Abstract
Physical obstructions are becoming increasingly recognized as major factors influencing the migrations, population structures, spawning success and recruitment of freshwater organisms. This paper presents a simple but effective method, intended for use by environmental managers, government agencies and conservation bodies, of rapidly assessing and prioritizing barriers to the migrations of diadromous fishes and lampreys for passage improvements. A prioritization matrix was developed using information on fish stock status, the passage efficiency of fishes at individual structures, the distance from the tidal limit and the passability of downstream barriers, and the quantity and quality of habitat upstream of each structure. Importantly, the ‘Likelihood of access’ was incorporated into the matrix to account for passage efficiency at downstream barriers. Barriers ranked as the highest priority for passage improvements were those characterized by poor fish stocks upstream, low passage efficiency, easy passage from downstream, and a large quantity and high quality of habitat upstream. Prioritization of migration barriers should ensure that access improvements are targeted to achieve optimum benefits.
Keywords: Barrier, Habitat connectivity, Habitat fragmentation, Migration, Obstruction, Rehabilitation
Introduction
Migration is an integral component of the life cycle of many organisms, involving the movement of individuals between habitats according to ontogenetic or temporal requirements. Increasing awareness of the prevalence and magnitude of fish migrations, including by species previously considered sedentary, has led to concerns over the possible impacts of barriers on population demographics (Baras and Lucas 2001; Nunn et al. 2008, 2010). Man-made structures such as weirs, sluices and barrages may impede or prevent access of individuals to essential habitats, potentially affecting the distribution, population structures, spawning success and recruitment of many species. The impacts of migration barriers are often most apparent in diadromous species, as they frequently move large distances between marine and freshwater environments and may encounter numerous man-made obstacles during migration. Barriers have been implicated in the dramatic decline in recruitment of the European eel (Anguilla anguilla (L.)) in the past three decades, for example, and can also have adverse impacts on other diadromous species, as well as species restricted to fresh water (Baras and Lucas 2001; Limburg and Waldman 2009; Renaud 1997; White and Knights 1997).
An understanding of the types, characteristics and impacts of barriers to fish migration is crucial if action is to be taken to address bottlenecks to recruitment. Many watercourses have numerous potential barriers, however, and there are invariably only limited resources available for mitigation activities, which could compromise their ecological status or potential [e.g. under the EU Water Framework Directive (2000/60/EEC)]. The aim of this study was thus to develop a simple, effective method of assessing and prioritizing migration barriers for passage improvements, to optimize the benefits to fishes, lampreys and other migratory organisms. The method is demonstrated using case studies on Atlantic salmon (Salmo salar L.), European eel and river lamprey (Lampetra fluviatilis (L.)) by prioritizing (1) barriers for installation of fish passes (to increase longitudinal connectivity) and (2) outfalls for retrofitting ‘fish-friendly’ flap-gates (to increase lateral connectivity).
Materials and Methods
The study was a cartography- and field-based examination of barriers in the Humber basin and tidal River Trent, England. The Humber is one of the largest catchments in the UK (>26 000 km2), draining one-fifth of the land area of England, and the Trent is the largest river in the Humber basin (~280 km length, ~10 500 km2 catchment). The industrial heritage of much of the Humber basin means that there is a large number of potential barriers to fish migration, and the vast majority of watercourses discharging to the tidal Trent have flap-gates at their outfalls, largely for flood defence. Sixty-seven potential barriers (mostly weirs) to the longitudinal migration of Atlantic salmon, European eel and river lamprey in the Humber basin were assessed, while 129 potential barriers (mostly flap-gates) to the lateral migration of European eel were surveyed in the tidal Trent between Cromwell Weir (tidal limit) and the Humber Estuary.
Decisions about which barriers should be targeted for passage improvements were made using a prioritization matrix. General information for each structure (e.g. structure name, type and purpose, catchment, watercourse, latitude and longitude, channel width, upstream land use, distance from tidal limit) was collated in a spreadsheet. Each structure was then scored on a scale of 1 (smallest potential benefits following passage improvements) to 5 (greatest potential benefits following passage improvements) for the following parameters: (1) ‘Fish stock status’; (2) ‘Passage efficiency’; (3) ‘Likelihood of access’; (4) ‘Habitat quantity’; and (5) ‘Habitat quality’. All the parameters were scored on the same scale to ensure that each was given equal weight in the prioritization process.
‘Fish stock status’ was defined as the status of the stock of the target species (diadromous fishes or lampreys) upstream of each structure, up to the next structure, with the poorest stocks scoring highest (Table 1). Note that ‘Fish stock status’ refers to the ‘non-migratory’ life periods of the target species that inhabit rivers (e.g. larval and juvenile Atlantic salmon, juvenile European eel, larval river lamprey). ‘Passage efficiency’ was an estimate of the percentage of the target species that successfully pass individual structures (in normal flow conditions during the migration period), with the lowest efficiencies scoring highest (1 = >95, 2 = 66–95, 3 = 36–65, 4 = 6–35, 5 = ≤5% passage efficiency). ‘Likelihood of access’ was an estimate of the difficulty of passage by the target species upstream to each structure; a function of the distance from the tidal limit and the passage efficiency at downstream barriers, where the latter was a product of the individual passage efficiencies at downstream barriers (e.g. 25% × 80% × 100% × 50% = 10%), with the easiest passages scoring highest (Table 2). Note that the ‘Likelihood of access’ score of a given structure will necessarily be lower than those of any barriers downstream. ‘Habitat quantity’ was an estimate of the quantity (river length, including tributaries if appropriate) of habitat upstream of each structure, up to the next structure, with the greatest quantities of habitat scoring highest (Table 3). ‘Habitat quality’ was an estimate of the quality of habitat upstream of each structure, up to the next structure; a function of water quality and physical habitat characteristics, with the highest quality habitats scoring highest (Table 4). Note that ‘Habitat quality’ refers to the requirements of the life period of the target species that migrates into rivers (e.g. spawning habitat for adult Atlantic salmon and river lamprey, nursery habitat for juvenile European eel). The output of the prioritization matrix was the ‘Barrier score’ (B), derived as:
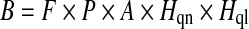 |
where F was the ‘Fish stock status’, P was the ‘Passage efficiency’, A was the ‘Likelihood of access’, Hqn was the ‘Habitat quantity’ and Hql was the ‘Habitat quality’. B ranges from 1 (1 × 1 × 1 × 1 × 1) to 3125 (5 × 5 × 5 × 5 × 5). The structures were then ranked according to B, identifying priority barriers for passage improvements (priority barriers had the highest B). For the Humber basin, where there were multiple target species, a composite B was produced by summing the B of individual species (see Table 5).
Table 1.
Determination of ‘Fish stock status’ for Atlantic salmon, European eel and river lamprey
| Score | Density (#100 m−2) | Status | ||
|---|---|---|---|---|
| Salmon | Eel | Lamprey | ||
| 1 | >5 | >20 | >10 | Very good |
| 2 | 3–5 | 11–20 | 6–10 | Good |
| 3 | 1–2 | 5–10 | 1–5 | Moderate |
| 4 | <1 | <5 | <1 | Poor |
| 5 | 0 | 0 | 0 | Very poor |
Note that the class boundaries can be adjusted (standardized against the maximum value in the study) to suit particular species (e.g. rare vs. common) or study areas (e.g. low vs. high productivity), and that the metric refers to the ‘non-migratory’ life periods of the target species that inhabit rivers (e.g. larval and juvenile Atlantic salmon, juvenile European eel, larval river lamprey)
Table 2.
Determination of ‘Likelihood of access’
| Distance from tidal limit (km) | |||
|---|---|---|---|
| <10 | 10–20 | >20 | |
| Passage efficiency at downstream barriers | |||
| Low (<30%) | 3 | 2 | 1 |
| Moderate (30–70%) | 4 | 3 | 2 |
| High (>70%) | 5 | 4 | 3 |
Note that the class boundaries can be adjusted (standardized against the maximum value in the study) to suit particular study areas (e.g. small vs. large catchments), and that the score of a given structure will necessarily be lower than those of any barriers downstream
Table 3.
Determination of ‘Habitat quantity’
| Score | River length (km) | |
|---|---|---|
| Humber | Trent | |
| 1 | <5 | <1 |
| 2 | 5–10 | 1–5 |
| 3 | 11–15 | 6–10 |
| 4 | 16–20 | 11–15 |
| 5 | >20 | >15 |
Note that the class boundaries can be adjusted (standardized against the maximum value in the study) to suit particular study areas (e.g. small vs. large catchments), and that tributaries can be included in the estimates if appropriate
Table 4.
Determination of ‘Habitat quality’
| Water qualitya | |||
|---|---|---|---|
| Good | Fair | Poor | |
| Physical habitat | |||
| Poor (little or no suitable habitat present) | 3 | 2 | 1 |
| Fair (sub-optimal habitat present) | 4 | 3 | 2 |
| Good (‘optimal’ habitat present) | 5 | 4 | 3 |
ae.g. chemical General Quality Assessment (GQA) grades A–B (good), C–D (fair), E–F (poor). Note that the metric refers to the habitat requirements of the life period of the target species that migrates into rivers (e.g. spawning habitat for adult Atlantic salmon and river lamprey, nursery habitat for juvenile European eel)
Table 5.
Priority barriers in the Humber basin for longitudinal passage improvements for Atlantic salmon, European eel and river lamprey
| River | Barrier | Species | F | P | A | Hqn | Hql | B | ΣB | Rank |
|---|---|---|---|---|---|---|---|---|---|---|
| Ouse | Naburn | Salmon | 3 | 4 | 5 | 5 | 3 | 900 | 2650 | 1 |
| Eel | 2 | 5 | 5 | 5 | 4 | 100 | ||||
| Lamprey | 2 | 5 | 5 | 5 | 3 | 750 | ||||
| Don | Sprotbrough | Salmon | 5 | 4 | 5 | 3 | 2 | 600 | 2250 | 2 |
| Eel | 4 | 5 | 5 | 3 | 3 | 900 | ||||
| Lamprey | 5 | 5 | 5 | 3 | 2 | 750 | ||||
| Trent | Cromwell | Salmon | 4 | 4 | 5 | 2 | 3 | 480 | 2030 | 3 |
| Eel | 4 | 5 | 5 | 2 | 4 | 800 | ||||
| Lamprey | 5 | 5 | 5 | 2 | 3 | 750 | ||||
| Derwent | Barmby | Salmon | 4 | 3 | 5 | 4 | 3 | 720 | 1560 | 4 |
| Eel | 2 | 3 | 5 | 4 | 4 | 480 | ||||
| Lamprey | 2 | 3 | 5 | 4 | 3 | 360 | ||||
| Wharfe | Tadcaster | Salmon | 3 | 4 | 5 | 2 | 4 | 480 | 1280 | 5 |
| Eel | 2 | 5 | 5 | 2 | 4 | 400 | ||||
| Lamprey | 2 | 5 | 5 | 2 | 4 | 400 | ||||
| Trent | Newark | Salmon | 4 | 2 | 3 | 3 | 3 | 216 | 1053 | 6 |
| Eel | 4 | 3 | 3 | 3 | 4 | 432 | ||||
| Lamprey | 5 | 3 | 3 | 3 | 3 | 405 | ||||
| Aire | Knottingley | Salmon | 5 | 3 | 4 | 3 | 2 | 360 | 954 | 7 |
| Eel | 4 | 3 | 3 | 3 | 3 | 324 | ||||
| Lamprey | 5 | 3 | 3 | 3 | 2 | 270 | ||||
| Aire | Chapel Haddlesey | Salmon | 5 | 2 | 5 | 2 | 2 | 200 | 860 | 8 |
| Eel | 4 | 3 | 5 | 2 | 3 | 360 | ||||
| Lamprey | 5 | 3 | 5 | 2 | 2 | 300 | ||||
| Trent | Averham | Salmon | 4 | 2 | 2 | 3 | 3 | 144 | 702 | 9 |
| Eel | 4 | 3 | 2 | 3 | 4 | 288 | ||||
| Lamprey | 5 | 3 | 2 | 3 | 3 | 270 | ||||
| Aire | Beal | Salmon | 5 | 1 | 4 | 2 | 2 | 80 | 344 | 10 |
| Eel | 4 | 2 | 3 | 2 | 3 | 144 | ||||
| Lamprey | 5 | 2 | 3 | 2 | 2 | 120 |
F fish stock status, P passage efficiency, A likelihood of access, Hqn habitat quantity, Hql habitat quality, B barrier score, ΣB composite barrier score
Note that the class boundaries for F, A and Hqn can be adjusted (standardized against the maximum value in the study) to suit particular species (e.g. rare vs. common) or study areas (e.g. small vs. large catchments, low vs. high productivity), ensuring that there are no redundant classes in the metrics. It should also be noted that estimates of P may vary between species (see Table 5), depending upon the characteristics of individual barriers. Whenever possible, all parameters should be scored using empirical data (e.g. from surveys [F, Hql], maps/GIS software [Hqn] or existing tools [P, A; see Kemp and O’Hanley 2010]); if no empirical data are available, ‘expert judgement’ can be used following site visits.
Independent prioritizations using expert judgement were conducted for European eel in the Humber basin by 12 assessors, with each assessor scoring the structures with which they were familiar and with each structure scored by a minimum of three assessors. Mean (±S.D.), minimum and maximum B were then calculated for each structure to examine variations in B and the relative importance (rank) of barriers between assessors. In addition, for each structure, variations in the parameter (F, P, A, Hqn, Hql) scores between assessors were calculated using the coefficient of variation (CV = [100 s]/m, where s was the parameter standard deviation and m was the parameter mean).
Results
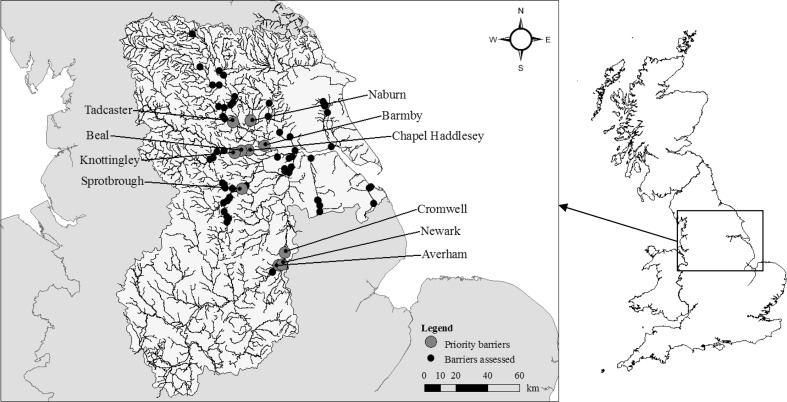
In the Humber basin, B ranged from 10 (5 × 1 × 2 × 1 × 1) to 1000 (2 × 5 × 5 × 5 × 4) for the 67 potential barriers to the longitudinal migration of Atlantic salmon, European eel and river lamprey. The structures prioritized for installation of fish passes were the weirs at Naburn (River Ouse), Sprotbrough (Don) and Cromwell (Trent), Barmby Barrage (Derwent), and Tadcaster (Wharfe), Newark (Trent), Knottingley (Aire), Chapel Haddlesey (Aire), Averham (Trent) and Beal (Aire) Weirs (Table 5; Fig. 1). Naburn was ranked as highest priority for salmon and eel, whereas Naburn, Sprotbrough and Cromwell were highest priorities for river lamprey. Naburn had comparatively good salmon, eel and river lamprey stocks upstream, but was ranked as highest priority (from the composite B) because of the large quantity of habitat that would become available following passage improvements (Table 5). By contrast, Sprotbrough was ranked highly due to a combination of the poor status of salmon, eel and river lamprey stocks upstream, the low passage efficiency of the weir by salmon, eel and river lamprey, and the location of the weir downstream of other major barriers. The same factors resulted in the high ranks of Cromwell, Newark, Knottingley, Chapel Haddlesey, Averham and Beal, whereas Barmby and Tadcaster were ranked highly because of the large quantity and high quality, respectively, of habitat that would become available following passage improvements.
Fig. 1.
Barriers in the Humber basin assessed for longitudinal passage improvements for Atlantic salmon, European eel and river lamprey
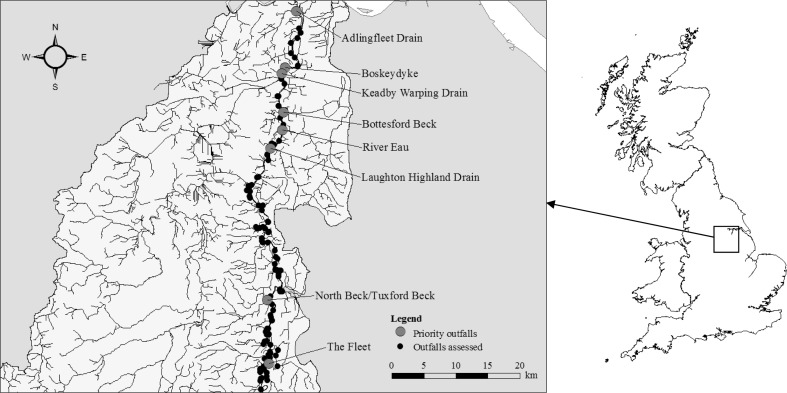
In the tidal Trent, B ranged from 40 (4 × 2 × 5 × 1 × 1) to 960 (4 × 4 × 5 × 3 × 4) for the 129 potential barriers to the lateral migration of European eel. Bottesford Beck and the River Eau were ranked as top priorities for retrofitting ‘fish-friendly’ flap-gates, followed by Boskeydyke, Keadby Warping Drain, Adlingfleet Drain, the Fleet, Laughton Highland Drain and North Beck/Tuxford Beck (Table 6; Fig. 2). Bottesford Beck and the River Eau were ranked as the highest priorities due to a combination of the poor status of the eel stocks upstream, the low passage efficiency of the outfalls by eel, the location of the outfalls downstream of other major barriers, and the comparatively large quantity and high quality of habitat that would become available to eel following passage improvements.
Table 6.
Priority barriers in the tidal River Trent for lateral passage improvements for European eel
| Watercourse | F | P | A | Hqn | Hql | B | Rank |
|---|---|---|---|---|---|---|---|
| Bottesford Beck | 4 | 4 | 5 | 3 | 4 | 960 | 1 |
| River Eau | 4 | 4 | 5 | 3 | 4 | 960 | 1 |
| Boskeydyke | 4 | 5 | 5 | 2 | 3 | 600 | 3 |
| Keadby Warping Drain | 4 | 4 | 5 | 2 | 3 | 480 | 4 |
| Adlingfleet Drain | 4 | 4 | 5 | 2 | 3 | 480 | 4 |
| The Fleet | 4 | 3 | 5 | 2 | 3 | 360 | 6 |
| Laughton Highland Drain | 4 | 3 | 5 | 2 | 2 | 240 | 7 |
| North Beck/Tuxford Beck | 4 | 3 | 5 | 2 | 2 | 240 | 7 |
F fish stock status, P passage efficiency, A likelihood of access, Hqn habitat quantity, Hql habitat quality, B barrier score
Fig. 2.
Barriers in the tidal River Trent assessed for lateral passage improvements for European eel
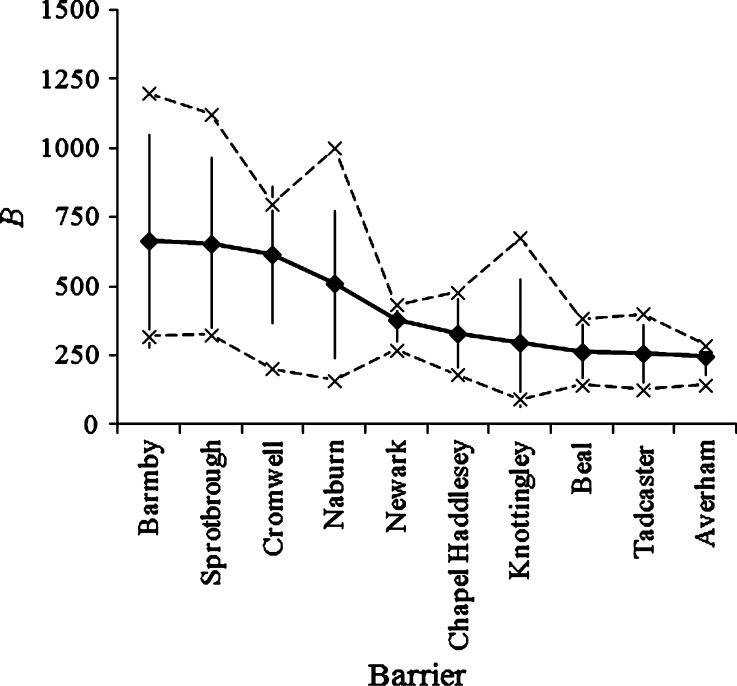
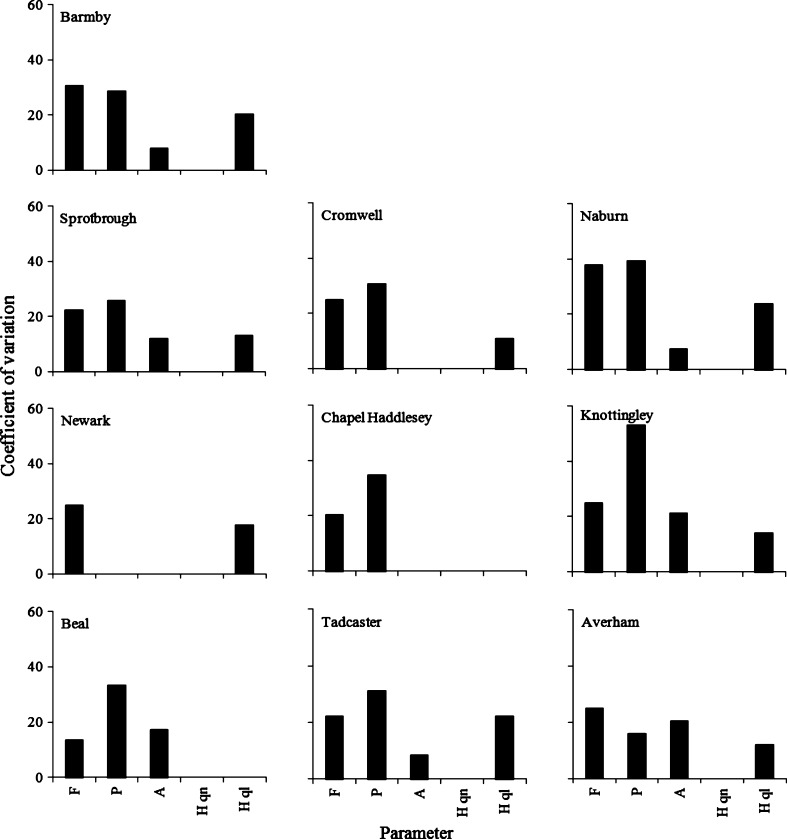
Using an additive (B = F + P + A + Hqn + Hql) rather than a multiplicative (B = F × P × A × Hqn × Hql) formula made little difference to the ranking and prioritization of the barriers. It was considered, however, that the wide range in B produced by the multiplicative formula (potential range 1–3125) identified the high- and low-priority barriers for passage improvements more effectively than did the narrower range of the additive formula (potential range 5–25). The choice of class boundaries made little difference to the ranking and prioritization of the barriers, as each was standardized against their maximum values. As anticipated, there was some variation in the estimates of B between assessors, but there was generally good concordance in the relative importance (rank) of the barriers (Fig. 3). The greatest sources of between-assessor differences in B were generally the estimates of F and P (Fig. 4). By contrast, there was less variation in the estimates of A, Hqn and Hql between assessors (Fig. 4).
Fig. 3.
Mean (±S.D.), minimum and maximum barrier scores (B) for priority barriers in the Humber basin for longitudinal passage improvements for European eel
Fig. 4.
Variations in parameter scores between assessors for priority barriers in the Humber basin for longitudinal passage improvements for European eel. Fish stock status (F), passage efficiency (P), likelihood of access (A), habitat quantity (Hqn), habitat quality (Hql)
Discussion
Migration barriers have been identified as one of the main factors contributing to the decline of many fish and lamprey populations worldwide (Baras and Lucas 2001; Limburg and Waldman 2009; Renaud 1997). Impacts are invariably observed most rapidly and dramatically when watercourses are dammed, but the cumulative effects of large numbers of smaller obstructions can also be significant (Lucas et al. 2009; Nunn et al. 2008). Tools are available to mitigate the impacts of barriers, thereby facilitating the migration of fishes up- and downstream or into adjacent watercourses (Cowx 1998; FAO 2002; Larinier 1998, 2008). Options include barrier removal, fish passes, moveable weirs and so-called fish-friendly flap-gates. A frequent problem, however, is either that it is not possible to remove the large numbers of barriers found in many river systems or that there are insufficient funds with which to mitigate the cumulative impacts of multiple barriers. As such, prioritization of the removal or mitigation of migration barriers is necessary.
A weakness of many prioritization methods is that barriers are assessed independently, without consideration of potential barriers downstream (Kemp and O’Hanley 2010). In addition, many are complicated, laborious or require large amounts of detailed information to be collected on a site-by-site basis, or are too rigid to be easily applied to certain situations. This study presents a simple but effective method, intended for use by environmental managers, government agencies and conservation bodies, of rapidly assessing and prioritizing fish- and lamprey-migration barriers for passage improvements. A prioritization matrix was developed using information on fish stock status, the passage efficiency of fishes at individual structures, the difficulty of passage upstream to each structure, and the quantity and quality of habitat upstream of each structure. Importantly, the ‘Likelihood of access’ was incorporated into the matrix to account for passage efficiency at downstream barriers. Barriers ranked as the highest priority for passage improvements were those characterized by poor fish stocks upstream, low passage efficiency, easy passage from downstream, and a large quantity and high quality of habitat upstream, as these represent the greatest opportunity to optimize benefits for fishes, lampreys and other migratory organisms. As such, structures ranked as the highest priority may not necessarily be the most significant migration barriers. Conversely, major barriers only a short distance downstream of another obstruction may only be ranked as medium or low priority.
Scoring and ranking methods are an established tool for prioritizing fish-passage improvements, in spite of their frequent reliance on subjective data or expert judgement (Kemp and O’Hanley 2010). Similar methods are also widely used in the risk assessment of non-native species (e.g. Copp et al. 2009; Tricarico et al. 2010). The matrix developed here is rapid to use, requires only limited data, and is sufficiently flexible to be applied to a range of scenarios. For example, the class boundaries for many of the parameters can be adjusted to suit particular species or study areas, the matrix can be applied in situations where there is more than one target species or river basin, and if no empirical data are available (as is often the case) it is possible to use expert judgement (following site visits). If necessary, the matrix can be used to short-list key barriers prior to more extensive and objective assessment or cost-benefit analysis. Although the matrix is inherently subjective, it is still effective at prioritizing passage improvements and, moreover, is more likely than complex methods to be adopted by the personnel tasked with assessing migration barriers in the field. Furthermore, the subjectivity of the matrix is not necessarily a weakness, as it is the importance of each barrier relative to other barriers, rather than the absolute ‘passability’ of the barriers per se, that is important when attempting to prioritize passage improvements. Indeed, it is unnecessary to precisely quantify each of the parameters required for the matrix, even if it was possible and cost effective in terms of time and resources, if they are to be subsequently assigned to categories or ranked.
In the current study, the matrix was used to prioritize passage improvements for two anadromous and one catadromous species. Ten barriers (nine weirs, one barrage) in the Humber basin were prioritized for the installation of fish passes, and eight barriers (flapped outfalls) in the tidal River Trent were considered suitable for retrofitting ‘fish-friendly’ flap-gates. Some of these barriers were ranked as high priority because of the large quantity and high quality of habitat that would become available following passage improvements, whereas others were ranked highly due to a combination of the poor status of the stocks upstream, the low passage efficiency at the barriers by the target species, and their location downstream of other major barriers. The choice of class boundaries made little difference to the ranking and prioritization of the barriers, as each was standardized against their maximum values. Moreover, even if the absolute values of B do change, either because of the choice of class boundaries or differences in expert judgement between assessors, the rank of the majority of barriers, as here, is unlikely to alter substantially. Notwithstanding, slight changes may occur if two similarly scored barriers fall either side of a class boundary, although this will always be a possibility when class boundaries are employed.
Prior to any rehabilitation scheme, it is prudent to conduct surveys so that the benefits of remediation activities can be identified and quantified. Similarly, although expert judgement can be used in the prioritization matrix, empirical data will increase the objectivity of the assessment. Data on some species are sparse and sometimes inaccurate, however, as many surveys target particular species or families, and because non-target species are frequently only recorded in terms of presence/absence or using a measure of approximate abundance (e.g. on a logarithmic scale). This is particularly the case for large river systems, which are often difficult to sample in the middle and lower reaches because of excessive channel widths and water depths. In addition, many smaller watercourses, which may also be important habitats for migratory fishes and lampreys, are rarely surveyed. As such, populations in many areas are likely to have been underestimated. Specific surveys may therefore be required to quantify the status of target populations before passage improvements are undertaken (Cowx et al. 2009; Moser et al. 2007), and surveys of passage efficiency and habitat quality will also reduce the subjectivity of the assessment (Kemp and O’Hanley 2010; Raven et al. 1998). Indeed, the greatest sources of between-assessor differences in B were generally the estimates of F and P. It may also be possible to incorporate confidence rankings for estimates based upon expert judgement, as is being developed for risk assessment of non-native species (e.g. Copp et al. 2009; Tricarico et al. 2010).
Acknowledgments
The authors would like to thank the Environment Agency for partly funding both case studies, and Steve Axford, Barry Byatt, Paul Frear, Tim Jacklin, Mike Lee, Alex Lumsdon, Joel Rawlinson, Darren Rollins, Dan Smallwood and Neil Trudgill for conducting independent prioritizations. The prioritization matrix was developed, with permission, from an early draft of the (unpublished) Environment Agency’s National Fish Pass Prioritization Project. The views expressed in the paper are those of the authors and not necessarily those of the Environment Agency. The paper benefited greatly from the constructive criticism of two anonymous referees.
Biographies
A. D. Nunn
is a Post-Doctoral Researcher at the University of Hull. Research interests include the ecology and rehabilitation of floodplain ecosystems, the migration of aquatic organisms, and the conservation and management of designated species.
I. G. Cowx
is a Professor of Applied Fisheries Science at the University of Hull and Director of Hull International Fisheries Institute (HIFI). Research interests include inland fisheries management and rehabilitation.
References
- Baras E, Lucas MC. Impacts of man’s modifications of river hydrology on the migration of freshwater fishes: A mechanistic perspective. Ecohydrology and Hydrobiology. 2001;1:291–304. [Google Scholar]
- Copp GH, Vilizzi L, Mumford J, Fenwick GV, Godard MJ, Gozlan RE. Calibration of FISK, an invasiveness screening tool for nonnative freshwater fishes. Risk Analysis. 2009;29:457–467. doi: 10.1111/j.1539-6924.2008.01159.x. [DOI] [PubMed] [Google Scholar]
- Cowx IG. Review of fish passage facilities in the UK: Issues and options for future development. In: Jungwith M, Schmutz S, Weiss S, editors. Fish migration and fish bypasses. Oxford: Fishing News Books, Blackwell Science; 1998. pp. 220–235. [Google Scholar]
- Cowx IG, Harvey JP, Noble RA, Nunn AD. Establishing survey and monitoring protocols for the assessment of conservation status of fish populations in river special areas of conservation in the UK. Aquatic Conservation: Marine and Freshwater Ecosystems. 2009;19:96–103. doi: 10.1002/aqc.968. [DOI] [Google Scholar]
- Fish passes—Design, dimensions and monitoring. Rome: FAO; 2002. [Google Scholar]
- Kemp PS, O’Hanley JR. Procedures for evaluating and prioritising the removal of fish passage barriers: A synthesis. Fisheries Management and Ecology. 2010;17:297–322. [Google Scholar]
- Larinier M. Upstream and downstream fish passage experience in France. In: Jungwith M, Schmutz S, Weiss S, editors. Fish migration and fish bypasses. Oxford: Fishing News Books, Blackwell Science; 1998. pp. 127–145. [Google Scholar]
- Larinier M. Fish passage experience at small-scale hydro-electric power plants in France. Hydrobiologia. 2008;609:97–108. doi: 10.1007/s10750-008-9398-9. [DOI] [Google Scholar]
- Limburg KE, Waldman JR. Dramatic declines in North Atlantic diadromous fishes. BioScience. 2009;59:955–965. doi: 10.1525/bio.2009.59.11.7. [DOI] [Google Scholar]
- Lucas MC, Bubb DH, Jang MH, Ha K, Masters JEG. Availability of and access to critical habitats in regulated rivers: Effects of low-head barriers on threatened lampreys. Freshwater Biology. 2009;54:621–634. doi: 10.1111/j.1365-2427.2008.02136.x. [DOI] [Google Scholar]
- Moser ML, Butzerin JM, Dey DB. Capture and collection of lampreys: The state of the science. Reviews in Fish Biology and Fisheries. 2007;17:45–56. doi: 10.1007/s11160-006-9037-3. [DOI] [Google Scholar]
- Nunn AD, Harvey JP, Noble RAA, Cowx IG. Condition assessment of lamprey populations in the Yorkshire Ouse catchment, north-east England, and the potential influence of physical migration barriers. Aquatic Conservation: Marine and Freshwater Ecosystems. 2008;18:175–189. doi: 10.1002/aqc.863. [DOI] [Google Scholar]
- Nunn AD, Copp GH, Vilizzi L, Carter MG. Seasonal and diel patterns in the migrations of fishes between a river and a floodplain tributary. Ecology of Freshwater Fish. 2010;19:153–162. doi: 10.1111/j.1600-0633.2009.00399.x. [DOI] [Google Scholar]
- Raven PJ, Holmes NTH, Dawson FH, Everard M. Quality assessment using River Habitat Survey data. Aquatic Conservation: Marine and Freshwater Ecosystems. 1998;8:477–499. doi: 10.1002/(SICI)1099-0755(199807/08)8:4<477::AID-AQC299>3.0.CO;2-K. [DOI] [Google Scholar]
- Renaud CB. Conservation status of Northern Hemisphere lampreys (Petromyzontidae) Journal of Applied Ichthyology. 1997;13:143–148. doi: 10.1111/j.1439-0426.1997.tb00114.x. [DOI] [Google Scholar]
- Tricarico E, Vilizzi L, Gherardi F, Copp GH. Calibration of FI-ISK, an invasiveness screening tool for nonnative freshwater invertebrates. Risk Analysis. 2010;30:285–292. doi: 10.1111/j.1539-6924.2009.01255.x. [DOI] [PubMed] [Google Scholar]
- White EM, Knights B. Dynamics of upstream migration of the European eel, Anguilla anguilla (L.), in the Rivers Severn and Avon, England, with special reference to the effects of man-made barriers. Fisheries Management and Ecology. 1997;4:311–324. doi: 10.1046/j.1365-2400.1997.00050.x. [DOI] [Google Scholar]