Abstract
Purpose
Overexpression of HER2/neu in breast cancer is correlated with poor prognosis. It may vary between primary tumors and metastatic lesions and change during the treatment. Therefore, there is a need for new means to assess HER2/neu expression in vivo. In this work, we used 68Ga-labeled DOTA-ZHER2:2891-Affibody to monitor HER2/neu expression in a panel of breast cancer xenografts.
Methods
DOTA-ZHER2:2891-Affibody molecules were labeled with 68Ga. In vitro binding was characterized by a receptor saturation assay. Biodistribution and PET imaging studies were conducted in athymic nude mice bearing subcutaneous human breast cancer tumors with three different levels of HER2/neu expression. Nonspecific uptake was analyzed using non-HER2-specific Affibody molecules. Signal detected by PET was compared with ex vivo assessment of the tracer uptake and HER2/neu expression.
Results
68Ga-DOTA-ZHER2:2891-Affibody probe showed high binding affinity to MDA-MB-361 cells (KD = 1.4±0.19 nM). In vivo biodistribution and PET imaging studies demonstrated high radioactivity uptake in HER2/neu-positive tumors. Tracer was eliminated quickly from the blood and normal tissues, resulting in high tumor-to-blood ratios. The highest normal tissue concentration of radioactivity was seen in the kidneys (227±14 %ID/g). High-contrast PET images of HER2/neu-overexpressing tumors were recorded as soon as 1 hour after tracer injection. Good correlation was observed between PET imaging, biodistribution estimates of tumor tracer concentration, and the receptor expression.
Conclusion
These results suggest that PET imaging using 68Ga-DOTA-ZHER2:2891-Affibody is sensitive enough to detect different levels of HER2/neu expression in vivo.
Keywords: Affibody molecules, Molecular imaging, HER2/neu, PET, Ga-68
INTRODUCTION
The human epidermal growth factor receptor (HER2/neu; c-erbB2) is a 185 kDa glycoprotein with tyrosine kinase activity that plays an important role in regulating tumor cell survival, proliferation, maturation, and mobility [1]. It has also been shown to be involved in angiogenesis [2]. Amplification and/or overexpression of HER2/neu have been detected in approximately 20% of invasive breast carcinomas and in a significant fraction of ovary, lung, stomach, and bladder cancers. In breast cancer, HER2/neu overexpression is usually associated with increased tumor aggressiveness, resistance to therapies, high rates of recurrence, and increased mortality [3].
HER2/neu expression is routinely determined by immunohistochemistry (IHC) or fluorescence in situ hybridization (FISH) at the time of diagnosis of the primary tumor. However, the HER2/neu status of the primary tumor does not always reflect that of metastatic lesions, and these expression levels might change during the course of the treatment [4-5]. Even though HER2/neu testing is common, there are problems associated with its sensitivity and specificity, potentially confounding the selection of appropriate patients for anti-HER2/neu treatment. Therefore, there is a need for methods that can assess global in vivo HER2/neu expression non-invasively and reproducibly. Molecular imaging modalities offer a strategy for determining HER2/neu expression and localizing HER2/neu positive lesions in vivo, including inaccessible distant metastasis [6].
Currently available HER2/neu-targeted ligands include full length monoclonal antibodies, Fab-fragments, F(ab’)2-fragments, diabodies, minibodies, Affibody molecules, and peptides that can be labeled with either a contrast agent for Magnetic Resonance Imaging (MRI), a fluorescent dye for Optical Imaging, positron-emitting radionuclides for Positron Emission Tomography (PET), or gamma emitting radionuclides for Single Photon Emission Computed Tomography (SPECT) [7-8]. Among these, PET is the most sensitive human-scale diagnostic imaging modality and provides the highest spatial resolution among radionuclide techniques especially when combined with Computed Tomography (CT).
Gallium-68, a metallic positron emitter, is of particular interest for PET imaging since it can be obtained from a generator, thereby, providing a convenient alternative to cyclotron-produced PET isotopes, such as 18F and 124I [9].
The stable oxidation state of gallium in aqueous solutions is +3 and it forms thermodynamically stable complexes with ligands containing oxygen, nitrogen, and sulfur donor atoms [9]. A variety of chelators have been developed to allow the formation of stable 68Ga (+3) complexes. 1,4,7,10-tetraazacyclododecane- N,N’,N’’,N’’’-tetraacetic acid (DOTA) is the most commonly used macrocyclic chelating agent for trivalent radiometals. Complexes of gallium with DOTA have been found to be stable in vivo and have been used in clinical studies [9-10].
The site-specific coupling of DOTA derivative during peptide synthesis provides a homogenous conjugate with well-defined chemical and biological properties. Gallium-68 also has attractive physical properties. It decays 89% of the time by positron emission and 11% by electron capture. Its half-life (68 min) matches the pharmacokinetics of radiopharmaceuticals of low molecular weight (less than 60 kDa) that are most suitable for imaging applications due to their fast blood clearance, rapid diffusion and target localization [11]. A potential disadvantage of 68Ga is the relatively high energy of the emitted positrons leading to a mean range in water of 2.9 mm that can reduce spatial resolution compared to PET images obtained with other potential positron labels, e.g. 18F (mean range 0.6 mm) [12].
Among all the HER2/neu-targeting agents, Affibody molecules seem to be the most promising tracers for imaging of HER2/neu receptor expression [13]. Affibody molecules were originally derived from the B-domain in the immunoglobulin-binding region of staphylococcal protein A. The B-domain is a relatively short 58-amino acid peptide (6.5 kDa) that is folded into a three-helical bundle scaffold structure [14].
Application of HER2/neu-specific Affibody molecules, labeled with a variety of radionuclides including 18F, 64Cu, 111In, 90Y, 177Lu [14-15], and, lately, also with 68Ga, for diagnostic and therapeutic purposes have been widely investigated [16] . These studies demonstrated the high tumor to background ratio in HER2/neu imaging due to rapid blood clearance and high-affinity, tumor-specific targeting. In addition, our recent work confirmed the potential of Affibody-based tracers for monitoring HER2/neu receptor changes in murine xenografts after therapeutic interventions [17].
Here, we report the first imaging studies with 68Ga-DOTA-ZHER2:2891-Affibody probe in a panel of breast cancer xenografts with different HER2/neu expression levels as verified by flow cytometry and ELISA assay. Our results indicate that this tracer would enable non-invasive assessment of HER2/neu expression level in vivo by PET imaging.
MATERIAL and METHODS
General
The 68Ge/68Ga generator (IGG100), in which the parent isotope 68Ge (1850 MBq) is adsorbed on a TiO2 column, was procured from Eckert & Ziegler Isotope Products (Berlin, Germany). Analytical grade chemicals were used. Water was distilled and deionized (18 MΩ/cm2) using a Milli-Q water filtration system (Millipore Corp., Milford, MA). For radiolabeling of Affibody molecules, the 68Ge/68Ga generator was coupled to an automated Modular Lab (Eckert & Ziegler Gmbh., Berlin, Germany). The labeling reactions were carried out in 3 mL glass vials (Wheaton Science Products, Millville, NJ). Strata–XC cartridges, 30 mg (Phenomenex, Torrance, CA), were used for pre-purification of the eluted 68Ga. DOTA-ZHER2:2891 (ABY025) and non-HER2-specific Affibody (His6-ZTaq-Cys) were kindly provided by our Cooperative Research and Development Agreement partner: Affibody, AB, Stockholm, Sweden. His6-ZTaq-Cys Affibody was conjugated with DOTA according to the method reported by Ahlgren et al. 2008 [18].
Radiolabeling
The 68Ge/68Ga generator was eluted with dilute hydrochloric acid (0.1 M HCl solution) to obtain 68Ga in 10 mL of 0.1 M HCl solution. The 68Ga solution was immediately transferred to a Strata XC cartridge (cationic polymeric sorbent), air-dried for a few seconds, and then treated with 0.7 mL of 0.02 M HCl, 98% acetone solution (a solution of 0.78 mM HCl with 97.56 mL of acetone) to release the 68Ga. Pre-purification of the generator eluent was performed to reduce any trace metallic impurities and limit 68Ge breakthrough. DOTA-ZHER2:2891 (30 μg) was added to 0.5 mL of 0.25 M ammonium acetate buffer (pH 3.9). Purified 68Ga eluate (400 MBq) was added to this solution and heated at 80 °C for 15 minutes.
The radiochemical purity of the labeled conjugate was determined by reverse-phase, high-performance liquid chromatography (RP-HPLC) (Beckmann-Coulter, System Gold) equipped with a UV166 detector (Beckmann Coulter Inc, Brea, CA) using a C-18 column (Agilent Extend, C-18, 4.6 ×150 mm), eluted with 0.1% TFA in water (solvent A) and 0.1 % TFA in acetonitrile (solvent B). The HPLC gradient was 20% - 40% of solvent B over 16 minutes with a flow rate of 1 mL/min. Monitoring of radioactivity during the HPLC run was performed using an inline radio-detector (Flow-Count radio-HPLC Detector System, Bioscan, Washington DC). The reaction mixture was then applied to a NAP-10 column (5 kDa cut-off), and eluted with phosphate-buffered saline (PBS) to obtain 68Ga-DOTA-ZHER2:2891. The purity of the filtrate was determined by RP-HPLC.
Cell Lines
Human breast (BT474, MDA-MB-361, and MCF7) that express different levels of HER2/neu were purchased from the American Type Culture Collection (Rockville, MD). The cells were cultured either in RPMI 1640 (BT474) or in Dulbecco modified Eagle (MCF7, MDA-MB-361) medium supplemented with 10% (v/v) heat-inactivated fetal bovine serum (GIBCO) and penicillin/streptomycin (a 100 U/mL concentration of each). Cells were grown as a monolayer at 37°C in a humidified atmosphere containing 5% CO2.
Fluorescence-Activated Cell Sorter (FACS) Analysis
For HER2/neu detection, cells were cultured overnight in T75 flasks (Sarstedt). The following day cells were rinsed twice with (PBS) and detached with cell stripper (Cellgro). For every sample, 1×106 cells were collected, and incubated for 45 min on ice with anti-mouse Neu(24D2)-fluorescein isothiocyanate monoclonal antibody or with IgG1-fluorescein isothiocyanate mouse isotypic control (Santa Cruz Biotechnology, Inc.). After antibody staining, all cells were washed with PBS and resuspended in 0.3 mL of PBS for data acquisition.
All solutions were kept on ice. Flow cytometry was performed using a LSRII (BD Biosciences, San Jose, CA). FACSDiVa software (BD Biosciences, San Jose, CA) was used for data acquisition and FlowJo software for analysis (Tree Star Inc.). For each sample, 20,000 events were recorded, and the population corresponding to single cells was gated and analyzed as a histogram plot. Results are reported as mean fluorescence intensity.
Enzyme-Linked Immunosorbent Assay (ELISA)
The HER2/neu protein level in tissue lysates was measured according to the procedure reported previously [17]. Briefly, the tumor tissue was homogenized on ice, using a Polytron homogenizer, and lysed in resuspension buffer provided with the ELISA kit (Calbiochem). Cell debris was removed through centrifugation of the suspensions at 13,000 rpm (4°C) for 15 min. The supernatants were collected and stored at -80°C. Before use, the protein concentration was estimated by the bicinchoninic acid assay kit (Pierce), according to the manufacturer’s protocol. Then the HER2/neu protein level was measured by an ELISA kit, according to the manufacturer’s recommended procedure. Results were expressed in nanograms of HER2/neu per milligram of protein. The data are presented as the mean of 2 replicates, with each experiment repeated 3 times.
In Vitro Cell-Binding Assays
The in vitro binding characteristics of the 68Ga-DOTA-ZHER2:2891-Affibody was evaluated using saturation cell-binding assays. The day before the experiments, MDA-MB-361 cells were seeded in six-well plates at concentration of 5×105 cells per ml. For receptor saturation analysis, the cells were incubated (4°C, 2 h) with increasing concentrations (0.06–220 nM) of 68Ga-DOTA-ZHER2:2891-Affibody, either alone or with an additional 50-fold excess of non-labeled ZHER2:2891-Affibody molecules. Then, the cells were washed with cold phosphate-buffered saline (PBS, pH 7.0), trypsinized, and the cell-associated radioactivity was measured using a γ-counter (1480 Wizard 3, Automatic Gamma Counter, Perkin-Elmer, Waltham, MA). Specific binding was plotted against the total molar concentration of added 68Ga-DOTA-ZHER2:2891-Affibody. The KD value and the concentration of the tracer required to saturate the receptors (Bmax) were analyzed by non-linear regression using GraphPad Prism5 (GraphPad Software, San Diego, CA). The Bmax value (nM) was converted to the number of HER2/neu receptors per cell. The assay was performed in duplicate and repeated in 2 separate experiments.
Tumor Model
All animal studies were conducted in accordance with the principles and procedures outlined in the Guide for the Care and Use of Laboratory Animals, and were approved by the Institutional Animal Care and Use Committee of the National Institutes of Health. Cells suspended in Matrigel (BD Bioscience) were implanted subcutaneously into the shoulder region of 5- to 7-week old female athymic nude mice: 5×106 MDA-MB-361, MCF7 or 8×106 BT474 cells. For MCF7 and BT474 cells estrogen pellets (1.72 mg; Innovative Research of America) were implanted 24 hours before breast cancer cells inoculation and remained in place until the end of the study. Tumors (150–200 cm3) developed after 3–5 weeks.
Biodistribution
68Ga-DOTA-ZHER2:2891-Affibody (3.7–6.7 MBq, 10-13 μg, 100 μL) was injected into the tail vein of mice bearing BT474 tumors. Mice were euthanized, and their major organs were dissected and weighed at 1 and 3 hours after injection. Four mice were used for each data point. Radioactivity uptake in the tissues was measured, along with a standard of the injected dose, using a γ-counter. The results were expressed as percentage injected dose per gram of tissue (%ID/g).
To study the blood kinetics and the stability of 68Ga-DOTA-ZHER2:2891-Affibody in vivo, the blood was collected in vials containing 30 μl of heparin at 15, 30, 45, 60, 120, and 180 minutes post-injection. The samples were weighed, and the radioactivity was measured by γ-counter. Then, the blood was centrifuged (10 min at 10,000×g) and fractions of plasma (200–300 μl) were separated on NAP-10 columns. The mean concentrations of radioactivity in the blood (% ID/g) were plotted against time after injection, and the curve was fitted by single exponential curve.
To confirm the specificity of 68Ga-DOTA-ZHER2:2891-Affibody binding in vivo, one group of mice bearing BT474 (very high HER2/neu expression) was injected with the non-HER2-specific Affibody molecules, His6-ZTaq-Cys-DOTA-68Ga. One hour later, mice (n = 4) were euthanized and their organs analyzed as described above. This time point was chosen since it showed the optimal tumor-to-background ratio for the binding of the labeled HER2-specific Affibody molecules.
PET/CT Imaging
PET scans were performed using the NIH Advanced Technology Laboratory Animal Scanner (ATLAS). CT images were obtained with the CT component of the NanoSPECT/CT scanner from Bioscan (Bioscan, Inc., Washington, DC). Spatially registered and fused PET and CT images were obtained by using a custom-designed adapter to the ATLAS bed support that allowed the Bioscan imaging bed (and the animals) to be reproducibly transferred between machines that had been previously calibrated for accurate spatial registration. The mice were anesthetized using isoflurane/O2 (1.5-2.0 % v/v), placed prone in the center of the field of view of the scanner, and given an injection of 68Ga-DOTA-ZHER2:2891-Affibody (3.7–6.7 MBq, 10-13 μg, 100 μL) via the lateral tail vein. Whole-body scanning was performed with a 2-cm axial field of view (3 bed positions each of 10 min) at 1, 2, 3 hours after radiotracer injection and/or a 10-min emission scan (single field of view) was recorded 1 hour post injection, both with a 100- to 700-keV energy window. The images were reconstructed with a 2-dimensional ordered-subsets expectation maximum algorithm (2D-OSEM). No correction was applied for attenuation or scatter. For each scan, regions of interest (ROI) were manually drawn over the tumor and normal tissue. The maximum counts per pixel within the tumor or normal tissues were obtained from multiple regions of interest (counts/s/cm3). The results were calculated as %ID/g using a calibration constant obtained from scanning the 68Ga source, assuming a tissue density of 1 g/ml, and dividing by the injected dose, decay-corrected to the time of scanning.
The CT component of the nanoSPECT/CT was configured to acquire image data with the X-Ray tube high voltage set to 65 keV, a sampling time of 1500 ms, and pixel size of 200 microns. The CT images were reconstructed using the Fast Cone Beam FBP algorithm with a Shepp Logan and a frequency cut off at 100% of the Nyquist frequency. ATLAS and CT images were spatially registered and fused as described above.
Statistics
Statistical analysis was performed using GraphPad Prism5. Correlations between PET quantification and biodistribution data were made using Pearson correlation test (p < 0.05). A p-value of less than 0.05 was considered as significant.
RESULTS
HER2/neu expression
Table 1 presents relation of HER2/neu expression in the tumor xenografts used in this study to in vitro binding of fluorescence-labeled Affibody molecules to the corresponding cell-lines in vitro. There is a statically significant linear correlation (R2>0.99) between the obtained values.
Table 1.
Expression of HER2/neu in breast carcinoma cells and tissue lysates measured by flow cytometry and ELISA assay respectively.
| FACS/ cell lysates |
ELISA/ tissue lysates |
||||
|---|---|---|---|---|---|
| Cells only | IgG1 | Neu(24D2) | Neu(24D2) - background |
ng/mg | |
| BT474 | 113 | 104 | 9 599 | 9 486 | 260 ± 18.2 |
| MDA-MB-361 | 113 | 114 | 2 993 | 2 879 | 67 ± 4.1 |
| MCF7 | 60 | 61 | 283 | 222 | 11 ± 0.9 |
Radiolabeling of 68Ga-DOTA- ZHER2:2891 Affibody
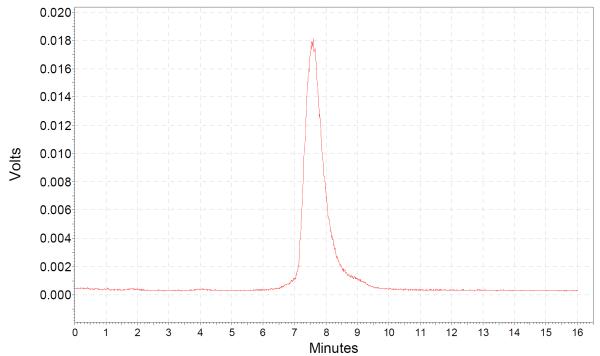
The elution profile of the 68Ga-DOTA- ZHER2:2891-Affibody reaction mixture showed RCP exceeding 90% with the product eluting at a retention time of 7.2 minutes. Specific activity of the radiolabeled conjugate was in the range of 2.8 – 5.2 GBq/nmol. Uncomplexed 68Ga eluted at 1.8 minutes. The elution profile of the purified 68Ga-DOTA-ZHER2:2891 Affibody showed the presence of a single peak (retention time of 7.2 minutes) corresponding to the isolated product (Fig.1.).
Fig.1.
HPLC radiochromatogram of purified 68Ga-DOTA-ZHER2:2891 Affibody.
HER2/neu Binding Saturation
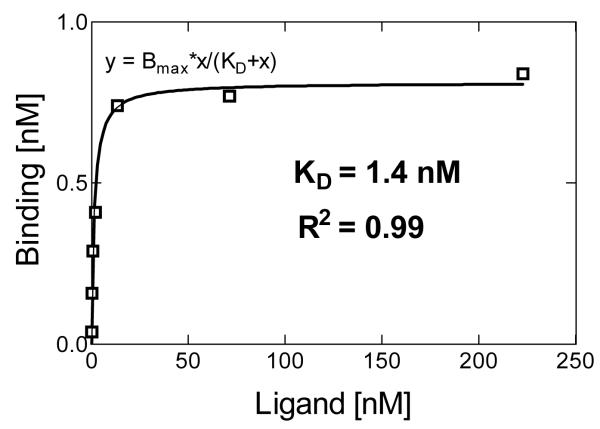
In vitro binding of 68Ga-DOTA-ZHER2:2891-Affibody to HER2/new on MDA-MB-361 cells was investigated using saturation binding assay. In a control experiment, a large excess of non-labeled Affibody molecules was added in order to saturate HER2/neu receptors and demonstrate binding specificity. A representative saturation binding curve is shown in Fig.2. The data indicated a single class of high-affinity binding sites that had a mean KD value of 1.4±0.19 nM. The Bmax value, expressed as the number of HER2/neu receptors per cell, was calculated to be (9.7±0.02 )×105.
Fig.2.
Representative results of saturation assay using MDA-MB-361 cells (concentration of radiolabeled Affibody molecule versus concentration bound). The KD of 68Ga-DOTA-ZHER2:2891 Affibody was determined to be 1.4 nM.
Biodistribution
Tumor and normal tissue uptake of 68Ga-DOTA-ZHER2:2891-Affibody in mice bearing tumors with very high HER2/neu expression (BT474) at the time points 1 and 3 hours post injection is shown in Table 2. There was very high (31±2.1 %ID/g) accumulation of the radioactivity in the tumors as early as 1 hour post injection. At that time, tumor-to-blood activity ratios were 27±4.4 and increased to 91.8±13.7 three hours later.
Table 2.
Biodistribution results for 68Ga- DOTA-ZHER2:2891 and His6-ZTaq-Cys-DOTA-68Ga Affibody injection in nude mice bearing BT474, mean ± SD (n = 4).
| 68Ga- DOTA-ZHER2:2891 | His6-ZTaq-Cys-DOTA- 68Ga |
||
|---|---|---|---|
|
| |||
| 1 h after injection | 3 h after injection | 1 h after injection | |
| Blood | 1.13±0.39 | 0.28±0.0235 | 1.2±0.36 |
|
| |||
| Heart | 0.87±0.32 | 0.3±0.04 | |
|
| |||
| Lungs | 1.9±0.49 | 0.56±0.07 | |
|
| |||
| Kidney | 223±26 | 227±14.2 | 172.2±12.5 |
|
| |||
| Spleen | 0.9±0.22 | 0.49±0.03 | |
|
| |||
| Liver | 2.04±0.25 | 1.7±0.49 | 1.3±0.2 |
|
| |||
| Pancreas | 0.6±0.05 | 0.38±0.031 | |
|
| |||
| Tumor | 31±2.1 | 25.7±2.2 | 1.1±0.4 |
|
| |||
| Bone | 1.2±0.5 | 0.47±0.09 | |
|
| |||
| Small intestine | 0.6±0.2 | 0.43±0.09 | |
|
| |||
| Muscle | 0.4±0.09 | 0.14±0.02 | 0.2±0.1 |
|
| |||
| Ratios: | |||
| T/Blood | 27± 4.4 | 91.8± 13.5 | |
| T/Muscle | 91.8± 13.7 | 128± 41 | |
Notably, the highest normal tissue concentration of radioactivity was seen in the kidneys. The uptake ranged from 223±26 %ID/g to 227%±14.2 ID/g. The tracer uptake in all other organs was low over the entire time course of the experiment.
The in vivo HER2/neu binding specificity was evaluated using non-HER2-specific Affibody molecules. One hour post injection the tumor-associated radioactivity in mice bearing BT474 tumors was comparable to that of surrounding tissues: 1.1±0.4 %ID/g in the tumor, 1.3±0.2 %ID/g in the liver and 0.2±0.1%ID/g in the muscles. Notably, the tumor uptake was 31-fold lower than observed in mice receiving the HER2/neu-specific 68Ga-DOTA-ZHER2:2891-Affibody. This difference confirmed that the tracer accumulation in these tumors was HER2/neu-dependent.
Blood Kinetics
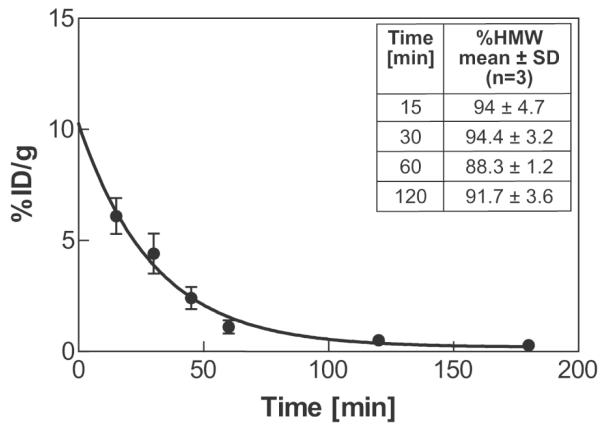
The mean radioactivity in the blood expressed as %ID/g is shown in Fig.3 as a function of time after administration of 68Ga-DOTA-ZHER2:2891-Affibody. The serum clearance was rapid with an elimination half-life of 20.8 min. The blood plasma was also evaluated for metabolic stability of 68Ga-labeled Affibody. To distinguish radiolabeled Affibody (high molecular weight) from small-molecule metabolites (low molecular weight), plasma samples were eluted through NAP-10 columns (5 kDa cut-off) with PBS. Reference analysis of a solution containing 68Ga-DOTA-ZHER2:2891-Affibody molecules on the NAP10 column resulted in 97% of the radioactivity eluted in the high molecular weight (HMW) fractions. The majority of radioactivity from plasma samples was also associated with the HMW fractions, indicating that the tracer was still present in the blood at 2 hours post injection (Fig.3. - Table insert).
Fig.3.
Blood kinetics. The squares represent % ID/g in the blood with an exponential curve fit. Insert Table: average HMW fractions of the plasma-associated radioactivity. Each point represents mean ± SD (n = 3).
PET/CT Imaging
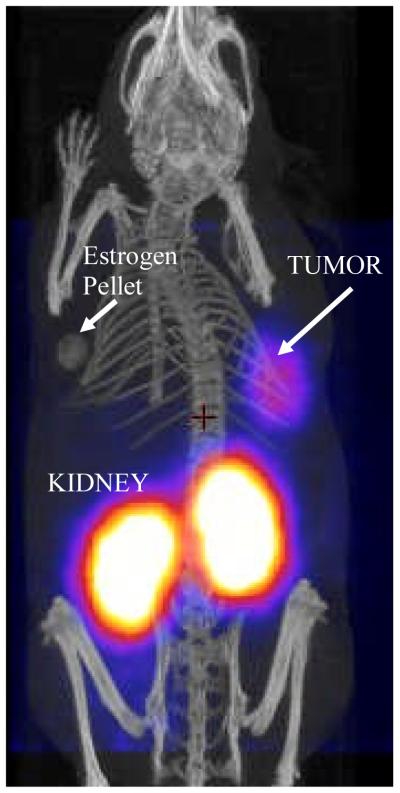
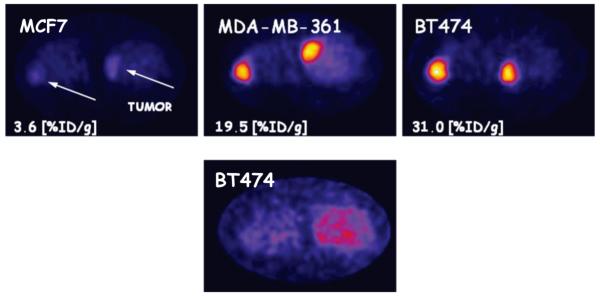
A representative PET/CT whole-body image of a mouse bearing BT474 tumor, obtained 2 hours post tracer injection, is shown in Fig.4. The axial sections comparing mice with different levels of HER2/neu expression are shown in Fig.5.
Fig.4.
Posterior whole-body fusion PET/CT image of representative athymic mouse implanted subcutaneously in the right shoulder with BT474 breast cancer cells two hours post tracer injection.
Fig.5.
Axial sections one hour post 68Ga-DOTA-ZHER2:2891 Affibody injection in mice bearing tumors with different HER2/neu expression Mcf7< MDA-MB-361 < BT474 (upper panel). Non-specific binding of His6-ZTaq-Cys-DOTA-68Ga in nude mice bearing BT474 (lower panel).
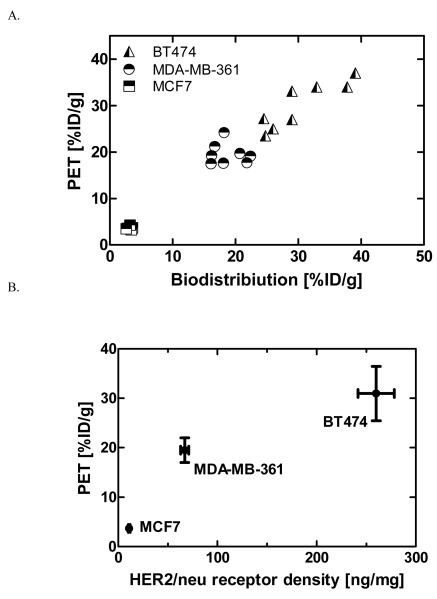
High contrast images were recorded 1 hour post injection, indicating high tracer uptake in tumors expressing high (BT474) and medium (MDA-MB-361) levels of HER2/neu as compared with the low-expressing tumors (MCF7). The radioactivity uptake in the tumors was significantly higher than in normal tissues except for the kidneys. The ROI values ranged from 31±7.0 %ID/g for BT474 to 18.8±2.5 %ID/g for MDA-MB-361. We observed poor, but still detectable signal from MCF7 tumors, the ROI value was 3.2±0.4 %ID/g. Notably, we found a strong and significant correlation between %ID/g values obtained from biodistribution and PET quantification studies (R2 = 0.94, p < 0.0001) for all tumor bearing mice (Fig.6A). Both, tracer accumulation and the signal detected in tumor by PET depended on the receptor expression, as assessed by ELISA (Fig.6B).
Fig.6.
Correlation between: A. PET quantification and biodistribution data (p < 0.0001; R2=0.95) B. HER2/neu expression measured ex vivo and 68Ga-DOTA-ZHER2:2891 Affibody uptake in vivo (R2 = 0.84); in mice bearing tumors with different HER2/neu expression (n = 5-8).
Discussion
Molecular imaging has great potential for characterization of therapeutically important targets in malignancies [19], including HER2/neu [6, 20]. Because HER2/neu expression levels in metastases may be quite different from the primary tumor [21], assessment of the specific expression of these receptors on cancer cells in various locations may be useful in selecting appropriate therapy [22]. However, the development of HER2/neu-specific imaging probes for routine clinical use has not yet been successful.
In our previous work [15], we labeled Affibody molecules with 18F - widely available and a nearly pure (97%) positron emitter with a relatively short, but clinically useful, half-life of 110 minutes. However, 18F production requires a cyclotron, and the radiolabeling procedures involving this radionuclide require several low-yielding steps that are often time consuming. Therefore, in this study, we evaluated the potential of 68Ga-DOTA-ZHER2:2891-Affibody [23] as an imaging agent in xenografts with different HER2/neu expression levels.
Gallium-68 presents an attractive alternative as a radionuclide for PET imaging. It can be obtained from commercially available 68Ge-68Ga generators, which have a half-life of 271 days and, thus, can provide an almost constant supply of 68Ga for over one year. The labeling procedures are well established and relatively straightforward. Also, when prorated, the unit cost of 68Ga from generators is reasonable when amortized over a year, which makes it even more attractive for busy nuclear medicine departments, particularly those with limited or no access to cyclotrons.
Most of the efforts in developing tracers for HER2/neu focused on using anti-HER2/neu antibodies. However, antibodies are rather bulky proteins typically characterized by limited tumor penetration and slow clearance from the circulation. A successful molecular imaging probe requires high affinity and specificity binding to tumor-associated structures with minimal non-specific accumulation in normal tissues and rapid clearance from the background. Optimally, it should easily penetrate the tumor parenchyma and rapidly clear from the blood to increase the tumor to background ratio. Recently, significant progress has been made in protein display technology that has led to the discovery of many small proteins and peptides [24-25]. Among them, Affibody molecules have been shown to meet most of the aforementioned requirements providing a suitable platform for development of new imaging probes [14, 26].
Our current results demonstrated the high affinity of 68Ga-DOTA-ZHER2:2891-Affibody for HER2/neu receptors on MDAMB-361 cells in vitro. The KD was similar to those found with other Affibody radioconjugates and was comparable to antibodies used clinically for imaging and therapy (e.g., the reported affinity of trastuzumab to HER2/neu is 5 nM) [27].
The in vivo pharmacokinetics data were also promising. The elimination half-life estimated by measuring radioactivity in blood samples at several time points after injection was approximately 21 min. This relatively fast clearance resulted in high tumor-to-blood ratios as well as high-contrast PET images of 68Ga-DOTA-ZHER2:2891-Affibody molecules as early as 1 hour post tracer injection. It is noteworthy that the obtained tumor-to-blood ratios were higher than for other imaging probes including 68Ga-DOTA-MUT-DS, 68Ga-ABY-002, 68Ga-labeled DOTA-F(ab’)2 fragment of Herceptin, as well as full length antibodies and antibody fragments labeled with 124I, 89Zr and, 86Y [28-31].
Because of the fast blood clearance, the images showed high tumor accumulation as compared with other tissues (except the kidneys) in the high- and intermediate-expressing tumors. In highly HER2/neu-expressing BT474 tumors the uptake reached 31±7.0 %ID/g as soon as 1 hour post tracer injection, and is was higher than the accumulation levels recently described by Ren et al. [30] and Tolmachev et a [16] for SKOV3 tumors. The absolute tumor accumulation reported by these authors was only 4.12±2.2 %ID/g (2 hours post injection of an Affibody-based, 2-helix 68Ga-DOTA-MUT-DS molecule) and 8.9±1.0 %ID/g (45 min post injection of 68Ga-ABY-002). It should be noted that SKOV3 cells express even more HER2/neu receptors than BT474 (ELISA results from our laboratory: 335 ng/mg for SKOV3 versus 260 ng/mg for BT474). These results are in line with our previous data obtained with radio-fluorinated probe [17]. It should be stressed that correction for the uptake for nonspecific binding of His6-ZTaq-Cys-DOTA-68Ga was not necessary since the accumulation of this non-HER2-specific tracer was approximately at the same level as surrounding tissues, and tumors were not detectable. We have previously shown a strong association between the uptake of 18F-FBEM– ZHER2:342-Affibody tracer in the panel of breast cancer tumor xenografts with different HER2/neu expression [17]. Also, McLarty et al., using mice bearing tumors with a wide range of receptor levels, showed a correlation between 111In-DTPA-trastuzumab accumulation and HER2/neu density in individual tumors [32].
In this work, we investigated the correlation between HER2/neu expression determined by PET imaging in vivo and the biodistribution data for each particular tumor model following injection of 68Ga-DOTA-ZHER2:2891-Affibody and by comparison to HER2/neu protein concentration measured in the same tumors ex vivo by ELISA. Based on our experience with 18F-labeled Affibody molecules and considering the short half-life of gallium, the biodistribution and imaging studies were done at 1 and 3 hours post tracer injection. There was no indication of label instability since the tracer was rapidly cleared from the blood and liver. By analyzing the biodistribution and imaging data, we were also able to correlate the tracer uptake with number of HER2/neu receptors. Unlike results reported for antibodies by McLarty et al. [32], who had to apply appropriate corrections for non-specific tumor uptake and blood flow, we found a strong linear correlation between PET values [%ID/g] and biodistribution data that also corresponded to HER2/neu level in each tumor. It is important to note that using gallium-labeled agents the estimation of receptor expression may be limited for the very low receptor expressing tumors. This is due to the greater positron range of 68Ga (1.9 MeV) compared for example to 18F that could lead to poorer spatial resolution and erroneous assignment of the ROI. The intrinsic resolution of small-animal PET scanners has improved to a level where the positron range of the radionuclide eventually could influence the effective spatial resolution. Jorn van Dalen et al. showed that the image resolution of a PET scanner with a 1.5 mm intrinsic system resolution is decreased by using PET emitters with large positron ranges such as 68Ga and 124I [33]. This could impact: (i) the detection of small lesions, (ii) assessment of low receptor expression, (iii) the ability to distinguish lesions that are close together, as well as (iv) quantification of tumor uptake. Therefore, the positron energy and range must be considered when planning small-animal imaging. On the other hand from clinical point of view, Yang et al. demonstrated that the conventional FWHM of 18F and that of 68Ga are indistinguishable in soft tissues (3.01 mm vs. 3.09 mm) [34]. This implies that with the spatial resolution of 5-7 mm for current clinical scanners, image quality using 68Ga-labeled tracers should be comparable to 18F-labeled agents.
We observed very high radioactivity accumulation in the kidney. These values are comparable to those previously reported for gallium-labeled Affibody molecules [16]. Use of 68Ga as well as other metal positron emitters including 64Cu and 89Zr, leads to residualization that results in trapping of radionuclides in lysosomes after internalization and degradation of the tracer [35-36]. Because of this phenomenon, the kidney is the major dose-limiting organ for this tracer. However, this is not a major diagnostic limitation since renal lesions are uncommon in breast cancer.
Positively charged amino acids, such as lysine and arginine are now commonly used for renal protection in clinical trials of peptide receptor-targeted radionuclide therapy and may be useful in this setting as well [37]. Studies of the possible effect of positively charged amino acids on accumulation of radioactivity in the kidney following 68Ga-DOTA-ZHER2:2891 Affibody administration are, however, outside the scope of this paper. In conclusion, our results indicate that differences in HER2/neu expression levels can be detected in vivo by PET imaging with 68Ga-DOTA-ZHER2:2891-Affibody. This generator-produced agent may, therefore, be useful in the characterization HER2/neu-positive tumors as an alternative to 18F-labeled compounds especially where access to a cyclotron is limited.
Acknowledgements
The authors appreciate the support of Affibody AB. We owe special thanks to Michael Green for continuous constructive discussions. We also appreciate technical assistance of Ilya Lyakhov, Monika Kuban, and Alesia Holly.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organization imply endorsement by the U.S. Government.
This research was supported in part by the Center for Cancer Research, an Intramural Research Program of the National Cancer Institute, Imaging Probe Development Center, National Heart, Lung, and Blood Institute, and by Breast Cancer Research Stamp Fund awarded through competitive peer review, and was funded in part with Federal funds from the National Cancer Institute, National Institutes of Health, under Contracts N01-CO-12400 and N01-CO-12401.
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest [16].
References
- 1.Tagliabue E, Balsari A, Campiglio M, Pupa SM. HER2 as a target for breast cancer therapy. Expert Opin Biol Ther. 2010;10:711–24. doi: 10.1517/14712591003689972. [DOI] [PubMed] [Google Scholar]
- 2.Dean-Colomb W, Esteva FJ. Her2-positive breast cancer: herceptin and beyond. Eur J Cancer. 2008;44:2806–12. doi: 10.1016/j.ejca.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 3.Pohlmann PR, Mayer IA, Mernaugh R. Resistance to Trastuzumab in Breast Cancer. Clin Cancer Res. 2009;15:7479–91. doi: 10.1158/1078-0432.CCR-09-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allison M. The HER2 testing conundrum. Nat Biotechnol. 2010;28:117–9. doi: 10.1038/nbt0210-117. [DOI] [PubMed] [Google Scholar]
- 5.Dowsett M, Hanna WM, Kockx M, Penault-Llorca F, Ruschoff J, Gutjahr T, et al. Standardization of HER2 testing: results of an international proficiency-testing ring study. Mod Pathol. 2007;20:584–91. doi: 10.1038/modpathol.3800774. [DOI] [PubMed] [Google Scholar]
- 6.Capala J, Bouchelouche K. Molecular imaging of HER2-positive breast cancer - a step toward an individualized “Image and Treat” strategy. Current Opinion in Oncology. 2010 doi: 10.1097/CCO.0b013e32833f8c3a. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wester HJ. Nuclear imaging probes: from bench to bedside. Clin Cancer Res. 2007;13:3470–81. doi: 10.1158/1078-0432.CCR-07-0264. [DOI] [PubMed] [Google Scholar]
- 8.Fass L. Imaging and cancer: A review. Mol Oncol. 2008;2:115–52. doi: 10.1016/j.molonc.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fani M, Andre JP, Maecke HR. Ga-68-PET: a powerful generator-based alternative to cyclotron-based PET radiopharmaceuticals. Contrast Media Mol I. 2008;3:53–63. doi: 10.1002/cmmi.232. [DOI] [PubMed] [Google Scholar]
- 10.Maecke HR, Hofmann M, Haberkorn U. 68Ga-Labeled Peptides in Tumor Imaging. J Nucl Med. 2005;46:172S–8. [PubMed] [Google Scholar]
- 11.Al-Nahhas A, Win Z, Szyszko T, Singh A, Khan S, Rubello D. What can gallium-68 PET add to receptor and molecular imaging? Eur J Nucl Med Mol Imaging. 2007;34:1897–901. doi: 10.1007/s00259-007-0568-1. [DOI] [PubMed] [Google Scholar]
- 12.Partridge M, Spinelli A, Ryder W, Hindorf C. The effect of beta(+) energy on performance of a small animal PET camera. Nucl Instrum Meth A. 2006;568:933–6. [Google Scholar]
- 13.Baum RP, Prasad V, Muller D, Schuchardt C, Orlova A, Wennborg A, et al. Molecular imaging of HER2-expressing malignant tumors in breast cancer patients using synthetic 111In- or 68Ga-labeled affibody molecules. J Nucl Med. 2010;51:892–7. doi: 10.2967/jnumed.109.073239. [DOI] [PubMed] [Google Scholar]
- 14.Lofblom J, Feldwisch J, Tolmachev V, Carlsson J, Stahl S, Frejd FY. Affibody molecules: Engineered proteins for therapeutic, diagnostic and biotechnological applications. Febs Letters. 2010;584:2670–80. doi: 10.1016/j.febslet.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 15.Kramer-Marek G, Kiesewetter DO, Martiniova L, Jagoda E, Lee SB, Capala J. [18F]FBEM-Z(HER2:342)-Affibody molecule-a new molecular tracer for in vivo monitoring of HER2 expression by positron emission tomography. Eur J Nucl Med Mol Imaging. 2008;35:1008–18. doi: 10.1007/s00259-007-0658-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tolmachev V, Velikyan I, Sandstrom M, Orlova A. A HER2-binding Affibody molecule labelled with 68Ga for PET imaging: direct in vivo comparison with the 111In-labelled analogue. Eur J Nucl Med Mol Imaging. 37:1356–67. doi: 10.1007/s00259-009-1367-7. [DOI] [PubMed] [Google Scholar]
- 17.Kramer-Marek G, Kiesewetter DO, Capala J. Changes in HER2 expression in breast cancer xenografts after therapy can be quantified using PET and (18)F-labeled affibody molecules. J Nucl Med. 2009;50:1131–9. doi: 10.2967/jnumed.108.057695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahlgren S, Orlova A, Rosik D, Sandstrom M, Sjoberg A, Baastrup B, et al. Evaluation of maleimide derivative of DOTA for site-specific labeling of recombinant affibody molecules. Bioconjug Chem. 2008;19:235–43. doi: 10.1021/bc700307y. [DOI] [PubMed] [Google Scholar]
- 19.Blasberg RG. Molecular imaging and cancer. Mol Cancer Ther. 2003;2:335–43. [PubMed] [Google Scholar]
- 20.Tolmachev V. Imaging of HER-2 overexpression in tumors for guiding therapy. Curr Pharm Des. 2008;14:2999–3019. doi: 10.2174/138161208786404290. [DOI] [PubMed] [Google Scholar]
- 21.Zidan J, Dashkovsky I, Stayerman C, Basher W, Cozacov C, Hadary A. Comparison of HER-2 overexpression in primary breast cancer and metastatic sites and its effect on biological targeting therapy of metastatic disease. Br J Cancer. 2005;93:552–6. doi: 10.1038/sj.bjc.6602738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McLarty K, Reilly RM. Molecular imaging as a tool for personalized and targeted anticancer therapy. Clin Pharmacol Ther. 2007;81:420–4. doi: 10.1038/sj.clpt.6100096. [DOI] [PubMed] [Google Scholar]
- 23.Feldwisch J, Tolmachev V, Lendel C, Herne N, Sjoberg A, Larsson B, et al. Design of an optimized scaffold for affibody molecules. J Mol Biol. 2010;398:232–47. doi: 10.1016/j.jmb.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Miao Z, Levi J, Cheng Z. Protein scaffold-based molecular probes for cancer molecular imaging. Amino Acids. 2010 doi: 10.1007/s00726-010-0503-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eigenbrot C, Ultsch M, Dubnovitsky A, Abrahmsen L, Hard T. Structural basis for high-affinity HER2 receptor binding by an engineered protein. Proc Natl Acad Sci U S A. 2010;107:15039–44. doi: 10.1073/pnas.1005025107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahlgren S, Tolmachev V. Radionuclide molecular imaging using Affibody molecules. Curr Pharm Biotechnol. 2010;11:581–9. doi: 10.2174/138920110792246609. [DOI] [PubMed] [Google Scholar]
- 27.De Lorenzo C, Cozzolino R, Carpentieri A, Pucci P, Laccetti P, D’Alessio G. Biological properties of a human compact anti-ErbB2 antibody. Carcinogenesis. 2005;26:1890–5. doi: 10.1093/carcin/bgi146. [DOI] [PubMed] [Google Scholar]
- 28.Perk LR, Visser GW, Vosjan MJ, Walsum M Stigter-van, Tijink BM, Leemans CR, et al. (89)Zr as a PET surrogate radioisotope for scouting biodistribution of the therapeutic radiometals (90)Y and (177)Lu in tumor-bearing nude mice after coupling to the internalizing antibody cetuximab. J Nucl Med. 2005;46:1898–906. [PubMed] [Google Scholar]
- 29.Smith-Jones PM, Solit DB, Akhurst T, Afroze F, Rosen N, Larson SM. Imaging the pharmacodynamics of HER2 degradation in response to Hsp90 inhibitors. Nat Biotechnol. 2004;22:701–6. doi: 10.1038/nbt968. [DOI] [PubMed] [Google Scholar]
- 30.Ren G, Zhang R, Liu Z, Webster JM, Miao Z, Gambhir SS, et al. A 2-helix small protein labeled with 68Ga for PET imaging of HER2 expression. J Nucl Med. 2009;50:1492–9. doi: 10.2967/jnumed.109.064287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orlova A, Wallberg H, Stone-Elander S, Tolmachev V. On the selection of a tracer for PET imaging of HER2-expressing tumors: direct comparison of a 124I-labeled affibody molecule and trastuzumab in a murine xenograft model. J Nucl Med. 2009;50:417–25. doi: 10.2967/jnumed.108.057919. [DOI] [PubMed] [Google Scholar]
- 32.McLarty K, Cornelissen B, Scollard DA, Done SJ, Chun K, Reilly RM. Associations between the uptake of 111In-DTPA-trastuzumab, HER2 density and response to trastuzumab (Herceptin) in athymic mice bearing subcutaneous human tumour xenografts. Eur J Nucl Med Mol Imaging. 2009;36:81–93. doi: 10.1007/s00259-008-0923-x. [DOI] [PubMed] [Google Scholar]
- 33.van Dalen EV Jorn, Peter Laverman, Wouter Vogel, Wim Oyen. Frans Corstens and Otto Boerman. Effect of the positron range on the spatial resolution of a new generation pre-clinical PET-scanner using F-18, Ga-68, Zr-89 and I-124. 2008;49(Supplement1):404P. [Google Scholar]
- 34.David J. Yang AAaEEK. Tumor Specific Imaging Using Tc-99m and Ga-68 Labeled Radiopharmaceuticals. Current Medical Imaging Review. 2005;1:25–34. [Google Scholar]
- 35.Aerts HJ, Dubois L, Perk L, Vermaelen P, van Dongen GA, Wouters BG, et al. Disparity between in vivo EGFR expression and 89Zr-labeled cetuximab uptake assessed with PET. J Nucl Med. 2009;50:123–31. doi: 10.2967/jnumed.108.054312. [DOI] [PubMed] [Google Scholar]
- 36.Tolmachev V, Nilsson FY, Widstrom C, Andersson K, Rosik D, Gedda L, et al. 111In-benzyl-DTPA-ZHER2:342, an affibody-based conjugate for in vivo imaging of HER2 expression in malignant tumors. J Nucl Med. 2006;47:846–53. [PubMed] [Google Scholar]
- 37.Rolleman EJ, Bernard BF, Breeman WA, Forrer F, de Blois E, Hoppin J, et al. Molecular imaging of reduced renal uptake of radiolabelled [DOTA0,Tyr3]octreotate by the combination of lysine and Gelofusine in rats. Nuklearmedizin. 2008;47:110–5. doi: 10.3413/nukmed-0069. [DOI] [PubMed] [Google Scholar]