Abstract
A three-component condensation of 5-amino-3-methylpyrazole, tetronic acid and various aromatic, heteroaromatic and aliphatic aldehydes leads to the formation of dihydropyridopyrazole analogues of a cytotoxic lignan podophyllotoxin. This new heterocyclic scaffold-based library allows a drastic reduction of the structural complexity of the natural product with the retention of its potent cytotoxic properties. Similarly to podophyllotoxin, the presented analogues induce apoptosis in Jurkat cells.
Keywords: Multicomponent synthesis, Antimitotic agents, Podophyllotoxin
Podophyllotoxin (1), an antimitotic cyclolignan isolated from plants of the genus Podophyllum, has served as a useful lead agent in the development of new anticancer drugs.1 Its semisynthetic derivatives, etoposide (2) and teniposide, are currently used in clinic for the treatment of a variety of cancers. Due to the structural complexity of podophyllotoxin, arising from the presence of four stereogenic carbons in ring C (Figure 1), most of the structure-activity relationship (SAR) studies have been performed by derivatization of the parent natural product rather than by de novo chemical synthesis.2 In this connection, Itokawa and Takeya made an important contribution to the field by demonstrating that greatly simplified 4-aza-2,3-didehydropodophyllotoxins 3a and 3b retain most of the cytotoxicity associated with the parent lignan.3 Importantly, removal of the stereocenters at C-2 and C-3 solves the problem of epimerization at C-2 that has plagued the clinical development of podophyllotoxin and its stereochemically complex derivatives, because the rapidly formed in vivo cis-lactone metabolite is inactive.4 Later, Giorgi-Renault and Husson disclosed a multicomponent one-step synthesis of these promising anticancer drug leads.5 Very recently, Tu and co-workers reported a multicomponent reaction leading to the formation of N-substituted dihydropyridines 4, although no biological data for these compounds were provided.6
Figure 1.
Structures of Podophyllotoxin (1), Etoposide (2) and 4-Aza-2,3-didehydro Analogues 3 and 4.
Inspection of the extensive published SAR data of podophyllotoxin analogues2 reveals that the intact ring A is not essential for antimitotic activity. In addition, the hydroxyl at C-4 can be repositioned to C-5 leading to the structural type of α- and β-peltatins, also isolated from Podophyllum plants (5a and 5b in Figure 2). Although the latter natural products have potent antitumor activity, their severe toxicity resulted in unacceptable clinical trial outcomes.7 Furthermore, phenolic hydroxyl commonly reduces drug bioavailability by making compounds susceptible to oxidation and glucuronidation, and this problem is often addressed with bioisosteric replacement of the phenolic ring with nitrogen-containing heterocycles, such as pyrazoles and triazoles.8 This line of reasoning, together with the success of a structural simplification exemplified by dihydropyridines 3a and 3b, has led us to explore dihydropyridopyrazole (6) analogues of podophyllotoxin. This novel heterocyclic scaffold represents a most significant structural departure from the natural lead compound. Notwithstanding, we report in this Letter that analogues 6 retain a considerable portion podophyllotoxin’s cytotoxicity. In addition, we show that, similarly to podophyllotoxin and etoposide, our compounds induce apoptosis in Jurkat cells, a model for human T-cell leukemia in vitro.
Figure 2.
Structures of α-Peltatin (5a), β-Peltatin (5b) and the Library of Analogues 6.
To streamline the preparation of analogues 6 we devised a multicomponent route involving the condensation of 5-amino-3-methylpyrazole, tetronic acid and a desired aldehyde (Figure 3). Although either (or both) dihydropyridine 6 or dihydropyrimidine 7 type products could be expected, we were encouraged by a number of literature reports describing related processes that resulted in the formation of thermodynamically more stable dihydropyridines.9 Indeed, we found that the desired library of analogues can be synthesized in a straightforward manner by refluxing the three above-mentioned components with Et3N in ethanol. In all cases dihydropyridopyrazoles 6 precipitate as the reaction mixtures are allowed to cool to room temperature without any traces of compounds 7. The yields of recrystallized products are given in Table.10
Figure 3.
Multicomponent Synthesis of Analogue Library 6.
Table.
Synthesis and Cytotoxicity Data for Compounds 6
| Analo- gue |
R | Synthetic yield % |
% Cell viability viabilitya |
||
|---|---|---|---|---|---|
| HeLa | MCF7-AZ | Jurkat | |||
| 1 | n/a | n/a | 19 ±5 | 55 ±3 | 18 ±5 |
| 2 | n/a | n/a | 91 ±2 | 76 ±2 | 75 ±5 |
| 3a | n/a | n/a | 53 ±5 | 58 ±4 | 35 ±6 |
| 3b | n/a | n/a | 54 ±6 | 52 ±3 | 54 ±1 |
| 6a | 3,4,5-tri-MeO-Ph | 80 | 50 ±2 | 58 ±4 | 47 ±2 |
| 6b | 3,4-di-MeO-Ph | 82 | 73 ±3 | 95 ±4 | 88 ±2 |
| 6c | 4-MeO-Ph | 72 | 73 ±7 | 82 ±3 | 94 ±4 |
| 6d | 4-F3CO-Ph | 67 | 97 ±2 | 84 ±3 | 97 ±6 |
| 6e | 4-MeS-Ph | 79 | 45 ±2 | 95 ±4 | 87 ±4 |
| 6f | 3-F-Ph | 63 | 99 ±1 | 76 ±2 | 84 ±2 |
| 6g | 3-Cl-Ph | 78 | 43 ±4 | 54 ±3 | 76 ±4 |
| 6h | 3,4-di-Cl-Ph | 75 | 88 ±1 | 56 ±3 | 71 ±4 |
| 6i | 3-Br-Ph | 76 | 51 ±3 | 63 ±4 | 68 ±3 |
| 6j | 2-Br-Ph | 58 | 57 ±4 | 100 ±3 | 90 ±1 |
| 6k | 4-Br-Ph | 83 | 62 ±3 | 92 ±2 | 90 ±1 |
| 6l | 3,5-di-Br-4-HO-Ph | 52 | 58 ±7 | 60 ±4 | 58 ±3 |
| 6m | 3-Br-4-EtO-5-MeO-Ph | 76 | 52 ±2 | 49 ±4 | 28 ±5 |
| 6n | 4-AcO-3-Br-5-MeO-Ph | 73 | 47 ±2 | 46 ±2 | 36 ±6 |
| 6o | 3-Br-4-Me2N-Ph | 75 | 55 ±3 | 59 ±3 | 34 ±15 |
| 6p | Ph | 83 | 74 ±4 | 84 ±3 | 87 ±5 |
| 6q | 2-O2N-Ph | 47 | 71 ±4 | 99 ±2 | 64 ±1 |
| 6r | 4-HO-3-MeO-5-O2N-Ph | 76 | 78 ±3 | 89 ±5 | 90 ±2 |
| 6s | Me | 37 | 70 ±4 | 97 ±2 | 78 ±5 |
| 6t | 54 | 95 ±5 | 91 ±4 | 89 ±6 | |
| 6u |  |
61 | 58 ±2 | 100 ±1 | 94 ±2 |
| 6v | 84 | 82 ±4 | 98 ±3 | 74 ±4 | |
| 6w | 92 | 98 ±2 | 100 ±2 | 82 ±5 | |
| 6x | 55 | 94 ±1 | 100 ±1 | 92 ±5 | |
| 6y | 59 | 69 ±4 | 96 ±2 | 88 ±9 | |
| 6z | 78 | 84 ±4 | 100 ±3 | 85 ±6 | |
| 6aa | 57 | 72 ±6 | 100 ±4 | 97 ±3 | |
| 6bb | 92 | 56 ±2 | 99 ±4 | 96 ±4 | |
% Remaining cell viability after 48 hours of treatment with indicated compounds at the final concentration of 5 µM relative to 100% DMSO control ±% s. d. from two experiments.
Analogues 6 were evaluated for cytotoxicity against three cancer cell lines, HeLa, MCF7-AZ and Jurkat as models for human cervical and breast adenocarcinomas and T-cell leukemia respectively. The corresponding cells were treated with compounds 6 at final concentrations of 5 and 50 µM for 48 hours and cell viability was assessed through measurements of mitochondrial dehydrogenase activities using MTT method.11 The 5 µM data, including those of podophyllotoxin, etoposide, 3a and 3b are shown in Table.12
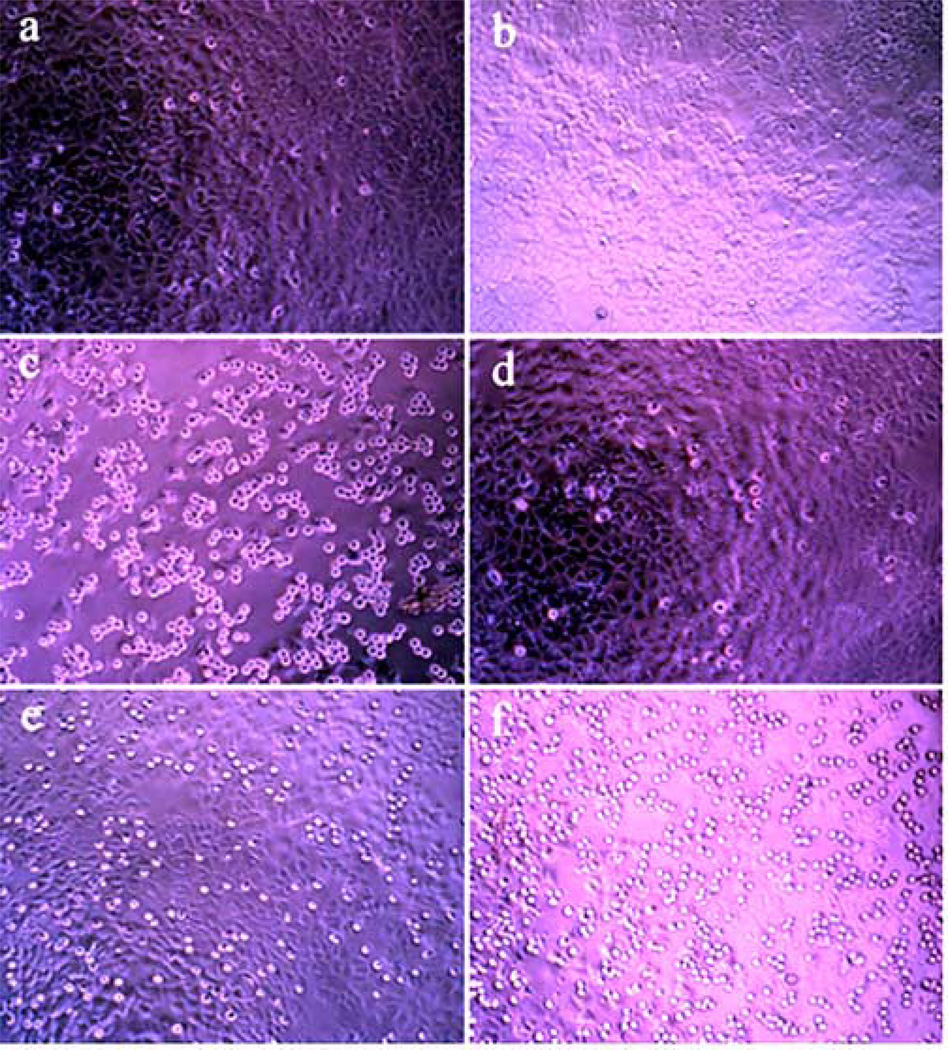
In addition to the potent cytotoxicity of 6a, whose ring E is the same as that of podophyllotoxin, 3a and 3b, the pronounced effect of m-bromo substituent is noteworthy. Thus, all of the most potent analogues, namely 6i, 6l, 6m, 6n, and 6o, have bromine at the meta-position of the aromatic ring E. The other notable observations are marginal toxicity of etoposide in our assays13 and surprising potency of 6s, whose significant structural dissimilarity with podophyllotoxin points to the possibility of a different mechanism of action for this analogue. It is generally accepted that cytotoxicity of podophyllotoxin is attributable to the inhibition of tubulin polymerization, while etoposide’s target is topoisomerase II. Although further mechanistic studies for analogues 6 are underway in our laboratories, various observations point to the anti-tubulin mechanism for these compounds. For example, rounding up of dead cells, the phenomenon that is commonly observed with tubulin-binding antimitotic agents that cause disruption of the cytoskeleton,14 is clearly seen when cells are treated with podophyllotoxin (Figure 4c), but not etoposide (Figure 4b). The extent of this effect correlates perfectly with the potency of analogues 6 (Figure 4d, 4e and 4f).
Figure 4.
Detachment and rounding up of MCF7-AZ cells after 48 hours of treatment with selected compounds at indicated concentrations. (a) DMSO control; (b) Etoposide (50 µM, 57% cell viability); (c) Podophyllotoxin (5 µM, 55% cell viability); (d) Inactive analogue 6b (5 µM, 95% cell viability); (e) Moderately potent analogue 6f (5 µM, 76% cell viability); (f) Highly potent analogue 6n (5 µM, 46% cell viability).
Lastly, analogues 6a and 6i, together with podophyllotoxin and etoposide, were tested for their ability to induce apoptosis in Jurkat cells in a flow cytometric annexin-V / propidium iodide assay (Figure 5). For all four compounds we observed a similar, time-dependent increase in the proportion of cells undergoing apoptosis with the maximum of 70–75% occurring after 48 hours of treatment. These data provide an excellent foundation for further investigation of compounds 6 as promising anticancer leads.
Figure 5.
Induction of apoptosis in Jurkat cells treated for an indicated number of hours with DMSO control, podophyllotoxin (5 µM), etoposide (50 µM), 6a (5 µM) and 6i (5 µM) in flow cytometric annexin-V / propidium iodide assay. □ 12 hours;  24 hours;
24 hours;  36 hours;
36 hours;  48 hours;
48 hours;  60 hours;
60 hours;  72 hours. Error bars represent data from two experiments.
72 hours. Error bars represent data from two experiments.
Investigation of the mechanism of action underlying the cytotoxicity of the reported dihydropyridopyrazoles and SAR studies, including substitutions of rings A and B with other heterocycles, are in progress and will be reported in due course.
Acknowledgments
US National Institutes of Health (CA-99957 and RR-16480) are gratefully acknowledged for partial financial support of this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.For reviews of the pharmaceutical action of podophyllotoxin, preclinical development and clinical trials of drugs derived from the parent lignan, see: Bohlin L, Rosen B. Drug Discov. Today. 1996;1:343. Imbert TF. Biochimie. 1998;80:207. doi: 10.1016/s0300-9084(98)80004-7.
- 2.For reviews of SAR studies, see: You YJ. Curr. Pharm. Des. 2005;11:1695. doi: 10.2174/1381612053764724. Gordaliza M, Castro MA, Corral JMM, San Feliciano A. Curr. Pharm. Des. 2000;6:1811. doi: 10.2174/1381612003398582.
- 3.(a) Hitotsuyanagi Y, Kobayashi M, Fukuyo M, Takeya K, Itokawa H. Tetrahedron Lett. 1997;38:8295. [Google Scholar]; (b) Hitotsuyanagi Y, Fukuyo M, Tsuda K, Kobayashi M, Ozeki A, Itokawa H, Takeya K. Bioorg. Med. Chem. Lett. 2000;10:315. doi: 10.1016/s0960-894x(99)00693-9. [DOI] [PubMed] [Google Scholar]
- 4.Gensler WJ, Murthy CD, Trammell MH. J. Med. Chem. 1977;20:635. doi: 10.1021/jm00215a004. [DOI] [PubMed] [Google Scholar]
- 5.Tratrat C, Giorgi-Renault S, Husson HP. Org. Lett. 2002;4:3187. doi: 10.1021/ol0200908. [DOI] [PubMed] [Google Scholar]
- 6.Tu S, Zhang Y, Jia R, Jiang B, Zhang J, Ji S. Tetrahedron Lett. 2006;47:6521. [Google Scholar]
- 7.Jardine I. In: Anticancer Agents Based on Natural Products. Cassady JM, Douros JD, editors. New York: Academic; 1980. pp. 319–351. [Google Scholar]; (b) Ayres DC, Loike JD. Lignans, Chemical, Biological and Clinical Properties. Cambridge: Cambridge University Press; 1990. [Google Scholar]
- 8.(a) Wright JL, Gregory TF, Kesten SR, Boxer PA, Serpa KA, Meltzer LT, Wise LD. J. Med. Chem. 2000;43:3408. doi: 10.1021/jm000023o. [DOI] [PubMed] [Google Scholar]; (b) Wilkening RR, Ratcliffe RW, Fried AK, Meng D, Sun W, Colwell L, Lambert S, Greenlee M, Nilsson S, Thorsell A, Mojena M, Tudela C, Frisch K, Chan W, Birzin ET, Rohrer SP, Hammond ML. Bioorg. Med. Chem. Lett. 2006;16:3896. doi: 10.1016/j.bmcl.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 9.(a) Quiroga J, Insuasty B, Hormaza A, Saitz C, Jullian C. J. Heterocyclic Chem. 1998;35:575. [Google Scholar]; (b) Quiroga J, Mejía D, Insuasty B, Abonía R, Nogueras M, Sánchez A, Cobo J, Low JN. Tetrahedron. 2001;57:6947. [Google Scholar]; (c) Drizin I, Holladay MW, Yi L, Zhang HQ, Gopalakrishnan S, Gopalakrishnan M, Whiteaker KL, Buckner SA, Sullivan JP, Carroll WA. Bioorg. Med. Chem. Lett. 2002;12:1481. doi: 10.1016/s0960-894x(02)00207-x. [DOI] [PubMed] [Google Scholar]
- 10.General procedure for dihydropyridopyrazole 6 synthesis: A mixture of 5-amino-3-methylpyrazole (1 mmol), tetronic acid (1 mmol), triethylamine (0.05 mL) and a corresponding aldehyde (1 mmol) in EtOH (4 mL) was refluxed for 0.5–3 hours. The reaction mixture was allowed to cool to room temperatrure, the precipitated product was collected by filtration and washed with EtOH (3 mL). In most cases the product was very pure as judged by NMR analysis. When an impurity was present, the product was recrystallized from DMF/H2O. Selected Characterization Data: Compound 6a: 80%; mp 266–267 °C (DMF/H2O); 1H NMR (DMSO-d6) δ 11.90 (s, 1H), 10.13 (s, 1H), 6.47 (s, 2H), 4.84 (m, 3H), 3.68 (s, 9H), 1.91 (s, 3H); 13C NMR (DMSO-d6) δ 172.6, 160.6, 153.1, 147.6, 141.5, 136.7, 136.3, 105.4, 102.8, 96.1, 65.4, 60.5, 56.3, 35.3, 10.3. Anal. Calcd for C18H19N3O5: C, 60.50; H, 5.36; N, 11.76. Found: C, 60.33; H, 5.26; N, 11.53. Compound 6h: 75%; mp 287–289 °C (DMF/H2O); 1H NMR (DMSO-d6) δ 11.98 (s, 1H), 10.21 (s, 1H), 7.17–7.53 (m, 3H), 4.83 (m, 3H), 1.82 (s, 3H);13C NMR (DMSO-d6) δ 172.5, 160.8, 147.5, 146.8, 136.9, 131.2, 130.9, 130.1, 129.2, 128.7, 102.1, 95.4, 65.6, 34.3, 10.1. Anal. Calcd for C15H11Cl2N3O2: C, 53.59; H, 3.30; N, 12.50. Found: C, 53.33; H, 3.15; N, 12.37. Compound 6i: 76%; mp 281–283 °C (DMF/H2O); 1H NMR (DMSO-d6) δ 11.98 (s, 1H), 10.21 (s, 1H), 7.19–7.37 (m, 4H), 4.60 (m, 3H), 1.83 (s, 3H); 13C NMR (DMSO-d6) δ 172.5, 160.7, 148.5, 147.5, 136.8, 130.9, 129.6, 127.4, 122.1, 102.5, 95.7, 65.5, 39.5, 10.1. Anal. Calcd for C15H12BrN3O2: C, 52.04; H, 3.49; N, 12.14. Found: C, 52.26; H, 3.36; N, 12.09. Compound 6m: 76%; mp 268 –270 °C (DMF/H2O); 1H NMR (DMSO-d6) δ 11.96 (s, 1H), 10.16 (s, 1H), 6.97 (s, 1H), 6.82 (s, 1H), 4.86 (m, 3H), 3.86 (q, J = 7.1 Hz, 2H), 3.76 (s, 3H), 1.88 (s, 3H), 1.27 (t, J = 6.9 Hz, 3H);13C NMR (DMSO- d6) δ 172.8, 160.8, 153.5,147.9, 143.7, 143.1, 136.8, 123.3, 117.5, 112.7, 102.5, 95.7, 68.9, 65.5, 56.6, 34.6, 16.0, 10.2. Anal. Calcd for C18H18BrN3O4: C, 51.44; H, 4.32; N, 10.0. Found: C, 51.07; H, 4.11; N, 9.85.
- 11.Mosmann T. J. Immunol. Methods. 1983;65:55. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 12.Although the cell-killing effect of many analogues 6 is much more pronounced at 50 µM, the direct comparison of the cytotoxicity data is impeded by limited solubility of a number of these compounds at this concentration.
- 13.These data are consistent with the fact that average cytotoxicity of etoposide in the NCI in vitro 60 cell line screen is between two and three orders of magnitude lower than that of podophyllotoxin.
- 14.See for example, Pirali T, Busacca S, Beltrami L, Imovilli D, Paglai F, Miglio G, Massarotti A, Verotta L, Tron GC, Sorba G, Genazzani AA. J. Med. Chem. 2006;49:5372. doi: 10.1021/jm060621o.