Abstract
BACKGROUND:
The lower limb nociceptive flexion reflex (NFR) is commonly used to assess the function of the nociceptive system. Currently, there is a lack of standardized stimulation procedures to determine the NFR threshold, making comparisons of thresholds across studies difficult.
OBJECTIVES:
To assess and compare the within- and between-session reliability of NFR threshold when elicited from two common stimulation locations: the medial arch of the foot (while standing) and the sural nerve (while seated).
METHODS:
A staircase procedure was used to determine NFR threshold in 20 healthy participants twice within one session and once more in a separate session approximately four days later. At both sessions, NFR threshold was determined from both medial arch and sural nerve stimulation. Comparisons of NFR threshold, reliability and participant discomfort ratings were made between the two stimulation locations.
RESULTS:
NFR thresholds were statistically equivalent at the two stimulation locations, but there were more nonresponders and ratings of participant discomfort were significantly higher during stimulation over the sural nerve. Within-session reliability measures were superior for stimulation over the sural nerve; however, between-session measures were more reliable using stimulation over the medial arch of the foot.
CONCLUSIONS:
The authors recommend stimulation over the medial arch of the foot while standing as the preferred location for eliciting the lower limb NFR, particularly if measurements are to be compared across multiple sessions.
Keywords: Flexion reflex, Lower limb, Reliability
Abstract
HISTORIQUE :
Le réflexe nociceptif de flexion (RNF) des jambes est souvent utilisé pour évaluer la fonction du système nociceptif. Il n’existe pas d’intervention de stimulation standardisée pour déterminer les seuils du RNF, ce qui complique la comparaison des seuils entre les études.
OBJECTIFS :
Évaluer et comparer la fiabilité du seuil de RNF pendant et entre les séances lorsqu’il est suscité par deux foyers de stimulation courants : la région médiane de la voûte plantaire (en position debout) et le nerf saphène (en position assise).
MÉTHODOLOGIE :
Les chercheurs ont utilisé la méthode de l’escalier pour déterminer le seuil de RNF de 20 participants en bonne santé deux fois pendant une séance et une fois de plus lors d’une séance distincte tenue environ quatre jours plus tard. Lors des deux séances, ils ont déterminé le seuil de RNF par la stimulation de la région médiane de la voûte plantaire et du nerf saphène. Ils ont comparé le seuil de RNF, la fiabilité et l’inconfort des participants entre les deux foyers de stimulation.
RÉSULTATS :
Les seuils de RNF étaient statistiquement équivalents aux deux foyers de stimulation, mais le nombre de non-répondants et les évaluations d’inconfort étaient considérablement plus élevées pendant la stimulation du nerf saphène. Les mesures de fiabilité au sein d’une même séance étaient supérieures lors de la stimulation du nerf saphène, mais les mesures entre les séances étaient plus fiables au moyen de la stimulation de la région médiane de la voûte plantaire.
CONCLUSIONS :
Les auteurs recommandent de privilégier la stimulation de la région médiane de la voûte plantaire en position debout pour obtenir un RNF des jambes, notamment si les mesures doivent être comparées dans le cadre de multiples séances.
The nociceptive flexion reflex (NFR) is widely used to study aspects of nociceptive transmission and modulation in spinal pathways involved in pain processing. The NFR is characterised by a graded withdrawal response to noxious stimulation that functions to move the limb away from actual or potential damage (1,2). Experimentally, this multisegmental spinal response is commonly elicited by electrocutaneous stimulation of Aδ and C afferents innervating the foot, with the resultant flexor muscle activity recorded via electromyography (EMG) (1,3–5).
The European Federation of Neurological Societies guidelines (6) recommend the NFR threshold as the most reliable measure in assessing treatment efficacy in neuropathic pain. However, there have been few attempts to standardize testing procedures or to examine the error associated with the NFR threshold to inform its practical use as a measurement tool. Tørring et al (7) concluded that the NFR threshold elicited through electrical stimulation of the posterior tibial nerve was highly reproducible between two days of testing; however, they did not perform any formal statistical analysis of reliability. Two additional studies used Pearson’s correlation analyses to examine the intra- and intersession reliability of the NFR threshold elicited by stimulation of the sural nerve (5,8). Both studies reported acceptable intra- and intersession values, but the usefulness of Pearson’s correlation coefficient as a measure of reliability has been questioned due to its inability to detect systematic bias (9). More recently, more robust reliability analyses of the NFR threshold in healthy participants (10) and in patients with chronic low back pain (11) have reported high reliability. In both of these studies, the NFR reflex was elicited using stimulation of the sural nerve with the participants in a seated or supine position. Several studies evaluated the NFR by stimulating the plantar aspect of the foot and/or while participants were in a standing posture (3,4,12). The standing position represents a more natural posture for the activation of lower limb nociceptors and a larger, less painful and more functionally appropriate response has been elicited compared with the traditional seated position (3,13). Given the discomfort commonly associated with eliciting the NFR, these factors would provide obvious benefits for participants.
No study has yet conducted a reliability analysis of NFR threshold following stimulation over the medial arch of the foot or while in a standing posture. Therefore, the purpose of the present study was to establish and compare the intra- and intersession reliability of the NFR threshold via electrical stimulation of the retromalleolar pathway of the sural nerve and the medial arch of the foot. A further aim was to compare subjective pain ratings between the two stimulation locations to identify the most comfortable stimulation position for participants.
METHODS
Participants
Twenty healthy, pain-free individuals (10 male, 10 female) between 20 and 33 years of age were recruited for the study (mean age 23.1 years; height 1.72 m; weight 70.2 kg). Participants with a history of back injury, knee injury or lower limb surgery were excluded. The study was conducted with the approval of the institutional ethics committee and all participants provided informed written consent.
Experimental protocol
The NFR threshold was evaluated using stimulation at two sites: over the medial arch of the foot while standing on the contralateral leg; and over the sural nerve while in a seated position. For each of these positions, the NFR threshold was evaluated twice (5 min apart; measurement A and B) and then once more on a separate session an average of four days later (measurement C). For each participant, the order of stimulation positions tested was randomized for the first session but remained the same between the first and second sessions. Reflex testing was conducted at the same time of day in the two sessions to minimize circadian variations in the NFR threshold (14). Participants were asked to refrain from taking analgesic medication for 24 h before reflex testing, and to refrain from consuming caffeine, alcohol and nicotine, and undertaking strenuous exercise for 4 h before reflex testing (5,8,15).
Stimulating electrode placement
The retromalleolar pathway of the sural nerve and the medial arch of the foot were shaved, abraded and cleansed with alcohol. A Nicolet bar electrode with 9 mm gold cups and 30 mm interelectrode distance (DO Weaver & Co, USA) was secured to the site and covered with an elastic bandage. Placement of the electrode over the sural nerve was confirmed via electrical stimulation at low intensity, resulting in a tingling sensation distal to the electrode in the sural nerve distribution. Electrode placement over the medial arch was standardized by placing the electrode 2 cm proximal to the first metatarsophalangeal joint on the plantar aspect of the foot. The anode was positioned inferiorly at both testing sites.
The NFR was elicited using a train of five rectangular pulses of 1 ms duration with a 3 ms interpulse interval (5,8). Each train of stimuli was separated by a random interval of 4 s to 8 s to decrease stimulus predictability and improve participant comfort (5,8). Stimulation was delivered using a DS7A constant current stimulator (Digitimer Ltd, UK).
Electromyography
Disposable silver-silver chloride (Ag-AgCl) recording electrodes were placed on the biceps femoris (BF) muscle on the participant’s dominant leg, 10 cm superior to the popliteal fossa. A ground electrode was placed on the anterior surface of the ipsilateral tibia. Impedance of <10,000 ohms was verified at each electrode site (5,10). EMG signals were amplified, filtered (10 Hz to 1000 Hz; AMT-8, Bortec Biomedical, Canada) and sampled at 2000 Hz using a Micro 1401 data acquisition board and Signal software (Cambridge Electronic Design, United Kingdom).
Electrocutaneous stimulation procedure
For sural nerve stimulation, the participants were seated in a chair with the hip and knee flexed to 90°. For stimulation over the medial arch of the foot, the participants stood on a wooden carpeted block (150 cm × 40 cm × 26 cm) on their nondominant leg while holding onto a rail for balance. The dominant (stimulated) leg hung in a relaxed position without touching the ground. Participants were asked to look straight ahead during testing and to relax their dominant leg muscles as much as possible.
To acclimatize participants to stimulation, each session began with 10 stimulus trains at varying intensities. The NFR threshold was then determined using an up-down staircase method (5). Specifically, the stimulation intensity was increased from 0 mA in 4 mA increments until an NFR was observed. The intensity was then decreased in 2 mA steps until an NFR was no longer evident. The intensity was further increased and decreased four more times in 1 mA increments until an NFR appeared and disappeared two additional times. The final four stimulation intensities were recorded and averaged to calculate the NFR threshold.
The presence of an NFR response was defined using an NFR interval peak z-score of BF EMG activity, derived using the following formula (5):
The NFR interval peak refers to the peak EMG activity of the BF muscle during the poststimulation window of 85 ms to 150 ms. This window was chosen to avoid signal contamination via nonnociceptive reflexes and startle or involuntary responses (16). The baseline mean and SD of BF EMG activity was recorded −65 ms to −5 ms before stimulation. A z-score ≥10.32 was considered to be a true NFR response (5).
Subjective pain ratings
During the testing procedure, participants were asked to rate each stimulus using a 0 to 100 intensity rating scale (5), with anchors of 0 (no sensation), 25 (uncomfortable), 50 (painful), 75 (very painful) and 100 (maximum tolerable pain). Testing was discontinued if a participant reported a rating of 100. For each of the final four stimuli used to calculate NFR threshold, the stimulus intensity rating was recorded. These were then averaged to determine the subjective pain intensity rating at NFR threshold.
Statistical analysis
NFR thresholds were analyzed using a two-way (session × stimulus location) repeated measures ANOVA to check for systematic bias. The within-session (measurement A and B) and between-session (measurement A and C) reliability of the NFR threshold was evaluated using intraclass correlation coefficient (ICC), SEM and Bland-Altman agreement methods (17). To assess the relationship between NFR thresholds obtained from sural nerve and medial arch stimulation, the same reliability analyses was also performed on measurement A data from the two stimulation locations. ICC analysis was performed using SPSS version 17 (IBM Corporation, USA) statistical software with a two-way, mixed effects model with terms of absolute agreement (18). The SEM, which provides a measure of precision of threshold scores, was determined using the following formula (19):
in which SD is the measurement standard deviation and r is the reliability coefficient (ie, ICC) of the measure. The SEM was expressed as a coefficient of variation (CVSEM) to provide an indication of measurement precision relative to absolute threshold values. Bland-Altman graphs were constructed by plotting the difference between threshold measurements against the mean of the threshold differences (17). Bias and the 95% limits of agreement were calculated for each plot (17).
RESULTS
The NFR threshold from sural nerve stimulation was not able to be determined in three of the 20 participants due to maximum tolerable pain being reached before a reflex was evident. NFR thresholds were determined in all 20 participants using medial arch stimulation. One participant was not retested with sural nerve stimulation due to the presence of a minor cutaneous abrasion at the stimulation site that appeared in the four days between testing sessions. Thus, reliability data for medial arch stimulation are from all 20 participants, while intra- and intersession reliability data for medial arch stimulation are from 17 and 16 participants, respectively.
NFR thresholds
The mean (± SD) NFR thresholds for medial arch stimulation were 19.6±12.6, 22.6±15.1 and 19.5±12.5 mA for measurement A, B and C, respectively. The mean NFR thresholds for sural nerve stimulation were 19.8±13.9, 21.7±16.1 and 19.9±11.4 mA for measurement A, B and C, respectively. The ANOVA did not reveal significant main effects of session (F[2, 30]=0.9; P=0.4) or stimulus location (F[1, 15]=0.08; P=0.8) or a significant interaction effect between these factors (F[2, 30]=0.2; P=0.8).
NFR reliability
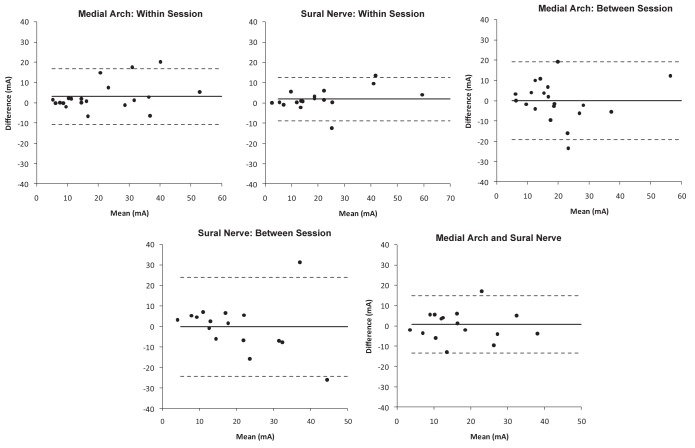
The ICC, SEM, bias and limits of agreement data are presented in Table 1. The within-session ICC values for medial arch and sural nerve stimulation are both classified as ‘high’ according to Vincent (20). Bland-Altman plots for the within-session data showing 95% limits of agreement are presented in the upper panel of Figure 1. For medial arch stimulation there was a bias of 3.0 mA (95% limits of agreement −10.8 mA to 16.9 mA). For sural nerve stimulation there was a bias of 2.8 mA (95% limits of agreement −8.8 mA to 12.6 mA).
TABLE 1.
Within- and between-session reliability data for medial arch and sural nerve stimulation
| Medial arch | Sural nerve | |
|
| ||
| Within-session | ||
|
| ||
| ICC (lower bound–upper bound) | 0.922 (0.794–0.969) | 0.964 (0.902–0.987) |
| SEM | 3.5 mA | 2.6 mA |
| CVSEM | 17.9% | 13.4% |
| Bias ± 95% limits of agreement | 3.0±13.9 mA | 1.9±10.7 mA |
| Between-session | ||
|
| ||
| ICC (lower bound–upper bound) | 0.826 (0.554–0.931) | 0.720 (0.169–0.903) |
| SEM | 5.2 mA | 7.6 mA |
| CVSEM | 26.8% | 37.7% |
| Bias ± 95% limits of agreement | 0.1±19.2 mA | 0.2±24.2 mA |
| Between stimulus location | ||
|
| ||
| ICC (lower bound–upper bound) | 0.926 (0.795–0.973) | |
| SEM | 3.5 mA | |
| CVSEM | 18.3% | |
| Bias ± 95% limits of agreement | 0.7±14.1 mA | |
CV Coefficient of variation; ICC Intraclass correlation coefficient
Figure 1).
Bland-Altman plots for nociception flexion reflex threshold obtained from medial arch and sural nerve stimulation. The upper plots show data from within the same session (measurement A, B), the middle plots show data from between sessions (measurement A, C), and the lower plot shows measurement A data from medial arch and sural nerve stimulation. The solid horizontal line indicates the bias and the dotted lines indicate the 95% limits of agreement
The between-session ICC value for medial arch stimulation is classified as ‘moderate’, while that obtained for sural nerve stimulation is ‘questionable’. Bland-Altman graphs for between-session data showing 95% limits of agreement are presented in the middle panel of Figure 1. For medial arch stimulation there was a bias of −0.05 mA (95% limits of agreement −19.3 mA to 19.2 mA). For sural nerve stimulation there was a bias of −1.72 mA (95% limits of agreement −24.3 mA to 24.0 mA).
The ICC value for comparison of measurement A data from medial arch and sural nerve stimulation is ‘high’. The Bland-Altman plot showing 95% limits of agreement is presented in the lower panel of Figure 1. There was a bias of 0.7 mA (95% limits of agreement −13.5 mA to 14.8 mA).
Subjective pain ratings
The mean subjective pain rating at NFR threshold during medial arch stimulation (27±16) was significantly lower than that reported during sural nerve stimulation (37±19 [P=0.01]). Thirteen of the 17 participants in whom the NFR threshold could be determined from both stimulation sites had lower pain ratings for medial arch stimulation compared with sural nerve stimulation.
DISCUSSION
NFR threshold reliability
We reported high within-session reliability of the NFR threshold following stimulation both at the sural nerve and over the medial arch of the foot. The between-session reliability values were lower for both stimulation locations but were still at a moderate level for stimulation over the medial arch of the foot. The interpretation of ICC values varies within the literature. Some consider values >0.75 to 0.8 to convey good-excellent reliability (21,22), while others consider any value exceeding 0.6 to be acceptable (23). We adopted the more stringent criteria of Vincent (20), who defines an ICC >0.9 as ‘high’, between 0.8 and 0.9 as ‘moderate’, and between 0.7 and 0.8 as ‘questionable’ reliability. What may be more important in terms of using the NFR threshold to monitor changes over time or detect differences between groups are the SEM values and 95% limits of agreement. In the present study, the 95% limits of agreement for medial arch stimulation indicate that a person with an unchanged NFR threshold would have a one in 20 chance of being 13.9 mA (within session) or 19.2 mA (between sessions) above or below their baseline threshold when retested. The 95% limits of agreement have been criticized for being overly conservative and markedly influenced by the sample size (24). For this reason, the SEM is favoured for providing estimates of test-retest variability (19,25); however, the reverse criticism applies for this measure because it covers only approximately 68% of the variability, is also sensitive to population heterogeneity and is only appropriate for data that do not show heteroscedasticity. Our within-session SEM values were <4 mA (20%) for the two stimulation locations and <8 mA (40%) between sessions. As a comparison, differences in NFR threshold between people with chronic pain conditions and healthy populations have ranged from 23% to 50% (26–28), while changes in NFR threshold over time following treatment interventions for acute and chronic pain have ranged from 20% to 87% (29–32).
Compared with previous studies that have examined the reliability of the NFR threshold, our ICCs are lower and CVSEM higher than those reported in healthy and in chronic pain populations (10,11). One reason for this may be the thresholding procedure we adopted. We used a staircase method of determining threshold described by Rhudy and France (5), whereas the other studies used a modified version of this method that uses smaller step increments and requires the delivery of multiple stimuli at each intensity level. The delivery of multiple stimuli at each intensity may elicit a more consistent estimate of threshold than what can be obtained using a single stimulus. An additional factor contributing to our lower reliability measures may be our definition of an NFR response. Rhudy and France (5) provided one of the only direct comparisons of criteria defining the presence of an NFR. They determined that a z-score reflecting the peak EMG activity in the NFR time period relative to background EMG was the best criterion to identify the presence of a response, which is the definition that we adopted in the current study.
In a subsequent investigation, France et al (33) reported that although the NRF interval peak z-score had a high sensitivity and specificity in identifying the presence of reflex, it was associated with a substantial SD, indicating a large variability between individuals. A large between-subject variability was present in our threshold values and is evident in other studies that have used the same criteria for determining the presence of a reflex (26). A large interindividual SD reduces ICC and inflates SEM values; thus, it may be more suitable to use a less variable reflex definition criteria, such as an NFR interval z-score based on the mean NFR EMG rather than peak EMG (33). Finally, the inclusion of female participants may have increased our NFR threshold variability. Tasorelli et al (34) showed significant modulation of NFR threshold across the menstrual cycle, which led others to control for menstrual cycle phase during NFR testing (35,36) or to exclude female participants (10).
Comparison between stimulation locations
To our knowledge, the present study was the first to examine the reliability of the NFR threshold obtained from stimulation over the medial arch of the foot. While our within-session ICC and CVSEM values were slightly lower compared with sural nerve stimulation, between-session reliability was higher for the medial arch location. Importantly, the subjective pain ratings indicated that participants found stimulation over the medial arch more comfortable, even though the NFR threshold was not significantly different from that obtained using stimulation of the sural nerve. In fact, the reliability analyses of NFR threshold across the two stimulation sites indicated very good agreement, suggesting that stimulation at the two locations assesses similar components of the nociceptive system. The pain ratings elicited during medial arch stimulation were lower, even after removal of three participants in whom an NFR threshold was unable to be determined using sural nerve stimulation due to maximum tolerable pain ratings, suggesting that the difference in subjective pain between the two conditions may be even greater.
In addition to differences in the location of the stimulus, our two conditions also involved different postures – the medial arch was stimulated while in a standing position and the sural nerve was stimulated while seated. In an earlier study that examined the NFR in seated and standing postures, Rossi and Decchi (3) found a larger NFR size while standing (stimulated limb unloaded) than sitting with a comparable foot position. Subsequently, Andersen et al (13) reported a higher pain threshold, determined using pain intensity ratings during NFR stimulation, while standing compared with seated. The standing posture also elicited a larger NFR in the biceps femoris and a greater knee flexion response. Both postures in that study involved stimulation at the medial arch of the foot. While these studies suggest the standing posture may elicit a larger NFR response or a lower threshold, there was no evidence in our data of a significant difference in NFR threshold between the two stimulation locations, even though the subjective pain ratings were lower at the medial arch. The finding that subjective pain ratings were different between the two stimulus locations while NFR thresholds were similar supports reports that these two variables can often be dissociated and may provide complimentary assessments of the nociceptive system rather than being interchangeable (15,37–39).
NFR thresholds and nonresponders
In a study involving 300 healthy participants, Neziri et al (40) reported a mean NFR threshold of 16.2 mA using stimulation over the sural nerve. Using similar stimulation parameters, our NFR thresholds were approximately 3 mA to 4 mA higher for both stimulation locations. Therefore, our thresholds were comparable given the slight differences in procedures used to determine threshold. We were not able to obtain threshold values at the sural nerve in three of our 20 participants (15%) because of high subjective pain ratings. Neziri et al (40) did not report any participants from whom they were unable to obtain a threshold. However, previous studies using similar stimulation techniques have reported an inability to determine threshold in 8% to 15% of participants (26,28,41), which is very similar to our nonresponse rate. It should be considered that our study involved a population of healthy young adults. In people with chronic pain conditions, the NFR threshold is routinely reduced in comparison with healthy participants (42–44). Thus, our results concerning the presence of nonresponders and the subjective pain ratings associated with stimulation may not be generalized to populations with long-term pain.
CONCLUSIONS
Assessing the NFR threshold during standing using stimulation of the medial arch of the foot may have advantages over the traditional method of assessing NFR threshold using stimulation of the sural nerve while seated. Although the threshold values were comparable, medial arch stimulation was more comfortable, gave rise to fewer non-responders and superior reliability across sessions. Our reliability values were lower and measurement error higher than that described using other threshold determination techniques. We suggest that using multiple stimuli at each intensity and an NFR interval z-score based on the mean EMG may lead to more reliable threshold values both within and across sessions.
Acknowledgments
This project was funded by an Auckland University of Technology Faculty of Health and Environmental Sciences summer studentship award to KJ.
REFERENCES
- 1.Willer J. Comparative study of perceived pain and nociceptive flexion reflex in man. Pain. 1977;3:69–80. doi: 10.1016/0304-3959(77)90036-7. [DOI] [PubMed] [Google Scholar]
- 2.Skljarevski V, Ramadan N. The nociceptive flexion reflex in humans. Pain. 2002;96:3–8. doi: 10.1016/s0304-3959(02)00018-0. [DOI] [PubMed] [Google Scholar]
- 3.Rossi A, Decchi B. Flexibility of lower limb reflex responses to painful cutaneous stimulation in standing humans: Evidence of load-dependent modulation. J Physiol. 1994;481:521–32. doi: 10.1113/jphysiol.1994.sp020460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersen O, Sonnenborg F, Arendt-Nielsen L. Modular organization of human leg withdrawal reflexes elicited by electrical stimulation of the foot sole. Muscle Nerve. 1999;22:1520–30. doi: 10.1002/(sici)1097-4598(199911)22:11<1520::aid-mus6>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 5.Rhudy JL, France CR. Defining the nociceptive flexion reflex (NFR) threshold in human participants: A comparison of different scoring criteria. Pain. 2007;128:244–53. doi: 10.1016/j.pain.2006.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cruccu G, Anand P, Attal N, et al. EFNS guidelines on neuropathic pain assessment. Eur J Neurol. 2004;11:153–62. doi: 10.1111/j.1468-1331.2004.00791.x. [DOI] [PubMed] [Google Scholar]
- 7.Tørring J, Pedersen E, Klemar B. Standardisation of the electrical elicitation of the human flexor reflex. J Neurol Neurosurg Psychiatry. 1981;44:129–32. doi: 10.1136/jnnp.44.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.French DJ, France CR, France JL, Arnott LF. The influence of acute anxiety on assessment of nociceptive flexion reflex thresholds in healthy young adults. Pain. 2005;114:358–63. doi: 10.1016/j.pain.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 9.Bates BT, Zhang S, Dufek JS, Chen FC. The effects of sample size and variability on the correlation coefficient. Med Sci Sports Exerc. 1996;28:386–91. doi: 10.1097/00005768-199603000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Micalos PS, Drinkwater EJ, Cannon J, Arendt-Nielsen L, Marino FE. Reliability of the nociceptive flexor reflex (RIII) threshold and association with pain threshold. Eur J Appl Physiol. 2009;105:55–62. doi: 10.1007/s00421-008-0872-x. [DOI] [PubMed] [Google Scholar]
- 11.Biurrun Manresa J, Neziri A, Curatolo M, Arendt-Nielsen L, Andersen O. Test-retest reliability of the nociceptive withdrawal reflex and electrical pain thresholds after single and repeated stimulation in patients with chronic low back pain. Eur J Appl Physiol. 2011;111:83–92. doi: 10.1007/s00421-010-1634-0. [DOI] [PubMed] [Google Scholar]
- 12.Decchi B, Zalaffi A, Spidalieri R, Arrigucci U, Di Troia A, Rossi A. Spinal reflex pattern to foot nociceptive stimulation in standing humans. Electroencephalogr Clin Neurophysiol. 1997;105:484–9. doi: 10.1016/s0924-980x(97)00048-9. [DOI] [PubMed] [Google Scholar]
- 13.Andersen OK, Spaich EG, Madeleine P, Arendt-Nielsen L. Gradual enlargement of human withdrawal reflex receptive fields following repetitive painful stimulation. Brain Res. 2005;1042:194–204. doi: 10.1016/j.brainres.2005.02.039. [DOI] [PubMed] [Google Scholar]
- 14.Sandrini G, Alfonsi E, Bono G, Facchinetti F, Montalbetti L, Nappi G. Circadian variations of human flexion reflex. Pain. 1986;25:403–10. doi: 10.1016/0304-3959(86)90245-9. [DOI] [PubMed] [Google Scholar]
- 15.France CR, France JL, Absi M, Ring C, McIntyre D. Catastrophizing is related to pain ratings, but not nociceptive flexion reflex threshold. Pain. 2002;99:459–63. doi: 10.1016/s0304-3959(02)00235-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dowman R. Possible startle response contamination of the spinal nociceptive withdrawal reflex. Pain. 1992;49:187–97. doi: 10.1016/0304-3959(92)90142-X. [DOI] [PubMed] [Google Scholar]
- 17.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10. [PubMed] [Google Scholar]
- 18.Shrout P, Fleiss J. Intraclass correlations: Uses in assessing rater reliability. Psychol Bull. 1979;86:420–8. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 19.Atkinson G, Nevill AM. Statistical methods for assessing measurement error (reliability) in variables relevant to sports medicine. Sports Med. 1998;26:217–38. doi: 10.2165/00007256-199826040-00002. [DOI] [PubMed] [Google Scholar]
- 20.Vincent J. Statistics in Kinesiology. Champaign: Human Kinetics Books; 1994. [Google Scholar]
- 21.Fleiss J. The design and analysis of clinical experiments. New York: John Wiley; 1986. [Google Scholar]
- 22.Portney LG, Watkins MP. Foundations of clinical research: Applications to practice. Norwalk: Appleton & Lange; 1993. [Google Scholar]
- 23.Chinn S. Repeatability and method comparison. Thorax. 1991;46:454–6. doi: 10.1136/thx.46.6.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hopkins WG. Measures of reliability in sports medicine and science. Sports Med. 2000;30:1–15. doi: 10.2165/00007256-200030010-00001. [DOI] [PubMed] [Google Scholar]
- 25.Wyrwich KW, Tierney WM, Wolinsky FD. Further evidence supporting an SEM-based criterion for identifying meaningful intra-individual changes in health-related quality of life. J Clin Epidemiol. 1999;52:861–73. doi: 10.1016/s0895-4356(99)00071-2. [DOI] [PubMed] [Google Scholar]
- 26.Sterling M, Hodkinson E, Pettiford C, Souvlis T, Curatolo M. Psychologic factors are related to some sensory pain thresholds but not nociceptive flexion reflex threshold in chronic whiplash. Clin J Pain. 2008;24:124–30. doi: 10.1097/AJP.0b013e31815ca293. [DOI] [PubMed] [Google Scholar]
- 27.Banic B, Petersen-Felix S, Andersen OK, et al. Evidence for spinal cord hypersensitivity in chronic pain after whiplash injury and in fibromyalgia. Pain. 2004;107:7–15. doi: 10.1016/j.pain.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Langemark M, Bach FW, Jensen TS, Olesen J. Decreased nociceptive flexion reflex threshold in chronic tension-type headache. Arch Neurol. 1993;50:1061–4. doi: 10.1001/archneur.1993.00540100056015. [DOI] [PubMed] [Google Scholar]
- 29.Sterling M, Pedler A, Chan C, Puglisi M, Vuvan V, Vicenzino B. Cervical lateral glide increases nociceptive flexion reflex threshold but not pressure or thermal pain thresholds in chronic whiplash associated disorders: A pilot randomised controlled trial. Man Ther. 2010;15:149–53. doi: 10.1016/j.math.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 30.Ang DC, Chakr R, Mazzuca S, France CR, Steiner J, Stump T. Cognitive-behavioral therapy attenuates nociceptive responding in patients with fibromyalgia: A pilot study. Arthritis Care Res. 2010;62:618–23. doi: 10.1002/acr.20119. [DOI] [PubMed] [Google Scholar]
- 31.Willer JC, Bergeret S, Gaudy JH. Epidural morphine strongly depresses nociceptive flexion reflexes in patients with postoperative pain. Anesthesiology. 1985;63:675–80. doi: 10.1097/00000542-198512000-00019. [DOI] [PubMed] [Google Scholar]
- 32.Willer JC, De Broucker T, Bussel B, Roby-Brami A, Harrewyn JM. Central analgesic effect of ketoprofen in humans: Electrophysiological evidence for a supraspinal mechanism in a double-blind and cross-over study. Pain. 1989;38:1–7. doi: 10.1016/0304-3959(89)90065-1. [DOI] [PubMed] [Google Scholar]
- 33.France CR, Rhudy JL, McGlone S. Using normalized EMG to define the nociceptive flexion reflex (NFR) threshold: Further evaluation of standardized NFR scoring criteria. Pain. 2009;145:211–8. doi: 10.1016/j.pain.2009.06.022. [DOI] [PubMed] [Google Scholar]
- 34.Tassorelli C, Sandrini G, Cecchini AP, Nappi RE, Sances G, Martignoni E. Changes in nociceptive flexion reflex threshold across the menstrual cycle in healthy women. Psychosom Med. 2002;64:621–6. doi: 10.1097/01.psy.0000021945.35402.0d. [DOI] [PubMed] [Google Scholar]
- 35.Serrao M, Rossi P, Sandrini G, et al. Effects of diffuse noxious inhibitory controls on temporal summation of the RIII reflex in humans. Pain. 2004;112:353–60. doi: 10.1016/j.pain.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 36.Baad-Hansen L, Poulsen HF, Jensen HM, Svensson P. Lack of sex differences in modulation of experimental intraoral pain by diffuse noxious inhibitory controls (DNIC) Pain. 2005;116:359–65. doi: 10.1016/j.pain.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 37.Roy M, Lebuis A, Peretz I, Rainville P. The modulation of pain by attention and emotion: A dissociation of perceptual and spinal nociceptive processes. Eur J Pain. 2011;15:641.e1–641.e10. doi: 10.1016/j.ejpain.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 38.Goffaux P, de Souza JB, Potvin S, Marchand S. Pain relief through expectation supersedes descending inhibitory deficits in fibromyalgia patients. Pain. 2009;145:18–23. doi: 10.1016/j.pain.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 39.Terkelsen AJ, Andersen OK, Hansen PO, Jensen TS. Effects of heterotopic- and segmental counter-stimulation on the nociceptive withdrawal reflex in humans. Acta Physiol Scand. 2001;172:211–7. doi: 10.1046/j.1365-201x.2001.00856.x. [DOI] [PubMed] [Google Scholar]
- 40.Neziri AY, Andersen OK, Petersen-Felix S, et al. The nociceptive withdrawal reflex: Normative values of thresholds and reflex receptive fields. Eur J Pain. 2010;14:134–41. doi: 10.1016/j.ejpain.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 41.Page GD, France CR. Objective evidence of decreased pain perception in normotensives at risk for hypertension. Pain. 1997;73:173–80. doi: 10.1016/S0304-3959(97)00111-5. [DOI] [PubMed] [Google Scholar]
- 42.Courtney CA, Lewek MD, Witte PO, Chmell SJ, Hornby TG. Heightened flexor withdrawal responses in subjects with knee osteoarthritis. J Pain. 2009;10:1242–9. doi: 10.1016/j.jpain.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 43.Leroux A, Belanger M, Boucher JP. Pain effect on monosynaptic and polysynaptic reflex inhibition. Arch Phys Med Rehabil. 1995;76:576–82. doi: 10.1016/s0003-9993(95)80514-1. [DOI] [PubMed] [Google Scholar]
- 44.Desmeules JA, Cedraschi C, Rapiti E, et al. Neurophysiologic evidence for a central sensitization in patients with fibromyalgia. Arthritis Rheum. 2003;48:1420–29. doi: 10.1002/art.10893. [DOI] [PubMed] [Google Scholar]