely to respond or by eliminating patients with a very low chance of response. For example, although epidermal growth factor receptor (EGFR) expression as measured by immunohistochemistry is not helpful in selecting patients for anti-EGFR monoclonal antibody therapy in advanced colorectal carcinoma, excluding those with mutated K-ras has become helpful in clinical practice125. In performing studies developing predictive biomarkers, one must remember that prospective validation is important, as development of a “targeted” therapy may be thwarted by a sub-optimal biomarker.
Although the initially investigated form of RIT utilized radiolabeled antibodies against carcinoembryonic antigen for solid tumors, the most studied form of radioimmunotherapy to date uses targeting of the CD20 antigen (I131 tositumomab or Y90 ibritumomab tiuxetan) in non- Hodgkin’s lymphoma, demonstrating safety and efficacy in phase I-III trials that resulted in FDA approval. While mostly used in the setting of relapsed disease, it appears that these therapies may have their greatest impact in the minimal disease setting126-131. RIT for solid tumor malignancies has been slower to develop. Reasons for this are multi-faceted, including lack of specific antigens and antibodies optimized for RIT, difficulties in stably linking radionuclides to existing mAb’s, shortfalls in existing (and readily available) radionuclides, and difficulty in clinical use (coordination between different specialties)132. However, clinical trials utilizing RIT in solid tumor malignancies have been increasing; on a recent query on clinicaltrials.gov, at least 28 clinical trials utilizing RIT for solid tumors were identified.
Choice of radionuclides
The physical and chemical characteristics of available radionuclides must be considered in choosing the radionuclide to be used for radioimmunotherapy. Currently, 3 beta emitting radionuclide, Iodine-131 [131I], Yttrium-90 [90Y] and Lutetium-177 [177Lu] are readily available to radiolabel antibodies (Table 5). Based upon the physical properties of each radionuclide, there may be more optimal tumor types and clinical situations for each one133. 131I has been used as a radio-therapeutic agent for over 60 years. In addition to its characteristic beta particle emission, a gamma photon is emitted that can be quantified and imaged. Iodine chemistry is well understood and most organic compounds can be readily iodinated. However, following binding to PSMA, the antibody is internalized followed by hydrolysis of portions of the immunoglobulin molecule. Iodinated fragments easily diffuse across the cell membrane. Although it is more difficult to bind a metal atom to immunoglobulins, if the molecule is internalized and digested, the metallic label is insoluble and remains intracellular. Yttrium-90 [90Y] and Lutetium-177 [177Lu] are radiometals that decay by beta emission. 90Y is a pure beta emitter with half-life less than 3 days. 177Lu emits both a beta particle and a gamma photon enabling imaging to be performed using the treatment dose (as opposed to using 111In followed by 90Y). The half-life of 177Lu is 6.7 days. Due to longer physical half-life of 177Lu, as compared to 90Y, the tumor residence times are higher. As a result, higher activities (more mCi amounts) of 177Lu labeled agents can be administered with comparatively less myelosuppression.
Table 5.
Radionuclides for RIT
| Radionuclide | Physical half-life |
Decay Type |
Particle Energy (MeV) |
Range in tissue (mm) |
Gamma Energy (MeV) |
|---|---|---|---|---|---|
| 131Iodine | 8 days | β,γ | 0.61 Max 0.20 Average |
2.4 0.4 |
0.364 |
| 90Yttrium | 2.7 days | β | 2.3 Max 0.94 Average |
12.0 2.7 |
none |
| 177Lutetium | 6.7 days | β,γ | 0.50 Max 0.15 average |
2.2 0.2 |
0.113-0.208 |
The beta particles emitted from 90Y is more energetic than those of 177Lu [Max: 2.3 MeV vs Max: 0.5 MeV]. In general, lower energy favors more effective energy transfer and radiobiologic effect for micrometastases while it is believed that higher energy beta emission (such as from 90Y) may be more effective for use in targeted irradiation of larger tumors. Large tumors could receive an adequate radiation absorbed dose from low energy beta emitting radiotracers if there is sufficient intra-tumoral distribution. In a clinical situation such as in metastatic prostate carcinoma, micrometastatic involvement of bone marrow may be the basis for recurrent disease following treatment of larger recognized lesions. Lower energy beta emission and, consequently, shorter range in tissue would result in less radiation delivered to surrounding normal tissue. A combination of radionuclides providing low and high-energy beta emissions would seem to be worthwhile. This concept has been confirmed in animal studies but has not yet been evaluated in humans.
Prostate cancer and RIT
Prostate cancer is an ideal solid tumor malignancy for which RIT may be utilized. It is a radiosensitive tumor with typical distribution to sites with high exposure to circulating antibodies (bone marrow and lymph nodes). Although sometimes clinically problematic, early readouts of efficacy can be examined using serum prostate specific antigen (PSA) levels. In pre-clinical and clinical prostate cancer settings, radionuclides have been linked to antibodies and/or peptides against mucin, gangioside (L6), Lewis Y (Ley), adenocarcinoma-associated antigens, and prostate specific membrane antigen (PSMA)14,122,123,134-141. Of these, prostate specific membrane antigen is the most specific and will be discussed in further detail in this review.
Prostate specific membrane antigen is a non-secreted type II membrane protein. It is expressed on the luminal surface of normal prostate epithelial cells, and its expression increases in prostate carcinoma142-146, being expressed on all prostate cancers in pathology studies147. PSMA has been validated as an in vivo target for imaging utilizing radiolabeled mAb 7E11 (CYT-356, capromab)148,149. However, subsequent clinical treatment studies were disappointing138,139. Molecular mapping revealed that 7E11 targets a portion of the PSMA molecule that is within the cell’s interior and not exposed on the outer cell surface150,151 and cannot bind to viable cells142,151. Recognition of these features led to the development of mAbs to the exposed, extracellular domain of PSMA which in theory would have the potential to significantly improve in vivo targeting likely resulting in enhanced imaging and therapeutic benefit151,152. These antibodies (J591, J415, J533 and E99) demonstrated high affinity binding to viable PSMA-expressing LNCaP cells in tissue culture and are rapidly internalized151,153..
J591 is a deimmunized IgG monoclonal antibody (mAb) which binds the external portion of PSMA followed by rapid internalization151,153,154. Phase I clinical trials of radiolabeled J591 were performed using Yttrium-90 (90Y) or Lutetium-177 (177Lu) linked to J591 via a DOTA chelate in patients with metastatic castration-resistant prostate cancer (CRPC)140,141. Each of these studies was designed to deliver a single-dose of radiolabeled J591 intravenously followed by planar gamma camera imaging +/− SPECT (in the case of 90Y-J591, imaging was performed after 111In-J591 administration). These trials defined the maximum tolerated dose (MTD) and further refined dosimetry, pharmacokinetics, and HAHA of the radiolabeled mAb conjugates and demonstrated preliminary evidence of anti-tumor activity. As expected, based upon the physical properties as described above, the MTD of single-dose 177Lu-J591 was higher (70 mCi/m2) than that of 90Y-J591 (17.5 mCi/m2)140,141
A phase II study was subsequently performed with 177Lu-J591, confirming safety, efficacy, and tumor targeting ability155. In this study, men with progressive metastatic CRPC received a single-dose of 177Lu-J591 intravenously followed by imaging one week later. As demonstrated in the phase I studies, myelosuppression was the most significant toxicity, mostly manifested by thrombocytopenia. The majority (94%) demonstrated accurate targeting of known sites of metastatic disease. Efficacy was confirmed, with the majority of subjects experiencing declines in PSA.
In aggregate, these trials provide support that radiolabeled J591 is well-tolerated with reversible myelosuppression, accurately targets prostate cancer metastatic sites, demonstrates efficacy, and is non-immunogenic. However, as discussed above, there are limitations of RIT for solid tumors, and the physical properties of 177Lu should be sub-optimal in treating the population treated to date (men with progressive metastatic CRPC were treated, many of whom had bulky disease). Additional studies to improve the therapeutic profile were therefore activated.
A Department of Defense sponsored study utilizing fractionated dose 177Lu-J591 has recently been completed with initial results presented156. Men with progressive metastatic CRPC received 2 fractionated doses two weeks apart. Doses were escalated in cohorts of 3-6 subjects, with cohort 1 receiving 20 mCi/m2 x2 and each successive cohort undergoing dose escalation by 5 mCi/m2 per dose (10 mCi/m2 cumulative dose increase per cohort). The primary endpoint was to determine dose-limiting toxicity (DLT) and the cumulative maximum tolerated dose (MTD) of fractionated 177Lu -J591 RIT with pharmacokinetics and dosimetry and secondary endpoints of efficacy. Dose limiting toxicity is defined as severe thrombocytopenia (platelet count < 15 or need for > 3 platelet transfusions in 30 days), grade 4 neutropenia > 7 days, febrile neutropenia, or grade > 2 non-hematologic toxicity. Twenty-eight subjects received treatment with cumulative doses of up to 90 mCi/m2 (highest planned dose). The median age was 72 years with median baseline PSA 49 ng/mL; the majority had Eastern Cooperative Oncology Group (ECOG) performance status 1 and had bone metastases. The study confirmed the hypothesis that fractionated dose would allow higher cumulative doses of 177Lu-J591 to be administered with less toxicity with evidence of anti-tumor activity.
Following progression on primary hormonal therapy, chemotherapy can offer symptomatic improvement as well as incremental survival benefit9,157. However, responses are transient and all men eventually suffer from progression of disease. As described above, single-agent anti-PSMA-based RIT has demonstrated efficacy in the treatment of metastatic CRPC, but the results are limited, and all men treated to date with mature follow up have suffered from progression of disease. The combination of taxane chemotherapy with radiotherapy has been used in several diseases because of the radiosensitizing effects of taxane-based chemotherapy158-160. The combination of taxane chemotherapy with radioimmunotherapy has also been studied in pre-clinical and early clinical studies122,123,161. In addition to favorable results from fractionated radioimmunotherapy and the radiosensitizing effects of taxane-based chemotherapy, it is hypothesized that the additional debulking by chemotherapy will overcome some of the limits imposed by the physical characteristics of 177Lu. Based upon these data, a phase I trial of docetaxel and prednisone with escalating doses of fractionated 177Lu-J591 is ongoing162.
As discussed above, the most studied form of RIT to date targets the CD20 antigen (131Itositumomab and 90Y ibritumomab tiuxetan) in non-hodgkin’s lymphoma. While approved in the relapsed setting, it appears that these therapies have their greatest impact in the minimal disease setting126-130,163. The vast majority of relapses after local therapy for prostate cancer are initially “biochemical” only, i.e. with a rising PSA despite no evidence of cancer on imaging164,165, affecting approximately 50,000 men per year in the United States alone. Although there is no proven overall survival benefit in a prospective randomized trial, radiotherapy as a salvage regimen can lead to long-term survival in selected individuals166-169. Unfortunately, most subsequently suffer systemic progression because of subclinical micrometastatic disease outside of the radiation field.
Based on the demonstrated ability of J591-based therapy to successfully target known sites of disease and apparent clinical efficacy in the advanced setting, it is now under investigation in the salvage setting. “Targeted radiotherapy” in the form of radioimmunotherapy is an attractive option with the possibility being a higher yield therapy in the minimal disease (biochemical only) setting. The primary objective of this trial is to prevent or delay radiographically evident metastatic disease. Radiolabeled J591 imaging will also be explored as a possible way to detect sites of disease in these patients with biochemical relapse and no evidence of disease on standard scans (99mTc-MDP bone scans and computed tomography or magnetic resonance imaging)170.
References
- 1.Damber JE, Aus G. Prostate cancer. Lancet. 2008;371:1710. doi: 10.1016/S0140-6736(08)60729-1. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 3.Barry MJ. Screening for prostate cancer--the controversy that refuses to die. N Engl J Med. 2009;360:1351. doi: 10.1056/NEJMe0901166. [DOI] [PubMed] [Google Scholar]
- 4.Klotz L. Active surveillance for prostate cancer: a review. Curr Urol Rep. 2010;11:165. doi: 10.1007/s11934-010-0110-z. [DOI] [PubMed] [Google Scholar]
- 5.Choi M, Hung AY. Technological advances in radiation therapy for prostate cancer. Curr Urol Rep. 2010;11:172. doi: 10.1007/s11934-010-0102-z. [DOI] [PubMed] [Google Scholar]
- 6.Tannock IF, Osoba D, Stockler MR, Ernst DS, Neville AJ, Moore MJ, et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points. J Clin Oncol. 1996;14:1756. doi: 10.1200/JCO.1996.14.6.1756. [DOI] [PubMed] [Google Scholar]
- 7.Kantoff PW, Halabi S, Conaway M, Picus J, Kirshner J, Hars V, et al. Hydrocortisone with or without mitoxantrone in men with hormone-refractory prostate cancer: results of the cancer and leukemia group B 9182 study. J Clin Oncol. 1999;17:2506. doi: 10.1200/JCO.1999.17.8.2506. [DOI] [PubMed] [Google Scholar]
- 8.Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr., Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 9.Tannock IF, de WR, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 10.Zarour L, Alumkal J. Emerging therapies in castrate-resistant prostate cancer. Curr Urol Rep. 2010;11:152. doi: 10.1007/s11934-010-0104-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lassi K, Dawson NA. Emerging therapies in castrate-resistant prostate cancer. Curr opin oncol. 2009;21:260. doi: 10.1097/CCO.0b013e32832a1868. [DOI] [PubMed] [Google Scholar]
- 12.Smith-Jones PM. Radioimmunotherapy of prostate cancer. Q J Nucl Med Mol Imaging. 2004;48:297. [PubMed] [Google Scholar]
- 13.Bouchelouche K, Capala J. ‘Image and treat’: an individualized approach to urological tumors. Curr opin oncol. 2010;22:274. doi: 10.1097/CCO.0b013e3283373d5c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tagawa ST, Beltran H, Vallabhajosula S, Goldsmith SJ, Osborne J, Matulich D, et al. Anti-prostate-specific membrane antigen-based radioimmunotherapy for prostate cancer. Cancer. 2010;116:1075. doi: 10.1002/cncr.24795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bouchelouche K, Choyke PL, Capala J. Prostate specific membrane antigen- a target for imaging and therapy with radionuclides. Discov Med. 2010;9:55. [PMC free article] [PubMed] [Google Scholar]
- 16.Turkbey B, Albert PS, Kurdziel K, Choyke PL. Imaging localized prostate cancer: current approaches and new developments. AJR Am J Roentgenol. 2009;192:1471. doi: 10.2214/AJR.09.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelloff GJ, Choyke P, Coffey DS. Challenges in clinical prostate cancer: role of imaging. AJR Am J Roentgenol. 2009;192:1455. doi: 10.2214/AJR.09.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bouchelouche K, Capala J, Oehr P. Positron emission tomography/computed tomography and radioimmunotherapy of prostate cancer. Curr opin oncol. 2009;21:469. doi: 10.1097/CCO.0b013e32832d56e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oehr P, Bouchelouche K. Imaging of prostate cancer. Curr opin oncol. 2007;19:259. doi: 10.1097/CCO.0b013e3280ad439b. [DOI] [PubMed] [Google Scholar]
- 20.Turkbey B, Pinto PA, Choyke PL. Imaging techniques for prostate cancer: implications for focal therapy. Nat Rev Urol. 2009;6:191. doi: 10.1038/nrurol.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Powles T, Murray I, Brock C, Oliver T, Avril N. Molecular positron emission tomography and PET/CT imaging in urological malignancies. Eur Urol. 2007;51:1511. doi: 10.1016/j.eururo.2007.01.061. [DOI] [PubMed] [Google Scholar]
- 22.Jana S, Blaufox MD. Nuclear medicine studies of the prostate, testes, and bladder. Semin Nucl Med. 2006;36:51. doi: 10.1053/j.semnuclmed.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Bouchelouche K, Oehr P. Positron emission tomography and positron emission tomography/computerized tomography of urological malignancies: an update review. J Urol. 2008;179:34. doi: 10.1016/j.juro.2007.08.176. [DOI] [PubMed] [Google Scholar]
- 24.Effert PJ, Bares R, Handt S, Wolff JM, Bull U, Jakse G. Metabolic imaging of untreated prostate cancer by positron emission tomography with 18fluorine-labeled deoxyglucose. J Urol. 1996;155:994. [PubMed] [Google Scholar]
- 25.Yeh SD, Imbriaco M, Larson SM, Garza D, Zhang JJ, Kalaigian H, et al. Detection of bony metastases of androgen-independent prostate cancer by PET-FDG. Nucl Med Biol. 1996;23:693. doi: 10.1016/0969-8051(96)00044-3. [DOI] [PubMed] [Google Scholar]
- 26.Shreve PD, Grossman HB, Gross MD, Wahl RL. Metastatic prostate cancer: initial findings of PET with 2-deoxy-2-[F-18]fluoro-D-glucose. Radiology. 1996;199:751. doi: 10.1148/radiology.199.3.8638000. [DOI] [PubMed] [Google Scholar]
- 27.Hofer C, Laubenbacher C, Block T, Breul J, Hartung R, Schwaiger M. Fluorine-18-fluorodeoxyglucose positron emission tomography is useless for the detection of local recurrence after radical prostatectomy. Eur Urol. 1999;36:31. doi: 10.1159/000019923. [DOI] [PubMed] [Google Scholar]
- 28.Oyama N, Akino H, Suzuki Y, Kanamaru H, Sadato N, Yonekura Y, et al. The increased accumulation of [18F]fluorodeoxyglucose in untreated prostate cancer. Jpn J Clin Oncol. 1999;29:623. doi: 10.1093/jjco/29.12.623. [DOI] [PubMed] [Google Scholar]
- 29.Liu IJ, Zafar MB, Lai YH, Segall GM, Terris MK. Fluorodeoxyglucose positron emission tomography studies in diagnosis and staging of clinically organ-confined prostate cancer. Urology. 2001;57:108. doi: 10.1016/s0090-4295(00)00896-7. [DOI] [PubMed] [Google Scholar]
- 30.Oyama N, Akino H, Suzuki Y, Kanamaru H, Ishida H, Tanase K, et al. FDG PET for evaluating the change of glucose metabolism in prostate cancer after androgen ablation. Nucl Med Commun. 2001;22:963. doi: 10.1097/00006231-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Oyama N, Akino H, Suzuki Y, Kanamaru H, Miwa Y, Tsuka H, et al. Prognostic value of 2-deoxy-2-[F-18]fluoro-D-glucose positron emission tomography imaging for patients with prostate cancer. Mol Imaging Biol. 2002;4:99. doi: 10.1016/s1095-0397(01)00065-6. [DOI] [PubMed] [Google Scholar]
- 32.Morris MJ, Akhurst T, Osman I, Nunez R, Macapinlac H, Siedlecki K, et al. Fluorinated deoxyglucose positron emission tomography imaging in progressive metastatic prostate cancer. Urology. 2002;59:913. doi: 10.1016/s0090-4295(02)01509-1. [DOI] [PubMed] [Google Scholar]
- 33.Chang CH, Wu HC, Tsai JJ, Shen YY, Changlai SP, Kao A. Detecting metastatic pelvic lymph nodes by 18F-2-deoxyglucose positron emission tomography in patients with prostate-specific antigen relapse after treatment for localized prostate cancer. Urol Int. 2003;70:311. doi: 10.1159/000070141. [DOI] [PubMed] [Google Scholar]
- 34.Schoder H, Herrmann K, Gonen M, Hricak H, Eberhard S, Scardino P, et al. 2-[18F]fluoro-2-deoxyglucose positron emission tomography for the detection of disease in patients with prostate-specific antigen relapse after radical prostatectomy. Clin Cancer Res. 2005;11:4761. doi: 10.1158/1078-0432.CCR-05-0249. [DOI] [PubMed] [Google Scholar]
- 35.Macapinlac HA, Humm JL, Akhurst T, Osman I, Pentlow K, Shangde C, et al. Differential Metabolism and Pharmacokinetics of L-[1-(11)C]-Methionine and 2-[(18)F] Fluoro-2-deoxy-D-glucose (FDG) in Androgen Independent Prostate Cancer. Clin Positron Imaging. 1999;2:173. doi: 10.1016/s1095-0397(99)00015-1. [DOI] [PubMed] [Google Scholar]
- 36.Nunez R, Macapinlac HA, Yeung HW, Akhurst T, Cai S, Osman I, et al. Combined 18F-FDG and 11C-methionine PET scans in patients with newly progressive metastatic prostate cancer. J Nucl Med. 2002;43:46. [PubMed] [Google Scholar]
- 37.Fricke E, Machtens S, Hofmann M, van den HJ, Bergh S, Brunkhorst T, et al. Positron emission tomography with 11C-acetate and 18F-FDG in prostate cancer patients. Eur J Nucl Med Mol Imaging. 2003;30:607. doi: 10.1007/s00259-002-1104-y. [DOI] [PubMed] [Google Scholar]
- 38.Oyama N, Akino H, Kanamaru H, Suzuki Y, Muramoto S, Yonekura Y, et al. 11C-acetate PET imaging of prostate cancer. J Nucl Med. 2002;43:181. [PubMed] [Google Scholar]
- 39.Oyama N, Miller TR, Dehdashti F, Siegel BA, Fischer KC, Michalski JM, et al. 11C-acetate PET imaging of prostate cancer: detection of recurrent disease at PSA relapse. J Nucl Med. 2003;44:549. [PubMed] [Google Scholar]
- 40.Picchio M, Messa C, Landoni C, Gianolli L, Sironi S, Brioschi M, et al. Value of [11C]choline-positron emission tomography for re-staging prostate cancer: a comparison with [18F]fluorodeoxyglucose-positron emission tomography. J Urol. 2003;169:1337. doi: 10.1097/01.ju.0000056901.95996.43. [DOI] [PubMed] [Google Scholar]
- 41.Larson SM, Morris M, Gunther I, Beattie B, Humm JL, Akhurst TA, et al. Tumor localization of 16beta-18F-fluoro-5alpha-dihydrotestosterone versus 18F-FDG in patients with progressive, metastatic prostate cancer. J Nucl Med. 2004;45:366. [PubMed] [Google Scholar]
- 42.Watanabe H, Kanematsu M, Kondo H, Kako N, Yamamoto N, Yamada T, et al. Preoperative detection of prostate cancer: a comparison with 11C-choline PET, 18F-fluorodeoxyglucose PET and MR imaging. J Magn Reson Imaging. 2010;31:1151. doi: 10.1002/jmri.22157. [DOI] [PubMed] [Google Scholar]
- 43.DeGrado TR, Baldwin SW, Wang S, Orr MD, Liao RP, Friedman HS, et al. Synthesis and evaluation of (18)F-labeled choline analogs as oncologic PET tracers. J Nucl Med. 2001;42:1805. [PubMed] [Google Scholar]
- 44.Hara T, Kosaka N, Kishi H. PET imaging of prostate cancer using carbon-11-choline. J Nucl Med. 1998;39:990. [PubMed] [Google Scholar]
- 45.Kotzerke J, Prang J, Neumaier B, Volkmer B, Guhlmann A, Kleinschmidt K, et al. Experience with carbon-11 choline positron emission tomography in prostate carcinoma. Eur J Nucl Med. 2000;27:1415. doi: 10.1007/s002590000309. [DOI] [PubMed] [Google Scholar]
- 46.de Jong IJ, Pruim J, Elsinga PH, Vaalburg W, Mensink HJ. Visualization of prostate cancer with 11C-choline positron emission tomography. Eur Urol. 2002;42:18. doi: 10.1016/s0302-2838(02)00129-x. [DOI] [PubMed] [Google Scholar]
- 47.de Jong IJ, Pruim J, Elsinga PH, Vaalburg W, Mensink HJ. 11C-choline positron emission tomography for the evaluation after treatment of localized prostate cancer. Eur Urol. 2003;44:32. doi: 10.1016/s0302-2838(03)00207-0. [DOI] [PubMed] [Google Scholar]
- 48.de Jong IJ, Pruim J, Elsinga PH, Vaalburg W, Mensink HJ. Preoperative staging of pelvic lymph nodes in prostate cancer by 11C-choline PET. J Nucl Med. 2003;44:331. [PubMed] [Google Scholar]
- 49.Sutinen E, Nurmi M, Roivainen A, Varpula M, Tolvanen T, Lehikoinen P, et al. Kinetics of [(11)C]choline uptake in prostate cancer: a PET study. Eur J Nucl Med Mol Imaging. 2004;31:317. doi: 10.1007/s00259-003-1377-9. [DOI] [PubMed] [Google Scholar]
- 50.Farsad M, Schiavina R, Castellucci P, Nanni C, Corti B, Martorana G, et al. Detection and localization of prostate cancer: correlation of (11)C-choline PET/CT with histopathologic step-section analysis. J Nucl Med. 2005;46:1642. [PubMed] [Google Scholar]
- 51.Yamaguchi T, Lee J, Uemura H, Sasaki T, Takahashi N, Oka T, et al. Prostate cancer: a comparative study of 11C-choline PET and MR imaging combined with proton MR spectroscopy. Eur J Nucl Med Mol Imaging. 2005;32:742. doi: 10.1007/s00259-004-1755-y. [DOI] [PubMed] [Google Scholar]
- 52.Yoshida S, Nakagomi K, Goto S, Futatsubashi M, Torizuka T. 11C-choline positron emission tomography in prostate cancer: primary staging and recurrent site staging. Urol Int. 2005;74:214. doi: 10.1159/000083551. [DOI] [PubMed] [Google Scholar]
- 53.Martorana G, Schiavina R, Corti B, Farsad M, Salizzoni E, Brunocilla E, et al. 11C-choline positron emission tomography/computerized tomography for tumor localization of primary prostate cancer in comparison with 12-core biopsy. J Urol. 2006;176:954. doi: 10.1016/j.juro.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 54.Reske SN, Blumstein NM, Neumaier B, Gottfried HW, Finsterbusch F, Kocot D, et al. Imaging prostate cancer with 11C-choline PET/CT. J Nucl Med. 2006;47:1249. [PubMed] [Google Scholar]
- 55.Rinnab L, Mottaghy FM, Blumstein NM, Reske SN, Hautmann RE, Hohl K, et al. Evaluation of [11C]-choline positron-emission/computed tomography in patients with increasing prostate-specific antigen levels after primary treatment for prostate cancer. BJU Int. 2007;100:786. doi: 10.1111/j.1464-410X.2007.07083.x. [DOI] [PubMed] [Google Scholar]
- 56.Rinnab L, Blumstein NM, Mottaghy FM, Hautmann RE, Kufer R, Hohl K, et al. 11C-choline positron-emission tomography/computed tomography and transrectal ultrasonography for staging localized prostate cancer. BJU Int. 2007;99:1421. doi: 10.1111/j.1464-410X.2007.06776.x. [DOI] [PubMed] [Google Scholar]
- 57.Scher B, Seitz M, Albinger W, Tiling R, Scherr M, Becker HC, et al. Value of 11C-choline PET and PET/CT in patients with suspected prostate cancer. Eur J Nucl Med Mol Imaging. 2007;34:45. doi: 10.1007/s00259-006-0190-7. [DOI] [PubMed] [Google Scholar]
- 58.Testa C, Schiavina R, Lodi R, Salizzoni E, Corti B, Farsad M, et al. Prostate cancer: sextant localization with MR imaging, MR spectroscopy, and 11C-choline PET/CT. Radiology. 2007;244:797. doi: 10.1148/radiol.2443061063. [DOI] [PubMed] [Google Scholar]
- 59.Rinnab L, Mottaghy FM, Simon J, Volkmer BG, de Petriconi R, Hautmann RE, et al. [11C]Choline PET/CT for targeted salvage lymph node dissection in patients with biochemical recurrence after primary curative therapy for prostate cancer. Preliminary results of a prospective study. Urol Int. 2008;81:191. doi: 10.1159/000144059. [DOI] [PubMed] [Google Scholar]
- 60.Schiavina R, Scattoni V, Castellucci P, Picchio M, Corti B, Briganti A, et al. (11)C-choline positron emission tomography/computerized tomography for preoperative lymph-node staging in intermediate-risk and high-risk prostate cancer: comparison with clinical staging nomograms. Eur Urol. 2008;54:392. doi: 10.1016/j.eururo.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 61.Li X, Liu Q, Wang M, Jin X, Liu Q, Yao S, et al. C-11 choline PET/CT imaging for differentiating malignant from benign prostate lesions. Clin Nucl Med. 2008;33:671. doi: 10.1097/RLU.0b013e318184b3a0. [DOI] [PubMed] [Google Scholar]
- 62.Rinnab L, Simon J, Hautmann RE, Cronauer MV, Hohl K, Buck AK, et al. [(11)C]choline PET/CT in prostate cancer patients with biochemical recurrence after radical prostatectomy. World J Urol. 2009;27:619. doi: 10.1007/s00345-009-0371-7. [DOI] [PubMed] [Google Scholar]
- 63.Castellucci P, Fuccio C, Nanni C, Santi I, Rizzello A, Lodi F, et al. Influence of trigger PSA and PSA kinetics on 11C-Choline PET/CT detection rate in patients with biochemical relapse after radical prostatectomy. J Nucl Med. 2009;50:1394. doi: 10.2967/jnumed.108.061507. [DOI] [PubMed] [Google Scholar]
- 64.Winter A, Uphoff J, Henke RP, Wawroschek F. First Results of [11C]choline PET/CT-guided secondary lymph node surgery in patients with PSA failure and single lymph node recurrence after radical retropubic prostatectomy. Urol Int. 2010;84:418. doi: 10.1159/000296298. [DOI] [PubMed] [Google Scholar]
- 65.Fuccio C, Castellucci P, Schiavina R, Santi I, Allegri V, Pettinato V, et al. Role of 11C-choline PET/CT in the restaging of prostate cancer patients showing a single lesion on bone scintigraphy. Ann Nucl Med. 2010;24:485. doi: 10.1007/s12149-010-0390-x. [DOI] [PubMed] [Google Scholar]
- 66.Kotzerke J, Volkmer BG, Glatting G, van den HJ, Gschwend JE, Messer P, et al. Intraindividual comparison of [11C]acetate and [11C]choline PET for detection of metastases of prostate cancer. Nuklearmedizin. 2003;42:25. [PubMed] [Google Scholar]
- 67.Kwee SA, Coel MN, Lim J, Ko JP. Prostate cancer localization with 18fluorine fluorocholine positron emission tomography. J Urol. 2005;173:252. doi: 10.1097/01.ju.0000142099.80156.85. [DOI] [PubMed] [Google Scholar]
- 68.Schmid DT, John H, Zweifel R, Cservenyak T, Westera G, Goerres GW, et al. Fluorocholine PET/CT in patients with prostate cancer: initial experience. Radiology. 2005;235:623. doi: 10.1148/radiol.2352040494. [DOI] [PubMed] [Google Scholar]
- 69.Kwee SA, Wei H, Sesterhenn I, Yun D, Coel MN. Localization of primary prostate cancer with dual-phase 18F-fluorocholine PET. J Nucl Med. 2006;47:262. [PubMed] [Google Scholar]
- 70.Cimitan M, Bortolus R, Morassut S, Canzonieri V, Garbeglio A, Baresic T, et al. [(18)F]fluorocholine PET/CT imaging for the detection of recurrent prostate cancer at PSA relapse: experience in 100 consecutive patients. Eur J Nucl Med Mol Imaging. 2006 doi: 10.1007/s00259-006-0150-2. [DOI] [PubMed] [Google Scholar]
- 71.Hacker A, Jeschke S, Leeb K, Prammer K, Ziegerhofer J, Sega W, et al. Detection of pelvic lymph node metastases in patients with clinically localized prostate cancer: comparison of [18F]fluorocholine positron emission tomography-computerized tomography and laparoscopic radioisotope guided sentinel lymph node dissection. J Urol. 2006;176:2014. doi: 10.1016/j.juro.2006.07.037. [DOI] [PubMed] [Google Scholar]
- 72.Heinisch M, Dirisamer A, Loidl W, Stoiber F, Gruy B, Haim S, et al. Positron emission tomography/computed tomography with F-18-fluorocholine for restaging of prostate cancer patients: meaningful at PSA < 5 ng/ml? Mol Imaging Biol. 2006;8:43. doi: 10.1007/s11307-005-0023-2. [DOI] [PubMed] [Google Scholar]
- 73.Husarik DB, Miralbell R, Dubs M, John H, Giger OT, Gelet A, et al. Evaluation of [(18)F]-choline PET/CT for staging and restaging of prostate cancer. Eur J Nucl Med Mol Imaging. 2008;35:253. doi: 10.1007/s00259-007-0552-9. [DOI] [PubMed] [Google Scholar]
- 74.Igerc I, Kohlfurst S, Gallowitsch HJ, Matschnig S, Kresnik E, Gomez-Segovia I, et al. The value of 18F-choline PET/CT in patients with elevated PSA-level and negative prostate needle biopsy for localisation of prostate cancer. Eur J Nucl Med Mol Imaging. 2008;35:976. doi: 10.1007/s00259-007-0686-9. [DOI] [PubMed] [Google Scholar]
- 75.Kwee SA, Thibault GP, Stack RS, Coel MN, Furusato B, Sesterhenn IA. Use of step-section histopathology to evaluate 18F-fluorocholine PET sextant localization of prostate cancer. Mol Imaging. 2008;7:12. [PubMed] [Google Scholar]
- 76.Steuber T, Schlomm T, Heinzer H, Zacharias M, Ahyai S, Chun KF, et al. [F(18)]-fluoroethylcholine combined in-line PET-CT scan for detection of lymph-node metastasis in high risk prostate cancer patients prior to radical prostatectomy: Preliminary results from a prospective histology-based study. Eur J Cancer. 2010;46:449. doi: 10.1016/j.ejca.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 77.Beheshti M, Imamovic L, Broinger G, Vali R, Waldenberger P, Stoiber F, et al. 18F choline PET/CT in the preoperative staging of prostate cancer in patients with intermediate or high risk of extracapsular disease: a prospective study of 130 patients. Radiology. 2010;254:925. doi: 10.1148/radiol.09090413. [DOI] [PubMed] [Google Scholar]
- 78.Beheshti M, Vali R, Waldenberger P, Fitz F, Nader M, Hammer J, et al. The use of F-18 choline PET in the assessment of bone metastases in prostate cancer: correlation with morphological changes on CT. Mol Imaging Biol. 2009;11:446. doi: 10.1007/s11307-009-0217-0. [DOI] [PubMed] [Google Scholar]
- 79.Poulsen MH, Bouchelouche K, Gerke O, Petersen H, Svolgaard B, Marcussen N, et al. [(18)F]-fluorocholine positron-emission/computed tomography for lymph node staging of patients with prostate cancer: preliminary results of a prospective study. BJU Int. 2010 doi: 10.1111/j.1464-410X.2009.09191.x. [DOI] [PubMed] [Google Scholar]
- 80.Vees H, Buchegger F, Albrecht S, Khan H, Husarik D, Zaidi H, et al. 18F-choline and/or 11C-acetate positron emission tomography: detection of residual or progressive subclinical disease at very low prostate-specific antigen values (<1 ng/mL) after radical prostatectomy. BJU Int. 2007;99:1415. doi: 10.1111/j.1464-410X.2007.06772.x. [DOI] [PubMed] [Google Scholar]
- 81.Beheshti M, Vali R, Waldenberger P, Fitz F, Nader M, Loidl W, et al. Detection of bone metastases in patients with prostate cancer by (18)F fluorocholine and (18)F fluoride PET-CT: a comparative study. Eur J Nucl Med Mol Imaging. 2008;35:1766. doi: 10.1007/s00259-008-0788-z. [DOI] [PubMed] [Google Scholar]
- 82.Apolo AB, Pandit-Taskar N, Morris MJ. Novel tracers and their development for the imaging of metastatic prostate cancer. J Nucl Med. 2008;49:2031. doi: 10.2967/jnumed.108.050658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ponde DE, Dence CS, Oyama N, Kim J, Tai YC, Laforest R, et al. 18F-fluoroacetate: a potential acetate analog for prostate tumor imaging--in vivo evaluation of 18F-fluoroacetate versus 11C-acetate. J Nucl Med. 2007;48:420. [PubMed] [Google Scholar]
- 84.Kato T, Tsukamoto E, Kuge Y, Takei T, Shiga T, Shinohara N, et al. Accumulation of [11C]acetate in normal prostate and benign prostatic hyperplasia: comparison with prostate cancer. Eur J Nucl Med Mol Imaging. 2002;29:1492. doi: 10.1007/s00259-002-0885-3. [DOI] [PubMed] [Google Scholar]
- 85.Kotzerke J, Volkmer BG, Neumaier B, Gschwend JE, Hautmann RE, Reske SN. Carbon-11 acetate positron emission tomography can detect local recurrence of prostate cancer. Eur J Nucl Med Mol Imaging. 2002;29:1380. doi: 10.1007/s00259-002-0882-6. [DOI] [PubMed] [Google Scholar]
- 86.Sandblom G, Sorensen J, Lundin N, Haggman M, Malmstrom PU. Positron emission tomography with C11-acetate for tumor detection and localization in patients with prostate-specific antigen relapse after radical prostatectomy. Urology. 2006;67:996. doi: 10.1016/j.urology.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 87.Wachter S, Tomek S, Kurtaran A, Wachter-Gerstner N, Djavan B, Becherer A, et al. 11C-acetate positron emission tomography imaging and image fusion with computed tomography and magnetic resonance imaging in patients with recurrent prostate cancer. J Clin Oncol. 2006;24:2513. doi: 10.1200/JCO.2005.03.5279. [DOI] [PubMed] [Google Scholar]
- 88.Albrecht S, Buchegger F, Soloviev D, Zaidi H, Vees H, Khan HG, et al. (11)C-acetate PET in the early evaluation of prostate cancer recurrence. Eur J Nucl Med Mol Imaging. 2007;34:185. doi: 10.1007/s00259-006-0163-x. [DOI] [PubMed] [Google Scholar]
- 89.Seppala J, Seppanen M, Arponen E, Lindholm P, Minn H. Carbon-11 acetate PET/CT based dose escalated IMRT in prostate cancer. Radiother Oncol. 2009;93:234. doi: 10.1016/j.radonc.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 90.Oka S, Hattori R, Kurosaki F, Toyama M, Williams LA, Yu W, et al. A preliminary study of anti-1-amino-3-18F-fluorocyclobutyl-1-carboxylic acid for the detection of prostate cancer. J Nucl Med. 2007;48:46. [PubMed] [Google Scholar]
- 91.Schuster DM, Votaw JR, Nieh PT, Yu W, Nye JA, Master V, et al. Initial experience with the radiotracer anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid with PET/CT in prostate carcinoma. J Nucl Med. 2007;48:56. [PubMed] [Google Scholar]
- 92.Toth G, Lengyel Z, Balkay L, Salah MA, Tron L, Toth C. Detection of prostate cancer with 11C-methionine positron emission tomography. J Urol. 2005;173:66. doi: 10.1097/01.ju.0000148326.71981.44. [DOI] [PubMed] [Google Scholar]
- 93.Dehdashti F, Picus J, Michalski JM, Dence CS, Siegel BA, Katzenellenbogen JA, et al. Positron tomographic assessment of androgen receptors in prostatic carcinoma. Eur J Nucl Med Mol Imaging. 2005;32:344. doi: 10.1007/s00259-005-1764-5. [DOI] [PubMed] [Google Scholar]
- 94.Even-Sapir E, Mishani E, Flusser G, Metser U. 18F-Fluoride positron emission tomography and positron emission tomography/computed tomography. Semin Nucl Med. 2007;37:462. doi: 10.1053/j.semnuclmed.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 95.Even-Sapir E, Metser U, Mishani E, Lievshitz G, Lerman H, Leibovitch I. The detection of bone metastases in patients with high-risk prostate cancer: 99mTc-MDP Planar bone scintigraphy, single- and multi-field-of-view SPECT, 18F-fluoride PET, and 18F-fluoride PET/CT. J Nucl Med. 2006;47:287. [PubMed] [Google Scholar]
- 96.Kramer-Marek G, Kiesewetter DO, Martiniova L, Jagoda E, Lee SB, Capala J. [18F]FBEM-Z(HER2:342)-Affibody molecule-a new molecular tracer for in vivo monitoring of HER2 expression by positron emission tomography. Eur J Nucl Med Mol Imaging. 2008;35:1008. doi: 10.1007/s00259-007-0658-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kramer-Marek G, Kiesewetter DO, Capala J. Changes in HER2 Expression in Breast Cancer Xenografts After Therapy Can Be Quantified Using PET and 18F-Labeled Affibody Molecules. J Nucl Med. 2009;50:1131. doi: 10.2967/jnumed.108.057695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Baum RP, Prasad V, Muller D, Schuchardt C, Orlova A, Wennborg A, et al. Molecular imaging of HER2-expressing malignant tumors in breast cancer patients using synthetic 111In- or 68Ga-labeled affibody molecules. J Nucl Med. 2010;51:892. doi: 10.2967/jnumed.109.073239. [DOI] [PubMed] [Google Scholar]
- 99.Rajinikanth A, Manoharan M, Soloway CT, Civantos FJ, Soloway MS. Trends in Gleason score: concordance between biopsy and prostatectomy over 15 years. Urology. 2008;72:177. doi: 10.1016/j.urology.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 100.Oyama N, Akino H, Kanamaru H, Suzuki Y, Muramoto S, Yonekura Y, et al. 11C-acetate PET imaging of prostate cancer. J Nucl Med. 2002;43:181. [PubMed] [Google Scholar]
- 101.Kelloff GJ, Choyke P, Coffey DS. Challenges in clinical prostate cancer: role of imaging. AJR Am J Roentgenol. 2009;192:1455. doi: 10.2214/AJR.09.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Akin O, Hricak H. Imaging of prostate cancer. Radiol Clin North Am. 2007;45:207. doi: 10.1016/j.rcl.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 103.Harisinghani MG, Barentsz J, Hahn PF, Deserno WM, Tabatabaei S, van de Kaa CH, et al. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. N Engl J Med. 2003;348:2491. doi: 10.1056/NEJMoa022749. [DOI] [PubMed] [Google Scholar]
- 104.Bouchelouche K, Capala J, Oehr P. Positron emission tomography/computed tomography and radioimmunotherapy of prostate cancer. Curr opin oncol. 2009;21:469. doi: 10.1097/CCO.0b013e32832d56e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Albrecht S, Buchegger F, Soloviev D, Zaidi H, Vees H, Khan HG, et al. (11)C-acetate PET in the early evaluation of prostate cancer recurrence. Eur J Nucl Med Mol Imaging. 2006 doi: 10.1007/s00259-006-0163-x. [DOI] [PubMed] [Google Scholar]
- 106.Fricke E, Machtens S, Hofmann M, van den HJ, Bergh S, Brunkhorst T, et al. Positron emission tomography with 11C-acetate and 18F-FDG in prostate cancer patients. Eur J Nucl Med Mol Imaging. 2003;30:607. doi: 10.1007/s00259-002-1104-y. [DOI] [PubMed] [Google Scholar]
- 107.Seppala J, Seppanen M, Arponen E, Lindholm P, Minn H. Carbon-11 acetate PET/CT based dose escalated IMRT in prostate cancer. Radiother Oncol. 2009;93:234. doi: 10.1016/j.radonc.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 108.Ibrahim T, Flamini E, Mercatali L, Sacanna E, Serra P, Amadori D. Pathogenesis of osteoblastic bone metastases from prostate cancer. Cancer. 2010;116:1406. doi: 10.1002/cncr.24896. [DOI] [PubMed] [Google Scholar]
- 109.Helyar V, Mohan HK, Barwick T, Livieratos L, Gnanasegaran G, Clarke SE, et al. The added value of multislice SPECT/CT in patients with equivocal bony metastasis from carcinoma of the prostate. Eur J Nucl Med Mol Imaging. 2010;37:706. doi: 10.1007/s00259-009-1334-3. [DOI] [PubMed] [Google Scholar]
- 110.Schirrmeister H, Guhlmann A, Elsner K, Kotzerke J, Glatting G, Rentschler M, et al. Sensitivity in detecting osseous lesions depends on anatomic localization: planar bone scintigraphy versus 18F PET. J Nucl Med. 1999;40:1623. [PubMed] [Google Scholar]
- 111.Grant FD, Fahey FH, Packard AB, Davis RT, Alavi A, Treves ST. Skeletal PET with 18F-fluoride: applying new technology to an old tracer. J Nucl Med. 2008;49:68. doi: 10.2967/jnumed.106.037200. [DOI] [PubMed] [Google Scholar]
- 112.Even-Sapir E, Metser U, Mishani E, Lievshitz G, Lerman H, Leibovitch I. The detection of bone metastases in patients with high-risk prostate cancer: 99mTc-MDP Planar bone scintigraphy, single- and multi-field-of-view SPECT, 18F-fluoride PET, and 18F-fluoride PET/CT. J Nucl Med. 2006;47:287. [PubMed] [Google Scholar]
- 113.Denardo GL, Schlom J, Buchsbaum DJ, Meredith RF, O’Donoghue JA, Sgouros G, et al. Rationales, evidence, and design considerations for fractionated radioimmunotherapy. Cancer. 2002;94:1332. doi: 10.1002/cncr.10304. [DOI] [PubMed] [Google Scholar]
- 114.O’Donoghue JA, Sgouros G, Divgi CR, Humm JL. Single-dose versus fractionated radioimmunotherapy: model comparisons for uniform tumor dosimetry. J Nucl Med. 2000;41:538. [PubMed] [Google Scholar]
- 115.Shen S, Duan J, Meredith RF, Buchsbaum DJ, Brezovich IA, Pareek PN, et al. Model prediction of treatment planning for dose-fractionated radioimmunotherapy. Cancer. 2002;94:1264. doi: 10.1002/cncr.10295. [DOI] [PubMed] [Google Scholar]
- 116.Kroger LA, Denardo GL, Gumerlock PH, Xiong CY, Winthrop MD, Shi XB, et al. Apoptosis-related gene and protein expression in human lymphoma xenografts (Raji) after low dose rate radiation using 67Cu-2IT-BAT-Lym-1 radioimmunotherapy. Cancer Biother Radiopharm. 2001;16:213. doi: 10.1089/10849780152389401. [DOI] [PubMed] [Google Scholar]
- 117.Vriesendorp HM, Shao Y, Blum JE, Quadri SM, Williams JR. Fractionated intravenous administration of 90Y-labeled B72. GYK-DTPA immunoconjugate in beagle dogs. Nucl Med Biol. 1993;20:571. doi: 10.1016/0969-8051(93)90025-p. [DOI] [PubMed] [Google Scholar]
- 118.Buchsbaum D, Khazaeli MB, Liu T, Bright S, Richardson K, Jones M, et al. Fractionated radioimmunotherapy of human colon carcinoma xenografts with 131I-labeled monoclonal antibody CC49. Cancer Res. 1995;55:5881s. [PubMed] [Google Scholar]
- 119.Denardo GL, DeNardo SJ, Lamborn KR, Goldstein DS, Levy NB, Lewis JP, et al. Low-dose, fractionated radioimmunotherapy for B-cell malignancies using 131I-Lym-1 antibody. Cancer Biother Radiopharm. 1998;13:239. doi: 10.1089/cbr.1998.13.239. [DOI] [PubMed] [Google Scholar]
- 120.Steffens MG, Boerman OC, Oyen WJ, Kniest PH, Witjes JA, Oosterhof GO, et al. Intratumoral distribution of two consecutive injections of chimeric antibody G250 in primary renal cell carcinoma: implications for fractionated dose radioimmunotherapy. Cancer Res. 1999;59:1615. [PubMed] [Google Scholar]
- 121.Hindorf C, Linden O, Stenberg L, Tennvall J, Strand SE. Change in tumor-absorbed dose due to decrease in mass during fractionated radioimmunotherapy in lymphoma patients. Clin Cancer Res. 2003;9:4003S. [PubMed] [Google Scholar]
- 122.O’Donnell RT, DeNardo SJ, Miers LA, Lamborn KR, Kukis DL, Denardo GL, et al. Combined modality radioimmunotherapy for human prostate cancer xenografts with taxanes and 90yttrium-DOTA-peptide-ChL6. Prostate. 2002;50:27. doi: 10.1002/pros.10029. [DOI] [PubMed] [Google Scholar]
- 123.Richman CM, DeNardo SJ, O’Donnell RT, Yuan A, Shen S, Goldstein DS, et al. High-dose radioimmunotherapy combined with fixed, low-dose paclitaxel in metastatic prostate and breast cancer by using a MUC-1 monoclonal antibody, m170, linked to indium-111/yttrium-90 via a cathepsin cleavable linker with cyclosporine to prevent human anti-mouse antibody. Clin Cancer Res. 2005;11:5920. doi: 10.1158/1078-0432.CCR-05-0211. [DOI] [PubMed] [Google Scholar]
- 124.Kelly MP, Lee FT, Smyth FE, Brechbiel MW, Scott AM. Enhanced efficacy of 90Y-radiolabeled anti-Lewis Y humanized monoclonal antibody hu3S193 and paclitaxel combined-modality radioimmunotherapy in a breast cancer model. J Nucl Med. 2006;47:716. [PubMed] [Google Scholar]
- 125.Allegra CJ, Jessup JM, Somerfield MR, Hamilton SR, Hammond EH, Hayes DF, et al. American Society of Clinical Oncology provisional clinical opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol. 2009;27:2091. doi: 10.1200/JCO.2009.21.9170. [DOI] [PubMed] [Google Scholar]
- 126.Kaminski MS, Estes J, Zasadny KR, Francis IR, Ross CW, Tuck M, et al. Radioimmunotherapy with iodine (131)I tositumomab for relapsed or refractory B-cell non-Hodgkin lymphoma: updated results and long-term follow-up of the University of Michigan experience. Blood. 2000;96:1259. [PubMed] [Google Scholar]
- 127.Kaminski MS, Zelenetz AD, Press OW, Saleh M, Leonard J, Fehrenbacher L, et al. Pivotal study of iodine I 131 tositumomab for chemotherapy-refractory low-grade or transformed low-grade B-cell non-Hodgkin’s lymphomas. J Clin Oncol. 2001;19:3918. doi: 10.1200/JCO.2001.19.19.3918. [DOI] [PubMed] [Google Scholar]
- 128.Press OW, Unger JM, Braziel RM, Maloney DG, Miller TP, Leblanc M, et al. A phase 2 trial of CHOP chemotherapy followed by tositumomab/iodine I 131 tositumomab for previously untreated follicular non-Hodgkin lymphoma: Southwest Oncology Group Protocol S9911. Blood. 2003;102:1606. doi: 10.1182/blood-2003-01-0287. [DOI] [PubMed] [Google Scholar]
- 129.Kaminski MS, Tuck M, Estes J, Kolstad A, Ross CW, Zasadny K, et al. 131I-tositumomab therapy as initial treatment for follicular lymphoma. N Engl J Med. 2005;352:441. doi: 10.1056/NEJMoa041511. [DOI] [PubMed] [Google Scholar]
- 130.Leonard JP, Coleman M, Kostakoglu L, Chadburn A, Cesarman E, Furman RR, et al. Abbreviated chemotherapy with fludarabine followed by tositumomab and iodine I 131 tositumomab for untreated follicular lymphoma. J Clin Oncol. 2005;23:5696. doi: 10.1200/JCO.2005.14.803. [DOI] [PubMed] [Google Scholar]
- 131.Press OW, Unger JM, Braziel RM, Maloney DG, Miller TP, Leblanc M, et al. Phase II trial of CHOP chemotherapy followed by tositumomab/iodine I-131 tositumomab for previously untreated follicular non-Hodgkin’s lymphoma: five-year follow-up of Southwest Oncology Group Protocol S9911. J Clin Oncol. 2006;24:4143. doi: 10.1200/JCO.2006.05.8198. [DOI] [PubMed] [Google Scholar]
- 132.Divgi C. Editorial: what ails solid tumor radioimmunotherapy? Cancer Biother Radiopharm. 2006;21:81. doi: 10.1089/cbr.2006.21.81. [DOI] [PubMed] [Google Scholar]
- 133.O’Donoghue JA, Bardies M, Wheldon TE. Relationships between tumor size and curability for uniformly targeted therapy with beta-emitting radionuclides. J Nucl Med. 1995;36:1902. [PubMed] [Google Scholar]
- 134.Meredith RF, Bueschen AJ, Khazaeli MB, Plott WE, Grizzle WE, Wheeler RH, et al. Treatment of metastatic prostate carcinoma with radiolabeled antibody CC49. J Nucl Med. 1994;35:1017. [PubMed] [Google Scholar]
- 135.O’Donnell RT, DeNardo SJ, Yuan A, Shen S, Richman CM, Lara PN, et al. Radioimmunotherapy with (111)In/(90)Y-2IT-BAD-m170 for metastatic prostate cancer. Clin Cancer Res. 2001;7:1561. [PubMed] [Google Scholar]
- 136.Kelly MP, Lee FT, Tahtis K, Smyth FE, Brechbiel MW, Scott AM. Radioimmunotherapy with alpha-particle emitting 213Bi-C-functionalized trans-cyclohexyl-diethylenetriaminepentaacetic acid-humanized 3S193 is enhanced by combination with paclitaxel chemotherapy. Clin Cancer Res. 2007;13:5604s. doi: 10.1158/1078-0432.CCR-07-1071. [DOI] [PubMed] [Google Scholar]
- 137.Schneider DW, Heitner T, Alicke B, Light DR, McLean K, Satozawa N, et al. In vivo biodistribution, PET imaging, and tumor accumulation of 86Y- and 111In-antimindin/RG-1, engineered antibody fragments in LNCaP tumor-bearing nude mice. J Nucl Med. 2009;50:435. doi: 10.2967/jnumed.108.055608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Deb N, Goris M, Trisler K, Fowler S, Saal J, Ning S, et al. Treatment of hormone-refractory prostate cancer with 90Y-CYT-356 monoclonal antibody. Clin Cancer Res. 1996;2:1289. [PubMed] [Google Scholar]
- 139.Kahn D, Austin JC, Maguire RT, Miller SJ, Gerstbrein J, Williams RD. A phase II study of [90Y] yttrium-capromab pendetide in the treatment of men with prostate cancer recurrence following radical prostatectomy. Cancer Biother Radiopharm. 1999;14:99. doi: 10.1089/cbr.1999.14.99. [DOI] [PubMed] [Google Scholar]
- 140.Milowsky MI, Nanus DM, Kostakoglu L, Vallabhajosula S, Goldsmith SJ, Bander NH. Phase I trial of yttrium-90-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 for androgen-independent prostate cancer. J Clin Oncol. 2004;22:2522. doi: 10.1200/JCO.2004.09.154. [DOI] [PubMed] [Google Scholar]
- 141.Bander NH, Milowsky MI, Nanus DM, Kostakoglu L, Vallabhajosula S, Goldsmith SJ. Phase I trial of 177lutetium-labeled J591, a monoclonal antibody to prostate-specific membrane antigen, in patients with androgen-independent prostate cancer. J Clin Oncol. 2005;23:4591. doi: 10.1200/JCO.2005.05.160. [DOI] [PubMed] [Google Scholar]
- 142.Horoszewicz JS, Kawinski E, Murphy GP. Monoclonal antibodies to a new antigenic marker in epithelial prostatic cells and serum of prostatic cancer patients. Anticancer Res. 1987;7:927. [PubMed] [Google Scholar]
- 143.Israeli RS, Powell CT, Fair WR, Heston WD. Molecular cloning of a complementary DNA encoding a prostate-specific membrane antigen. Cancer Res. 1993;53:227. [PubMed] [Google Scholar]
- 144.Israeli RS, Powell CT, Corr JG, Fair WR, Heston WD. Expression of the prostate-specific membrane antigen. Cancer Res. 1994;54:1807. [PubMed] [Google Scholar]
- 145.Troyer JK, Beckett ML, Wright GL., Jr. Detection and characterization of the prostate-specific membrane antigen (PSMA) in tissue extracts and body fluids. Int J Cancer. 1995;62:552. doi: 10.1002/ijc.2910620511. [DOI] [PubMed] [Google Scholar]
- 146.Sokoloff RL, Norton KC, Gasior CL, Marker KM, Grauer LS. A dual-monoclonal sandwich assay for prostate-specific membrane antigen: levels in tissues, seminal fluid and urine. Prostate. 2000;43:150. doi: 10.1002/(sici)1097-0045(20000501)43:2<150::aid-pros10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 147.Bostwick DG, Pacelli A, Blute M, Roche P, Murphy GP. Prostate specific membrane antigen expression in prostatic intraepithelial neoplasia and adenocarcinoma: a study of 184 cases. Cancer. 1998;82:2256. doi: 10.1002/(sici)1097-0142(19980601)82:11<2256::aid-cncr22>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 148.Kahn D, Williams RD, Manyak MJ, Haseman MK, Seldin DW, Libertino JA, et al. 111Indium-capromab pendetide in the evaluation of patients with residual or recurrent prostate cancer after radical prostatectomy. The ProstaScint Study Group. J Urol. 1998;159:2041. doi: 10.1016/S0022-5347(01)63239-7. [DOI] [PubMed] [Google Scholar]
- 149.Kahn D, Williams RD, Haseman MK, Reed NL, Miller SJ, Gerstbrein J. Radioimmunoscintigraphy with In-111-labeled capromab pendetide predicts prostate cancer response to salvage radiotherapy after failed radical prostatectomy. J Clin Oncol. 1998;16:284. doi: 10.1200/JCO.1998.16.1.284. [DOI] [PubMed] [Google Scholar]
- 150.Troyer JK, Beckett ML, Wright GL., Jr. Location of prostate-specific membrane antigen in the LNCaP prostate carcinoma cell line. Prostate. 1997;30:232. doi: 10.1002/(sici)1097-0045(19970301)30:4<232::aid-pros2>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 151.Liu H, Moy P, Kim S, Xia Y, Rajasekaran A, Navarro V, et al. Monoclonal antibodies to the extracellular domain of prostate-specific membrane antigen also react with tumor vascular endothelium. Cancer Res. 1997;57:3629. [PubMed] [Google Scholar]
- 152.Yao D, Trabulsi EJ, Kostakoglu L, Vallabhajosula S, Joyce MA, Nanus DM, et al. The utility of monoclonal antibodies in the imaging of prostate cancer. Semin Urol Oncol. 2002;20:211. doi: 10.1053/suro.2002.36250. [DOI] [PubMed] [Google Scholar]
- 153.Liu H, Rajasekaran AK, Moy P, Xia Y, Kim S, Navarro V, et al. Constitutive and antibody-induced internalization of prostate-specific membrane antigen. Cancer Res. 1998;58:4055. [PubMed] [Google Scholar]
- 154.Bander NH, Trabulsi EJ, Kostakoglu L, Yao D, Vallabhajosula S, Smith-Jones P, et al. Targeting metastatic prostate cancer with radiolabeled monoclonal antibody J591 to the extracellular domain of prostate specific membrane antigen. J Urol. 2003;170:1717. doi: 10.1097/01.ju.0000091655.77601.0c. [DOI] [PubMed] [Google Scholar]
- 155.Tagawa ST, Jeske S, Milowski MI, Vallabahajosula S, Goldsmith SJ, Matulich D, Kaplan I, Bander NH, Nanus DM. Phase II trial of 177Lutetium radiolabeled anti-prostate-specific membrane antigen (PSMA) monoclonal antibody J591 (177Lu-J591) in patients with metastatic castrate-resistent prostate cancer. Cancer Biother.Radiopharm. 2008;23(4):525. Ref Type: Abstract. [Google Scholar]
- 156.Tagawa ST, Vallabahajosula S, Osborne J. Phase I trial of fractionated-dose 177lutetium radiolabeled anti-prostate-specific membrane antigen (PSMA) monocloncal antibody J591 (177Lu-J591) in patients (pts) with metastatic castration-resistent prostate cancer (metCRPC) J Clin.Oncol. 2010;28 Ref Type: Abstract. [Google Scholar]
- 157.Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr., Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 158.Choy H, Rodriguez FF, Koester S, Hilsenbeck S, Von Hoff DD. Investigation of taxol as a potential radiation sensitizer. Cancer. 1993;71:3774. doi: 10.1002/1097-0142(19930601)71:11<3774::aid-cncr2820711147>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 159.Tishler RB, Schiff PB, Geard CR, Hall EJ. Taxol: a novel radiation sensitizer. Int J Radiat Oncol Biol Phys. 1992;22:613. doi: 10.1016/0360-3016(92)90888-o. [DOI] [PubMed] [Google Scholar]
- 160.Hennequin C, Giocanti N, Balosso J, Favaudon V. Interaction of ionizing radiation with the topoisomerase I poison camptothecin in growing V-79 and HeLa cells. Cancer Res. 1994;54:1720. [PubMed] [Google Scholar]
- 161.Kelly MP, Lee FT, Smyth FE, Brechbiel MW, Scott AM. Enhanced efficacy of 90Y-radiolabeled anti-Lewis Y humanized monoclonal antibody hu3S193 and paclitaxel combined-modality radioimmunotherapy in a breast cancer model. J Nucl Med. 2006;47:716. [PubMed] [Google Scholar]
- 162.Beltran H, Vallabahajosula S, Kelly WK. Phase I dose escalation study of Docetaxel/Prednisone and fractionated 177Lu-J591 for metastatic castrate resistent prostate cancer (metCRPC) J Clin.Oncol. 2010;28 Ref Type: Abstract. [Google Scholar]
- 163.Press OW, Unger JM, Braziel RM, Maloney DG, Miller TP, Leblanc M, et al. Phase II trial of CHOP chemotherapy followed by tositumomab/iodine I-131 tositumomab for previously untreated follicular non-Hodgkin’s lymphoma: five-year follow-up of Southwest Oncology Group Protocol S9911. J Clin Oncol. 2006;24:4143. doi: 10.1200/JCO.2006.05.8198. [DOI] [PubMed] [Google Scholar]
- 164.Moul JW. Prostate specific antigen only progression of prostate cancer. J Urol. 2000;163:1632. [PubMed] [Google Scholar]
- 165.Scher HI, Eisenberger M, D’Amico AV, Halabi S, Small EJ, Morris M, et al. Eligibility and outcomes reporting guidelines for clinical trials for patients in the state of a rising prostate-specific antigen: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 2004;22:537. doi: 10.1200/JCO.2004.07.099. [DOI] [PubMed] [Google Scholar]
- 166.Pazona JF, Han M, Hawkins SA, Roehl KA, Catalona WJ. Salvage radiation therapy for prostate specific antigen progression following radical prostatectomy: 10-year outcome estimates. J Urol. 2005;174:1282. doi: 10.1097/01.ju.0000173911.82467.f9. [DOI] [PubMed] [Google Scholar]
- 167.Buskirk SJ, Pisansky TM, Schild SE, Macdonald OK, Wehle MJ, Kozelsky TF, et al. Salvage radiotherapy for isolated prostate specific antigen increase after radical prostatectomy: evaluation of prognostic factors and creation of a prognostic scoring system. J Urol. 2006;176:985. doi: 10.1016/j.juro.2006.04.083. [DOI] [PubMed] [Google Scholar]
- 168.Stephenson AJ, Shariat SF, Zelefsky MJ, Kattan MW, Butler EB, Teh BS, et al. Salvage radiotherapy for recurrent prostate cancer after radical prostatectomy. JAMA. 2004;291:1325. doi: 10.1001/jama.291.11.1325. [DOI] [PubMed] [Google Scholar]
- 169.Ward JF, Zincke H, Bergstralh EJ, Slezak JM, Blute ML. Prostate specific antigen doubling time subsequent to radical prostatectomy as a prognosticator of outcome following salvage radiotherapy. J Urol. 2004;172:2244. doi: 10.1097/01.ju.0000145262.34748.2b. [DOI] [PubMed] [Google Scholar]
- 170.Tagawa ST, Osborne J, Christos PJ. A randomized phase II trial of 177Lu radiolabeled monocloncal antibody J591 (177Lu-J591) and ketoconazole in patients (pts) with high-risk castrate biochemically relapsed prostate cancer (PC) after local therapy. J Clin.Oncol. 2010;28 Ref Type: Abstract. [Google Scholar]