Abstract
Sigma1 receptor (sigma1R), a significant protein, has been found to be frequently upregulated in human tumor cells and tissues. It has been demonstrated that sigma1R is involved in proliferation and adhesion of cancer cells. However, the significance of sigma1R expression in esophageal squamous cell carcinoma (ESCC) remains unclear. In this article, by a series of methods, the authors examined the expression of sigma1R protein in ESCC cell lines and tissues. Flow cytometry indicated intense staining of sigma1R in ESCC cells. Immunocytochemistry staining demonstrated that sigma1R was mainly distributed in cytoplasm and nucleus in ESCC cell lines. Western blotting was performed to characterize the relative expression of sigma1R in different ESCC cell lines. Moreover, different levels of sigma1R were presented from normal epithelium to carcinoma by immunohistochemistry analysis, which demonstrated that sigma1R was highly expressed in tumors. Association analysis showed significant correlations between total sigma1R protein levels and pathologic TNM (pTNM) classification of tumors (r=0.216, p=0.011). Furthermore, the sigma1R in the nucleus was significantly correlated with pTNM classification and lymph node metastasis (r=0.263, p=0.002, and r=0.269, p=0.002, respectively). These data indicated that sigma1R may serve as a potential predictive factor for pTNM classification and tumor development in ESCC.
Keywords: esophageal squamous cell carcinoma, sigma1 receptor, pathologic TNM classification
Esophageal carcinoma (EC) is one of the most common malignant tumors in the world, the origin of which has been related to many factors (Stoner and Gupta 2001). Esophageal squamous cell carcinoma (ESCC), one of the two main histological types of esophageal cancer, is the predominant histopathological type in East Asian countries with high mortality rate (Shimada et al. 2003; Parkin et al. 2005). So far, the methods of diagnosis and treatment of the disease are numerous, but a short-lived survival indicates its poor prognosis (Reed 1999). Thus, exploration of novel molecular markers for prognostication is required in clinical diagnosis and therapy.
The sigma receptor (sigmaR) was first postulated as a novel opioid receptor by Martin et al. (1976). Subsequent studies centering on its pharmacology and behavior and the cloning of the sigma1R subtype in 1996 revealed indeed that the “sigma-binding site” corresponded to a 25-kDa protein that was unrelated to known mammalian proteins but possessed weak homology with fungal sterol isomerase (Hanner et al. 1996; Kekuda et al. 1996). And now the sigmaR is viewed as an independent receptor family. Aydar et al. (2002) have demonstrated that the sigma1R has two transmembrane segments that are localized to the plasma membrane with the NH2 and COOH termini on the cytoplasmic side of the membrane. A variety of subcellular localization experiments have suggested sigma1R localized in the plasma membrane, the membranes of the endoplasmic reticulum, and the nucleus (Hanner et al. 1996; Aydar et al. 2002; Hayashi et al. 2003).
Endogenous ligands for sigma receptors are not defined yet. Nevertheless, the receptors have high affinity with psychoactive drugs, such as haloperidol, phencyclidine, and benzomorphans (Quirion et al. 1992; Hanner et al. 1996). Pentazocine and (+)-SKF10047 bind only to sigma1R (Quirion et al. 1992). Although we know little about the transduction mechanisms of sigma1R, it has been demonstrated that the functions of the receptor are diversified. Sigma1R was shown to be involved in regulation of dysphrenia, neurotransmitter release, learning and memory processes, and so on (Su 1993; Ishiguro et al. 1998). In addition, there was increasing evidence that the sigma1R could play a significant role in cancer biology (Crawford and Bowen 2002). Earlier reports indicated the sigma1R was highly expressed in various human cancer tissues, including small- and non-small-cell lung carcinoma, large-cell carcinoma, renal carcinoma, colon carcinoma, sarcoma, brain tumors, breast cancer, melanoma, glioblastoma, neuroblastoma, and prostate cancer (Thomas et al. 1990; Bem et al. 1991; John et al. 1995; Vilner et al. 1995; Moody et al. 2000; Crawford et al. 2002). Previous research showed that sigma1R participated in some biological behavior such as proliferation, adhesion, and cell death of tumors (Brent and Pang 1995; Aydar et al. 2006). However, the expression of sigma1R and its biological relevance were not identified in ESCC. In this report, we aimed to determine the expression of sigma1R in ESCC and evaluate whether sigma1R could be regarded as a novel biomarker that might contribute to the prognosis of ESCC and pathogenesis of the disease.
Materials and Methods
Cell Lines and Specimen Collection
Human ESCC cell lines KYSE180, KYSE150, and EC109 were cultured in 1640 medium (Thermo, Waltham, MA) plus 10% fetal calf serum. All cells were maintained at 37C in a humidified 5% CO2 atmosphere. These cell lines were previously established from primary human esophageal squamous cell carcinomas (CICAMS 1976; Shimada et al. 1992) and provided by professor Dong Xie (Institute for Nutritional Sciences of Chinese Academy of Sciences, China).
For the retrospective study, archival formalin-fixed, paraffin-embedded specimens from 143 patients were obtained from the Department of Pathology of Shantou Central Hospital from 1987 to 1997. The samples of 143 patients including 64 men and 79 women (median age, 54 years) had undergone tissue microarray (TMA) construction before immunohistochemical staining. Information on sex, age, stage of disease, and histopathologic parameters were retrieved from the medical records. Patients’ data are summarized in Table 1. To evaluate the expression of sigma1R in the esophagus tissues, we chose 18 primary ESCC patients who were from Shantou Central Hospital and affiliated Hospital of Medical College of Shantou University for routine pathological sections. The routine pathological sections included normal esophagus tissues, precancerous lesions, and/or tumor tissues. The number of normal esophagus tissue, low-grade dysplasia, high-grade dysplasia, and carcinoma was 12, 18, 8, and 18, respectively.
Table 1.
Basic Information of the Patients by Clinical Characteristics
| Clinical Parameters | No. | Five-Year Survival Rate (%) | p a |
|---|---|---|---|
| Age (year) | |||
| ≤54 | 106 | 34.2 | 0.822 |
| >54 | 37 | 29.6 | |
| Gender | |||
| Male | 64 | 37.2 | 0.783 |
| Female | 79 | 28.6 | |
| Tumor size | |||
| ≤3cm | 31 | 44.0 | 0.049 |
| 3–5cm | 79 | 35.9 | |
| >5cm | 33 | 15.0 | |
| Differentiation grade | |||
| G1 | 26 | 58.4 | 0.057 |
| G2 | 99 | 28.7 | |
| G3 | 18 | 19.6 | |
| Invasive depth | |||
| T2 | 17 | 35.3 | 0.305 |
| T3 | 120 | 34.8 | |
| T4 | 6 | 0 | |
| Lymph node metastasis | |||
| N0 | 77 | 49.7 | 0.000 |
| N1+N2+N3 | 66 | 12.4 | |
| pTNM classification | |||
| IA+IB | 4 | 50.0 | 0.000 |
| IIA+IIB | 71 | 47.2 | |
| IIIA+IIIB+IIIC | 49 | 23.5 | |
| IV | 19 | 5.3 | |
| Treatmentb | |||
| Only surgery | 85 | 38.8 | 0.467 |
| Surgery + chemo | 26 | 15.6 | |
| Surgery + radio | 25 | 28.9 | |
| Surgery + chemo + radio | 7 | 26.8 | |
Kaplan-Meier curves (long-rank test); each p value is two-tailed and significance level is 0.05.
Surgery + Chemo, surgery combining chemotherapy; Surgery + Radio, surgery combining radiotherapy; Surgery + Chemo + Radio, surgery combining chemotherapy and radiotherapy.
All of the tumors were confirmed as ESCC by the pathologists in Clinical Pathology Department of the Hospital, and the cases were classified according to the seventh edition of the tumor-node-metastasis (TNM) classification of the International Union Against Cancer. Evaluation of tumor differentiation was based on histological criteria of the guidelines of the World Health Organization’s Pathological Classification of Tumors. The study was approved by the Ethics Committee of the Center Hospital of Shantou City, the local ethics committee, and only patients with written informed consent were included.
Flow Cytometry
Approximately 2×105 cells were seeded on the 25-cm2 culture flask and incubated overnight. Cells were collected to 1.5-ml centrifuge tube after disposed by pancreatic enzymes. After being washed with PBS, cells were fixed with 70% alcohol for 30 min and then treated with 100% methanol at −10C for 5 min. Cells were subsequently incubated with a blocking solution (1%BSA in PBS) for 30 min and incubated with anti-rabbit sigma1R polyclonal antibody (1:10, Abgent, San Diego, CA) for 1.5 hr. Cells were washed and incubated with FITC-conjugated anti-rabbit IgG (1:50, Thermo, Waltham, MA) as a secondary antibody for 40 min in dark place. Afterward, the cells were resuspended in PBS and examined the immunoreaction with a flow cytometer (FACSCalibur CV<2, BD, Franklin Lakes, NJ). All experiments were carried out in triplicate.
Immunocytochemistry Staining
In sum, 1×104 cells were seeded on 24-well plates and incubated for 24 hr. After being washed with PBS, cells were fixed with 100% methanol at −10C for 15 min. Cells were subsequently incubated with a blocking solution (1%BSA in PBS) for 60 min and incubated with anti-rabbit sigma1R polyclonal antibody (1:50) overnight at 4C. Afterward, cells were washed and disposed with 3rd Gen IHC Detection Kit (Invitrogen, San Francisco, CA). Cells were rinsed well with distilled water. Finally, slides were counterstained with hematoxylin, dehydrated, and then mounted with neutral balsam. The negative control was prepared by substituting PBS for the primary antibodies. Experiments were repeated in triplicate.
Western Blotting Analysis
Total cells lysates were prepared in 1×Laemmli Sample Buffer (Bio-Rad, Contra Costa, CA). The lysates were then centrifuged for 5 min (12,000 rpm, 4C). The protein concentration was estimated by the Pierce 660 nm Protein Assay. An equal amount of tissues lysates (50 µg) was electrophoresed on 12% polyacrylamide gel with 40-V voltage for 30 min, followed by 60-V voltage for 3 hr, respectively. Then the lysates were transferred to PVDF membranes (Millipore, Bedford, MA). The membranes were blocked with 5% skim milk-phosphate-buffered saline Tween (0.01M PBS, 0.05% Tween 20) for 1 hr and incubated at room temperature for 1.5 hr with anti-rabbit sigma1R polyclonal antibody (1:50). The membrane was subsequently incubated at room temperature for 1.5 hr with anti-mouse or anti-rabbit peroxidase-conjugated secondary antibodies (1:2000, Santa Cruz Biotechnology, Santa Cruz, CA) and analyzed using Western Blotting Luminol Reagent (Santa Cruz Biotechnology, Santa Cruz, CA). Image acquisition and quantitative analysis were carried out using the FluorChem 8900 image analysis system (Alpha Innotech, Miami, FL). To verify the relative amounts of protein in each lane, the level of beta-actin as an internal control was measured with anti-beta-actin monoclonal antibody (1:1000, Sigma, St. Louis, MO).
TMA Construction
TMA construction of esophageal carcinoma tissue has been described (Zhang et al. 2008). Briefly, TMAs for immunohistochemistry were generated from samples selected from those specimens with more tissue available for persistent correlative studies. In advance, representative regions of each tissue were singled out of hematoxylin-stained and eosin-stained sections and marked on the individual paraffin blocks. Two tissue cores at least were acquired from each specimen, measuring 1.8 mm in diameter and 1.0 to 3.0 mm in length depending on the depth of tissue in the donor block. Each core was precisely arrayed into a new paraffin block. These microarrays were serially sectioned (4 µm) and stained with hematoxylin and eosin to ensure tissue sampling and completeness. The unstained sections were baked overnight at 56C in preparation for immunohistochemistry staining.
Immunohistochemistry Staining
The sections were dewaxed in xylene and rehydrated in a series of graded alcohols. After that, slides were submerged in a Peroxidase Quenching Solution containing one part of 30% hydrogen peroxide to nine parts of absolute methanol for 10 min. After rinsing in PBS, antigen retrieval from the tissue was carried out by autoclaving in 0.01 M sodium citrate buffer (pH 6.0) at 120C for 3 min. Next, sections were blocked in 10% normal goat serum for 10 min at room temperature (RT) and then incubated overnight at 4C with anti-rabbit sigma1R polyclonal antibody (1:50). Then, the sections were subjected to immunostaining in the PV-9000 2-step plus Poly-HRP Anti-Mouse/Rabbit IgG Detection System (ZSGB-BIO, Beijing, China) and the Liquid DAB Substrate Kit (Invitrogen, San Francisco, CA). Samples were rinsed well with distilled water. Subsequently, slides were counterstained with Maye’s hematoxylin, dehydrated, and mounted.
The immunohistochemical staining results were assigned a mean score considering both the intensity of staining and the proportion of tumor cells showing unequivocal positive reaction. Each section was independently assessed by two histopathologists without prior knowledge of patients’ data. Positive reactions were defined as those showing brown signals in the cell cytoplasm, nucleus, and membrane. For sigma1R, a staining index (values 0–12) was determined by multiplying the score for staining intensity with the score for positive area—the intensity of staining: 0, no staining; 1, weak staining; 2, moderate staining; and 3, strong staining; the tumor cells area: 1, positive staining in 0–25% of tumor cells; 2, positive staining in 25–50% of tumor cells; 3, positive staining in 51–75% of tumor cells; 4, positive staining in 75–100% of tumor cells. For statistical analyses, scores of 0–4 were considered − (weak staining), scores of 5–8 were considered + (moderate staining), and scores of 9–12 were considered ++ (intense staining).
Statistical Analysis
Associations of sigma1R expression and other clinicopathological characteristics, including age, gender, tumor size, tumor differentiation grade, invasive depth, lymph nodes metastasis, andTNM classification, were assessed with the Kendall’s tau-b test. Kaplan-Meier curves were constructed for overall survival (OS) analysis by a log-rank test. All statistical analyses were performed with SPSS 13.0 software (SPSS, Inc., Chicago, IL). Each p value is two-tailed, and significance level is 0.05.
Results
Sigma1R Expression in ESCC Cell Lines
We examined the level of sigma1R in KYSE180, KYSE150, and EC109 cells by several methods. Results showed that there were some differences in sigma1R expression depending on the cell lines. By flow cytometry, sigma1R was detected in these three ESCC cells (Fig. 1), and the fluorescence intensity was between 80 and 150. Data from Immunocytochemistry staining showed different subcellular localization of sigma1R in ESCC cells (Fig. 2A). In KYSE180 cells, the membrane was strongly stained, whereas in EC109 cells, positive staining of sigma1R was mainly in nucleus. In addition, we performed Western blot to identify the level of sigma1R protein in ESCC cells. The results demonstrated that the EC109, KYSE180, and KYSE150 cell lines all expressed sigma1R protein in 25 kDa (Fig. 2B). Further analysis indicated EC109 cell lines expressed most sigma1R.
Figure 1.
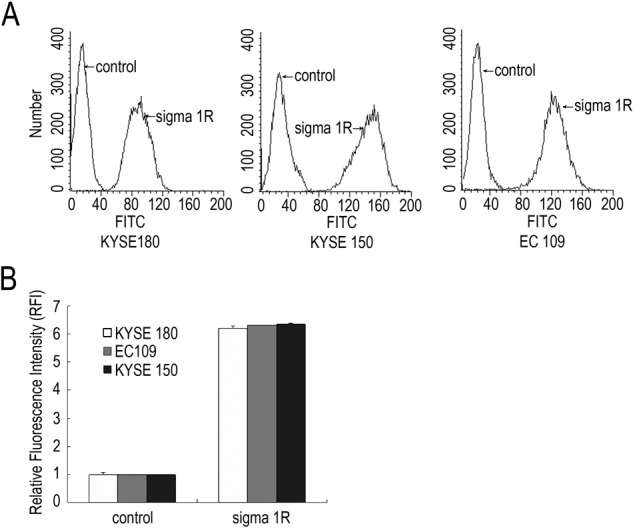
Flow cytometry analysis of sigma1R in three esophageal squamous cell carcinoma (ESCC) cell lines. A. Sigma1R expression in three cell lines. PBS replaced the primary antibody and was regarded as a negative control in preparation for flow cytometry. B. Relative fluorescence intensity. The experiments were repeated for three times, the value was expressed in Mean±SD.
Figure 2.
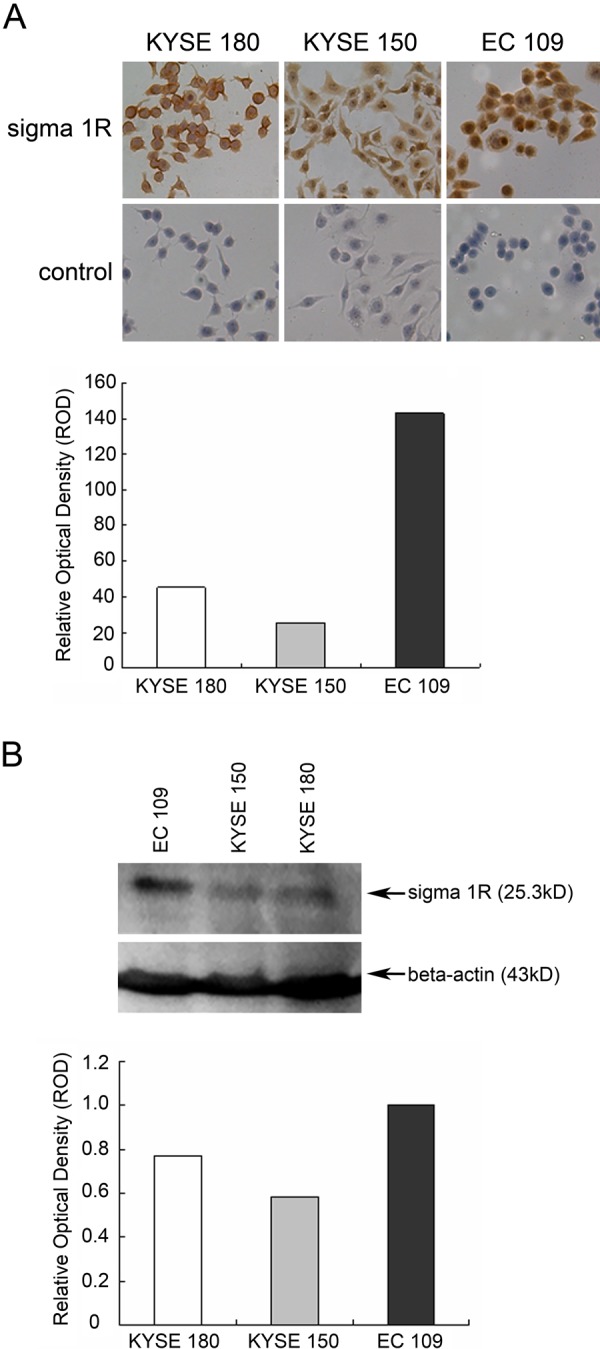
Immunostaining and immunoblotting analyses of sigma1R in ESCC cell lines. A. Immunocytochemistry analysis of sigma1R in three ESCC cells (DAB immunostaining). Original magnification (×200). B. Immunoblotting analysis of sigma1R expression in ESCC cells. Expression of beta-actin was simultaneously tested as an internal control. Densitometric analysis for immunocytochemistry analysis (A) and Western blot analysis (B) by Imagepro-plus and ImageJ, respectively. The relative optical density in ESCC cells was performed in the diagram.
Sigma1R Expression in the Progression From Normal Epithelium to Carcinoma
By immunohistochemistry analysis, we investigated the immunoreactivity of sigma1R in ESCC tissues. The immunostaining was mainly in cytoplasm in normal epithelium and precancerous lesions. We noticed that sigma1R nucleus and cytomembrane were strongly stained besides overexpression of cytoplasm in tumor tissues (Fig. 3). In ESCC, most of the cancer cells showed intense immunostaining (Fig. 3B), whereas weak to moderately positive signals were seen in normal epithelium of the esophagus (Fig. 3A). Positive immunostaining for sigma1R could be observed in a cytoplasmic pattern in the progression from normal epithelium to cancerous tissue. The positive rates from normal epithelium, low-grade dysplasia, and high-grade dysplasia to carcinoma were observed in 33.3% (4 of 12), 22.2% (4 of 18), 25.0% (2 of 8) and 61.1% (11 of 18), respectively (Fig. 3C). And there is statistically significant between low-grade dysplasia and ESCC (p=0.041). We conclude that sigma1R is upregulated in ESCC tissues.
Figure 3.
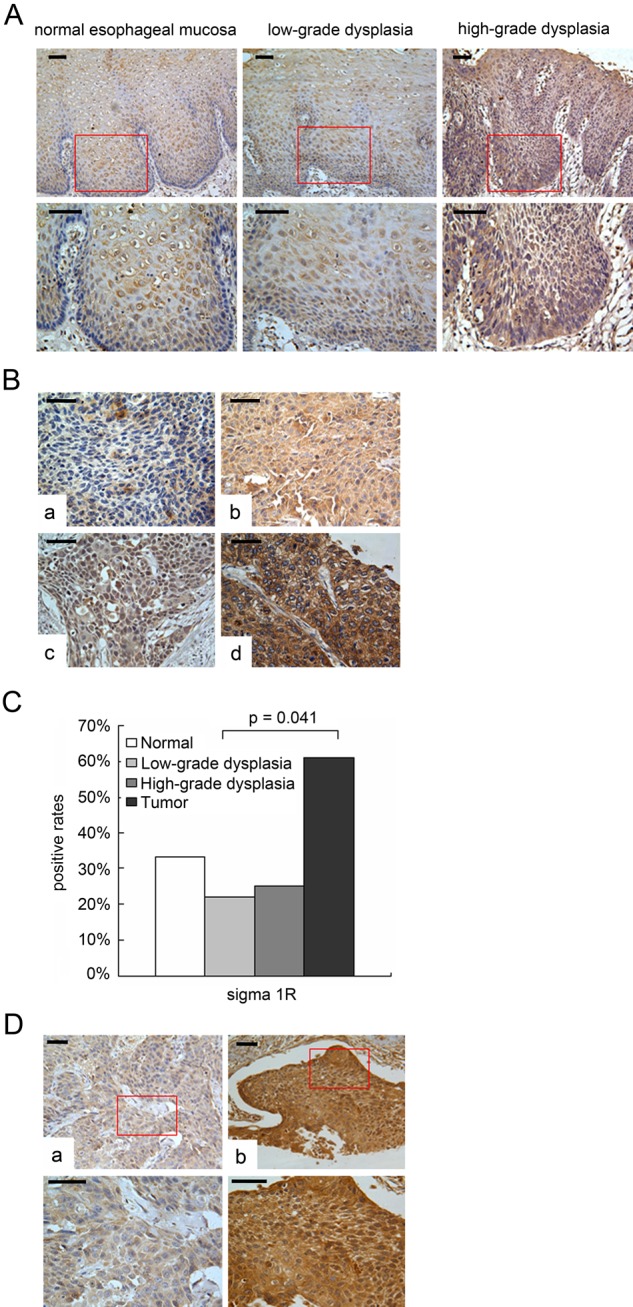
Immunohistochemistry analysis of sigma1R in normal esophagus and ESCC. A. Sigma1R staining in the progression from normal epithelia to precancerous lesions. The staining was mainly in cytoplasm from normal epithelia to precancerous lesions. The full-thickness epithelia showed a reduction of defined membranous staining for sigma1R. In normal epithelia and low-grade dysplasia, the immunostaining of sigma1R was dispersed and poorer compared with that in the high-dysplasia. B. Various staining in ESCC, negative (a), cytoplasmic staining (b), nucleonic staining (c), membranous staining (d). Scale bars = 50 µm. C. Positive rates of sigma1R in normal epithelia, low-grade dysplasia, high-grade dysplasia, and ESCC. Statistical significance was seen between low-grade dysplasia and ESCC (p=0.041). D. Immunohistochemical staining of sigma1R for pTNM classification I+II cases (a) and III+IV cases (b). Scale bars = 50 µm.
Correlations Between Sigma1R and Clinical Characteristics in ESCC
To obtain a better understanding of the clinical significance of sigma1R expression in ESCCs, we correlated its expression with a series of clinicopathological parameters in 143 cases (Table 2). High sigma1R expression was defined as strong intensity staining in greater than 50% of tumor cells. In these samples, 85 of 143 tumors were designated as cases of high sigma1R expression (85 of 143, 59.4%). A significant correlation was shown between sigma1R level and pathologic TNM (pTNM) classification of tumors (r=0.216, p=0.011). The positive rate of sigma1R was observed in 49.3% for grade I and II cases and 70.6% for grade III and IV cases (Fig. 3D). There were no significant correlations between sigma1R expression and other clinical parameters.
Table 2.
Association Between Sigma1R Expression and Clinical Pathological Parameters in ESCC
| Sigma1R Statusa | ||||
|---|---|---|---|---|
| Clinical Parameters | − | + | r b | p b |
| Age (year) | 0.087 | 0.31 | ||
| ≤54 | 29 | 35 | ||
| >54 | 29 | 50 | ||
| Gender | −0.130 | 0.173 | ||
| Male | 39 | 67 | ||
| Female | 19 | 18 | ||
| Tumor size | 0.158 | 0.053 | ||
| ≤3cm | 17 | 14 | ||
| 3–5cm | 31 | 48 | ||
| >5cm | 10 | 23 | ||
| Differentiation | −0.056 | 0.499 | ||
| G1 | 9 | 17 | ||
| G2 | 41 | 58 | ||
| G3 | 8 | 10 | ||
| Invasive depth | 0.122 | 0.166 | ||
| T2 | 8 | 9 | ||
| T3 | 50 | 70 | ||
| T4 | 0 | 6 | ||
| Lymph node metastasis | 0.165 | 0.061 | ||
| N0 | 37 | 40 | ||
| N1+N2 | 21 | 45 | ||
| pTNM classification | 0.216 | 0.011 | ||
| I+II | 38 | 37 | ||
| III+IV | 20 | 48 | ||
−, negative, scores of 0–8; +, positive, scores of 9–12.
Kendall’s tau-b test; r, Kendall tau coefficient value; each p value is two-tailed, and significance level is 0.05.
To address the relevance of sigma1R localizations in ESCC, we investigated the relationship between sigma1R subcellular distribution and clinicopathologic parameters (Table 3). A significant correlation was found between the membranous sigma1R and the tumor size (r=0.200, p=0.019). The intense staining was presented more frequently in bigger size of ESCC cases. Moreover, sigma1R in the nucleus was significantly correlated with pTNM classification and lymph node metastasis (r=0.263, p=0.002, and r=0.269, p=0.002, respectively). The positive rate of sigma1R was 27.3% for lymph node metastasis cases and 53.0% for lymph node metastasis cases.
Table 3.
Association Between Sigma1R Expression and Clinical Pathological Parameters in ESCC
| Clinical Parameters | Sigma1R Status | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytoplasma | Nucleusb | Membraneb | ||||||||||
| − | + | r c | p c | − | + | r c | p c | − | + | r c | p c | |
| Age (year) | ||||||||||||
| ≤54 | 30 | 34 | 0.077 | 0.398 | 37 | 27 | −0.056 | 0.606 | 56 | 8 | 0.200 | 0.024 |
| >54 | 31 | 48 | 50 | 29 | 56 | 23 | ||||||
| Gender | ||||||||||||
| Male | 42 | 64 | −0.104 | 0.249 | 63 | 43 | −0.049 | 0.696 | 83 | 23 | −0.001 | 1 |
| Female | 19 | 18 | 24 | 13 | 29 | 8 | ||||||
| Tumor size | ||||||||||||
| ≤3cm | 17 | 14 | 0.158 | 0.052 | 20 | 11 | 0.066 | 0.410 | 27 | 4 | 0.183 | 0.019 |
| 3–5cm | 34 | 45 | 49 | 30 | 64 | 15 | ||||||
| >5cm | 10 | 23 | 18 | 15 | 21 | 12 | ||||||
| Differentiation | ||||||||||||
| G1 | 9 | 17 | −0.061 | 0.484 | 14 | 12 | −0.048 | 0.581 | 20 | 6 | −0.094 | 0.271 |
| G2 | 44 | 55 | 62 | 37 | 75 | 24 | ||||||
| G3 | 8 | 10 | 11 | 7 | 17 | 1 | ||||||
| Invasive depth | ||||||||||||
| T2 | 9 | 8 | 0.149 | 0.086 | 12 | 5 | 0.116 | 0.186 | 13 | 4 | 0.014 | 0.985 |
| T3 | 52 | 68 | 73 | 47 | 95 | 25 | ||||||
| T4 | 0 | 6 | 2 | 4 | 4 | 2 | ||||||
| Lymph node metastasis | ||||||||||||
| N0 | 38 | 39 | 0.146 | 0.092 | 56 | 21 | 0.263 | 0.002 | 58 | 19 | −0.079 | 0.418 |
| N1+N2 | 23 | 43 | 31 | 35 | 54 | 12 | ||||||
| pTNM classification | ||||||||||||
| I+II | 40 | 35 | 0.227 | 0.007 | 55 | 20 | 0.269 | 0.002 | 56 | 19 | −0.093 | 0.313 |
| III+IV | 21 | 47 | 32 | 36 | 56 | 12 | ||||||
–, negative, scores of 0–8; +, positive scores of 9–12.
−, negative, scores of 0–4; +, positive, scores of 5–12.
Kendall’s tau-b test; r, Kendall tau coefficient value; each p value is two-tailed and significance level is 0.05.
Impact of Sigma1R Expression on the Overall Survival of ESCC Patients
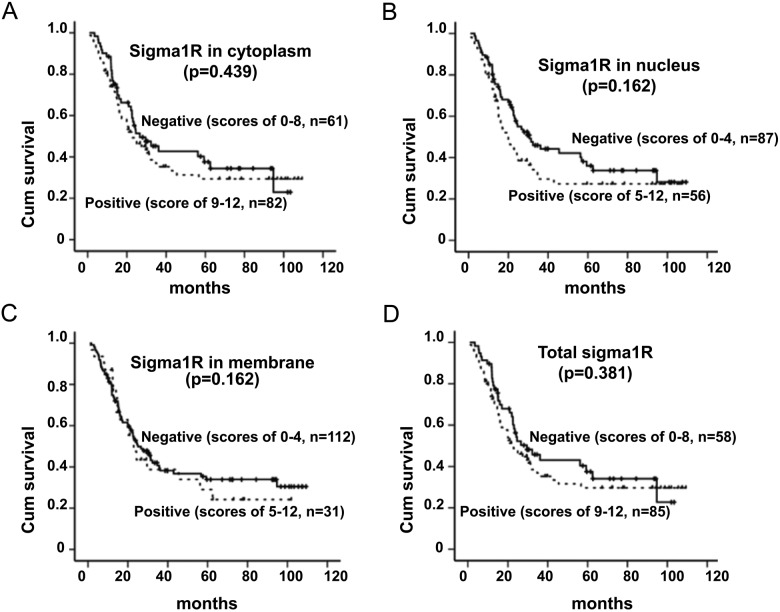
Kaplan-Meier survival analysis was used to obtain the impact of sigma1R expression on the overall survival of ESCC patients. The patients with sigma1R in high-expressed tumors had an overall five-year survival of 29.7% compared with 37.5% in low-expressed tumors. Nonetheless, no statistical significant difference was observed between sigma1R levels and the overall survival of ESCC (Fig. 4).
Figure 4.
Overall survival of 143 patients with ESCC versus sigma1R. Kaplan-Meier survival curve for (A) cytoplasmic sigma1R, (B) nuclear sigma1R, (C) membranous sigma1R, and total sigma1R.
Discussion
Sigma1R was cloned by Hanner (Hanner et al. 1996) and originally described in nervous system. Although initially demonstrated in neuronal tissues, sigma1R was observed in other organs, including the livers, kidneys, lungs, and the gonads (Wolfe et al. 1989; Hellewell et al. 1994). Subsequent studies have shown that sigma1R was upregulated in several types of cancer (Thomas et al. 1990; Bem et al. 1991; John et al. 1995; Vilner et al. 1995; Moody et al. 2000; Crawford et al. 2002). In this report we explored the expression of sigma1R in ESCC cell lines and normal and tumor tissues of esophagus.
A novel finding in the present study was that sigma1R had a high affinity with ESCC. We carried out flow cytometry, immunocytochemistry, Western blotting, and immunohistochemistry analysis to examine sigma1R expression in ESCC. Flow cytometry analysis showed that sigma1R staining in the three ESCC cells was a little different from that in other test methods. This contradiction may be due to the different survivable microenvironments between the cell lines and the cells in tissue specimens. However, all studies demonstrated that sigma1R was frequently expressed in ESCC cell lines. The subcellular localization was in plasma membrane, cytoplasm, and/or nucleus, which was consistent with previous research (Hanner et al. 1996; Aydar et al. 2002; Hayashi and Su 2003). Interestingly, nucleus was hyperchromatic in EC109 cells, which was shown in immunocytochemistry. It was hypothesized that sigma1R in nucleus might play a role in a certain regulation function. It was reported that the protein of sigma1R possessed weak homology with fungal sterol isomerase and was found to colocalize with human sterol isomerase around the nucleus (Hanner et al. 1996; Dussossoy et al. 1999). The two proteins took the same route in cell cycle and followed the nuclear envelope disassembly and reformation during mitosis (Georgatos et al. 1994; Yang et al. 1997). As we all know, sterol isomerase plays an important part in the dynamic organization of the membrane. It was proposed that sigma1R and human sterol isomerase might involve in reassembling the nuclear envelope and organizing membrane-bound karyoskeleton in cell cycle.
In our work, we investigated sigma1R expression in the progression from normal epithelium to carcinoma. Generally, the positive rates of nonneoplastic tissues were much lower than that of tumor tissues. Although the positive rates of nonneoplastic tissues were a bit unstable, which might be related to limited cases, the whole level was less than 35%. And no significant difference was observed in nonneoplastic tissues. The positive rate of ESCC was greater than 60% and was statistically significant between low-grade dysplasia and ESCC (p=0.041). We consider sigma1R upregulated in ESCC. A larger number of cases would be necessary to determine whether sigma1R is involved in early identification of ESCC. It is reported that some narcotic drugs, such as cocaine, can activate sigma1R expression (Guitart et al. 2004). In addition, oxygen and glucose deprivation are two important factors in inducing sigma1R (Ruscher et al. 2011). Esophagus is an organization connected with the outside. External factors may play a role in inducing sigma1R in ESCC. However, the reason for upregulation of sigma1R in ESCC is worth exploring in our future work.
pTNM classification is a significant factor to measure the prognosis of patients, and lymph node metastasis is an important negative prognostic indicator of ESCC, which is often related to the depth of invasion and distant metastasis (Wolfe et al. 1989; Christein et al. 2002). As the data show above, a significant correlation was observed between sigma1R expression level and pTNM classification (p=0.011), suggesting that sigma1R might play an important role in the development of ESCC. Increased nuclear sigma1R was positively associated with pTNM classification (p=0.200) and lymph node metastasis (p=0.200), indicating that nuclear sigma1R might contribute to tumor metastasis, resulting in malignant progression of ESCC. The results imply that sigma1R may serve as a potential predictive factor for pTNM classification and tumor progression in ESCC. Unfortunately, sigma1R failed to show association with the survival of ESCC patients. After all, our study can only be regarded as an exploratory.
Significant correlation was observed between nuclear sigma1R and lymph node metastasis (p=0.002), less so between the membranous sigma1R and tumor size (p=0.024). It was assumed that different signaling pathways might exist for subcellular distribution of sigma1R. As we mention above, sigma1R was likely to involve in nuclear division with human sterol isomerase in cell cycle (Georgatos et al. 1994; Yang et al. 1997). Further study is required to clarify the signaling in nucleus. According to previous studies of sigma1R, we found that sigma1R had two transmembrane segments with the NH2 and COOH termini on the cytoplasmic side of the membrane (Aydar et al. 2002). Sigma1R drugs were observed to combine with the receptor and play an important role in inhibition of proliferation and apoptosis of cancer cells (Brent and Pang 1995; Spruce et al. 2004). The effects were achieved by interactions with ion channels. Ca2+ channels and K+ channels were two important ion channels involved. Previous research clarified that ion channels participate in proliferation and metastasis in cancer cell lines (Fraser et al. 2000; Ouadid-Ahidouch et al. 2000; Yao and Kwan 1999). These results remind us that sigma1R may participate in tumor progression in ESCC. However, more work needs to be done to figure out the distinct involvement of sigma1R in ESCC.
In summary, our study has shown that sigma1R is upregulated in ESCC and associated with pTNM classification; in addition, nuclear sigma1R has a vital correlation with pTNM classification and lymph node metastasis, which might contribute to tumor malignant progression. Additional investigations are required to elucidate the functions and signal transduction of sigma1R in ESCC.
Acknowledgments
This work was supported by grants from the National High Technology Research and Development Program of China (N0.2006AA02A403) and the Natural Science Foundation of China-Guangdong Joint Fund (No.U0932001).
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- Aydar E, Onganer P, Perrett R, Djamgoz MB, Palmer CP. 2006. The expression and functional characterization of sigma (sigma) 1 receptors in breast cancer cell lines. Cancer Lett. 242:245–257 [DOI] [PubMed] [Google Scholar]
- Aydar E, Palmer CP, Klyachko VA, Jackson MB. 2002. The sigma receptor as a ligand-regulated auxiliary potassium channel subunit. Neuron. 34:399–410 [DOI] [PubMed] [Google Scholar]
- Bem WT, Thomas GE, Mamone JY, Homan SM, Levy BK, Johnson FE, Coscia CJ. 1991. Overexpression of sigma receptors in nonneural human tumors. Cancer Res. 51:6558–6562 [PubMed] [Google Scholar]
- Brent PJ, Pang GT. 1995. Sigma binding site ligands inhibit cell proliferation in mammary and colon carcinoma cell lines and melanoma cells in culture. Eur J Pharmacol. 278:151–160 [DOI] [PubMed] [Google Scholar]
- Christein JD, Hollinger EF, Millikan KW. 2002. Prognostic factors associated with resectable carcinoma of the esophagus. Am Surg. 68:258–262 [PubMed] [Google Scholar]
- CICAMS 1976. Establishment of an epithelial cell line from human esophageal carcinoma. Chin Med J. 2:357–364 [PubMed] [Google Scholar]
- Crawford KW, Bowen WD. 2002. Sigma-2 receptor agonists activate a novel apoptotic pathway and potentiate antineoplastic drugs in breast tumor cell lines. Cancer Res. 62:313–322 [PubMed] [Google Scholar]
- Dussossoy D, Carayon P, Belugou S, Feraut D, Bord A, Goubet C, Roque C, Vidal H, Combes T, Loison G, et al. 1999. Colocalization of sterol isomerase and sigma(1) receptor at endoplasmic reticulum and nuclear envelope level. Eur J Biochem. 263:377–386 [DOI] [PubMed] [Google Scholar]
- Fraser SP, Grimes JA, Djamgoz MB. 2000. Effects of voltage-gated ion channel modulators on rat prostatic cancer cell proliferation: comparison of strongly and weakly metastatic cell lines. Prostate. 44:61–76 [DOI] [PubMed] [Google Scholar]
- Georgatos SD, Meier J, Simos G. 1994. Lamins and lamin-associated proteins. Curr Opin Cell Biol. 6:347–353 [DOI] [PubMed] [Google Scholar]
- Guitart X, Codony X, Monroy X. 2004. Sigma receptors: biology and therapeutic potential. Psychopharmacology. 174:301–319 [DOI] [PubMed] [Google Scholar]
- Hanner M, Moebius FF, Flandorfer A, Knaus HG, Striessnig J, Kempner E, Glossmann H. 1996. Purification, molecular cloning, and expression of the mammalian sigma1-binding site. Proc Natl Acad Sci U S A. 93:8072–8077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Su TP. 2003. Sigma-1 receptors (sigma(1) binding sites) form raft-like microdomains and target lipid droplets on the endoplasmic reticulum: roles in endoplasmic reticulum lipid compartmentalization and export. J Pharmacol Exp Ther. 306:718–725 [DOI] [PubMed] [Google Scholar]
- Hellewell SB, Bruce A, Feinstein G, Orringer J, Williams W, Bowen WD. 1994. Rat liver and kidney contain high densities of sigma 1 and sigma 2 receptors: characterization by ligand binding and photoaffinity labeling. Eur J Pharmacol. 268:9–18 [DOI] [PubMed] [Google Scholar]
- Ishiguro H, Ohtsuki T, Toru M, Itokawa M, Aoki J, Shibuya H, Kurumaji A, Okubo Y, Iwawaki A, Ota K, et al. 1998. Association between polymorphisms in the type 1 sigma receptor gene and schizophrenia. Neurosci Lett. 257:45–48 [DOI] [PubMed] [Google Scholar]
- John CS, Bowen WD, Varma VM, McAfee JG, Moody TW. 1995. Sigma receptors are expressed in human non-small cell lung carcinoma. Life Sci. 56:2385–2392 [DOI] [PubMed] [Google Scholar]
- Kekuda R, Prasad PD, Fei YJ, Leibach FH, Ganapathy V. 1996. Cloning and functional expression of the human type 1 sigma receptor (hSigmaR1). Biochem Biophys Res Commun. 229:553–558 [DOI] [PubMed] [Google Scholar]
- Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE. 1976. The effects of morphine- and nalorphine-like drugs in the nondependent and morphine-dependent chronic spinal dog. J Pharmacol Exp Ther. 197:517–532 [PubMed] [Google Scholar]
- Moody TW, Leyton J, John C. 2000. Sigma ligands inhibit the growth of small cell lung cancer cells. Life Sci. 66:1979–1986 [DOI] [PubMed] [Google Scholar]
- Ouadid-Ahidouch H, Chaussade F, Roudbaraki M, Slomianny C, Dewailly E, Delcourt P, Prevarskaya N. 2000. KV1.1 K+ channels identification in human breast carcinoma cells: involvement in cell proliferation. Biochem Biophys Res Commun. 278:272–277 [DOI] [PubMed] [Google Scholar]
- Parkin DM, Bray F, Ferlay J, Pisani P. 2005. Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108 [DOI] [PubMed] [Google Scholar]
- Quirion R, Bowen WD, Itzhak Y, Junien JL, Musacchio JM, Rothman RB, Su TP, Tam SW, Taylor DP. 1992. A proposal for the classification of sigma binding sites. Trends Pharmacol Sci. 13:85–86 [DOI] [PubMed] [Google Scholar]
- Reed CE. 1999. Surgical management of esophageal carcinoma. Oncologist. 4:95–105 [PubMed] [Google Scholar]
- Ruscher K, Shamloo M, Rickhag M, Ladunga I, Soriano L, Gisselsson L, Toresson H, Ruslim-Litrus L, Oksenberg D, Urfer R, et al. 2011. The sigma-1 receptor enhances brain plasticity and functional recovery after experimental stroke. Brain. 134:732–746 [DOI] [PubMed] [Google Scholar]
- Shimada H, Nabeya Y, Okazumi S, Matsubara H, Shiratori T, Gunji Y, Kobayashi S, Hayashi H, Ochiai T. 2003. Prediction of survival with squamous cell carcinoma antigen in patients with resectable esophageal squamous cell carcinoma. Surgery. 133:486–494 [DOI] [PubMed] [Google Scholar]
- Shimada Y, Imamura M, Wagata T, Yamaguchi N, Tobe T. 1992. Characterization of twenty-one newly established esophageal cancer cell lines. Cancer. 69:277–284 [DOI] [PubMed] [Google Scholar]
- Spruce BA, Campbell LA, McTavish N, Cooper MA, Appleyard MV, O’Neill M, Howie J, Samson J, Watt S, Murray K, et al. 2004. Small molecule antagonists of the sigma-1 receptor cause selective release of the death program in tumor and self-reliant cells and inhibit tumor growth in vitro and in vivo. Cancer Res. 64:4875–4886 [DOI] [PubMed] [Google Scholar]
- Stoner GD, Gupta A. 2001. Etiology and chemoprevention of esophageal squamous cell carcinoma. Carcinogenesis. 22:1737–1746 [DOI] [PubMed] [Google Scholar]
- Su TP. 1993. Delineating biochemical and functional properties of sigma receptors: emerging concepts. Crit Rev Neurobiol. 7:187–203 [PubMed] [Google Scholar]
- Thomas GE, Szucs M, Mamone JY, Bem WT, Rush MD, Johnson FE, Coscia CJ. 1990. Sigma and opioid receptors in human brain tumors. Life Sci. 46:1279–1286 [DOI] [PubMed] [Google Scholar]
- Vilner BJ, John CS, Bowen WD. 1995. Sigma-1 and sigma-2 receptors are expressed in a wide variety of human and rodent tumor cell lines. Cancer Res. 55:408–413 [PubMed] [Google Scholar]
- Wolfe SA, Culp SG, De Souza EB. 1989. Sigma-receptors in endocrine organs: identification, characterization, and autoradiographic localization in rat pituitary, adrenal, testis, and ovary. Endocrinology. 124:1160–1172 [DOI] [PubMed] [Google Scholar]
- Yang L, Guan T, Gerace L. 1997. Integral membrane proteins of the nuclear envelope are dispersed throughout the endoplasmic reticulum during mitosis. J Cell Biol. 137:1199–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X, Kwan HY. 1999. Activity of voltage-gated K+ channels is associated with cell proliferation and Ca2+ influx in carcinoma cells of colon cancer. Life Sci. 65:55–62 [DOI] [PubMed] [Google Scholar]
- Zhang FR, Tao LH, Shen ZY, Lv Z, Xu LY, Li EM. 2008. Fascin expression in human embryonic, fetal, and normal adult tissue. J Histochem Cytochem. 56:193–199 [DOI] [PMC free article] [PubMed] [Google Scholar]