Abstract
The gC1qR (i.e., gC1q receptor, gC1q binding protein, p32, p33) is a multifunctional cellular protein that interacts with components of the complement, kinin, and coagulation cascades and select microbial pathogens. Enhanced gC1qR expression has been reported in adenocarcinomas arising in a variety of organs. The present study compared gC1qR expression in normal, inflammatory, dysplastic, and malignant tissue of epithelial and mesenchymal origin. gC1qR expression was visualized in tissue sections by immunohistochemistry using the 60.11 monoclonal antibody (i.e., IgG1 mouse monoclonal antibody directed against gC1qR) and the UltraVision LP Detection System. Sections were counterstained with hematoxylin and examined by light microscopy. Strongest gC1qR expression was noted in epithelial tumors of breast, prostate, liver, lung, and colon, as well as in squamous and basal cell carcinoma of the skin. However, increased gC1qR staining was appreciated also in inflammatory and proliferative lesions of the same cell types, as well as in normal continuously dividing cells. In contrast, tumors of mesenchymal origin generally stained weakly, with the exception of osteoblasts, which stained in both benign and malignant tissues. The data suggest that increased gC1qR expression may be a marker of benign and pathologic cell proliferation, particularly in cells of epithelial origin, with potential diagnostic and therapeutic applications.
Keywords: gC1qR, immunohistochemistry, inflammation, neoplasia
gC1qR/p33 (i.e., gC1q receptor, gC1q binding protein, p32, p33) is a ubiquitously expressed, highly anionic cellular protein of 33 kDA that was initially identified and characterized as a receptor for the globular heads of C1q (Ghebrehiwet et al. 1994; Ghebrehiwet and Peerschke 1998; Ghebrehiwet et al. 2001). Known alternatively as p33 and sometimes as p32 or HABP-1 (hyaluronic acid binding protein-1), it is localized predominantly to the mitochondrial matrix (Muta et al. 1997; Dedio et al. 1998; Matthews and Russell 1998; Majumdar et al. 2002) but is also found in other cellular compartments, including the endoplasmic reticulum and nucleus, as well as the cell surface, where it may be associated with lipid rafts (Braun et al. 2000; Kittleson et al. 2000; Soltys et al. 2000; Ghebrehiwet et al. 2001; Mahdi et al. 2001, 2002; Kim et al. 2011). Although the biologic significance of gC1qR’s multicompartmental distribution is yet to be elucidated, the relevance of gC1qR as an important modulator of ligands both inside and outside the cell is recognized increasingly.
Several binding partners have been identified for cell surface gC1qR, including C1q (Ghebrehiwet et al. 1994; Lynch et al. 1997), vitronectin (Lim et al. 1996), and high molecular weight kininogen (Herwald et al. 1996; Joseph et al. 1996, 1999). Interaction of these ligands with gC1qR leads to classical complement pathway activation with generation of inflammatory cytokines (Peterson et al. 1997; Ghebrehiwet et al. 2006), cell adhesion (Lim et al. 1996), and activation of the kinin system with production of bradykinin and ensuing vascular permeability (Ghebrehiwet et al. 2006), respectively. It has also been demonstrated that the C-terminal cytoplasmic tail of membrane type-1 metalloproteinase (MT1-MMP), a key enzyme primarily recruited to the leading edge of migrating tumor cells, binds gC1qR (Rozanov et al. 2002). Although a direct functional link between these two proteins remains to be established, this observation suggests that the transient association of gC1qR with MT1-MMP may be involved in mechanisms regulating the presentation of MT1-MMP at the tumor cell surface. In this regard, enhanced gC1qR expression has been described in human adenocarcinomas of the thyroid, colon, pancreas, stomach, esophagus, and lung relative to nonmalignant counterparts (Rubinstein et al. 2004).
Other studies have demonstrated increased cell surface and cytoplasmic gC1qR expression by breast cancer cell lines and a role for gC1qR in tumor metastasis, suggesting that gC1qR may represent a potential new diagnostic and therapeutic target (Fogal et al. 2008; Chen et al. 2009; Fogal et al. 2010; Sanchez-Martin et al. 2011). Since increased gC1qR expression has been described also in normal cells responding to reparative and inflammatory stimuli in vitro, such as human blood platelets activated by a variety of biochemical agonists (Peerschke et al. 2003) and endothelial cells stimulated by inflammatory cytokines or sheer stress (Guo et al. 1999; Yin et al. 2008), the present study compared gC1qR expression in normal, inflammatory, dysplastic, and malignant tissues of epidermal and mesenchymal origin, employing immunohistochemical techniques. Results demonstrate strong gC1qR expression in normal proliferating cells, in benign inflammatory lesions, and particularly in malignancies of epithelial derivation.
Methods
Sections from human tissue were obtained from archives in the Department of Pathology and Dermatopathology at Mount Sinai School of Medicine, according to Institutional Review Board–approved protocols. Immunohistochemical analysis was performed to examine gC1qR expression in inflammatory, benign, dysplastic, and neoplastic lesions and their normal counterparts. Archived paraffin-embedded tissue sections, 4 µm thick, were deparaffinized and rehydrated, treated with 3% hydrogen peroxide for 10 min to eliminate endogenous peroxidase activity, and processed for antigen retrieval with 10 mM citrate buffer, pH 6.0, using a pressure cooker (Pascal; Dako Cytomation) for 4 min at 125°C, followed by slow cooling. Sections were rinsed with phosphate buffer, pH 7.4 (137 mM NaCl, 2.7 mM potassium chloride, 4.2 mM sodium phosphate, and 1.5 mM potassium phosphate). gC1qR expression was probed using the 60.11 monoclonal antibody (i.e., IgG1 mouse monoclonal antibody directed against gC1qR). Tissue sections were incubated with 60.11 (0.33 µg/ml) diluted in Antibody Diluent Solution (Invitrogen Ltd., Camallio, CA) with 5% normal goat serum (Sigma-Aldrich Co., St. Louis, MO) for 2 hr at room temperature. The UltraVision LP Detection System HRP Polymer & DAP [diamino benzidine] Plus Chromogen (Thermo Fisher Scientific, Fremont CA) was used as secondary antibody according to manufacturer’s protocol. Immunoreactivity was visualized with diamino benzidine. Sections were counterstained with hematoxylin, dehydrated, and mounted. Stained sections were examined by light microscopy. A total of 175 cases were stained and examined as follows: breast (n=30), colon (n=40), skin (n=20), prostate (n=10), liver (n=10), lung (n=20), cartilage (n=5), adipose tissue (n=5), bone, cartilage and soft tissue tumors (n=20), and blood vessels (n=15). gC1qR expression was evaluated by at least two pathologists.
Results
gC1qR Expression in Tissues of Epithelial Origin
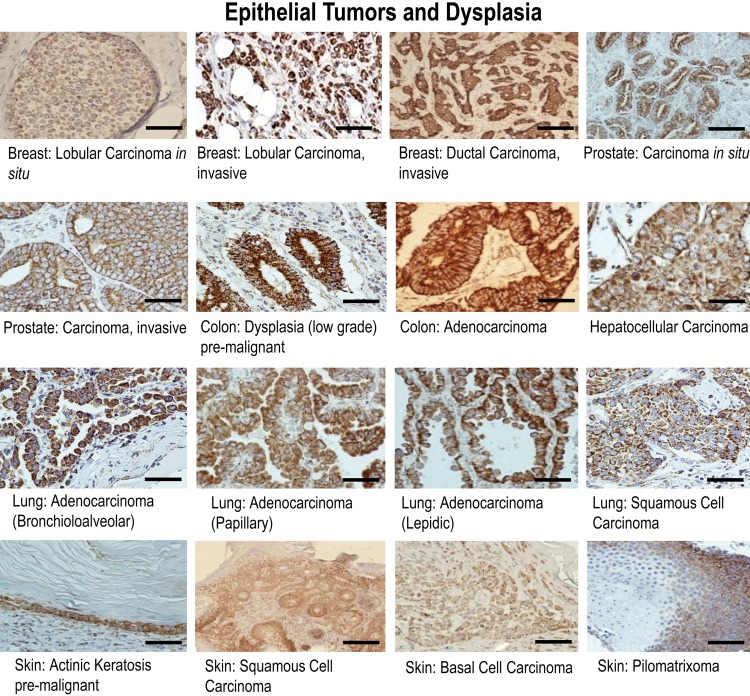
Strong gC1qR staining was observed in malignant as well as in benign pathological conditions. gC1qR expression was noted in in situ and invasive lobular and ductal carcinoma of the breast, carcinoma of the prostate, hepatocellular carcinoma of liver, adenocarcinoma of colon, and in lung adenocarcinoma subtypes (i.e., bronchioloalveolar, lepidic, and papillary carcinoma) and squamous cell carcinoma (Fig. 1). Staining was predominantly cytoplasmic, but membrane staining was also appreciated. Normal tissue analogues, without exception, did not stain as strongly as their malignant counterparts (Fig. 2). Strongest staining was seen in invasive tumors. For example, although strong gC1qR staining was observed in both in situ and invasive lobular carcinoma of the breast, the latter stained more strongly (Fig 1). Similarly, in carcinomas of the prostate, strongest gC1qR expression was noted in invasive tumors (Fig. 1). Adenocarcinomas and squamous cell carcinoma of the lung also stained strongly for gC1qR (Fig. 1). Premalignant lesions such as colonic dysplasia also stained strongly (Fig. 1). Biopsy samples of colonic epithelium taken from patients with inflammatory bowel disease—that is, either ulcerative colitis or Crohn’s disease—demonstrated moderate to marked expression of gC1qR (Fig. 1).
Figure 1.
Results of immunohistochemical staining of selected epithelial tumors and dysplasia for gC1qR with monoclonal antibody 60.11. gC1qR expression is represented by brown staining. Bars = 25 µm.
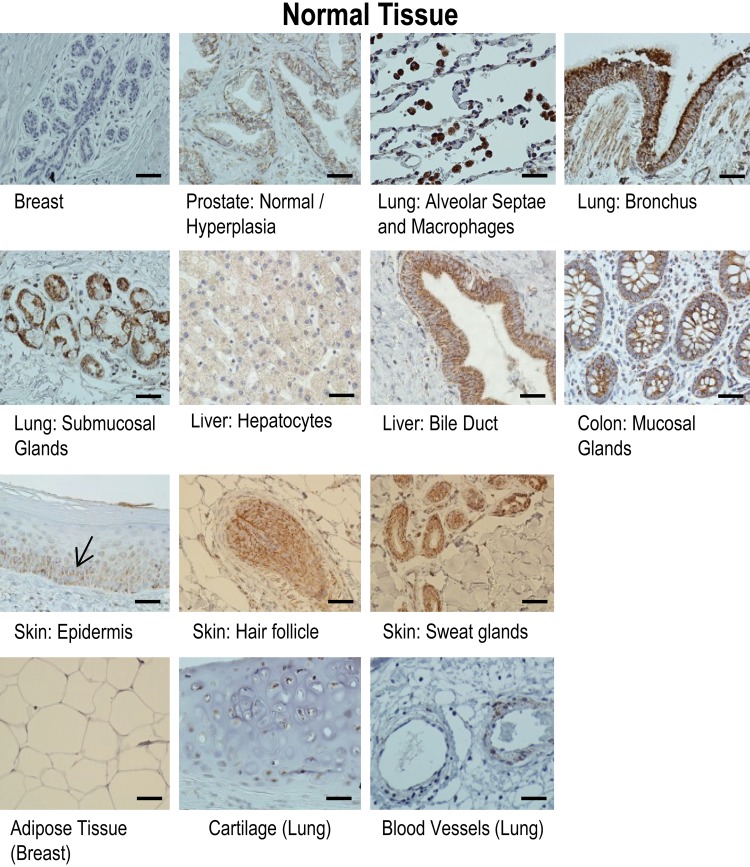
Figure 2.
Results of immunohistochemical staining of selected normal epithelial and mesenchymal tissue for gC1qR with monoclonal antibody 60.11. gC1qR expression is represented by brown staining. Note gC1qR staining of epithelium of breast and prostate, colonic mucosal glands, hepatocytes and liver bile duct, bronchial epithelium and submucosal glands of lung, alveolar macrophages, and the basal layer of normal epidermis, hair follicle, and sweat glands. In contrast, mesenchymally derived tissue (i.e., adipose tissue and cartilage) demonstrates none and weak staining (respectively), with more appreciable staining of blood vessels, particularly the endothelium. Bars = 25 µm.
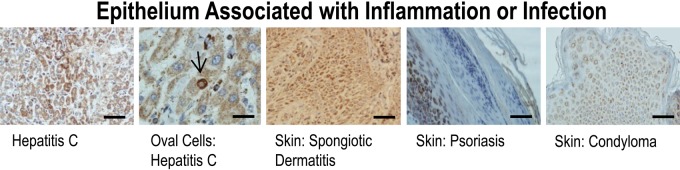
Inflammatory lesions also demonstrated robust gC1qR staining (Fig. 3). Tissue sections of both hepatitis B (not shown) and C (Fig. 3) had marked gC1qR staining. Furthermore, it was observed that oval cells of the liver, thought to be hepatic progenitor cells with the ability to proliferate and differentiate into either hepatocytes or biliary epithelial cells (Sun et al. 2006; Ma et al. 2001; Dabeva and Shafritz 1993), stained visibly above the staining background of hepatocytes in virally infected liver, that is, hepatitis C (Fig. 3).
Figure 3.
Results of immunohistochemical staining of epithelial tissues associated with inflammation or infection for gC1qR with monoclonal antibody 60.11. gC1qR expression is represented by brown staining. Hepatitis C, Skin: Spongiotic Dermatitis, Skin: Psoriasis, and Skin: Condyloma, Bars = 25 µm; Oval Cells: Hepatitis C, Bar = 10µm.
The same general patterns of gC1qR expression were exhibited in benign, proliferating, and malignant dermal lesions. Strong gC1qR expression was seen in keratinocytes of the basal layer of normal epidermis (i.e., the proliferative layer), with progressively diminished staining noted in the stratum spinosum, stratum granulosum, and stratum corneum (Fig. 2). Hair follicles (Fig. 2), whose matrix epithelium is thought to be the most proliferating cell type in the body, stained strongly, as did the basal cells of pilomatrixoma (Fig. 1), a benign tumor with differentiation toward hair cells. Inflammatory, hyperproliferative lesions, including psoriatic dermatitis (psoriasis) and spongiotic dermatitis, stained strongly (Fig. 3). Actinic keratosis (Fig. 1), a precursor lesion of squamous cell carcinoma, and condyloma (Fig. 3), a T-cell antigen induced hyperproliferative lesion, stained throughout the epidermis. In general, inflammatory conditions (Fig. 3) demonstrated compensatory hypertrophy of the squamous epithelium with increased gC1qR staining beyond the basal layer. In psoriasis, gC1qR staining was appreciably greater, as expected, given the accelerated cell turnover, with uniform epidermal acanthosis and parakeratosis in the stratum corneum.
Frank malignancies, including basal cell carcinoma and squamous cell carcinoma, demonstrated moderate to strong gC1qR staining (Fig. 1). Squamous cell carcinoma arising in actinic keratosis demonstrated strong staining in atypical keratinocytes with enlarged hyperchromatic nuclei infiltrating the dermis. Staining was seen throughout the diffusely infiltrating tumor, which spared the epidermis. Basal cell carcinoma, nodular cystic type, demonstrated strong staining of the dermal basaloid and peripherally palisading clusters.
gC1qR Expression in Tissues of Mesenchymal Origin
To evaluate whether tissue of mesenchymal origin demonstrated similar patterns of gC1qR expression as tissues of epithelial derivation, staining was performed on normal, benign, and malignant lesions of bone and cartilage, soft tissue, adipose tissues, and blood vessels. Osteoblasts and osteoclasts in normal bone (not shown) stained strongly, and this staining was consistent across the spectrum of bone lesions through malignant osteosarcoma (Figs. 4, 5). Osteoblastoma, a primary neoplasm of bone considered to be benign (Fig. 4) and composed of osteoid and primitive woven bone, demonstrated strong staining of osteoblasts lining the bony trabeculae, as did osteoid osteoma (Fig. 4), a diminutive version of the former. Osteosarcoma (Fig. 5), a highly malignant mesenchymal tumor of malignant osteoblastic differentiation, demonstrated the expected strong expression of gC1qR. Chordoma, a rare malignant bone tumor thought to arise from cellular remnants of notochord, demonstrated intermediate staining, predominantly perinuclear and cytoplasmic, of the characteristic physaliferous cells (Fig. 5).
Figure 4.
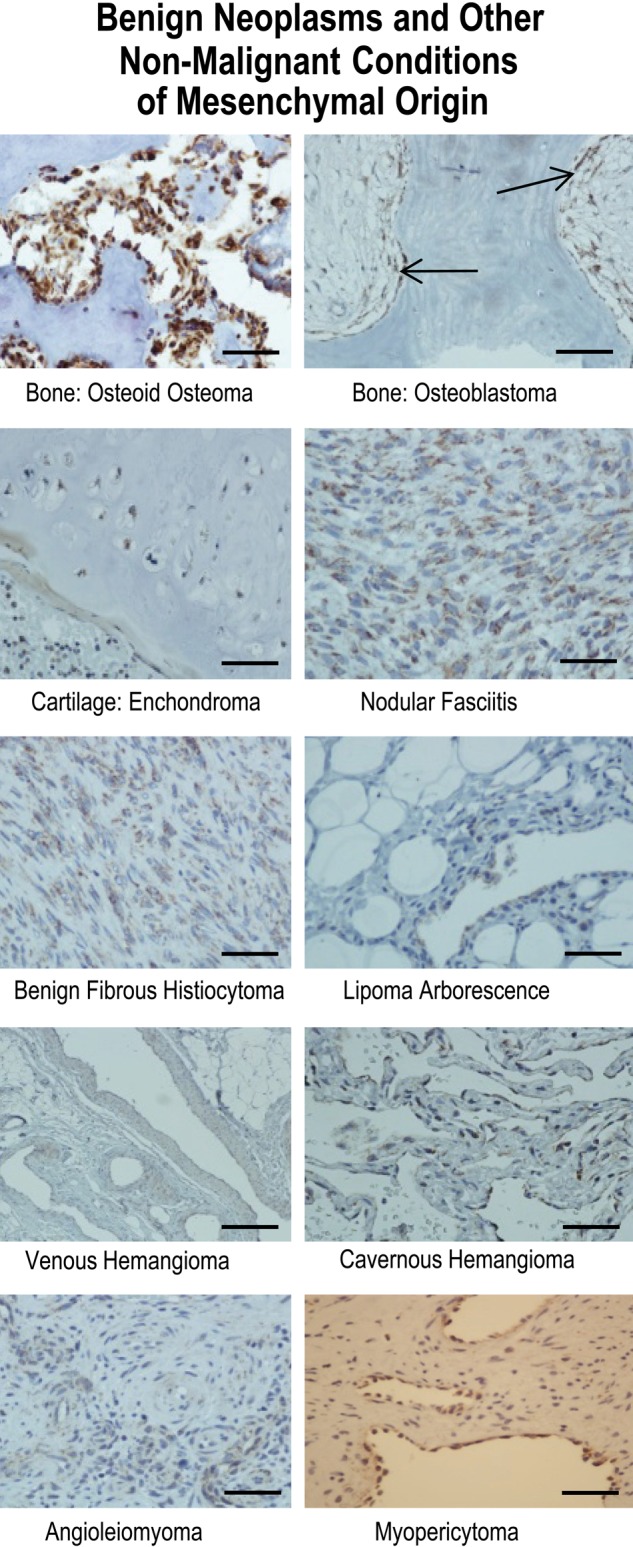
Results of immunohistochemical staining of benign neoplasms and other nonmalignant conditions of mesenchymal origin for gC1qR with monoclonal antibody 60.11. gC1qR expression is represented by brown staining. The arrows indicate location of osteoblasts lining bony trabeculae in osteoblastoma. Bars = 25 µm.
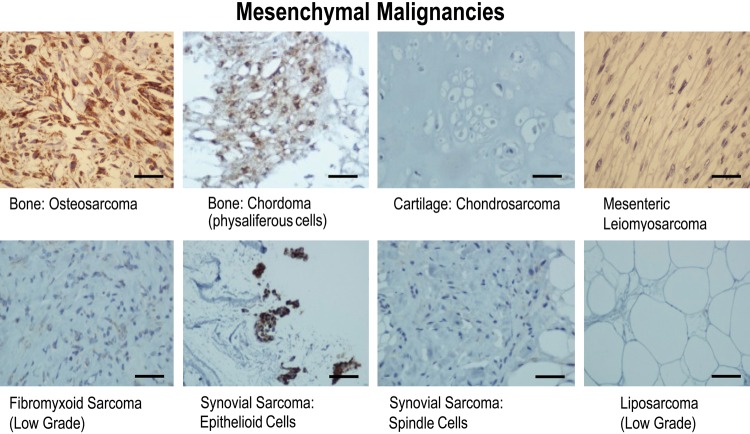
Figure 5.
Results of immunohistochemical staining of mesenchymal malignancies for gC1qR with monoclonal antibody 60.11. gC1qR expression is represented by brown staining. Bars = 25 µm.
Cartilaginous tissue marked the first significant departure in the predictive gC1qR staining paradigm of increased gC1qR expression in proliferating/malignant cells. Normal cartilage (Fig. 2), enchondroma (Fig. 4), an overgrowth or persistent growth of embryonal cartilage, and well-differentiated chondrosarcoma (Fig. 5), a low-grade malignancy of cartilage, all demonstrated similarly weak gC1qR expression in chondrocytes.
Soft tissue tumors with fibroblastic differentiation showed variable staining for gC1qR (Figs. 4, 5), which, on the whole, appeared weak relative to that seen in epithelial tissues. Nodular fasciitis, a benign reparative process with exuberant proliferation of fibroblasts, whose etiology is favored to be secondary to trauma, was associated with the strongest gC1qR expression (Fig. 4). Benign fibrous histiocytoma (Fig.4) demonstrated intermediate staining, while low-grade fibromyxoid sarcoma (Fig. 5), a malignant neoplasm, paradoxically demonstrated weak staining. Interestingly, synovial sarcoma (Fig. 5), a biphasic tumor purportedly of synovial origin, stained weakly in the spindle, mesenchymal component but more strongly in the epithelioid cells. Adipocytes were uniformly negative for gC1qR staining in normal adipose tissue (Fig. 2), benign lipoma (Fig. 4, lipoma arborescence), and in a well-differentiated liposarcoma (Fig. 5), a low-grade malignancy. In leiomyosarcoma, a malignant tumor of smooth muscle cells, gC1qR expression was equivocal or absent in the characteristic spindle cells (Fig. 5).
Vascular endothelial cells of benign neoplasms or malformations stained consistently but with variable intensity for gC1qR (Fig. 4). Venous hemangiomas, composed of thick-walled vessels with diminished smooth muscle organization when compared to normal vessels, exhibited gC1qR expression by endothelial and smooth muscle cells that was similar to counterparts in normal vessels (Fig. 2). In contrast, cavernous hemangioma, considered to be either a neoplastic or hamartomatous malformation with dilated vessels comprised of a single layer of flattened endothelium, demonstrated more pronounced gC1qR staining than normal vascular endothelium (Fig. 4). Myopericytoma and angioleiomyoma stained similarly to normal vessels (Fig. 4).
Discussion
Results of this study confirm the increased expression of gC1qR in adenocarcinoma of the breast, colon, and lung (Rubinstein et al. 2004) compared to normal tissue counterparts and expand these findings to include skin cancers, hepatocellular carcinoma, and other subtypes of adenocarcinomas. Moreover, strong gC1qR expression was observed in both normal tissue and benign conditions (i.e., inflammatory or proliferative lesions of epithelial derivation), suggesting that enhanced gC1qR expression occurs not only in dysplasia and malignancy but also in normal biological processes that are associated with cell proliferation and, in some cases, cell activation.
In contrast, gC1qR expression in malignant and benign conditions involving cells of mesenchymal derivation was, overall, considerably weaker when compared to tissues of epithelial derivation, in which malignant tissue, without exception, expressed gC1qR strongly. Only in tumors of osteoblastic proliferation, both benign and malignant, was there congruent expression with that of epithelial tumors. Osteosarcomas and, to a lesser extent, chordoma stained avidly with the gC1qR antibody, as did other benign lesions that rely on osteoblastic/osteoclastic activity.
The difference in gC1qR expression in epithelial and mesenchymal tumors suggests that gC1qR expression may be a phenotypic marker of epithelial tumors and could be informative in tumors undergoing epithelial to mesenchymal (Chaffer and Weinberg 2011) or mesenchymal to epithelial (Yang et al. 2010) transitions. It is interesting in this regard that in the present study, the plumper epithelioid cells in a case of biphasic synovial sarcoma, stained more strongly for gC1qR than the spindle cell mesenchymal component. Further studies are required to better understand the differential expression of gC1qR by tumors of different embryologic derivations and its potential pathobiological association.
Although the precise relationship between gC1qR expression and carcinogenesis is as yet unclear, several associations have emerged. A direct involvement of gC1qR in angiogenesis has been demonstrated in vivo (Bossi et al. 2011). gC1qR is directly involved in bradykinin generation, as a consequence of binding to high molecular weight kininogen (Joseph et al. 1996, 1999). Bradykinin is a potent vasoactive peptide that has been implicated in tumor metastasis and angiogenesis (Rubinstein et al. 2004). gC1qR is involved in complement activation (Ghebrehiwet et al. 2006), and targeting of tumor associated complement activation has been shown in animal models to inhibit tumor growth (Harris et al. 1997; Hakulinen and Meri 1998).
Moreover, data suggest a role for gC1qR in epithelial malignancies, making it a potential therapeutic and diagnostic target (Fogal et al. 2008, 2010; Sanchez-Martin et al. 2011). For example, changes in gC1qR expression levels were recently shown to coincide with specific stages of tumor progression in skin of mice treated with benzo[a]pyrene (Ghosh et al. 2004), and these results correlate with observations made in human skin lesions in the present study. Additionally, in a recent study of 63 patients with breast cancer, gC1qR mRNA expression levels correlated with axillary lymph node metastasis and poor patient survival (Chen et al. 2009). Indeed, immunohistochemical staining of breast cancer in the present study is consistent with these observations, demonstrating enhanced gC1qR staining in invasive as compared to in situ breast carcinoma. In addition, cell surface gC1qR has been described in lamellipodia in human lung carcinoma A549 cells, which exhibited reduced proliferation in culture, as well as diminished tumorigenic and metastatic activities in grafted mice after gC1qR depletion (Kim et al. 2011).
In summary, the present study provides evidence that gC1qR constitutes part of the immunohistochemical signature of epithelial malignancies. However, our data also demonstrate that gC1qR expression is not limited to malignant cells and tissues. gC1qR is strongly expressed in normal/benign proliferating and continuously dividing cells, as well as by cells present in inflammatory lesions, suggesting a potential link between gC1qR expression and cell replication and activation. The consistent and robust avidity of malignant epithelial cells for staining with the gC1qR antibody further suggests a role for gC1qR as a potential diagnostic marker for malignancies of epithelial derivation. In particular, its absence may have strong negative predictive value for ruling out a pathologic process involving epithelial cells.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by the Division of Translational Laboratory Medicine of the Mount Sinai School of Medicine, New York, New York, and grants from the National Institute of Allergy and Infectious Diseases, R01-AI 060886 and R01 AI-084178 (BG).
References
- Bossi F, Rizzi L, Bulla R, Tripodo C, Guarnotta C, Novati F, Ghebrehiwet B, Tedesco F. 2011. C1q induces in vivo angiogenesis and promotes wound healing. Molec Immunol. 48:1676 [Google Scholar]
- Braun L, Ghebrehiwet B, Cossart P. 2000. gC1q-R/p32, a C1q-binding protein is a novel receptor for Listeria monocytogenes. EMBO J. 19:1458–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffer CL, Weinberg RA. 2011. A perspective on cancer cell metastasis. Science. 331:1559–1564 [DOI] [PubMed] [Google Scholar]
- Chen Y-B, Jiang C-T, Zhang G-Q, Wang J-S, Pang D. 2009. Increased expression of hyaluronic acid binding protein 1 is correlated with poor prognosis in patients with breast cancer. J Surg Oncol. 100:382–386 [DOI] [PubMed] [Google Scholar]
- Dabeva MD, Shafritz DA. 1993. Activation, proliferation and differentiation of progenitor cells into hepatocytes in the D-galactosamine model of liver regeneration. Am J Pathol. 143:1606–1620 [PMC free article] [PubMed] [Google Scholar]
- Dedio J, Jahnen-Dechent W, Bachmann M, Muller-Esterl W. 1998. The multiligand–binding protein gC1qR, putative C1q receptor, is a mitochondrial protein. J Immunol. 160,3534–3542 [PubMed] [Google Scholar]
- Fogal V, Richardson AD, Karmali PP, Scheffler IE, Smith JW, Ruoslahti E. 2010. Mitochondrial p32 protein is a critical regulator of tumor metabolism via maintenance of oxidative phosphorylation. Molec Cell Biol. 30:1303–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogal V, Zhang L, Krajewski S, Ruoslahti E. 2008. Mitochondrial/cell surface protein p32/gC1qR as a molecular target in tumor cells and tumor stroma. Cancer Res. 68:7210–7218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghebrehiwet B, Cebada-Mora C, Tantral L, Jesty J, Peerschke EIB. 2006. gC1qR/p33 serves as a molecular bridge between the complement and contact systems and is an important catalyst for inflammation. Current Topics in Complement. Adv Exp Med Biol. 586:95–105 [DOI] [PubMed] [Google Scholar]
- Ghebrehiwet B, Lim BL, Kumar R, Feng X, Peerschke EIB. 2001. gC1q-R/p33, a member of a new class of multifunctional and multicompartmental cellular proteins is involved in inflammation and infection. Immunol Rev. 180:65–77 [DOI] [PubMed] [Google Scholar]
- Ghebrehiwet B, Lim BL, Peerschke EIB, Willis AC, Reid KBM. 1994. Isolation, cDNA cloning, and overexpression of a 33-kDa cell surface glycoprotein that binds to the globular heads of C1q. J. Exp Med. 179:1809–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghebrehiwet B, Peerschke EIB. 1998. Structure and function of gC1q-R a multiligand binding membrane protein. Immunobiol. 199:225–238 [DOI] [PubMed] [Google Scholar]
- Ghosh I, Chowdhury AR, Rajeswari MR, Datta K. 2004. Differential expression of hyaluronic acid binding protein 1 (HABP1)/P32/C1QBP during progression of epidermal carcinoma. Molec Cell Biochem. 267:133–139 [DOI] [PubMed] [Google Scholar]
- Guo W-X, Ghebrehiwet B, Weksler B, Schweitzer K, Peerschke EI. 1999. Up-regulation of endothelial cell binding proteins/receptors for complement component C1q by inflammatory cytokines. J Lab Clin Med. 133:541–550 [DOI] [PubMed] [Google Scholar]
- Hakulinen J, Meri S. 1998. Complement mediated killing of microtumors in vitro. Am J Pathol. 153:845–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris CL, Kans KS, Stevenson GT, Morgan BP. 1997. Tumour cell killing using chemically engineered antibody constructs specific for tumour cells and the complement inhibitor CD59. Clin Exp Immunol. 107:364–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herwald H, Dedio J, Kellner R, Loos M, Mueller-Esterl W. 1996. Isolation and characterization of the kininogen-binding protein p33 from endothelial cells. Identity with gC1q receptor. J Biol Chem. 271:13040–13047 [DOI] [PubMed] [Google Scholar]
- Joseph K, Ghebrehiwet B, Kaplan AP. 1999. Cytokeratin-1 and gC1q-R mediate high molecular weight kininogen binding to endothelial cells. Clin Immunol. 92:246–255 [DOI] [PubMed] [Google Scholar]
- Joseph K, Ghebrehiwet B, Peerschke EIB, Reid KBM, Kaplan AP. 1996. Identification of the zinc-dependent endothelial cell binding protein for high molecular weight kininogen and factor XII: identity with the receptor which binds to the globular “heads” of C1q (gC1q-R). Proc Natl Acad Sci U S A. 93:8552–8557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K-B, Yi J-S, Nguyen N, Lee J-H, Kwon Y-C, Ahn B-Y, Cho H, Kim YK, Yoo H-J, Lee J-S, et al. 2011. Cell-surface receptor for complement component C1q (gC1qR) is a key regulator for lamellipodia formation and cancer metastasis. J Biol Chem. 286:23093–23101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittleson DJ, Chianese-Bullock KA, Yao ZQ, Braciale TJ, Hahn YS. 2000. Interaction between complement receptor gC1q-R and hepatitis C virus core protein inhibits T-lymphocyte proliferation. J Clin Invest. 106:1239–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim BL, Preissner K, Ghebrehiwet B, Leigh LEA, Reid KBM. 1996. The binding proteins for globular “heads” of complement C1q, gC1q-R: Functional expression and characterization as a novel vitronectin binding factor. J Biol Chem. 271:26739–26744 [DOI] [PubMed] [Google Scholar]
- Lynch NJ, Reid KBM, van den Berg RH, Daha MR, Leigh LEA, Lim BL, Ghebrehiwet B, Schwaeble WJ. 1997. The murine homologues of gC1qBP, a 33 kDA protein that binds to the globular “heads” of C1q. FEBS Lett. 418:111–114 [DOI] [PubMed] [Google Scholar]
- Ma X, Qiu DK, Pend YS. 2001. Immunohistochemical study of hepatic oval cells in human chronic viral hepatitis. World J Gastroenterol. 7:238–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahdi F, Shariat-Madar Z, Figueroa CD, Schmaier AH. 2002. Factor XII interacts with multiprotein assembly of urokinase plasminogen activator receptor, gC1qR and cytokeratin 1 on endothelial cell membranes. Hemost Thromb Vasc Biol. 99:3585–3596 [DOI] [PubMed] [Google Scholar]
- Mahdi F, Shariat-Madar Z, Todd RF, III, Figueroa CD, Schmaier AH. 2001. Expression and localization of cytokeratin 1 and urokinase plasminogen activator receptor on endothelial cells. Blood. 97:2342–2350 [DOI] [PubMed] [Google Scholar]
- Majumdar M, Meenakshi J, Goswami SK, Datta K. 2002. Hyaluronan binding protein 1 (HBP1)/C1QBP/p32 is an endogenous substrate for MAP kinase and is translocated to the nucleus upon mitogenic stimulation. Biochem Biophys Res Commun. 291:829–837 [DOI] [PubMed] [Google Scholar]
- Matthews DA, Russell WC. 1998. Adenovirus core protein V interacts with p32—a protein which is associated with both the mitochondria and the nucleus. J Gen Virol. 79:1677–1685 [DOI] [PubMed] [Google Scholar]
- Muta T, Kang D, Kitajima S, Fujiwara T, Hamasaki N. 1997. p 32 protein, a splicing factor 2-associated protein, is localized in mitochondrial matrix and is functionally important in maintaining oxidative phosphorylation. J Biol Chem. 272:24363–24370 [DOI] [PubMed] [Google Scholar]
- Peerschke EI, Murphy TK, Ghebrehiwet B. 2003. Activation-dependent surface expression of gC1qR/p33 on human blood platelets. Thromb Haemost. 89:331–339 [PubMed] [Google Scholar]
- Peterson K, Zhang W, Lu PD, Keilbaugh SA, Peerschke EI, Ghebrehiwet B. 1997. The C1q binding membrane proteins cC1q-R and gC1q-R are released from activated cells. Subcellular localization and immunochemical characterization. Clin Immunol Immunopathol. 84:17–26 [DOI] [PubMed] [Google Scholar]
- Rozanov DV, Ghebrehiwet B, Ratnikov BI, Monosov EZ, Deryugina EI, Strongin A. 2002. The cytoplasmic tail peptide sequence of membrane type-1 matrix metalloproteinase (MT-MMP) directly binds to gC1q-R, a compartment-specific chaperone-like regulatory protein. FEBS Lett. 571:51–57 [DOI] [PubMed] [Google Scholar]
- Rubinstein DB, Stortchevoi A, Boosalis M, Ashfaq R, Ghebrehiwet B, Peerschke EIB, Calvo F, Guillaume T. 2004. Receptor for the globular heads of C1q (gC1q-R, p33, hyaluronan-binding protein) is preferentially expressed by adenocarcinoma cells. Int J Cancer. 110:741–750 [DOI] [PubMed] [Google Scholar]
- Sanchez-Martin D, Cuesta AM, Fogal V, Ruoslahti E, Alvarez-Vallina L. 2011. The multi-compartmental p32/gC1qR as a new target for antibody-based tumor targeting strategies. J Biol Chem. 286:5197–5203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltys BJ, Kang D, Gupta RS. 2000. Localization of P32 protein (gC1q-R) in mitochondria and at specific extramitochondrial locations in normal tissue. Histochem Cell Biol. 114:245–255 [DOI] [PubMed] [Google Scholar]
- Sun C, Jin X-L, Xiao J-C. 2006. Oval cells in hepatitis B virus-positive and hepatitis C virus-positive liver cirrhosis: Histological and ultrastructural study. Histopathology. 48:546–555 [DOI] [PubMed] [Google Scholar]
- Yang J, Eddy JA, Pan Y, Hategan A, Tabus I, Wang Y, Cogdell D, Price ND, Pollock RE, Lazar AJF, et al. 2010. Integrating proteomics and genomics analysis reveals a novel mesenchymal to epithelial reverting transition in leiomyosarcoma through regulation of Slug. Molec Cell Proteomics. 9:2405–2413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin W, Ghebrehiwet B, Weksler B, Peerschke EIB. 2008. Regulated complement deposition on the surface of human endothelial cells: Effect of tobacco smoke and shear stress. Thromb Res. 122:221–228 [DOI] [PMC free article] [PubMed] [Google Scholar]