Abstract
Over the past decade there has been a dramatic increase in referrals to specialty clinics, craniofacial centers, plastic surgeons, and neurosurgeons for assessment and treatment of deformational plagiocephaly (DP). Though considered a medically benign condition, preliminary reports suggest that DP may be associated with developmental problems. However, mechanisms to account for this association have not been hypothesized or empirically tested. Although treatment justifications often center on prevention of atypical appearance, little is known about the cosmetic outcomes of treated and untreated children. In this review we hypothesize different etiological pathways linking DP with neurodevelopment (e.g., environmental positioning limitations with and without underlying CNS pathology). We outline directions for research on incidence and prevalence, developmental outcomes, sex differences, determinants of treatment participation, and craniofacial appearance. Despite the paucity of existing research, preliminary findings suggest that children with this condition should be screened and monitored for developmental delays or deficits, as we await more conclusive information from future studies.
Index terms: plagiocephaly, neurodevelopment, “back to sleep”
Plagiocephaly is a broad term referring to cranial asymmetry.1 Plagiocephaly can result from the premature fusion of one or more cranial sutures (i.e., synostotic plagiocephaly or craniosynostosis), but is more commonly due to external forces shaping the infant’s skull, such as intra-uterine constraint, twinning, or invariant sleeping position.2–4 This is called “deformational plagiocephaly” (DP). Conditions that limit infants’ mobility, such as isolated torticollis, hypotonia, and cervical spine anomalies, are also associated with DP.2,5,6 “Positional plagiocephaly” is a more specific term, denoting external forces related to caregivers’ positioning of the infant during sleep or other activities. Prevalence estimates for DP vary widely, ranging from less than one percent to 48%.5,7–9 In part, this reflects a lack of accepted measurement procedures and diagnostic thresholds for differentiating normal variation from “plagiocephaly.” Although considered a minor and purely cosmetic condition by many clinicians, DP is of great concern to parents, who worry about the effect the condition might have on their child’s appearance, peer interactions, and development. As a result, DP referrals to pediatric plastic surgeons, neurosurgeons, and craniofacial centers have increased exponentially.10–13
Greater awareness of DP has been triggered by several factors including a dramatic increase in cases during the last decade, new technologies for measuring and modifying skull shape, and the American Academy of Pediatrics’ (AAP) “Back to Sleep” campaign.14 This campaign was initiated to reduce rates of sudden infant death syndrome (SIDS) by encouraging parents to position infants on their backs during sleep. The effort has been quite successful, resulting in changes in parenting practices and a corresponding decrease in SIDS in the United States.15–20 However, it is widely believed that an unintended “side effect” of this program is the exponential increase in the rate of DP.
There are few data on the effects of DP on infants’ neurodevelopment or other outcomes, perhaps because the condition has been considered so benign. However, recent studies of children with single-suture synostoses (once regarded as having no functional significance) indicate significantly elevated risk for developmental delays and subsequent learning disorders.21,22 These findings have prompted investigators to reconsider the neurodevelopmental implications of DP, with initial studies tentatively suggesting that children with DP also have elevated risk of developmental delays or deficits.23,24 Unfortunately, methodological problems limit the interpretation of these studies and the potential explanation for such findings are unclear: Could such deficits result from the plagiocephaly itself or neurological deficits unrelated to skull shape (e.g., muscle tone or motor development deficits/delays that predispose infants to maintain more static positions while sleeping)? Data on the cosmetic and social outcomes for children with DP are also quite limited, even though DP is thought of as a cosmetic condition and rationales for treatment often focus on “normalizing” children’s craniofacial appearance in order to prevent social stigma. At present, we know very little about how these children are perceived by objective raters, whether appearance normalizes over time with or without treatment, or how attractiveness ratings might relate to the severity of DP.
Further development of research and theory is needed to refine etiologic hypotheses and to provide clinicians and parents with accurate information regarding the association between plagiocephaly and infant development. Several factors indicate the need for rather immediate study of these issues including: the rapidly increasing rate of DP (and/or increasing awareness of and parental worry about the condition), the proliferation of associated medical treatments (helmets and craniofacial surgery), the implication of causal association with the “Back to Sleep” campaign, and the possibility of preventable developmental delays (e.g., through early identification and educational intervention). Moreover, there is concern that in the absence of clear-cut guidelines regarding DP, some parents will ignore the recommendations of the Back to Sleep program in an effort to prevent deformation of their infant’s skull.25
In this paper, we first provide an overview of the diagnosis, treatment, suspected pathogenesis, and prevalence of DP. This first section extends Rekate’s26 review of early research on the phenomenological aspects of DP, including several newer studies. In the second section of this review, we discuss critical issues pertaining to child development, including the association and possible causal linkages between DP and neurodevelopment, and the effects of parental concerns about facial appearance on initial identification. Given the paucity of research on these issues, we offer conceptual models and tentative hypotheses to guide future research. We conclude with a discussion of the clinical implications of these issues for developmental and behavioral practitioners.
DIAGNOSIS AND PATHOGENESIS
DP is a descriptive diagnosis that is potentially relevant for a very heterogeneous group of children with cranial asymmetry. The etiology, by definition, relates to pre- or post-natal “external forces” (i.e., surfaces that the fetus’/ infant’s head rests against) that are believed to mold and ultimately distort the shape of the infant’s skull.2 In utero, the fetus’ head can consistently rest against a hard surface, such as the maternal pubis, a uterine fibroid, or a twin.2,3 Other intrauterine factors that induce constraint and/or limit fetal mobility in utero include large fetal size, uterine anomalies, decreased amniotic fluid, and multiple gestation pregnancy; all of these factors have been associated with prenatal development of plagiocephaly.2–5,12,25 Once plagiocephaly is present, it may promote positional preference and/or induce neck muscle asymmetry.2,8 Conditions that restrict range of motion may contribute to the post-natal development of plagiocephaly. For example, cervical spine anomalies, muscular anomalies in the neck and shoulders, isolated torticollis, and generalized neurological or motor conditions can all restrict an infant’s ability to control his/ her head position and, therefore, elevate risk for DP (although as we detail below the causal sequencing of these factors is unclear).2,5,6,9 Premature infants have elevated risk for plagiocephaly, possibly due to limited mobility and/or inadequate bone mineralization.13,27 As we discuss in more detail below, there is emerging evidence that infants can develop DP as a result of forced positioning during sleep or other activities, and frequent exposure to devices that constrain mobility and/or force position, such as car seats.25
Initial Detection
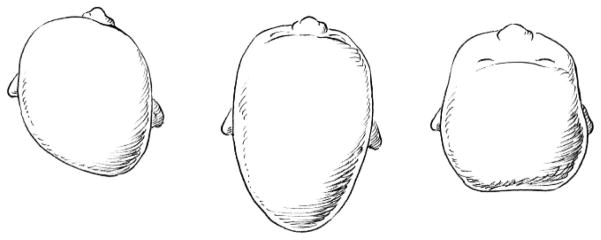
Parents and/or pediatricians typically notice an infant’s skull asymmetry at 2 to 3 months of age2,4 and diagnoses of DP are generally made by age 6 months.4,10,11 Typical manifestations include a parallelogram shaped skull, with flattening of the left or right occiput, ipsilateral frontal bossing, and contralateral occipital bulging.29,30 Other common presentations include brachycephaly (flattening of the central occiput) and dolichocephaly (flattening of both sides of the skull). Fig. 1 illustrates these skull shapes. Severity varies widely, ranging from mild flattening to notable asymmetry with secondary ipsilateral anterior ear displacement and/or mild mandibular and maxillary deformation.2,29–31 Currently, diagnosis is based upon visual inspection of the infant skull and radiographic evaluation (CT scans) as necessary to rule out synostosis.29,30 Various quantitative measurements of asymmetry have been described in the research literature,9,32,33 but until recently such measures had limited clinical utility due to the difficulty of obtaining accurate measurements, poor reliability, and/or undue expense of CT scans. Newer and less costly technologies are likely to change this, and researchers and many specialty clinics are now beginning to use three-dimensional surface imaging to calculate an index of cranial symmetry.33–35
FIGURE 1.
Top view of children’s skulls with various presentations of DP (from left to right: typical manifestation of DP, with flattening of the left occiput, ipsilateral bossing of the forehead, and contralateral occipital bulging; “dolichocephaly,” or bi-lateral skull flattening; and “brachycephaly,” or flattening of central occiput).
Prevalence and the “Back to Sleep” Program
Commonly cited incidence estimates for DP range from 0.3%7 to 48%9 in otherwise healthy infants. However, these figures are based on early studies that were not population-based and were limited in several respects (e.g., poorly defined diagnostic criteria). Well-controlled epidemiological research continues to be very limited, and a lack of widely agreed upon methods to establish asymmetry and define “caseness” makes it virtually impossible to make comparisons across studies. In one of the few population-based studies, Peitsch and colleagues8 investigated cranial asymmetry among otherwise healthy newborns, with results indicating that roughly 13% showed measurable evidence of posterior flattening of the skull shortly after birth (24 to 72 hours post-natal). Males were at greatest risk (2:1 male:female ratio), and variables such as primiparity, twinning, prolonged delivery, and use of assistive devices (forceps, vacuum extraction) conferred additional risk. Risk was not greater among any specific ethnic group. The authors propose that such initial skull flattening may lead to positional preference and become exacerbated over time, representing at least one pathway to DP in later childhood. In another population-based study conducted in the Netherlands by Boere-Boonekamp and Linden-Kuiper,5 the prevalence of DP was estimated at 9.9% in a sample of 7,609 infants ages 1–6 months. Among children with positional preference (i.e., head rotation to one side 75% of the time when in supine), 76% had visible occipital flattening and 34.8% had flattening of the forehead. In the controls, 4% had occipital flattening and 2% had frontal flattening. Unfortunately, as is often the case in this area of research, children were classified as having DP based solely on physicians’ visual inspection of the child’s head shape rather than objective measurements. As a result, it is impossible to determine how “cases” from this sample compare with those from other studies. In nearly all population-based and clinical samples, the prevalence of DP is significantly higher among males (1:5–1 to 3:1, male:female).5,6,8,12,25
Despite the difficulties in establishing the epidemiology of DP, there is broad consensus that diagnoses of DP have increased dramatically in the United States over the past 10 years, a trend that several authors have attributed to the AAP “Back to Sleep” campaign.10–13,36 This public health intervention was intended to reduce the incidence of sudden infant death syndrome (SIDS) by having parents routinely place infants on their backs during sleep rather than in a prone position, which was more common at the time. The intervention has been highly successful, leading to changes in parents’ infant positioning practices and a decrease in the rate of SIDS in the United States.15–20 Specifically, in 1992, 70% of parents reported placing their infant in the prone position for sleep; in 1996, the rate was reduced to 24%.19 Over this period, the incidence of SIDS decreased by roughly 40–50%.15–20 At the same time, the incidence of posterior DP increased exponentially.10–13,36 A similar trend is seen in other westernized countries that have implemented public health campaigns advocating supine sleep positioning (e.g., in the Netherlands5 and Australia6). Unfortunately, epidemiological studies from non-westernized societies are lacking, making it impossible to evaluate trends in cultures with other infant sleep-positioning practices (e.g., among those who swaddle their infants for sleep, or where prone sleep position continues to be more common).
Hutchinson et al.25 have published relatively compelling evidence suggesting that routine supine positioning is at least one causal factor in the development of DP. These authors conducted a case-control study examining various determinants of the condition. They found that parents of infants with plagiocephaly were more likely than controls to report supine positioning in their child’s first 6-weeks. In part, it appears that the association between positioning and DP may reflect parents’ over-interpretation of the AAP’s recommendations. That is, parents may have assumed that the prone position was to be avoided altogether during both sleep and waking periods.20 In addition to routine supine sleep positioning, Hutchinson et al. found that spending < 5-minutes per day in “tummy time” was a significant risk factor for DP.25
The observed increase in plagiocephaly diagnoses may also be attributed to increased awareness of the condition and the availability and promotion of previously unavailable treatments.26 Parents are naturally concerned about their infant’s craniofacial appearance and the possibility of abnormal or unattractive appearance in later life. Several web sites describing deformational and synostotic plagiocephaly refer to the social-psychological literature regarding the consequences of children’s abnormal or unattractive facial characteristics (see Langlois et al.,37 for a review), with the implication that adverse effects such as teasing, poor self-perception, and teacher bias are likely. Many of the same web sites also refer to dire neuropsychological consequences, presumably due to the effects of abnormal skull shape on brain development. As parents more often turn to the Internet for supplemental medical information and advice, they become increasingly vulnerable to these messages, with heightened scrutiny of their infant’s craniofacial appearance. It is likely that some parents are more vulnerable in this regard than others. Clinical observation suggests that a significant minority of parents seeking evaluations of plagiocephaly show elevated concerns about their infant’s health and appearance in general, perhaps related to their perceptions of responsibility for the problem (e.g., “I’ve distorted my baby’s head shape by the way I’ve put her to sleep”). There has been no empirical study of these issues and it is not known whether parents seeking evaluation are any more anxious or concerned than typical parents of neonates; nor whether parents of diagnosed infants who elect treatment are any different in this regard than those who decline. Regardless of the source of the observed increase in diagnosis and attention that plagiocephaly has received, pediatricians, neurosurgeons, pediatric plastic surgeons, and craniofacial centers have been deluged with requests for evaluations, prompting some craniofacial centers to develop specialized clinics. Some programs have found it necessary to hold “group screening days” in order to accommodate the growing number of requests for evaluations of DP.38
MEDICAL TREATMENT
Common treatments for DP include repositioning “coaching” for parents, combined with physical therapy and exercises to improve range of motion (generally used for younger infants).2,4,5,39,40 Specially designed orthotic helmets or bands are often used for infants 6-months or older, with more significant deformity.2,4,28,39,41 In both cases, the objective is to relieve pressure from the flattened region to allow the skull to “fill out” during brain growth. Given that most of the infant’s head growth takes place during the first year, early intervention is considered essential for optimal outcomes,42 although helmets are sometimes still used in the 12–18 month age range. Clarren et al.1 first described the use of orthotic helmets, and subsequent studies have documented improvements in skull shape with their use.2,28,41–43 However, controlled, randomized studies have not been conducted and there is some evidence that rates of improvement are comparable with repositioning and physical therapy, at least among infants with mild to moderate plagiocephaly.39,40 It is presumed that, without treatment, DP will resolve for some patients as they develop increasing mobility and brain growth helps to round their developing skull. Hair growth may also conceal cranial asymmetry.2,26 However, the extent to which the deformation changes (resolves or worsens) or becomes less notable with age is not known. There has been suspicion of adverse effects on dental malocclusion and difficulties with the temporomandibular joint. However, the comprehensive review of the literature by Rekate26 and our own literature search did not reveal any studies documenting these effects. Indeed, there are very few longitudinal studies documenting the course of untreated or treated DP.
ASSOCIATIONS BETWEEN PLAGIOCEPHALY AND NEURODEVELOPMENT
Although both deformational and synostotic plagiocephaly have long been recognized in the medical community, few studies have evaluated the neurodevelopmental sequelae of these disorders. In the late 1980’s, researchers began to seriously investigate the neurodevelopmental and behavioral correlates and outcomes of single-suture craniosynostosis and the issue has gained further attention over the past 10 years. For infants with DP, the condition has been viewed as conferring little or no risk aside from possible cosmetic problems and associated social-psychological risks (i.e., teasing and other forms of appearance-related social bias). Studies of neurodevelopmental outcomes for infants with DP have just begun to emerge. The results from these two lines of research are briefly reviewed below.
Single-suture Synostoses
Single-suture (or “isolated”) synostosis is the most common form of craniosynostosis,44 including fusions of the sagittal, metopic, left or right lambdoid sutures, and left or right coronal sutures. The incidence of any one of these suture fusions is about 1 in 2,000 live births, with sagittal fusions believed to be the most common.44,45 Genetics and environmental exposures (uterine constraint) have been implicated as causes for isolated craniosynostosis.46–49 For example, researchers are investigating mutations in fibroblast growth factor receptors 1–3, and TWIST, which have been found in some craniofacial syndromes and even in some samples of nonsyndromic, single-suture synostoses.47–49 Most cases of single-suture synostosis require a single surgery (cranioplasty) to release the fused suture and reshape the deformed calvaria.50 This surgery is preferentially performed within the first year of life in order to capitalize on the malleability of the infant’s skull–as well as the rapid growth of the infant’s brain–and to minimize secondary facial deformation.50,51 Speltz et al.52 reviewed studies of neurobehavioral outcomes and correlates in children with these disorders. Collectively, these studies examined over 1,000 infants, children, or adolescents with single-suture fusions, with most cases under 5 years at the time of assessment. The majority of studies have used standardized tests of cognition, language, or some other neuropsychological function; three studies21,53,54 used retrospective reviews of medical charts, including reports of previous standardized testing. Only 2 studies used a comparison (or control) group.55,56
Overall, findings suggest that isolated craniosynostosis is associated with a 3- to 5-fold increase in risk for cognitive deficits or learning/language disabilities,21,22,57 assuming a population base rate of roughly 10%.58 Case-control studies, however, have either revealed no differences55 or subtle differences in only a few specific neuropsychological domains (e.g., verbal reasoning and comprehension, auditory memory).56 No particular calvarial suture has yet been associated with a higher risk of problems, although this may primarily reflect a lack of statistical power in most reported comparisons of diagnostic subgroups (e.g., sagittal vs. metopic synostosis). These conclusions are tentative, given the several methodological limitations of this research (e.g., ascertainment factors, lack of control groups) and the age of the children studied—mostly infancy, toddlerhood, and preschool-age, developmental periods in which the reliable assessment of mild to moderate neurodevelopmental deficits is difficult. Several of these studies have also examined outcomes in relation to age of surgery, with the expectation of inverse correlation between age and developmental status, due to the effects of suture fusions on brain growth over time. The results of these quasi-experimental studies are equivocal, with most finding little relation between these two variables (e.g., Gewalli et al.59). Finally, it has been proposed that the genetic mechanisms implicated in the etiology of craniosynostosis (e.g., fibroblast growth factor receptors 1–3, TWIST) are associated with higher probability of developmental deficits or delays, a notion with preliminary support from animal (e.g., McDonald et al.60) and human studies (e.g., Johnson et al.61).
Deformational Plagiocephaly
As noted, DP has been considered a benign cosmetic condition with little interest in its neurobehavioral correlates. However, recently published findings have challenged this assumption. Miller and Clarren23 completed a chart review for 254 school-age patients who had been diagnosed with DP in the Craniofacial Center at Seattle Children’s Hospital when they were infants. Of the 181 families approached, 63 agreed to participate in a follow-up interview. Questions focused on whether children evidenced delays in infancy, academic delays, or need for special educational services. Twenty-five of the 63 children (39.6%) showed evidence of developmental delays. The siblings of these patients (who were not diagnosed with plagiocephaly) were used as controls, though they were not matched on gender or age. Miller and Clarren23 found that children with plagiocephaly were more likely to require special education services in school than their non-affected siblings (34.9% vs. 6.6%, respectively). Required services included speech therapy (n = 10), occupational therapy (n = 1), and physical therapy (n = 1). A significant sex difference emerged: only males were found to be more likely than their siblings to show developmental delays. Children whose DP occurred in the presence of other related pre-natal risk factors were at highest risk. Presence or absence of previous medical treatment (e.g. helmeting) for plagiocephaly did not affect the occurrence of problems.
Overall, the results of this study suggested that school-aged children with DP were at increased risk for developmental delays and required special education services more frequently than their non-affected siblings. This effect was only observed in males, and was most notable for those who manifested cranial asymmetry at birth. The study is limited in several respects. Because children’s development was not directly assessed, there are no data on the specific areas of deficit that prompted special education referral or the extent of deficits/delays observed. Moreover, as referral procedures can vary widely among school districts, referral bias and/or missed cases of neurodevelopmental problems are possible. It is also notable that the proportion of interviewed families among those receiving a diagnosis is relatively small (about 25%) and siblings were not matched on age or gender. Finally, the children from this sample had been diagnosed with DP between 1980 and 1991, before the introduction of the “Back to Sleep” campaign and the subsequent increase in rates of the condition. This raises the possibility of important cohort differences between this sample and more recently identified cases, which might be expected to influence the findings.
Panchal et al.24 examined neurodevelopment in infants with DP (n = 42) and single-suture craniosynostosis (n = 21). All infants were assessed using the mental development index (MDI) and psychomotor development index (PDI) of the Bayley Scales of Infant Development – Second Edition (BSID-II).62 Infants were tested at 10.9 and 8.4 months of age in the craniosynostosis and DP groups, respectively. Standard scores were used to compare infants with the normative sample. For children with craniosynostosis, PDI scores, but not MDI scores, were significantly lower than BSID-II test norms. None of these infants scored within the accelerated range of PDI scores and they were more likely than expected to score in the mildly to severely delayed range. Results from the DP sample are particularly intriguing. In this group, none of the infants scored within the accelerated range and they were more likely than expected to score within the mildly to severely delayed range on both the MDI and PDI. Subsequent analyses suggested that these findings were not accounted for by socioeconomic factors (using Medicaid coverage as an indicator of SES). These findings suggest that infants with DP are at increased risk for developmental delays in infancy, and the level of risk is comparable to or even greater than the risk level for infants with craniosynostosis. The study was limited by reliance on a single measure of neurodevelopment, lack of a control sample, and minimal description of cases, including clinical histories and risk factors for plagiocephaly. The authors did not report standard scores, therefore limiting comparisons with other child populations.
Hutchinson et al.25 asked mothers whether they perceived their children as being more or less active than other infants and whether they perceived any developmental delay. Relative to the mothers of control infants, these mothers were significantly more likely to describe their infants as being less active than usual during their first 6-weeks. These mothers were also more likely to report perceived developmental delays, particularly head lag problems (i.e., difficulty with head control) and delays in rolling over. This study has the advantage of including a control sample. However, given that investigation of neurodevelopment was not the primary objective of the study, these authors measured only maternal perceptions of infant activity and development, which may be biased by mothers’ awareness of their infants’ diagnostic status.
Taken together, the results of these studies very tentatively suggest that DP is associated with increased risk for developmental delay; however, a causal association should not be presumed. Although there may be adverse effects resulting from brain development in an asymmetric skull, it is also plausible that DP is merely a “marker” for other conditions that impede development (e.g., primary neuromuscular condition, environmental constraints; see below for discussion of possible pathways). Moreover, existing studies have several important limitations and there has not been a well-controlled study specifically designed to investigate neurodevelopmental outcomes for children with DP.
PLAGIOCEPHALY: CAUSE OR CORRELATE OF COGNITIVE DELAYS OR DEFICITS?
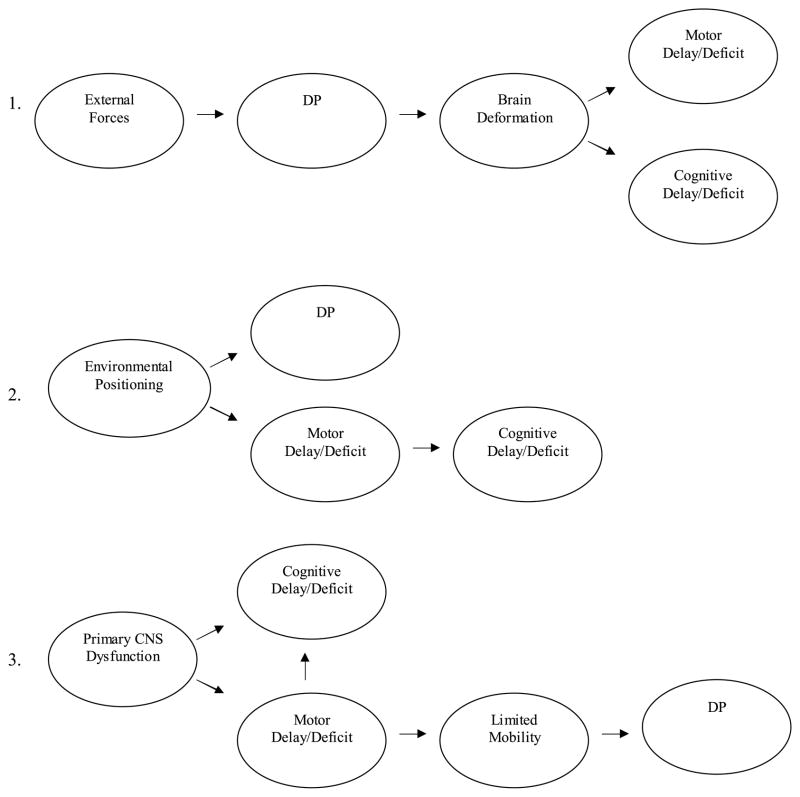
The preceding studies indicate an association between single-suture synostoses and neurodevelopment, and suggest the possibility of association between DP and neurodevelopment. However, in neither case do we know whether there are causal relations between skull deformity and neurodevelopment. There has been extensive discussion and debate about the possible linkages between single-suture synostoses and brain development, as well as the role that cranioplasty might play in the prevention or amelioration of neurodevelopmental delays or deficits.21,59,63,64 However, to date, little has been written about these issues with respect to DP and associated treatments. At least three “main effect” pathways, with varying degrees of plausibility, can be discerned with respect to the possible link between DP and neurodevelopment. These are not considered mutually exclusive and, as discussed below, multi-factorial combinations are a distinct possibility. The pathways, shown in Fig. 2 and described below, consider the separate and interrelated development of motor and cognitive abilities.
FIGURE 2.
Proposed developmental pathways accounting for association between DP and developmental.
“External forces” leading to skull deformation and ultimately, brain deformation. As presumed by some early studies of isolated craniosynostosis and current media accessible to parents, brain growth in an abnormally shaped skull (e.g., craniosynostosis or DP) could lead to structural abnormalities that are manifested by measurable developmental delays or deficits. Developmental problems are viewed in this pathway as directly related to DP, which itself could result from any one or more of the biomechanical or environmental factors described above–in this pathway, no particular “external force” is central to the hypothesis. Relevant to this pathway are neuroimaging studies showing cortical and subcortical abnormalities in cases of synostotic plagiocephaly.51,65–67 For example, Aldridge et al.65 using 3-D MRI found differences in subcortical morphology between infants with and without unilateral coronal synostosis, which is characterized by plagiocephalic head shape. Specifically, the anterior lateral ventricle on the “synostosed” (affected) side was displaced toward the midline and the anterior corpus callosum was compressed. Although it is theoretically possible that severe cases of DP could show similar subcortical dysmorphology, to our knowledge there is no supporting evidence. Moreover, MRI data involving cases of isolated sagittal and metopic synostosis indicate that many of the observed cortical and subcortical abnormalities found in these cases were not related in any obvious way to the shape of the synostosed skull.66 This observation leaves open the possibility that CNS dysfunction precedes or even causes cranial abnormalities such as plagiocephaly, as described in pathway 3 below.
Environmental positioning limitations leading to both DP and motor deficits. Post-natal environmental constraints – presumably imposed by caregivers –could independently produce DP and motor delays or deficits. For example, over-use of car seats or baby swings, or invariant sleep positioning could alter head shape and significantly limit an infant’s opportunities for motor development. Given the highly integrated and reciprocal development of cognition and motor skills in early infancy, such constraints on mobility could lead to delays or deficits in the development of “motor-driven” cognitive functions as well (e.g., cause and effect reasoning).68 In this pathway, DP is viewed as causally unrelated to development. In support of this hypothesis, several studies have shown that parent-reported sleep positioning and encouragement of awake time in prone are positively related to the infants’ motor development.69–72
CNS pathology leading to DP. In this pathway, CNS pathology is the primary casual agent, leading to specific neuromuscular deficits (e.g., torticollis, hypo- or hypertonia) and associated limitations in infant mobility and infant preference for static positions. Limitations in mobility and positioning would in turn elevate the risk of DP. Although environmental positioning contributes to skull deformation in this pathway, it is seen as having little if any causal effect on development. More specifically, cognitive delays or deficits in this model could result directly from CNS pathology and/or indirectly, as mediated by delay or deficits in motor development. Similarly, in a closely related variant of this pathway, CNS pathology might be seen as directly contributing to DP, without the mediating effect of motor delays or deficits. Support for this idea comes from animal studies finding that the CNS can influence skull shape by way of its physical and developmental connections with the intermediate dura.66,73
At present, pathways 2 and 3, in which DP is conceptualized as a correlate or “marker” of neurodevelopmental problems but not a cause, seem the most plausible. The combination of pathways 2 and 3 is also possible, in which primary CNS dysfunction and environmental positioning limitations contribute to DP and associated motor delays or deficits. And, as noted by Aldridge et al.,59 causal pathways may differ among affected individuals, depending upon genotype, developmental timing of specific events, and occurrence of specific environmental and biomechanical events. Further complicating our understanding of these pathways are the anticipated effects of neural plasticity, compensatory processes, behavioral adaptation, and environmental factors unrelated to positioning, all of which are known to moderate the effects of prenatal and postnatal/environmental CNS insults on neurobehavioral outcomes.74–76
Summary
We do not yet understand nor have a sound basis for making hypotheses about the specific mechanisms that link DP with neurobehavioral development. The direction of basic causal pathways is yet to be even tentatively established (i.e., do motor and other neurodevelopmental deficits lead to or follow from skull deformation?). Although there is the basis for hypothesizing a positive linear relation between plagiocephaly and neurobehavioral outcome, hypotheses regarding how or why the two may be linked are extremely tentative. Nonetheless, these pathways offer testable predictions for future research. For example, support for pathway 3 (primary CNS pathology) would be provided if cognitive deficits were observed among infants with both DP and diagnosable neuromuscular problems, but not among infants with only DP. Pathway 1 might be supported by (a) robust correlations between degree of cranial asymmetry and development, and (b) an association between treatment to address asymmetry (i.e., orthotic helmets) and later developmental outcomes. In other words, we would anticipate worse outcomes for those children with more severe asymmetry and there may be some developmental benefit of efforts to correct asymmetry early in the course of development. Finally, pathway 2 would be supported by findings of minimal differences between infants with and without DP when controlling for relevant contextual factors associated with environmental positioning (e.g., awake time in prone, amount of sleep time in supine, etc.).
Testing these hypotheses will require further precision in measurement strategies. For example, the operationalization of “asymmetry” has been a challenge in prior studies and, in clinical practice, often relies on clinician judgment. Newer measures, such as surface imaging technologies, are likely to result in more reliable and precise quantitative measurement. For example, the Starscanner™ and the 3dMD three-dimensional camera are devices that can generate in a matter of seconds a three-dimensional “wire mesh” representation of the skull, from which continuous measures of cranial asymmetry and other quantitative data can be derived with appropriate software.34
In addition to the broad developmental measures that have been used in previous research (e.g., the BSID-II), assessment strategies emerging from the developmental and experimental psychology literature are likely to be useful in identifying specific cognitive skills in young children that might be influenced by DP (e.g., Diamond et al.77). Ideally, these skills would be assessed at multiple time points to assess both cognitive functioning at a given point in time and developmental trends. Finally, the use of neuroimaging technology, such as MRI, may be used to assess underlying structural CNS pathology that precedes or follows from DP. A comparable approach has been used with children having craniosynostosis,65 and is likely to prove informative for those with DP.
DP AND PARENTAL CONCERNS ABOUT FACIAL APPEARANCE
In addition to distortion of skull shape, the facial appearance of children with DP may be affected adversely by secondary ear displacement and/or mandibular and maxillary deformations.26,31 Even in the absence of obvious facial differences, children’s overall attractiveness may be compromised by plagiocephalic head shape. Not surprisingly, one of the most frequently reported parental concerns regarding DP relates to the child’s craniofacial appearance and the possibility that he or she will be teased, embarrassed, or otherwise stigmatized because of the condition.78 Parents of infants with plagiocephaly often comment that their child will “hold them responsible” for how they look, which is often the primary motivation for seeking treatment.78
Studies of typical children have clearly demonstrated that facial “attractiveness” has significant impact on the perceptions and behaviors of adult caregivers,79–81 interactions with peers,82,83 and teachers’ perceptions of competence and aptitude.84,85 Nurses in the nursery and mothers of newborns have been observed to spend more time attending to typical infants with more attractive facial features.86 In research involving craniofacial populations, Speltz and colleagues87,88 have found that adult raters are adept at detecting even subtle facial anomalies in infants/toddlers and adjusting their attractiveness ratings accordingly. Specifically, in a sample of young children with cleft lip and palate (CLP), cleft palate only (CPO), and typical children, adults – not surprisingly – consistently rated those with CLP as less attractive than other groups. However, children with CPO, who have no visible disfigurement (such as cleft lip), were also rated as significantly less attractive than control children. Presumably raters were able to detect the very subtle facial anomalies associated with isolated cleft palate (mid-face retrusion, asymmetry).
Despite the high level of parent concern regarding the craniofacial appearance of children with DP, this aspect of the condition has received little attention by researchers. We are aware of only one study that examined attractiveness in very low birth weight (VLBW) infants with “post-natal headmolding,” or dolichocephaly (see Fig. 1).89 The authors evaluated attractiveness in three groups: VLBW infants with head molding, VLBW infants without the condition, and full-term newborns without bi-parietal narrowing of the skull. Raters included mothers of VLBW infants and mothers of full-term infants. Both groups of mothers rated infants with skull flattening as less “cute” than other infants. As might be expected, the mothers of VLBW infants rated those with skull flattening as more attractive than did the mothers of full-term infants.
Although speculative, these findings and the more general literature on facial appearance suggest that DP could affect a child’s attractiveness and have some yet undetermined effect on social responses and ultimately, the child’s psychological development. In our clinical work and pilot research, parents frequently report undesirable frontal facial appearance in their own infants with plagiocephaly. Whether such characteristics are noticeable by “judges” unfamiliar with the infant is unknown, an important factor in trying to gauge the potential vulnerability of plagiocephaly-related appearance to negative social responses.
DIRECTIONS FOR FUTURE RESEARCH
Empirical data are needed to guide clinicians’ diagnosis and treatment practices, and to better inform parents about the prognosis and associated features of DP.
Prospective Longitudinal Studies
Prospective, population-based epidemiological studies are particularly needed to establish the incidence and prevalence of DP. These studies are likely to be advanced by the time efficient surface imaging technologies described earlier, which could be used to develop normative data with non-referred infants. This would allow for further precision regarding the operational definition of “cases” across studies. Prospective studies can also provide information about the course of DP over time. Longitudinal data can address questions such as “when is the condition likely to resolve without intervention or using non-intrusive interventions, such as repositioning?”
Developmental Outcomes
Research investigating the association between DP and developmental outcomes is also needed, with only three previous studies of this issue to our knowledge.23–25 The primary question for this research is “does DP serve as a biological marker of subsequent developmental delays or persisting deficits, regardless of whether it exerts any causal effect on development?” Longitudinal studies that utilize well-matched control groups and objectively assess multiple developmental functions (e.g., cognition, learning, memory, motor skill, executive functioning, language, and behavioral adaptation) will provide the best answers to this question. Such studies could be combined with the prospective epidemiological research described above, assessing children’s development prior to and following the onset of DP to determine which children are at greatest risk for developing the condition and whether observable delays precede or follow the condition. Comparisons of DP cases with test norms (instead of control groups) are inadequate because they do not control for the many factors that bias the ascertainment of clinical samples (e.g., family social status, parent anxiety, provider variables). Several other potential confounds in the comparison of DP cases and controls would need to be examined including various prenatal exposures and environmental hazards, parent IQ, quality of care, infant positioning factors (e.g., time spent in supine), elective treatments for DP cases (e.g., helmet or band therapy), and special interventions to enhance development in either cases or controls (e.g., early intervention programs).
Given the reported prevalence of cranial asymmetry among otherwise healthy children, it will be necessary to rule out cases of undiagnosed plagiocephaly among controls and to clarify the threshold at which deformation (as quantified by, for example, surface imaging) is associated with developmental delay or deficit. Ideally, future research would combine standardized assessment of development, surface imaging, and neuroimaging to clarify the relation among functional impairment, skull asymmetry, and CNS pathology.
Sex Differences
Given the preponderance of males among ascertained cases of DP, analysis of sex differences and interaction between child sex and diagnostic status (DP vs. controls) is important in determining whether sex is uniquely associated with DP or more generally related to developmental course. Males have shown elevated risk of delayed development in a variety of high-risk samples (e.g., prematurity; drug exposure, high social risk) and it is possible that DP serves as a biologic marker of heightened developmental risk for males only.90
Determinants of Treatment Participation
Studies are needed to determine the factors most influential in parents’ decisions to pursue elective treatment for DP (e.g., helmet or band therapy). In our clinical experience and pilot work, roughly half of parents given the option of elective treatment choose to follow up. Severity of DP does not always appear to be the primary determining factor, with parent characteristics possibly contributing as well (e.g., parental anxiety about the child’s future, as affected by background stress). There has been no study of this issue to our knowledge, and it would be possible to examine the relative contribution of cranial asymmetry and parenting variables to treatment participation.
Craniofacial Appearance
Finally, as suggested above, studies regarding the association between objectively measured cranial asymmetry and subjective ratings of craniofacial appearance – including general attractiveness and frontal facial attractiveness –are needed to determine the validity of parents’ concerns in this regard. Key questions include: To what extent does skull shape affect measurable frontal facial features in children with varying severity of DP? Does skull shape contribute to social perceptions of “attractiveness” or is facial appearance the only significant determinant? To what extent does attractiveness normalize over the course of development with and without treatment?
IMPLICATIONS FOR CLINICAL CARE
The American Academy of Pediatrics (AAP) has provided a number of recommendations regarding the prevention and medical management of DP, including infant positioning strategies, mechanical adjustments, exercises, and use of orthotic helmets.36 However, the AAP’s recommendations have not specifically considered the neurodevelopmental implications of a positive DP diagnosis, presumably due to limited knowledge of neurodevelopmental correlates and outcomes. Developmental and behavioral pediatricians are in a unique position to provide consultation to colleagues in other disciplines/specialty areas, educate concerned parents, and participate in multi-disciplinary screening and assessments of these high risk children. Given emerging data suggestive of at least mild neurodevelopmental problems among infants with DP, and the fact that early detection and educational and psychomotor interventions are highly successful in preventing or reducing the impact of developmental delays or deficits,91 infants meeting diagnostic criteria for DP should be routinely screened and monitored for neurodevelopmental problems. The known association between neuromuscular disorders (e.g., limited range of motion, hypotonia) and DP indicate that it is especially important to screen and monitor motor skill development. When indicated, referral for physical or occupational therapy may not only help to minimize cranial deformation, but may also facilitate other areas of development, including cognitive ability.
SUMMARY AND CONCLUSIONS
The observed increase in DP likely reflects a combination of factors including the AAP’s “Back to Sleep” campaign, increased public awareness and sensitivity to infant skull shapes, and the continuing development of treatment alternatives. Although the condition has been considered relatively benign from medical and developmental perspectives, parents often report concern and show a great deal of interest in treatment options, raising the possibility that some parents may choose to ignore the recommendation for supine sleep positioning, despite the documented effectiveness of this public health intervention in preventing SIDS. The ambiguities of the diagnosis, combined with the availability of information (and misinformation) regarding potential adverse outcomes, are likely to heighten parents’ concern. Both of these factors suggest the need for further research on the phenomenology of DP and associated developmental outcomes.
Acknowledgments
The author wishes to thank Ray Sze, M.D., for his help with the illustration used in Figure 1.
References
- 1.Clarren SK, Smith DW, Hanson JW. Helmet treatment for plagiocephaly and congenital torticollis. J Pediatr. 1979;94:43–46. doi: 10.1016/s0022-3476(79)80347-9. [DOI] [PubMed] [Google Scholar]
- 2.Clarren SK. Plagiocephaly and torticollis: Etiology, natural history, and helmet treatment. J Pediatr. 1981;98:92–95. doi: 10.1016/s0022-3476(81)80549-5. [DOI] [PubMed] [Google Scholar]
- 3.Littlefield TR, Kelly KM, Pomatto JK, Beals SP. Multiple-birth infants at higher risk for development of deformational plagiocephaly. Pediatrics. 1999;103:565–569. doi: 10.1542/peds.103.3.565. [DOI] [PubMed] [Google Scholar]
- 4.Pollack IA, Losken HW, Fasick P. Diagnosis and management of posterior plagiocephaly. Pediatrics. 1997;99:180–185. doi: 10.1542/peds.99.2.180. [DOI] [PubMed] [Google Scholar]
- 5.Boere-Boonekamp MM, Linden-Kuiper LT. Positional preference: Prevalence in infants and follow-up after two years. Pediatrics. 2001;107:339–343. doi: 10.1542/peds.107.2.339. [DOI] [PubMed] [Google Scholar]
- 6.David DJ, Menard RM. Occipital plagiocephaly. Br J Plastic Surg. 2000;53:367–377. doi: 10.1054/bjps.2000.3329. [DOI] [PubMed] [Google Scholar]
- 7.Dunn PM. Congenital postural deformities. Br Med Bull. 1976;32:71–76. doi: 10.1093/oxfordjournals.bmb.a071327. [DOI] [PubMed] [Google Scholar]
- 8.Peitsch WK, Keefer CH, LaBrie RA, et al. Incidence of cranial asymmetry in healthy newborns. Pediatrics. 2002;110:E72. doi: 10.1542/peds.110.6.e72. [DOI] [PubMed] [Google Scholar]
- 9.Watson GH. Relation between side of plagiocephaly, dislocation of hip, scoliosis, bat ears, and sternomastoid tumors. Arch Dis Child. 1971;46:203–210. doi: 10.1136/adc.46.246.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Argenta LC, David LR, Wilson J, et al. An increase in infant cranial deformity with supine sleep positioning. J Craniofac Surg. 1996;7:5–11. doi: 10.1097/00001665-199601000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Kane AA, Mitchell LE, Craven KP, et al. Observations on a recent increase in plagiocephaly without synostosis. Pediatrics. 1996;97:877–885. [PubMed] [Google Scholar]
- 12.Mulliken JB, Van Der Woude DL, Hansen M, et al. Analysis of posterior plagiocephaly: Deformational versus synostotic. Plast Reconstr Surg. 1999;103:371–380. doi: 10.1097/00006534-199902000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Turk AE, McCarthy JG, Thorne CH, et al. The “back to sleep campaign” and DP: is there cause for concern? J Craniofac Surg. 1996;7:12–18. doi: 10.1097/00001665-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 14.American Academy of Pediatrics Task Force on Infant Positioning and SIDS. Pediatrics. 1992;89:1120–1126. [PubMed] [Google Scholar]
- 15.Pollack HA, Frohna JG. Infant sleep placement after the Back to Sleep campaign. Pediatrics. 2002;109:608–614. doi: 10.1542/peds.109.4.608. [DOI] [PubMed] [Google Scholar]
- 16.Spiers PS, Guntheroth WG. Recommendations to avoid the prone sleeping position and recent statistics for sudden infant death syndrome in the United States. Arch Pediatr Adolesc Med. 1994;148:141–146. doi: 10.1001/archpedi.1994.02170020027004. [DOI] [PubMed] [Google Scholar]
- 17.Willinger M, Hoffman HJ, Hartford RB. Infant sleep position and risk for sudden infant death syndrome: Report of meeting held January 1994, National Institutes of Health, Bethesda, MD. Pediatrics. 1994;93:814–819. [PubMed] [Google Scholar]
- 18.Willinger M, Hoffman HJ, Kuo-Tsung W, et al. Factors associated with the transition to nonprone sleep positions of infants in the United States. JAMA. 1998;280:329–335. doi: 10.1001/jama.280.4.329. [DOI] [PubMed] [Google Scholar]
- 19.American Academy of Pediatrics Task Force on Infant Positioning and SIDS. Positioning and sudden infant death syndrome (SIDS): Update. Pediatrics. 1996;98:1216–1218. [PubMed] [Google Scholar]
- 20.Task Force on Infant Sleep Position and Sudden Infant Death Syndrome. Changing concepts of sudden infant death syndrome: Implications for infant sleeping environment and sleep position. Pediatrics. 2000;105:650–656. doi: 10.1542/peds.105.3.650. [DOI] [PubMed] [Google Scholar]
- 21.Magge SN, Westerveld M, Pruzinsky T. Long-term neuropsychological effect of sagittal craniosynostosis on child development. J Craniofac Surg. 2002;13:99–104. doi: 10.1097/00001665-200201000-00023. [DOI] [PubMed] [Google Scholar]
- 22.Shipster C, Hearst D, Somerville A, Stackhouse J, Hayward R, Wade A. Speech, language, and cognitive development in children with isolated sagittal synostosis. Devel Med Child Neurol. 2003;45:34–43. [PubMed] [Google Scholar]
- 23.Miller RI, Clarren SK. Long-term developmental outcomes in patients with DP. Pediatrics. 2000;105:e26. doi: 10.1542/peds.105.2.e26. [DOI] [PubMed] [Google Scholar]
- 24.Panchal J, Amirsheybani H, Gurwitch R. Neurodevelopment in children with single-suture craniosynostosis and plagiocephaly without synostosis. Plast Reconstr Surg. 2001;108:1492–1500. doi: 10.1097/00006534-200111000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Hutchinson BL, Thompson JMD, Mitchell EA. Determinants of nonsynostotic plagiocephaly: A case-controlled study. Pediatrics. 2003;112:e316–e322. doi: 10.1542/peds.112.4.e316. [DOI] [PubMed] [Google Scholar]
- 26.Rekate HL. Occipital plagiocephaly: A critical review of the literature. J Neurosurg. 1998;89:24–30. doi: 10.3171/jns.1998.89.1.0024. [DOI] [PubMed] [Google Scholar]
- 27.Baum JD, Searles D. Head shape and size of pre-term low-birth-weight infants. Dev Med Child Neurol. 1971;13:576–581. doi: 10.1111/j.1469-8749.1971.tb08320.x. [DOI] [PubMed] [Google Scholar]
- 28.Teichbraeber JF, Ault JK, Baumgartner J, et al. Deformational posterior plagiocephaly: Diagnosis and treatment. Cleft Palate Craniofac J. 2002;39:582–586. doi: 10.1597/1545-1569_2002_039_0582_dppdat_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- 29.Huang MH, Gruss JS, Clarren SK. The differential diagnosis of posterior plagiocephaly: true lambdoid synostosis versus positional molding. Plast Reconstr Surg. 1996;98:765–774. doi: 10.1097/00006534-199610000-00001. discussion 775–776. [DOI] [PubMed] [Google Scholar]
- 30.Ehret FW, Whelan MF, Ellenbogen RG, Cunningham ML, Gruss JS. Differential diagnosis of the trapezoid-shaped head. Cleft Palate Craniofac J. 2004;41:13–19. doi: 10.1597/02-053. [DOI] [PubMed] [Google Scholar]
- 31.St John D, Mulliken JB, Kaban LB, et al. Anthropometric analysis of mandibular asymmetry in infants with deformational posterior plagiocephaly. J Oral Maxillofacial Surg. 2002;60:873–877. doi: 10.1053/joms.2002.33855. [DOI] [PubMed] [Google Scholar]
- 32.Glat PM, Freund RM, Spector JA, et al. A classification of plagiocephaly utilizing a three-dimensional computer analysis of cranial base landmarks. Ann Plast Surg. 1996;36:469–474. doi: 10.1097/00000637-199605000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Lo LJ, Marsh JL, Pilgram TK, et al. Plagiocephaly: Differential diagnosis based on endocranial morphology. Plast Reconstr Surg. 1996;97:282–291. doi: 10.1097/00006534-199602000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Littlefield TR, Kelly K, Cherney J, Beals SP, Pomatto JK. Development of a new three-dimensional cranial imaging system. J Craniofac Surg. 2004;15:175–181. doi: 10.1097/00001665-200401000-00042. [DOI] [PubMed] [Google Scholar]
- 35.Zonenshayn M, Kronberg E, Souweidane MM. J Neurosurg Spine. 2004;100:537–540. doi: 10.3171/ped.2004.100.5.0537. [DOI] [PubMed] [Google Scholar]
- 36.Persing J, James H, Swanson J Committee on Practice and Ambulatory Medicine; Section on Plastic Surgery; and Section on Neurological Surgery. Prevention and management of positional skull deformation in infants. Pediatrics. 2003;112:199–202. doi: 10.1542/peds.112.1.199. [DOI] [PubMed] [Google Scholar]
- 37.Langlois JH, Kalakanis L, Rubenstein AJ, et al. Maxims or myths of beauty: A meta-analytic and theoretical review. Psych Bull. 2000;126:390–423. doi: 10.1037/0033-2909.126.3.390. [DOI] [PubMed] [Google Scholar]
- 38.Hozjan I, Forrest C, Phillips J, et al. Positional plagiocephaly epidemic in infants: A novel approach to timely management. Paper presented at the Annual Meeting of the American Cleft Palate-Craniofacial Association; Greensboro, NC. March 2003. [Google Scholar]
- 39.Moss SD. Nonsurgical, nonorthotic treatment of occipital plagiocephaly: What is the natural history of the misshapen head? J Neurosurg. 1997;87:667–670. doi: 10.3171/jns.1997.87.5.0667. [DOI] [PubMed] [Google Scholar]
- 40.Hellbusch JL, Hellbusch LC, Bruneteau RJ. Active counterpositioning treatment of deformational occipital plagiocephaly. Nebr Med J. 1995;80:344–349. [PubMed] [Google Scholar]
- 41.Ripley CE, Pomatto J, Beals SP, et al. Treatment of positional plagiocephaly with dynamic orthotic cranioplasty. J Craniofac Surg. 1994;5:150–159. doi: 10.1097/00001665-199407000-00003. [DOI] [PubMed] [Google Scholar]
- 42.Kelly KM, Littlefield TR, Pomatto JK, et al. Importance of early recognition and treatment of DP with orthotic cranioplasty. Cleft Palate Craniofac J. 1999;36:127–130. doi: 10.1597/1545-1569_1999_036_0127_ioerat_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 43.Bruner TW, David LR, Gage HD, Argenta LC. Objective outcome analysis of soft shell helmet therapy in the treatment of deformational plagiocephaly. J Craniofac Surg. 2004;15:643–650. doi: 10.1097/00001665-200407000-00022. [DOI] [PubMed] [Google Scholar]
- 44.Singer S, Bower C, Southall P, et al. Craniosynostosis in Western Australia, 1980–1994: A population-based study. Am J Med Gen. 1999;83:382–387. doi: 10.1002/(sici)1096-8628(19990423)83:5<382::aid-ajmg8>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 45.Shuper A, Merlob P, Grunebaum M, et al. The incidence of isolated craniosynostosis in the newborn infant. Am J Dis Child. 1985;139:85–86. doi: 10.1001/archpedi.1985.02140030091038. [DOI] [PubMed] [Google Scholar]
- 46.Zeiger JS, Beaty TH, Hetmanski JB, Wang H, Scott AF, Kasch L, Raymond G, Jabs EW, VanderKolk C. Genetic and environmental risk factors for Sagittal Craniosynostosis. J Craniofacial Surg. 2002;13:602–606. doi: 10.1097/00001665-200209000-00002. [DOI] [PubMed] [Google Scholar]
- 47.Gripp KW, McDonald-McGinn DM, Gaudenz K, et al. Identification of a genetic cause for isolated unilateral coronal synostosis: A unique mutation in the fibroblast growth factor receptor 3. J Pediatr. 1998;132:714–716. doi: 10.1016/s0022-3476(98)70366-x. [DOI] [PubMed] [Google Scholar]
- 48.Moloney DM, Wall SA, Ashworth GJ, et al. Prevalence of Pro250Arg mutation of fibroblast growth factor receptor 3 in coronal Craniosynostosis. Lancet. 1997;349:1059–1062. doi: 10.1016/s0140-6736(96)09082-4. [DOI] [PubMed] [Google Scholar]
- 49.Muenke M, Gripp KW, McDonald-McGinn DM, et al. A unique point mutation in the fibroblast growth factor receptor 3 gene (FGFR3) defines a new craniosynostosis syndrome. Am J Hum Genet. 1997;60:555–564. [PMC free article] [PubMed] [Google Scholar]
- 50.Marsh JL, Jenny A, Galic M, et al. Surgical management of sagittal synostosis. A quantitative evaluation of two techniques. Neurosurg Clin North Am. 1991;2:629–640. [PubMed] [Google Scholar]
- 51.Marsh JL, Vannier MW. Cranial base changes following surgical treatment of craniosynostosis. Cleft Palate J. 1986;23(Suppl1):9–18. [PubMed] [Google Scholar]
- 52.Speltz ML, Kapp-Simon KA, Cunningham ML, et al. Single-suture craniosynostosis: A review of neurobehavioral research and theory. J Pediatr Psychol. 2004;29:651–668. doi: 10.1093/jpepsy/jsh068. [DOI] [PubMed] [Google Scholar]
- 53.Rozelle A, Marty-Grames L, Marsh JL. Speech-language disorders in nonsyndromic sagittal synostosis. Paper presented at the Annual American Cleft Palate-Craniofacial Association Conference; Tampa, FL. April 1995. [Google Scholar]
- 54.Sidoti EJ, Marsh JL, Marty-Grames L, et al. Long-term studies of metopic synostosis: frequency of cognitive impairment and behavioral disturbances. Plast Reconstr Surg. 1996;97:276–281. doi: 10.1097/00006534-199602000-00002. [DOI] [PubMed] [Google Scholar]
- 55.Speltz ML, Endriga MC, Mouradian WE. Presurgical and postsurgical mental and psychomotor development of infants with sagittal synostosis. Cleft Palate Craniofac J. 1997;34:374–379. doi: 10.1597/1545-1569_1997_034_0374_papmap_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 56.Virtanen R, Korhonen T, Fagerholm J, et al. Neurocognitive sequelae of scaphocephaly. Pediatrics. 1999;103:791–795. doi: 10.1542/peds.103.4.791. [DOI] [PubMed] [Google Scholar]
- 57.Kapp-Simon K, Speltz ML, Leroux B, et al. The Infant Learning Project: A Multi-site, Multi-disciplinary Study of Neurobehavioral Development in Isolated Synostosis. Paper presented at the Annual American Cleft Palate-Craniofacial Association Conference; Chicago, IL. March 2004. [Google Scholar]
- 58.Nietzel MT, Speltz ML, McCauley EA, Bernstein DA. Abnormal Psychology. Needham Heights, MA: Allyn and Bacon; 1998. [Google Scholar]
- 59.Gewalli F, Guimaraes-Ferreira JP, Sahlin P, et al. Mental development after modified pi procedure: Dynamic cranioplasty for sagittal synostosis. Ann Plast Surg. 2001;46:415–420. doi: 10.1097/00000637-200104000-00011. [DOI] [PubMed] [Google Scholar]
- 60.McDonald MP, Miller KM, Li C, Deng C, Crawley JN. Motor deficits in fibroblast growth factor receptor-3 null mutant mice. Behav Pharmacol. 2001;12:477–486. doi: 10.1097/00008877-200111000-00009. [DOI] [PubMed] [Google Scholar]
- 61.Johnson D, Wall SA, Mann S, Wilkie AO. A novel mutation, A1a315Ser, in FGFR2: A gene-environment interaction leading to craniosynostosis? Eur J Hum Genet. 2000;8:571–577. doi: 10.1038/sj.ejhg.5200499. [DOI] [PubMed] [Google Scholar]
- 62.Bayley N. The Bayley Scales of Infant Development. 2. San Antonio, TX: The Psychological Corporation; 1993. [Google Scholar]
- 63.Kapp-Simon KA, Figueroa A, Jocher C, et al. Longitudinal assessment of mental development of infants with nonsyndromic craniosynostosis with and without cranial release and reconstruction. Plast Reconstruc Surg. 1993;92:831–839. [PubMed] [Google Scholar]
- 64.Bottero L, Lajuenie E, Arnaud E, et al. Functional outcome after surgery for trignocephaly. Plast Reconstruc Surg. 1998;102:952–928. [PubMed] [Google Scholar]
- 65.Aldridge K, Marsh JL, Perlyn CA, Richmeister JT. Quantification of central nervous system dysmorphology in isolated craniosynostoses. Paper presented at the annual meeting of the American Cleft Palate-Craniofacial Association; Minneapolis, MN. April 2001. [Google Scholar]
- 66.Aldridge K, Marsh J, Govier D, Richmeister JT. Central nervous system phenotypes in craniosynostosis. J Anat. 2002;201(1):31–39. doi: 10.1046/j.1469-7580.2002.00074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fernbach SK, Feinstein KA. Radiologic evaluation of the child with craniosynostosis. Neurosurg Clin North Am. 1991;2:569–585. [PubMed] [Google Scholar]
- 68.Bremner JG. Motor abilities as causal agents in infant cognitive development. In: Savelsbergh G, editor. Advances in Psychology: The Development of Coordination in Infancy. Vol. 97. San Diego, CA: Elseviers Science; 1993. pp. 47–77. [Google Scholar]
- 69.Davis BE, Moon RY, Sachs HC, Ottolini MC. Effects of sleep position on infant motor development. Pediatrics. 1998;102:1135–1140. doi: 10.1542/peds.102.5.1135. [DOI] [PubMed] [Google Scholar]
- 70.Monson RM, Deitz J, Kartin D. Effects of awake positioning on motor performance of infants who slept supine. Pediatr Phys Ther. 2003;15:196–203. doi: 10.1097/01.PEP.0000096380.15342.51. [DOI] [PubMed] [Google Scholar]
- 71.Ratliff-Schaub K, Hunt CE, Crowell D, et al. Relationship between infant sleep position and motor development in preterm infants. J Dev Behav Pediatr. 2001;22:293–299. doi: 10.1097/00004703-200110000-00003. [DOI] [PubMed] [Google Scholar]
- 72.Salls JS, Silverman LN, Gatty CM. The relationship of infant sleep and play positioning to motor milestone achievement. Am J Occ Ther. 2002;56:577–580. doi: 10.5014/ajot.56.5.577. [DOI] [PubMed] [Google Scholar]
- 73.Vu HL, Panchal J, Parker EE, Levine NS, Francel P. The timing of physiologic closure of the metopic suture: A review of 159 patients using reconstructed 3D CT scans of the craniofacial region. J Craniofac Surg. 2001;12(6):527–532. doi: 10.1097/00001665-200111000-00005. [DOI] [PubMed] [Google Scholar]
- 74.Fletcher JM, Taylor HG. Neuropsychological approaches to children. J Clin Neuropsych. 1984;6:39–56. doi: 10.1080/01688638408401195. [DOI] [PubMed] [Google Scholar]
- 75.Pennington BF. Genes and behavior: Individual differences and human universals. In: Johnson MH, Munakata Y, Gilmore RO, editors. Brain Development and Cognition: A Reader. 2. Malden, MA: Blackwell Publishers; 2002. pp. 494–508. [Google Scholar]
- 76.Spreen O. The relationship between learning disability, emotional disorders, and neuropsychology: Some results and observations. In: Spreen O, Risser AH, Edgell D, editors. Developmental Neuropsychology. New York: Oxford University Press; 1989. [DOI] [PubMed] [Google Scholar]
- 77.Diamond A, Prevor MB, Callender G, Druin DP. Monographs of the Society for Research in Child Development. Chicago: University of Chicago Press; 1997. Prefrontal cortex cognitive deficits in children treated early and continuously for PKU; p. 272. [PubMed] [Google Scholar]
- 78.Collett B, Breiger D, King D, et al. Neurodevelopment in young children with deformational plagiocephaly. Paper presented at the Annual American Cleft Palate-Craniofacial Association Conference; Chicago, IL. March 2004. [Google Scholar]
- 79.Langlois JH, Ritter JM, Casey RJ, et al. Infant attractiveness predicts maternal behaviors and attitudes. Dev Psychol. 1995;31:464–472. [Google Scholar]
- 80.Ritter JM, Casey RJ, Langlois JH. Adults’ responses to infants varying in appearance of age and attractiveness. Child Dev. 1991;62:68–82. [PubMed] [Google Scholar]
- 81.Stephan CW, Langlois JH. Baby beautiful: Adult attributions of infant competence as a function of infant attractiveness. Child Dev. 1984;55:576–585. [PubMed] [Google Scholar]
- 82.Kapp-Simon KA, McGuire DE. Observed social interaction patterns in adolescents with and without craniofacial conditions. Cleft Palate Craniofac J. 1997;34:380–384. doi: 10.1597/1545-1569_1997_034_0380_osipia_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 83.Kapp-Simon KA, Simon DJ, Kristovich S. Self-perception, social skills, adjustment, and inhibition in young adolescents with craniofacial anomalies. Cleft Palate Craniofac J. 1992;29:352–356. doi: 10.1597/1545-1569_1992_029_0352_spssaa_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 84.Clifford MM, Walster E. Research note: The effect of physical attractiveness on teacher expectations. Soc Educ. 1973;46:248–258. [Google Scholar]
- 85.Richman L. The effects of facial disfigurement on teachers’ perception of ability in cleft palate children. Cleft Palate J. 1978;15:155–160. [PubMed] [Google Scholar]
- 86.Karraker KH. Adult attention to infants in a newborn nursery. Nurs Res. 1986;35:358–363. [PubMed] [Google Scholar]
- 87.Coy K, Speltz ML, Jones K. Facial appearance and attachment in infants with orofacial clefts: A replication. Cleft Palate Craniofac J. 2002;39:66–72. doi: 10.1597/1545-1569_2002_039_0066_faaaii_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- 88.Speltz ML, Endriga MC, Fisher PA, et al. Early predictors of attachment in infants with cleft lip and/or palate. Child Dev. 1997;68:12–25. [PubMed] [Google Scholar]
- 89.Kelley ML, Vannostrand TL, Shiflett CL, et al. Maternal perceptions of and sensitivity toward very low birthweight infants with and without post-natal head molding. Infant Ment Health J. 1996;17:358–374. [Google Scholar]
- 90.Stoelhorst GM, Rijken M, Martens SE, et al. Follow-Up Project on Prematurity - Developmental outcome at 18 and 24 months of age in very preterm children: A cohort study from 1996 to 1997. Early Hum Dev. 2003;72(2):83–95. doi: 10.1016/s0378-3782(03)00011-2. [DOI] [PubMed] [Google Scholar]
- 91.Shonkoff JP, Meisels SJ. Handbook of Early Childhood Intervention. 2. Saltham, MA: Brandeis Univ Heller Grad School; 2000. [Google Scholar]