Abstract
Rationale
Ca binding to the troponin complex represents a major portion of cytosolic Ca buffering. Troponin mutations that increase myofilament Ca sensitivity are associated with familial hypertrophic cardiomyopathy and confer a high risk for sudden death. In mice, Ca sensitization causes ventricular arrhythmias, but the underlying mechanisms remain unclear.
Objective
To test the hypothesis that myofilament Ca sensitization increases cytosolic Ca buffering, and to determine the resulting arrhythmogenic changes in Ca homeostasis in the intact mouse heart.
Methods and Results
Using cardiomyocytes isolated from mice expressing troponin T (TnT) mutants (TnT-I79N, TnT-F110I, TnT-R278C), we found that increasing myofilament Ca sensitivity produced a proportional increase in cytosolic Ca binding. The underlying cause was an increase in the cytosolic Ca binding affinity, whereas maximal Ca binding capacity was unchanged. The effect was sufficiently large to alter Ca handling in intact mouse hearts at physiological heart rates, resulting in increased end-diastolic [Ca] at fast pacing rates, and enhanced sarcoplasmic reticulum Ca content and release after pauses. Accordingly, action potential (AP) regulation was altered, with post-pause AP prolongation, afterdepolarizations and triggered activity. Acute Ca sensitization with EMD 57033 mimicked the effects of Ca sensitizing TnT mutants and produced pause-dependent ventricular ectopy and sustained ventricular tachycardia after acute myocardial infarction.
Conclusions
Myofilament Ca sensitization increases cytosolic Ca binding affinity. A major proarrhythmic consequence is a pause-dependent potentiation of Ca release, AP prolongation and triggered activity. Increased cytosolic Ca binding represents a novel mechanism of pause-dependent arrhythmia that may be relevant for inherited and acquired cardiomyopathies.
Keywords: myofilament Ca sensitivity, familial hypertrophic cardiomyopathy, Ca buffering, early afterdepolarizations, arrhythmia
INTRODUCTION
Familial Hypertrophic Cardiomyopathy (FHC) is a heterogeneous disease resulting from autosomal-dominant mutations in genes encoding cardiac contractile proteins.1 Sudden cardiac death (SCD) is the main culprit for the mortality in FHC patients,2 with ventricular tachycardia (VT) and/or ventricular fibrillation as the underlying mechanism for the SCD.3 Even though, in general, the degree of cardiac hypertrophy is an important risk factor for SCD,4 certain mutations in thin filament proteins confer a high risk of SCD even in the absence of marked cardiac hypertrophy.5 In particular, mutations in troponin T (TnT) are responsible for approximately 7% of FHC cases,6 but represent a major proportion of those referred for tertiary care.5–7 For these mutations, the risk of SCD seems to be less dependent on structural remodelling like hypertrophy and fibrosis,8, 9 suggesting that other mechanisms importantly contribute to arrhythmia susceptibility. In vitro, FHC-linked TnT mutations frequently increase myofilament Ca sensitivity of force development.10 We previously demonstrated that myofilament Ca sensitization increases the susceptibility for ventricular arrhythmias, and found a direct correlation between the degree of Ca sensitization and the risk for ventricular arrhythmias in TnT mutant mice.11 However, the underlying mechanisms remain unknown.12
The troponin complex represents a substantial portion of cytoplasmic Ca buffering, binding approximately 50% of Ca released from the sarcoplasmic reticulum (SR) during a typical heart beat.13 The effect of a TnT mutation on myofilament Ca sensitivity is likely due to an indirect action of TnT on Ca binding by troponin C (TnC), which is possible because TnT, TnC and troponin I (TnI) form a trimeric protein complex.14 Hence, we hypothesize that increased myofilament Ca sensitivity alters cytoplasmic Ca buffering. Based on experiments that introduced exogenous Ca buffers into myocytes,15 increased cytosolic Ca buffering should lead to reduced systolic Ca transient with a slow rate of decay and increased cytosolic [Ca] at the end of diastole.16 As the heart rate increases further, Ca may stay partly bound even at the end of diastole. On the other hand, when heart rate slows, or during a pause, the excessive Ca bound to the myofilaments during the preceding rapid beats may get mobilized and taken up into the sarcoplasmic reticulum (SR). Thus, we further hypothesize that as a consequence of increased cytosolic Ca buffering, Ca release after a pause will be increased. Increased Ca release after a brief pause is a phenomenon also observed in normal myocardium (the resulting increased contraction is often referred to as post-rest potentiation), but this process may be abnormally enhanced in the setting of increased cytosolic Ca buffering. Intracellular Ca regulates membrane currents and directly alters the membrane potential via the electrogenic Na Ca exchanger (NCX).9 Hence, an increased post-rest potentiation of Ca-sensitized hearts may cause pause-dependent action potential (AP) prolongation, afterdepolarizations and triggered activity.
To test these hypotheses, we examined the effect of both acutely and chronically increasing myofilament Ca sensitivity on myocyte Ca buffering, Ca cycling and action potential (AP). As chronic model we used transgenic mice expressing the Ca sensitizing human TnT mutant TnT-I79N. In FHC patients, this mutation is associated with a high rate of SCD at young age.17 This group was compared to mice expressing either the human wild-type TnT (WT) or the TnT-R278C mutant. TnT-R278C does not increase myofilament Ca sensitivity18 and is associated with a better prognosis.7,19 We also used a FHC-linked TnT mutation that causes intermediate Ca sensitization (TnT-F110I).11 As an acute model, we used the Ca sensitizer EMD57033, which has relatively specific myofilament Ca sensitizing properties.20, 21
As hypothesized, we find that Ca sensitizing TnT mutations increase cytosolic Ca binding and alter myocyte Ca handling with increased end-diastolic [Ca] at fast pacing rates and enhanced SR Ca content and release after pauses. AP regulation is altered with post-pause AP prolongation, afterdepolarizations and triggered activity. Acute Ca sensitization of non-transgenic hearts with EMD mimics the effects of Ca sensitizing TnT mutants and produces pause-dependent ventricular ectopy and sustained VT in mouse hearts with acute myocardial infarction (MI). Our findings suggest a novel mechanism of pause-dependent arrhythmia that could be relevant for inherited and acquired human cardiomyopathies with increased myofilament Ca sensitivity.
METHODS
A detailed methods section describing the mouse models, intracellular Ca, AP and ECG measurements, and data analysis is available online.
RESULTS
Myofilament Ca sensitization increases cytosolic Ca binding by altering its apparent Kd
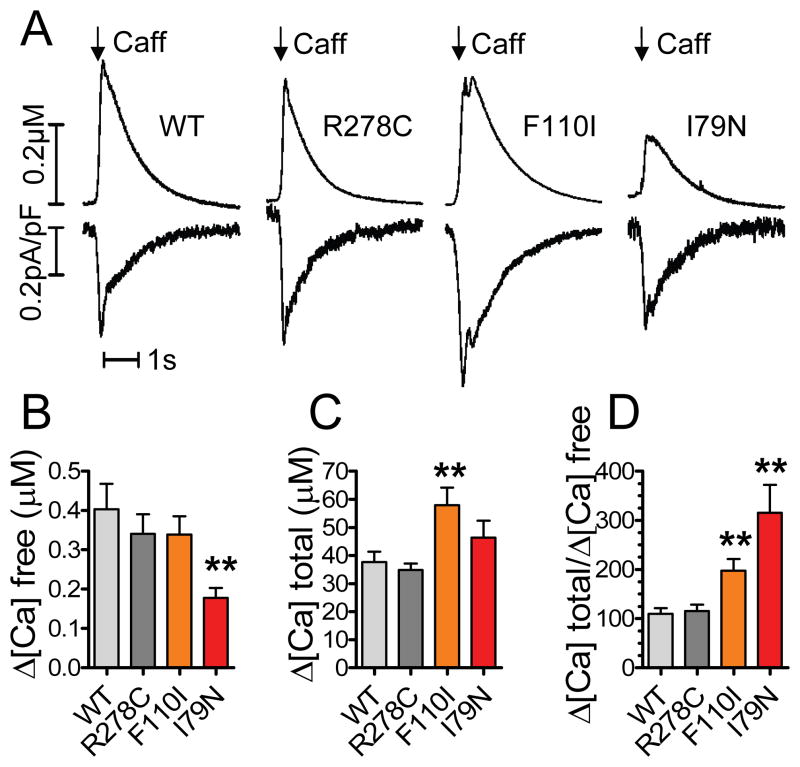
To test the hypothesis that increased myofilament Ca sensitivity changes cytosolic Ca binding, we quantified cytosolic Ca buffering in ventricular myocytes from transgenic mice with varying levels of myofilament Ca sensitivity.11, 18, 22 To measure cytosolic Ca buffering, caffeine was rapidly applied to release Ca from the SR (Figure 1A, upper trace). Integration of the NCX current (Figure 1A, lower trace) yielded the total amount of Ca released from the SR.23 We did not block cytosolic Ca removal via mitochondrial Ca uptake or sarcolemmal Ca-ATPase and therefore somewhat underestimate [Ca]total. Importantly though, the rates of non-NCX mediated Ca removal were not different between the mutations (Online Figure I) and are not expected to bias the result. SR Ca content was reduced by pretreatment with a low concentration of caffeine24 to achieve caffeine transients that match the amplitude of the Ca transient during a typical myocyte contraction.25 The rise in free cytosolic Ca was significantly smaller in myocytes expressing the Ca sensitizing TnT-I79N mutant compared to WT, even though the total amount of Ca released from the SR was the same (Figure 1A). On average, the ratio between the total amount of Ca released from the SR and the resulting peak change in [Ca]free (=Δ[Ca]total/Δ[Ca]free) was significantly higher in TnT-I79N myocytes (Figure 1B–D), indicating that significantly more Ca was bound in TnT-I79N compared to WT myocytes and myocytes expressing the non-sensitizing TnT-R278C mutant. Myocytes expressing the TnT-F110I mutant that has intermediate Ca sensitizing effects11 exhibited also an intermediate effect on cytosolic Ca binding (Figure 1D). At the same time, the concentrations of key Ca binding proteins SERCA, calsequestrin, phospholamban, NCX and calmodulin were not significantly different among the groups (Online Figure II).
Figure 1. Ca2+ sensitizing troponin T (TnT) mutants increase apparent cytosolic Ca2+ binding.
Cytosolic Ca2+ fluorescence was recorded from voltage-clamped myocytes loaded with the fluorescent indicator Fluo-4 (25 μM). A, Representative examples of mice expressing either human wild-type cardiac TnT (WT) or mutant TnT (TnT-R278C, TnT-F110I, TnT-I79N). Myofilament Ca2+ sensitivity was altered in the following order: TnT-R278C≤TnT-WT<TnT-F110I<TnT-I79N. Upper trace: Rapidly applied caffeine was used to release Ca2+ from the sarcoplasmatic reticulum (SR). Lower trace: Integration of the Na+ Ca2+ exchanger current yielded the total amount of Ca2+ released from the SR. B–D, Myocytes expressing the Ca2+ sensitizing TnT mutants show a higher net cytosolic Ca2+ binding calculated by a smaller rise in [Ca2+]free and an higher Δ[Ca2+]total. n=11–13 myocytes per group. **p<0.01 vs WT and TnT-R278C
We also used an independent method to confirm the effect of the TnT-I79N mutation on cytosolic Ca buffering (Online Figure III).26 Compared to non-transgenic myocytes (NTG), Ca-sensitized TnT-I79N myocytes exhibited much smaller rises in [Ca]free in response to approximately the same amount of total Ca influx into the cytosol, which was calculated by integrating the Ca current (Online Figure IIIA, middle trace). As before, we calculated the ratio between the increase in [Ca]total and the resulting change in [Ca]free for each myocyte. On average, cytosolic Ca binding capacity was significantly higher in TnT-I79N compared to NTG myocytes (Online Figure IIIB).
We next examined how sensitizing TnT mutants increase cytosolic Ca binding by calculating cytosolic Kd and Bmax for each myocyte using the protocol by Trafford et al.23 as illustrated in Figure 2A. Both the Ca sensitizing TnT-I79N and TnT-F110I mutants significantly lowered average Kd (Figure 2B), but did not change maximal cytosolic buffering capacity (Bmax). The Kd of the non-sensitizing TnT-R278C mutant was not significantly different from that of WT myocytes. Taken together, these data demonstrate that Ca sensitizing TnT mutants increase Ca buffering by lowering the apparent Kd for cytosolic Ca binding (presumably to TnC).
Figure 2. Ca2+ sensitizing TnT mutants alter the apparent Kd of cytosolic Ca2+ buffering.
Cytosolic buffering parameters (Kd and Bmax) were determined using the Ca2+ binding data presented in Figure 1. Δ[Ca2+]total was plotted as a function of Δ[Ca2+]free, fitted to a modified Michaelis-Menten equation and Bmax and Kd calculated for each myocyte. A, Representative buffering plots. B–C, Ca2+ sensitizing TnT-I79N and TnT-F110I mutants show significantly lowered average Kd, but did not change maximal cytosolic buffering capacity (Bmax). The non-sensitizing TnT-R278C mutant was not different from wild-type (WT) myocytes. n=9–10 myocytes per group. **p<0.01 vs TnT-R278C and WT. D, Cytosolic buffering curves calculated from experimental Kd and Bmax obtained in B and C. E, Predicted cytosolic buffering capacity as a function of steady-state end-diastolic [Ca2+]free. A simulated twitch Ca2+ increase of 30 μM (= Δ[Ca2+]total) was used to compare buffering capacity in the four groups based on the buffering curves from D. The cytosolic buffering capacity decreases as end-diastolic [Ca2+]free increases. Note that at the same low diastolic Ca2+ this results in increased buffering capacity in the TnT-I79N (a vs. b) and therefore expected decreased systolic Ca2+. But buffering capacity can be the same in I79N and WT when diastolic [Ca2+] is different (a vs. c).
A potential limitation is that cytosolic Ca buffering could be measured experimentally only at resting diastolic [Ca]free, which was on average 0.13 ± 0.08 μM, and not significantly different between the four groups of myocytes. To examine the effect of the TnT mutations on cytosolic buffering at different diastolic [Ca]free, we first modeled full buffering curves for all groups (Figure 2D) based on the average Kd and Bmax obtained experimentally (Figure 2B & C). Note that initially, the buffering curves of Ca-sensitized myocytes are much steeper compared to those of WT and R278C myocytes. Hence, more Ca can be buffered when end-diastolic [Ca]free is low. However, since the total number of Ca binding sites (Bmax) is unchanged, Ca binding will saturate more rapidly in Ca-sensitized compared to non-sensitized myocytes. As a result, the amount of Ca that can be bound by sensitized myofilaments is expected to diminish more rapidly than that of non-sensitized myofilaments as steady-state end-diastolic [Ca]free rises. To quantify this phenomenon, we next calculated for each genotype their capacity to buffer systolic Ca transients during a myocyte twitch as a function of end-diastolic [Ca]free (Figure 2E). Consistent with the experimental results of Figure 1D, cytosolic Ca buffering is increased in I79N and F110I myocytes at resting diastolic [Ca]free values (Figure 2E, points a & b). However, as steady-state end-diastolic [Ca]free rises (i.e., during rapid stimulation), the difference in buffering power between the groups is progressively reduced and may even reverse at high end-diastolic [Ca]free (Figure 2E). Taken together, the modeling results suggest that the net effect of changes in myofilament Ca binding affinity (Kd) on the Ca transient amplitude during a twitch will depend on the level of end-diastolic [Ca]free.
To test whether acute myofilament Ca sensitization can reproduce the effects of Ca sensitizing TnT mutants, we used the Ca sensitizer EMD57033 (Online Figure IVA). Measurements of the intracellular Ca buffering using the Trafford method23 yielded results that were comparable to TnT-I79N: significantly more Ca was bound in myocytes treated with EMD compared to vehicle-treated myocytes (Online Figure IVB). The increased buffering capacity can be attributed to an increased cytosolic Ca binding affinity (Kd), whereas maximal Ca binding capacity (Bmax) was unchanged (Online Figure IVB).
Ca sensitization prolongs Ca transients, increases end-diastolic [Ca] and potentiates post-pause SR Ca content and release
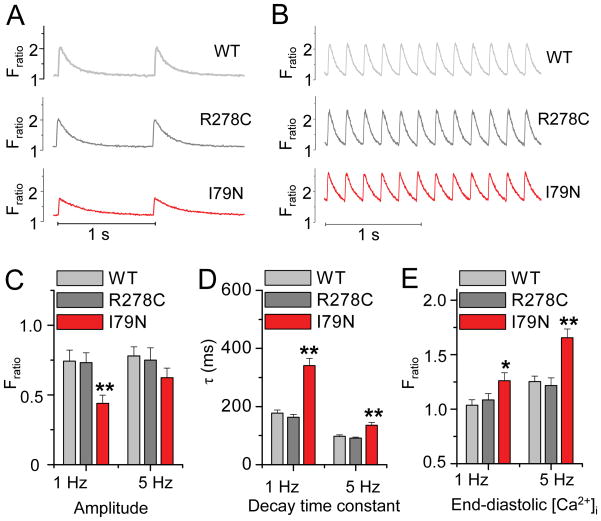
Increased cytosolic Ca buffering can be predicted to change intracellular Ca cycling during a twitch.16 We first studied the effect of Ca sensitization in field-stimulated intact myocytes. Consistent with their increased cytosolic Ca buffering (Figures 1 & 2), TnT-I79N myocytes exhibit depressed systolic Ca transients with slowed decay rates when paced at 1 Hz (Figure 3A,C,D). End-diastolic [Ca]free was modestly but significantly increased (Figure 3A,E). Ca transients of myocytes expressing the non-sensitizing TnT-R278C were not different from WT myocytes. At a faster pacing of 5 Hz, decay kinetics of TnT-I79N myocytes remain slower and end-diastolic [Ca] further increased compared to both WT and TnT-R278C (Figure 3B,D,E). Ca transient amplitude remained the same in TnT-WT and TnT-R278C, but increased significantly in the TnT-I79N group. As a result, I79N Ca transient amplitude was only modestly lower compared to the non-sensitized myocytes at 5 Hz, a difference that was no longer statistically significant (Figure 3B,C). The latter result is consistent with modelling predictions that the effect of the TnT-I79N mutation on cytosolic buffering is reduced when end-diastolic [Ca]free is increased (Figure 2E, points a and c).
Figure 3. Ca2+ sensitized TnT-I79N myocytes exhibit prolonged Ca2+ transients and increased end-diastolic Ca2+ concentrations.
Ca2+ transients were measures in field-stimulated ventricular myocytes, loaded with fura-2AM. A–B, Representative traces from three myocytes stimulated at 1 Hz (A) and 5 Hz (B). C–E, Average data. At 1 Hz, Ca2+ transients from the Ca2+ sensitized TnT-I79N myocytes have smaller amplitudes, slow decay kinetics and increased end-diastolic [Ca2+]. At a faster pacing rate of 5 Hz, the amplitude is nearly normalized, but decay kinetics remain significantly longer and end-diastolic [Ca2+] is further increased compared to both TnT-WT and TnT-R278C. *p<0.05, **p<0.01 compared to WT and R278C, n = 39–63 myocytes from 6–7 mice per group. Except for WT and R278C amplitude, all other parameter means measured at 5 Hz were significantly different from those measured at 1 Hz for each genotype (p<0.01, not indicated in the figure).
We next determined the effect of increased Ca binding affinity on intracellular Ca cycling in the intact heart at physiological heart rates. Hearts were subjected to pacing trains at cycle lengths ranging from 150 to 80 ms, which correspond to physiological heart rates of mice in vivo. To test the effect of a sudden change in heart rate, we introduced a 1000 ms pause followed by an extrastimulus (S2) and recorded Ca fluorescence (Figure 4A). Similar to the results in isolated myocytes, Ca removal from the cytosol is slowed and end-diastolic [Ca]free during the pacing train are significantly increased in TnT-I79N compared to WT and TnT-R278C hearts (Figure 4B,C). The systolic Ca transients during the pacing train (S1) were not statistically different among the groups (Figure 4D), but TnT-I79N hearts showed significantly increased S2 Ca transient amplitudes after the pause (Figure 4E). This post-pause potentiation of SR Ca release is even more evident when plotting the ratio of S1 and S2 (Figure 4F). Importantly, pretreatment of hearts with the myosin inhibitor blebbistatin, which both de-sensitizes and uncouples myofilaments,11 prevented the altered Ca handling in TnT-I79N hearts (Online Figure V).
Figure 4. TnT-I79N hearts have increased Ca2+ transients in response to an extrastimulus after a pause.
Ca2+ transients were measured from intact hearts using ratios of rhod-2 fluorescence. Hearts were subjected to rapid pacing at different pacing cycle length (S1), followed by a 1 second pause and an extrastimulus (S2 pulse). A, Representative trace demonstrating the pacing protocol, pacing cycle length 100 ms. B–F, Average data. Ca2+ removal from the cytosol is slowed (B) and end-diastolic Ca2+ concentrations are significantly increased in TnT-I79N (C). Ca2+ transients of TnT-I79N hearts have unchanged amplitude at steady state pacing (D), but show a significantly enhanced Ca2+ release after a pause (E, F). n=8–10 mice per group. *p<0.05, **p<0.01 TnT-I79N vs wild-type (WT).
L-type Ca currents that are responsible for triggering SR Ca release are not significantly different in TnT-I79N compared to TnT-WT myocytes.9 Hence, we hypothesized that increased SR Ca content may be the underlying cause for the pause-induced large Ca transient in the Ca-sensitized hearts. To test this hypothesis, voltage-clamped myocytes were stimulated from a holding potential of −70 mV with brief membrane depolarizations to 0 mV at 0.5 s cycle length (2 Hz). To measure steady-state SR Ca content during the pacing train, caffeine was applied 0.5 s (= pacing cycle length, [PCL]) after the last pacing stimulus (Figure 5A). To measure post-pause SR Ca content, caffeine was applied 4 s after the last stimulus (Figure 5A). SR Ca content was determined by measuring the NCX current integral in response to the caffeine application (Figure 5B). End-diastolic SR Ca content was not statistically different between TnT-I79N and TnT-WT myocytes during steady-state pacing (Figure 5C). After the pause, SR Ca content was significantly larger in TnT-I79N myocytes compared to TnT-WT myocytes (Figure 5C)
Figure 5. SR Ca2+ content is increased in TnT-I79N myocytes after a pause.
A, Experimental protocol. Voltage-clamped myocytes were stimulated with a pacing train (2 Hz, 20 s) of brief membrane depolarizations (0 mV, 50 ms) from a holding potential of − 70 mV. To measure end-diastolic SR Ca2+ content during steady-state pacing and after a pause, caffeine (10 mM) was applied either 0.5 s (= pacing cycle length) or 4 s following the last pacing stimulus. B, Representative NCX current records elicited by caffeine application. SR content was calculated from the NCX current integral. C, SR Ca2+ content is significantly increased in TnT-I79N vs TnT-WT myocytes only after a pause. N = 8–12 myocytes from 3–4 animals per genotype, *p<0.05.
Taken together, these data demonstrate that the main consequences of increased myofilament Ca binding affinity during physiological heart rates are an increased end-diastolic [Ca]free and a pause-dependent increase of SR Ca release.
Increasing Ca sensitivity causes action potential prolongation, early afterdepolarizations (EADs) and triggered activity after pauses
Since the myocyte membrane potential is closely linked to intracellular [Ca]free via the electrogenic NCX, we next examined the effect of enhanced post-rest potentiation of SR Ca release on the cardiac AP in the intact heart at physiological heart rates. Monophasic action potentials (MAP) were recorded using a pacing protocol analogous to the Ca transient measurements presented in Figure 4. TnT-I79N hearts were susceptible to induction of VT, which can be triggered after a pause (Figure 6A). Figure 6B shows representative examples of MAP recordings. Consistent with previous studies,9, 11 the S1 AP during steady-state pacing had a triangular shape in TnT-I79N hearts, but the overall action potential duration (measured as the duration at 90% repolarization, APD90) was not different among the groups (summary data not shown). On the other hand, the post-pause beat (S2) had a trend towards longer APD90 in TnT-I79N at normal pacing rates with a PCL of 150 ms. At fast pacing rates, the AP prolongation was more pronounced and reached statistical significance (Figure 6C). This post-pause AP prolongation became even more apparent when comparing the relative APD changes after every pause (Fig 6D), i.e. the relationship between the S1 and the S2 beat. Similarly, an acute increase in Ca sensitivity with EMD also resulted in a striking post-pause AP prolongation compared to both WT and TnT-R278C (Figure 6E,F).
Figure 6. Increasing Ca2+ sensitivity causes post-pause action potential prolongation.
Hearts were subjected to rapid pacing (S1) at different pacing cycle length (PCL), followed by a 500 ms pause and an extrastimulus (S2). A, Example of spontaneous ventricular tachycardia (VT) in a TnT-I79N mouse after a pause following fast pacing. B, Representative examples of monophasic action potentials recorded during the stimulation protocol, PCL 100 ms. C and D, Ca2+ sensitized TnT-I79N hearts show rate-dependent AP prolongation after the pause. Panel D shows the relative post-pause APD90 (S2) compared to pre-pause APD90 (S1). N=5–8 mice per group. E and F, Acutely increased Ca2+ sensitivity with EMD (3 μM) also causes rate-dependent AP prolongation after pauses. n=4–11 mice per group. *p<0.05, **p<0.01.
Prolonged APs can cause EADs and triggered arrhythmias.27 Thus, we investigated whether the post-pause AP prolongation in Ca-sensitized hearts increases the rate of EADs. Figure 7A shows a typical example of an EAD. Chronically increased Ca sensitivity in TnT-I79N resulted in an increased occurrence of EADs at fast pacing rates (Figure 7B). The mean takeoff potential of the EADs was around 80% repolarisation level (81% ± 2%, n=17). There was no difference in the incidence of EADs between TnT-R278C and WT. Application of EMD caused a significant increase in post-pause EADs already at normal heart rates with a PCL of 150 ms, at faster pacing rates this increase was even more dramatic (Figure 7C).
Figure 7. Ca2+ sensitization causes early afterdepolarizations (EADs) and triggers premature beats after pauses.
A, Pacing train with an extra stimulus (S2) after a 500 ms pause. Example record from a NTG heart treated with EMD (3 μM) shows an EAD followed by a triggered beat. Pacing cycle length (PCL) 100ms. B, At fast pacing rates, the incidence of EADs is increased in the Ca2+ sensitized TnT-I79N hearts. n = 4–11 mice per group. C, NTG hearts treated with EMD exhibit an increased rate of EADs compared to vehicle (VEH) treated NTG hearts and recordings after washout (WASH). n = 7–10 mice. D, In TnT-I79N hearts, the S2 beat frequently triggers premature beats. n = 4–11 mice per group. E, The incidence of triggered beats is increased by acute Ca2+ sensitization with EMD. n = 11–14 mice. *p<0.05, **p<0.01.
EADs were frequently followed by triggered activity (Figure 7A). Hence, we examined the occurrence of triggered beats following the post-pause beat. Triggered beats occurred more frequently in TnT-I79N at fast pacing rates (PCL 100 ms), while TnT-R278C was not statistically different from control (Figure 7D). Likewise, EMD significantly increased the incidence of triggered beats (Figure 7E) compared to baseline and washout recordings. Taken together, these results demonstrate that myofilament Ca sensitization increases the rate of EADs and triggered beats after pauses.
Increasing Ca sensitivity renders hearts with acute MI susceptible to pause-dependent ventricular ectopy and sustained VT
To explore the relevance of myofilament Ca sensitization as an arrhythmogenic mechanism in acquired heart disease, we next tested the effect of EMD in isolated hearts after inducing an acute MI by coronary ligation (Figure 8). Ca transients were recorded from the non-ischemic area at the base of the left ventricle. Analogous to the effects of Ca sensitizing TnT-mutants (Figure 4), EMD treatment slowed cytosolic Ca removal and caused an increase in end-diastolic [Ca]free (Figure 8A–C). Post-pause Ca transients were significantly larger in EMD-treated compared to vehicle-treated MI hearts. The large S2 Ca transient in EMD-treated hearts was frequently followed by either a single triggered premature beat or sustained VT (Figure 8A, lower panels). Compared to non-ischemic NTG control hearts, all MI hearts exhibit an increased incidence of ectopic beats during steady-state pacing (Figure 8D). Interestingly, EMD had no effect on the rate of ventricular ectopy during steady-state pacing (Figure 8D). Rather, we find that EMD selectively increased the incidence of post-pause ventricular ectopy (Figure 8E) and caused sustained VT in 5 out of 7 MI hearts examined (Figure 8F).
Figure 8. In hearts with acute MI, Ca2+ sensitization with EMD 57033 enhances post-pause Ca2+ transients and triggers sustained VT.
A, upper panels: Representative examples of simultaneously recorded ECG and Ca2+ fluorescence traces in the presence of EMD (3 μM) or vehicle (VEH). Lower panels: Pause-dependent triggered beats and sustained VT in EMD-treated MI hearts. B–C, EMD slowed cytosolic Ca2+ removal and increased post-pause Ca2+ release. D, Compared to non-ischemic hearts (NTG), MI hearts exhibit an increased incidence of ectopic beats during steady state pacing, which is not affected by EMD. E, Acute MI by itself does not increase the occurrence of post-pause ectopic beats compared to NTG; MI hearts treated with EMD exhibit a 3-fold increase in post-pause triggered beats. F, EMD causes pause-triggered sustained VT in MI hearts. Black arrows: pacing stimuli. *p<0.05 compared to NTG+VEH, # p<0.05 and ## p<0.01 compared to both MI+VEH and NTG+VEH, n = 7–9 hearts per group.
DISCUSSION
We report three major findings: (1) Both chronic and acute increases in myofilament Ca sensitivity (caused by TnT mutations or the drug EMD57033, respectively), produce a proportional increase in cytosolic Ca binding affinity (Figures 1 & 2, Online Figure IV). (2) The increased myofilament Ca binding results in increased end-diastolic [Ca]free during steady-state pacing and potentiates SR Ca release after brief pauses (Figures 3–5). (3) Ca-sensitized hearts exhibit altered AP regulation characterized by post-pause AP prolongation, afterdepolarizations and triggered activity (Figures 6–7), likely as a result of excessive post-rest potentiation of Ca release generated by cytosolic Ca accumulation during physiologic heart rates. Taken together, these observations suggest a novel mechanism for triggering ventricular ectopy that occurs as a direct consequence of increased myofilament Ca sensitivity. If a substrate able to support reentrant excitation is present, i.e., induced by rapid pacing (as reported previously11) or in the setting of an acute MI (Figure 8), the triggered beats can initiate sustained VT. Hence, our results are likely relevant for the pathogenesis of ventricular arrhythmia in inherited and acquired cardiomyopathies associated with increased myofilament Ca sensitivity.
Mechanism of increased cytosolic Ca buffering caused by myofilament Ca-sensitization
Since TnT by itself does not bind Ca in amounts sufficient to alter Ca buffering, finding increased Ca buffering in myocytes expressing TnT mutants may seem surprising. However, protein conformation changes in TnT can change Ca binding to TnC.28 Given the polymeric nature of the thin filament, even mutations in other thin filament proteins29 or changes in cross-bridge activity20 alter myofilament Ca sensitivity and likely change the Ca binding affinity (=Kd) of TnC in intact fibers. Given the fixed cytoplasmic volume of intact myocytes and the fact that TnC binds close to 50% of Ca released during a typical heart beat,13 even a small change in the Kd of TnC should result in a significant change in cytosolic [Ca]free during a physiological Ca release in the beating heart. On the other hand, the total number of Ca binding sites in the cytosol (=maximal buffering capacity, Bmax) did not change (Figure 2). This result is consistent with the finding that protein expression levels of TnT, TnC or TnI are not affected by the investigated TnT mutations.18, 30 The hypothesis that myofilament Ca sensitization can increase myofilament Ca binding is further supported by our experiments with EMD57033 (Online Figure IV). EMD binds to the C-lobe of TnC in a region that interacts with TnI as well as TnT with a Kd of approximately 8 μM.21 Previous reports did not show significantly increased TnC Ca binding in response to EMD,20 but differences in the experimental approach (e.g. equilibrium vs. dynamic) as well as the possibility that EMD only increases Ca binding at physiological [Ca]free may explain the different results.
Increased myofilament Ca buffering alters myocyte Ca homeostasis
In the intact myocytes, [Ca]free is determined at any given time point by the rate of sarcolemmal and SR Ca fluxes and the Ca buffering properties of the cytosol.31 As long as the net rate of Ca flux into the cytosol remains unchanged, systolic [Ca]free will be decreased in the context of increased Ca buffering. Other groups have shown that increasing maximal Ca binding capacity (Bmax) by Ca chelators can reduce Ca transient amplitude.16 Here we directly demonstrate that increasing cytosolic Ca binding affinity (= lower Kd) without changing Bmax, was similarly able to reduce Ca transient amplitude. The increased net Ca buffering is sufficient to lower systolic [Ca]free (Figure 1) and likely contributes to the decreased Ca transient amplitude in TnT-I79N cardiomyocytes at slow pacing rates (Figure 3A). Assuming that the increased myofilament Ca sensitivity affect TnC Ca “on” rates as well as “off” rates, the other consequence is slower Ca dissociation from the sensitized myofilaments during diastole. Consistent with this theory, we observed slow Ca transient decay rates in Ca-sensitized hearts, both in isolated cells and in whole hearts (Figures 3 and 5). Next, slow Ca decay rates can be predicted to lead to increased diastolic [Ca]free, once the diastolic interval is not sufficiently long to allow for complete cytosolic Ca removal. Again, our observations agree with this notion. Diastolic [Ca]free is increased both in isolated myocytes and in intact heart at physiological heart rates. A possible limitation is that the Ca indicators used to measure [Ca]free introduce exogenous cytosolic buffering that will further slow the Ca decay kinetics and will exacerbate the rate-dependent increase in end-diastolic [Ca]free. However, we previously reported that rapid pacing causes an excessive increase in end-diastolic pressure in indicator-free TnT mutant hearts,32 suggesting that the presence of Ca indicators was not responsible for this phenomenon.
A surprising finding was that increased myofilament buffering did not reduce Ca transient amplitude at physiological heart rates (Figure 3B and 4D). While we cannot exclude that other factor such as differences in sarcomere length13 or RyR2 SR Ca release channel activity33 are contributory, our modeling studies provide a plausible explanation: Myofilament Ca sensitization only changes Kd but not Bmax (Figure 2). Modeling the effect of an altered Kd predicts that the differences in cytosolic buffering are progressively reduced and may even reverse as end-diastolic [Ca]free rises (Figure 2E). Since end-diastolic [Ca]free is increased in Ca-sensitized muscle during steady-state pacing, finding unchanged Ca transient amplitudes suggests that the extra cytosolic Ca buffering provided by myofilament sensitization is largely saturated at physiological heart rates. This situation is illustrated in Figure 2E by the points a and c. On the other hand, the increased myofilament Ca accumulated during steady-state pacing is likely responsible for the exaggerated post-rest potentiation of Ca release (Figure 4).
Post-rest potentiation is a physiological phenomenon observed in mammalian cardiac muscle that describes a larger contraction upon restimulation of isolated myocardium after short periods of rest.34 The major underlying mechanisms are a shift of Ca from the cytosol into the SR resulting in larger SR load and also a more complete recovery of L-type Ca current and RyR2 refractoriness, together leading to enhanced Ca release after a pause.33 Post-rest potentiation is enhanced with faster pacing rates35 and can be abolished by pretreatment with ryanodine.36 Our findings support the hypothesis that an increased SR load was responsible for the enhanced post-rest potentiation of Ca-sensitized hearts: While SR load was the same during steady state, the SR load was significantly increased after a pause in Ca-sensitized TnT-I79N compared to WT myocytes (Figure 5). We interpret this result as follows: In a situation where cytosolic Ca affinity (i.e., Kd) is increased by myofilament sensitization, the extra Ca accumulated in the cytosol during the pacing train and bound to the myofilaments can be mobilized during a pause, of which a large fraction will be pumped into the SR.33 In addition, the increased end-diastolic [Ca]free found in Ca-sensitized cardiac muscle (Figures 5 & 8) will further increase post-rest potentation.
Myofilament sensitization as a cause of pause-dependent EADs and triggered arrhythmia
In mouse models expressing human TnT mutations associated with hypertrophic cardiomyopathy, the degree of Ca sensitization correlates with the risk for VT.9, 11 We reported previously reentrant activation pattern, likely produced as the result of increased CV dispersion and AP alternans.11 However, an additional triggering mechanism is still required to initiate VT. Here, we find that that Ca-sensitized hearts exhibit pause-dependent AP prolongation, EADsm and triggered activity (Figures 6–7). Cardiac AP repolarization is highly interconnected with intracellular [Ca]free.25, 37 Hence, the large post-pause S2 Ca transients found in Ca-sensitized hearts (Figure 4) presumably activate inward NCX current,38 thereby causing the striking AP prolongation shown in Figure 6. Prolonged AP durations and elevated intracellular [Ca] are also well-recognized mechanism for late EADs.25
Another form of pause-dependent arrhythmia is torsades de pointes that occurs in patients with the long QT syndrome (LQTS).39 A brief pause or slowing of the heart rate in the setting of baseline APD prolongation leads to an increased incidence of EADs and possibly torsades if the substrate is sufficiently primed.40–42 In LQTS, the physiologic post-pause APD prolongation in the setting of already prolonged APs causes triggered activity. In the case of increased myofilament Ca sensitivity, APD and QT are normal during steady-state pacing11 or in vivo.9, 22 Rather, as we show here, the APD prolongation after a pause is abnormally enhanced, leading to post-pause EADs and potentially VT. As increased myofilament Ca sensitivity is frequently found in hypertrophic cardiomyopathy,10 such a triggering mechanism could be life-threatening when paired with structural remodelling of the heart.
Potential implications
In this study, we present a new mechanism of how increased myofilament Ca sensitivity can render hearts susceptible to ventricular arrhythmia: increased cytosolic Ca buffering leads to pause-dependent increased SR Ca load and triggered activity. In addition to inherited cardiac diseases such as TnT-linked FHC, our findings have implications for more common acquired human cardiomyopathies. Increased myofilament Ca sensitivity is also found in animals post-myocardial infarction43 and in humans with heart failure;44 which are heart diseases with a high incidence of ventricular arrhythmias and sudden death.45 In both cases, the pathophysiology is extremely complex, but altered myocyte Ca regulation with increased end-diastolic [Ca] is one of the central findings.
Supplementary Material
Novelty and Significance.
What Is Known?
Familial hypertrophic cardiomyopathy (FHC) is an inherited disease caused by mutations in sarcomeric proteins that is associated with a high risk for ventricular arrhythmia and sudden death.
FHC-linked mutations often increase myofilament Ca sensitivity, which has been linked to increased arrhythmia susceptibility.
Myofilaments are the dominant cytosolic Ca buffer, binding about 50% of Ca entering the cytosol during a normal heart beat.
What New Information Does This Article Contribute?
Ca sensitizing FHC-mutants increase the cytosolic Ca binding affinity (Kd), and cause excess cytosolic Ca accumulation during physiological heart rates, which shifts into the sarcoplasmic reticulum (SR) during longer diastolic intervals.
The pause-dependent excessive SR Ca uptake and subsequent release causes action potential prolongation, early afterdepolarizations and triggered beats that can be prevented by myofilament Ca desensitization.
Acute Ca sensitization with EMD 57033 mimics the effects of Ca sensitizing FHC-mutants and produces pause-dependent ventricular arrhythmia after acute myocardial infarction (MI).
Increased myofilament Ca sensitivity is commonly caused by FHC mutations, but has also been described after MI. Both diseases are associated with a high risk for ventricular arrhythmia and sudden death. We previously found that increasing myofilament Ca sensitivity renders mouse hearts susceptible to ventricular tachycardia. However, the underlying cellular mechanisms remain unclear. Here, we report a novel arrhythmia triggering mechanism that is based on myofilament Ca sensitization. The initiating event is the increased cytosolic Ca binding affinity that leads to Ca accumulation in the cytosol during physiological heart rates. The accumulated Ca is mobilized and taken up by the SR during longer diastolic intervals or skipped heart beats (=pauses). The resulting post-pause excessive Ca release causes action potential prolongation, afterdepolarizations and triggered ventricular beats. Triggered beats lead to ventricular tachycardia in structurally abnormal hearts, e.g. after MI. To our knowledge, our findings provide the first direct evidence of the mechanism responsible for triggering ventricular arrhythmia in Ca sensitized hearts, which may contribute to sudden death risk in FHC and ischemic cardiomyopathy. We propose that increased cytosolic Ca binding affinity represents a heretofore unrecognized pause-dependent arrhythmia mechanism that could manifest itself in patients with normal QT duration during regular sinus rhythm.
Acknowledgments
SOURCES OF FUNDING
Funding support by NIH grants R01HL71670 (BCK, FCB), R01HL88635 (BCK), American Heart Association Established Investigator Award 0840071N (BCK) and Scientist Development Grant 10SDG2640109 (SH).
Non-standard abbreviations and acronyms
- AP
Action Potential
- APD
Action Potential Duration
- CV
Conduction Velocity (CV)
- EAD
Early Afterdepolarization
- FHC
Familial Hypertrophic Cardiomyopathy
- MAP
Monophasic Action Potential
- MI
Myocardial Infarction
- NCX
Na Ca exchanger
- NTG
Non-transgenic
- PCL
Pacing Cycle Length
- SCD
Sudden Cardiac Death
- SR
Sarcoplasmatic Reticulum
- TnC
Troponin C
- TnI
Troponin I
- TnT
Troponin T
- VT
Ventricular Tachycardia
- WT
Wild-type
Footnotes
DISCLOSURES
NONE
References
- 1.Marian AJ, Roberts R. The molecular genetic basis for hypertrophic cardiomyopathy. J Mol Cell Cardiol. 2001;33(4):655–670. doi: 10.1006/jmcc.2001.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maron BJ, Olivotto I, Spirito P, Casey SA, Bellone P, Gohman TE, Graham KJ, Burton DA, Cecchi F. Epidemiology of hypertrophic cardiomyopathy-related death: revisited in a large non-referral-based patient population. Circulation. 2000;102(8):858–864. doi: 10.1161/01.cir.102.8.858. [DOI] [PubMed] [Google Scholar]
- 3.Maron BJ, Shen WK, Link MS, Epstein AE, Almquist AK, Daubert JP, Bardy GH, Favale S, Rea RF, Boriani G, Estes NA, 3rd, Spirito P. Efficacy of implantable cardioverter-defibrillators for the prevention of sudden death in patients with hypertrophic cardiomyopathy [see comments] N Engl J Med. 2000;342(6):365–373. doi: 10.1056/NEJM200002103420601. [DOI] [PubMed] [Google Scholar]
- 4.Spirito P, Bellone P, Harris KM, Bernabo P, Bruzzi P, Maron BJ. Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N Engl J Med. 2000;342(24):1778–1785. doi: 10.1056/NEJM200006153422403. [DOI] [PubMed] [Google Scholar]
- 5.Varnava AM, Elliott PM, Baboonian C, Davison F, Davies MJ, McKenna WJ. Hypertrophic cardiomyopathy: histopathological features of sudden death in cardiac troponin T disease. Circulation. 2001;104(12):1380–1384. doi: 10.1161/hc3701.095952. [DOI] [PubMed] [Google Scholar]
- 6.Richard P, Charron P, Carrier L, Ledeuil C, Cheav T, Pichereau C, Benaiche A, Isnard R, Dubourg O, Burban M, Gueffet JP, Millaire A, Desnos M, Schwartz K, Hainque B, Komajda M. Hypertrophic cardiomyopathy: distribution of disease genes, spectrum of mutations, and implications for a molecular diagnosis strategy. Circulation. 2003;107(17):2227–2232. doi: 10.1161/01.CIR.0000066323.15244.54. [DOI] [PubMed] [Google Scholar]
- 7.Van Driest SL, Ellsworth EG, Ommen SR, Tajik AJ, Gersh BJ, Ackerman MJ. Prevalence and spectrum of thin filament mutations in an outpatient referral population with hypertrophic cardiomyopathy. Circulation. 2003;108(4):445–451. doi: 10.1161/01.CIR.0000080896.52003.DF. [DOI] [PubMed] [Google Scholar]
- 8.Maass AH, Ikeda K, Oberdorf-Maass S, Maier SK, Leinwand LA. Hypertrophy, fibrosis, and sudden cardiac death in response to pathological stimuli in mice with mutations in cardiac troponin T. Circulation. 2004;110(15):2102–2109. doi: 10.1161/01.CIR.0000144460.84795.E3. [DOI] [PubMed] [Google Scholar]
- 9.Knollmann BC, Kirchhof P, Sirenko SG, Degen H, Greene AE, Schober T, Mackow JC, Fabritz L, Potter JD, Morad M. Familial hypertrophic cardiomyopathy-linked mutant troponin T causes stress-induced ventricular tachycardia and Ca2+-dependent action potential remodeling. Circ Res. 2003;92(4):428–436. doi: 10.1161/01.RES.0000059562.91384.1A. [DOI] [PubMed] [Google Scholar]
- 10.Knollmann BC, Potter JD. Altered regulation of cardiac muscle contraction by troponin T mutations that cause familial hypertrophic cardiomyopathy. Trends Cardiovasc Med. 2001;11(5):206–212. doi: 10.1016/s1050-1738(01)00115-3. [DOI] [PubMed] [Google Scholar]
- 11.Baudenbacher F, Schober T, Pinto JR, Sidorov VY, Hilliard F, Solaro RJ, Potter JD, Knollmann BC. Myofilament Ca2+ sensitization causes susceptibility to cardiac arrhythmia in mice. J Clin Invest. 2008;118(12):3893–3903. doi: 10.1172/JCI36642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huke S, Knollmann BC. Increased myofilament Ca(2+)-sensitivity and arrhythmia susceptibility. J Mol Cell Cardiol. 2010 doi: 10.1016/j.yjmcc.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bers DM. Excitation and Contraction Coupling and Cardiac Contractile Force. 2. Dordrecht/Boston/London: Kluwer Academic Publishers; 2001. Calcium Sources and Sinks; pp. 39–56. [Google Scholar]
- 14.Solaro RJ. Troponin C - troponin I interactions and molecular signalling in cardiac myofilaments. Adv Exp Med Biol. 1995;382:109–115. doi: 10.1007/978-1-4615-1893-8_12. [DOI] [PubMed] [Google Scholar]
- 15.Diaz ME, Trafford AW, Eisner DA. The effects of exogenous calcium buffers on the systolic calcium transient in rat ventricular myocytes. Biophys J. 2001;80(4):1915–1925. doi: 10.1016/S0006-3495(01)76161-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ter Keurs HE, Boyden PA. Calcium and arrhythmogenesis. Physiol Rev. 2007;87(2):457–506. doi: 10.1152/physrev.00011.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watkins H, McKenna WJ, Thierfelder L, Suk HJ, Anan R, O’Donoghue A, Spirito P, Matsumori A, Moravec CS, Seidman JG, Seidman CE. Mutations in the genes for cardiac troponin T and alpha-tropomyosin in hypertrophic cardiomyopathy. N Engl J Med. 1995;332(16):1058–1064. doi: 10.1056/NEJM199504203321603. [DOI] [PubMed] [Google Scholar]
- 18.Hernandez O, Szczesna-Cordary D, Knollmann BC, Miller T, Bell M, Zhao J, Sirenko SG, Diaz Z, Guzman G, Xu Y, Wang Y, Kerrick WG, Potter JD. F110I and R278C troponin T mutations that cause familial hypertrophic cardiomyopathy affect muscle contraction in transgenic mice and reconstituted human cardiac fibers. J Biol Chem. 2005;280(44):37183–37194. doi: 10.1074/jbc.M508114200. [DOI] [PubMed] [Google Scholar]
- 19.Theopistou A, Anastasakis A, Miliou A, Rigopoulos A, Toutouzas P, Stefanadis C. Clinical features of hypertrophic cardiomyopathy caused by an Arg278Cys missense mutation in the cardiac troponin T gene. Am J Cardiol. 2004;94(2):246–249. doi: 10.1016/j.amjcard.2004.03.077. [DOI] [PubMed] [Google Scholar]
- 20.Solaro RJ, Gambassi G, Warshaw DM, Keller MR, Spurgeon HA, Beier N, Lakatta EG. Stereoselective actions of thiadiazinones on canine cardiac myocytes and myofilaments. Circ Res. 1993;73(6):981–990. doi: 10.1161/01.res.73.6.981. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Li MX, Spyracopoulos L, Beier N, Chandra M, Solaro RJ, Sykes BD. Structure of the C-domain of human cardiac troponin C in complex with the Ca2+ sensitizing drug EMD 57033. J Biol Chem. 2001;276(27):25456–25466. doi: 10.1074/jbc.M102418200. [DOI] [PubMed] [Google Scholar]
- 22.Knollmann BC, Blatt SA, Horton K, de Freitas F, Miller T, Bell M, Housmans PR, Weissman NJ, Morad M, Potter JD. Inotropic stimulation induces cardiac dysfunction in transgenic mice expressing a troponin T (I79N) mutation linked to familial hypertrophic cardiomyopathy. J Biol Chem. 2001;276(13):10039–10048. doi: 10.1074/jbc.M006745200. [DOI] [PubMed] [Google Scholar]
- 23.Trafford AW, Diaz ME, Eisner DA. A novel, rapid and reversible method to measure Ca buffering and time-course of total sarcoplasmic reticulum Ca content in cardiac ventricular myocytes. Pflugers Arch. 1999;437(3):501–503. doi: 10.1007/s004240050808. [DOI] [PubMed] [Google Scholar]
- 24.Trafford AW, Sibbring GC, Diaz ME, Eisner DA. The effects of low concentrations of caffeine on spontaneous Ca release in isolated rat ventricular myocytes. Cell Calcium. 2000;28(4):269–276. doi: 10.1054/ceca.2000.0156. [DOI] [PubMed] [Google Scholar]
- 25.Bers DM. Calcium and cardiac rhythms: physiological and pathophysiological. Circ Res. 2002;90(1):14–17. [PubMed] [Google Scholar]
- 26.Berlin JR, Bassani JW, Bers DM. Intrinsic cytosolic calcium buffering properties of single rat cardiac myocytes. Biophys J. 1994;67(4):1775–1787. doi: 10.1016/S0006-3495(94)80652-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.January CT, Moscucci A. Cellular mechanisms of early afterdepolarizations. Ann N Y Acad Sci. 1992;644:23–32. doi: 10.1111/j.1749-6632.1992.tb30999.x. [DOI] [PubMed] [Google Scholar]
- 28.Gomes AV, Venkatraman G, Davis JP, Tikunova SB, Engel P, Solaro RJ, Potter JD. Cardiac troponin T isoforms affect the Ca(2+) sensitivity of force development in the presence of slow skeletal troponin I: insights into the role of troponin T isoforms in the fetal heart. J Biol Chem. 2004;279(48):49579–49587. doi: 10.1074/jbc.M407340200. [DOI] [PubMed] [Google Scholar]
- 29.Bottinelli R, Coviello DA, Redwood CS, Pellegrino MA, Maron BJ, Spirito P, Watkins H, Reggiani C. A mutant tropomyosin that causes hypertrophic cardiomyopathy is expressed in vivo and associated with an increased calcium sensitivity [see comments] Circ Res. 1998;82(1):106–115. doi: 10.1161/01.res.82.1.106. [DOI] [PubMed] [Google Scholar]
- 30.Miller T, Szczesna D, Housmans PR, Zhao J, de Freitas F, Gomes AV, Culbreath L, McCue J, Wang Y, Xu Y, Kerrick WG, Potter JD. Abnormal contractile function in transgenic mice expressing a familial hypertrophic cardiomyopathy-linked troponin T (I79N) mutation. J Biol Chem. 2001;276(6):3743–3755. doi: 10.1074/jbc.M006746200. [DOI] [PubMed] [Google Scholar]
- 31.Bers DM. Calcium fluxes involved in control of cardiac myocyte contraction. Circ Res. 2000;87(4):275–281. doi: 10.1161/01.res.87.4.275. [DOI] [PubMed] [Google Scholar]
- 32.Sirenko SG, Potter JD, Knollmann BC. Differential effect of troponin T mutations on the inotropic responsiveness of mouse hearts--role of myofilament Ca2+ sensitivity increase. J Physiol. 2006;575(Pt 1):201–213. doi: 10.1113/jphysiol.2006.107557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bers DM. Excitation and Contraction Coupling and Cardiac Contractile Force. 2. Dordrecht/Boston/London: Kluwer Academic Publishers; 2001. Sarcoplasmic Reticulum; pp. 161–202. [Google Scholar]
- 34.Koch-Weser J, Blinks JR. The Influence of the Interval between Beats on Myocardial Contractility. Pharmacol Rev. 1963;15:601–652. [PubMed] [Google Scholar]
- 35.Allen DG, Jewell BR, Wood EH. Studies of the contractility of mammalian myocardium at low rates of stimulation. J Physiol. 1976;254(1):1–17. doi: 10.1113/jphysiol.1976.sp011217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Banijamali HS, Gao WD, MacIntosh BR, ter Keurs HE. Force-interval relations of twitches and cold contractures in rat cardiac trabeculae. Effect of ryanodine. Circ Res. 1991;69(4):937–948. doi: 10.1161/01.res.69.4.937. [DOI] [PubMed] [Google Scholar]
- 37.Carmeliet E. Intracellular Ca(2+) concentration and rate adaptation of the cardiac action potential. Cell Calcium. 2004;35(6):557–573. doi: 10.1016/j.ceca.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 38.Knollmann BC, Schober T, Petersen AO, Sirenko SG, Franz MR. Action potential characterization in intact mouse heart: steady-state cycle length dependence and electrical restitution. Am J Physiol Heart Circ Physiol. 2007;292(1):H614–621. doi: 10.1152/ajpheart.01085.2005. [DOI] [PubMed] [Google Scholar]
- 39.Viskin S, Fish R, Zeltser D, Belhassen B, Heller K, Brosh D, Laniado S, Barron HV. Arrhythmias in the congenital long QT syndrome: how often is torsade de pointes pause dependent? Heart. 2000;83(6):661–666. doi: 10.1136/heart.83.6.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vos MA, Gorenek B, Verduyn SC, van der Hulst FF, Leunissen JD, Dohmen L, Wellens HJ. Observations on the onset of torsade de pointes arrhythmias in the acquired long QT syndrome. Cardiovasc Res. 2000;48(3):421–429. doi: 10.1016/s0008-6363(00)00192-9. [DOI] [PubMed] [Google Scholar]
- 41.Roden DM, Anderson ME. The pause that refreshes, or does it? Mechanisms in torsades de pointes. Heart. 2000;84(3):235–237. doi: 10.1136/heart.84.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu J, Laurita KR. The mechanism of pause-induced torsade de pointes in long QT syndrome. J Cardiovasc Electrophysiol. 2005;16(9):981–987. doi: 10.1111/j.1540-8167.2005.40677.x. [DOI] [PubMed] [Google Scholar]
- 43.de Waard M, van der Velden J, Bito V, Ozdemir S, Biesmans L, Boontje N, Dekkers D, Schoonderwoerd K, Schuurbiers H, de Crom R, Stienen G, Sipido K, Lamers J, Duncker D. Early exercise training normalizes myofilament function and attenuates left ventricular pump dysfunction in mice with a lange myocardial infarction. Circ Res. 2007;100(7):1079–1088. doi: 10.1161/01.RES.0000262655.16373.37. [DOI] [PubMed] [Google Scholar]
- 44.van der Velden J, Papp Z, Zaremba R, Boontje NM, de Jong JW, Owen VJ, Burton PB, Goldmann P, Jaquet K, Stienen GJ. Increased Ca2+-sensitivity of the contractile apparatus in end-stage human heart failure results from altered phosphorylation of contractile proteins. Cardiovasc Res. 2003;57(1):37–47. doi: 10.1016/s0008-6363(02)00606-5. [DOI] [PubMed] [Google Scholar]
- 45.Tomaselli GF, Zipes DP. What causes sudden death in heart failure? Circ Res. 2004;95(8):754–763. doi: 10.1161/01.RES.0000145047.14691.db. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.