Abstract
Background
The use of general anaesthetics in young children and infants has raised concerns regarding the adverse effects of these drugs on brain development. Sevoflurane might have harmful effects on the developing brain; however, these effects have not been well investigated.
Methods
Postnatal day 7 (P7) Sprague–Dawley rats were continuously exposed to 2.3% sevoflurane for 6 h. We used the Fox battery test and Morris water maze (MWM) to examine subsequent neurobehavioural performance. Cleaved caspase-3 and neuronal nitric oxide synthase (nNOS) were quantified by immunoblotting, and the Nissl staining was used to observe the histopathological changes in the hippocampus.
Results
A single 6 h sevoflurane exposure at P7 rats resulted in increased cleaved caspase-3 expression and decreased nNOS levels in the hippocampus, and induced the loss of pyramidal neurones in the CA1 and CA3 subfields of the hippocampus at P7–8. These changes were accompanied by temporal retardation of sensorimotor reflexes. However, neither the Fox battery test at P1–21 nor the MWM test at P28–32 showed differences between the air- and sevoflurane-treated groups.
Conclusions
Although early exposure to sevoflurane increases activated caspase-3 expression and neuronal loss and decreases nNOS in the neonatal hippocampus, it does not affect subsequent neurobehavioural performances in juvenile rats.
Keywords: anaesthetic, sevoflurane; caspase 3; hippocampus; memory; neuronal nitric oxide synthase; sevoflurane
Editor's key points.
General anaesthesia is implicated in developmental neurotoxicity in neonatal animals and in subsequent cognitive dysfunction.
The effects of sevoflurane on cell death and neurobehavioural performance were studied in neonatal rats.
A single 6 h exposure produced early evidence of neuronal death in the hippocampus, but had no effect on subsequent neurobehavioural performance.
Animal and human brains grow rapidly during early development, making them especially vulnerable to environmental influences, including general anaesthetics. As anaesthetic and surgical technology advances, the administration of general anaesthetics to very young children, including premature babies, is becoming increasingly common. Accumulating evidence suggests that general anaesthetics administered to neonatal animals can have deleterious effects, including widespread degeneration of neurones and long-term abnormal social behaviour and cognitive dysfunction.1–3 This has raised significant concerns among anaesthesiologists, neuroscientists, parents, and the media regarding the safety of general anaesthetics in infants and neonates.
Sevoflurane is one of the most frequently used inhalation anaesthetics. It has been used during child labour4 and is especially suitable for infants and children because of its low blood gas partition coefficient, rapid onset and offset, aromatic odour, and low pungency into the airway.5 Several studies6,7 and case reports8 have shown epileptiform EEG and seizure activity during induction of anaesthesia with sevoflurane. A previous study reported that no significant neurodegeneration in primary cortical neurones was found in rats exposed to sevoflurane at clinically relevant concentrations.9 Conversely, Satomoto and colleagues3 showed that sevoflurane results in learning deficits and autism-like abnormal social behaviours in neonatal mice. Moreover, a study in human volunteers suggested that sevoflurane disrupts human emotional memory processing.10 These results raise the possibility that sevoflurane might be harmful to the developing brain. However, the effects of sevoflurane on immature brains have not been well explored.
Neuronal nitric oxide synthase (nNOS) is the predominant NOS isoform in the central nervous system (CNS). During neuronal development, nNOS is transcriptionally induced in CNS.11 Furthermore, nNOS in the hippocampus is localized at sites of neuronal proliferation and migration.12 Nitric oxide, the product of nNOS, and its downstream effectors regulate cell proliferation and differentiation in the developing nervous system by playing a neurotrophic role in neuronal cell–cell communication.13,14 In the hippocampus, nNOS plays an important role in synaptic plasticity and is involved in learning and memory.15
Recent evidence suggests that during the period of rapid brain development, enhanced degeneration of neurones triggered by anaesthetics is caused by increased intracellular free calcium,16 a potent activator of nNOS.17 Caspase-3, a member of the cysteine-aspartic acid protease (caspase) family, which is essential for normal brain development. The sequential activation of caspases plays a central role in the execution phase of apoptosis,18 and cleaved caspase-3 indicates the endogenous levels of activated caspase-3, a marker of cell apoptosis.19,20
To investigate the potential neurotoxicity induced by sevoflurane, we exposed neonatal rats to sevoflurane and assessed the protein expression levels of nNOS and cleaved caspase-3 in the developing hippocampus. We also examined neurobehavioural performance to determine whether a single sevoflurane exposure had deleterious effects on long-term cognitive function.
Methods
Animals
The use of animals in this study was approved by the Institutional Animal Care and Use Committee at Sun Yat-sen University (Guangzhou, Guangdong, China). All efforts were made to minimize the number of animals used and their suffering. Male Sprague–Dawley (sd) rats were obtained from the Experimental Animal Centre of Sun Yat-sen University. The rats were housed under a 12 h light–dark cycle (light from 07:00 to 19:00) at 20–22°C. In addition, the rats were given ad libitum access to water and food. A total of 19 litters consisting of 99 male pups were used in this study. Each experimental condition had its own group of littermate controls to minimize variability in the rate of apoptosis.21
Sevoflurane exposure
Rats at postnatal day 7 (P7, 16–17 g) were randomly divided into a sevoflurane-treated group (51 rats) and an air-treated control group (48 rats). Rats in the sevoflurane-treated group were placed in a plastic container and continuously exposed to 2.3% sevoflurane for 6 h using air as a carrier with a gas flow of 2 litre min−1. During sevoflurane exposure, the container was heated to 38°C (NPS-A3 heated device, Midea, Co., Guangdong, China). Sevoflurane, oxygen, and carbon dioxide in the chamber were monitored using a gas monitor (Detex-Ohmeda, Louisville, KY, USA). After 6 h, the rats were exposed to air only and, when able to move freely, were placed back into their maternal cages. During sevoflurane exposure, an investigator monitored respiratory frequency and skin colour; if signs of apnoea or hypoxaemia were detected, the rat was immediately exposed to air and excluded from the experiment. Rats in the control group were placed into the container and were exposed to air only for 6 h.
Arterial blood gas analysis
Arterial blood analysis was performed on P7–8 rats (16–17 g) from the sevoflurane- and air-treated groups.1,22 Arterial blood samples were obtained from the left cardiac ventricle immediately after removal from the maternal cage (0 h, n=3 in each subgroup) or after anaesthesia (6 h, n=3 in each subgroup). Samples were transferred into heparinized glass capillary tubes and analysed immediately by a blood gas analyser (Gem premier 3000). The pups were killed by decapitation at the time of blood sampling and the analysis of each sample was repeated at least three times.
Behavioural studies
The Fox battery test was used to assess the cerebral maturation of P1–21 rats,23,24 and the Morris water maze (MWM) was used to test spatial learning and memory performance in P28–32 rats.25,26
The Fox battery test
Fox battery tests were conducted on 12 rats from P1 to P21 (4–55 g) daily between 08:30 and 23:00 which corresponded to the rats' active period, as described in previous studies.23,24 At P7, rats were randomly divided into sevoflurane-treated (n=6) and the air-treated groups (n=6) that were exposed to sevoflurane or to air for 6 h, respectively. The Fox battery test was performed after the rats had fully recovered from anaesthesia and were able to move freely as described.23,24 The time of the appearance (days) of the eye opening, incisor eruption, limb grasp, crossed extensor reflex, negative geotaxis reflex, and gait reflex was recorded for each rat. Additionally, the time needed to achieve the righting reflex, the negative geotaxis reflex, and the gait reflex was recorded. The maximum angle at which the animals could maintain the position on an inclined board test for 5 s was also documented.
MWM test
Based on a previous study,25 we performed the MWM test on P28 (80–100 g) rats using the Water Maze Tracking System (TME; Chengdu, China) with minor modifications (1984).26 This test was conducted on both sevoflurane-treated (n=9) and air-treated groups (n=6). The MWM consisted of a grey circular tank (100 cm diameter, 50 cm in depth), which was surrounded by several visual cues. Immediately before the test, the tank was filled with water [22 (1)°C] to a height of 30 cm. The tank was equally divided into the target (T, where a plastic platform was submerged), right (R), opposite (O), and left (L) quadrants, with four starting locations that were equidistant from the rim. We conducted memory-acquisition trials (training) four times daily for 5 days. A single adaptation trial (without the platform) was performed and the rats were released into the pool for 60 s in the absence of any escape platform on day 0. On the following 4 days (days 1–4, Place Navigation), two blocks of tests (morning 08:30–11:00 and afternoon 14:30–15:00) were performed with four trials per block per day for each rat. In each trial, the rat was placed into the water facing the wall from one of the four starting points. The escape latency (time to find the submerged platform), the swimming route to reach the platform, and the swimming speed were measured by a computer-operated video tracking system. Once the rat had reached the platform, it was allowed to remain on the platform for 15 s for orientation purposes. Those rats that failed to independently find the escape platform within 60 s were placed on to the escape platform by the experimenter. The rats were removed afterwards to rest in a heated cage until the next trial. Four daily trials were averaged for each animal. On day 5, the memory retention tests (Spatial Probe) were performed in the absence of a submerged platform in the tank. The rat was placed into the water, facing the wall from one of the four starting points. Within 120 s, both the time spent in each quadrant and the swimming route were recorded. The rats were then removed from the tank and placed back into the heated cage.
Western blot analysis
Immunoblotting was performed on hippocampi obtained from 48 P7–8 rats (16–17 g) as previously described.27,28 Briefly, rats were killed by decapitation at 0, 2, 6, and 24 h after 6 h sevoflurane or air treatments, with six rats at each time point per treatment. The rat brain was quickly dissected, and the hippocampus was quickly removed and homogenized in 100 mg ml−1 RIPA Lysis Buffer (Shenergy Biocolor Co., China) with 1% (v/v) PMSF (Shenergy Biocolor Co., China). The homogenate was centrifuged at 13 000g for 20 min at 4°C, and the supernatant was separated and stored at −80°C until further use. The proteins extracted from the hippocampus were separated on a 10% gel by electrophoresis and transferred on to polyvinylidene fluoride membranes (Pall Co., USA). The blots were then incubated with anti-cleaved caspase-3 (1:1000, rabbit polyclonal, Asp175; Cell Signaling Technology, Inc., USA) or anti-β-actin (1:2000, mouse monoclonal; Santa Cruz Biotechnology, USA) antibodies. The changes in the protein expression levels of nNOS using an anti-nNOS antibody (1:500, mouse monoclonal; Santa Cruz Biotechnology, USA) were examined using the ECL-PLUS system (CWBIO, China) and imaged. The β-actin levels were used as a loading control. Optical density was measured by analysing scanned images using the Image J software (NIH, USA). Changes in protein expression ratio (compared with β-actin) were determined by optical density measurements (n=3 for each rat hippocampus sample).
Histopathological examination
Sevoflurane-treated (n=6) and air-treated (n=6) rats (P7–8, 16–17 g) were killed for the Nissl staining at 6 h after a 6 h exposure to either sevoflurane or air. Animals were anaesthetized with a lethal dose of 10% chloral hydrate and transcardially perfused with saline through the left cardiac ventricle until the liver and lungs were cleared of blood, followed by 4% paraformaldehyde in 0.1 M PB (NaH2PO4.2H2O 2.96 g, Na2HPO4.12H2O 29 g dissolved in 1000 ml water, PH 7.4). The perfusion lasted for 15–25 min. The brains were removed and incubated overnight in the same fixative. Paraffin blocks of brain tissue (0.5 mm thick) included sections of the hippocampus at different levels along the septotemporal axis and associated areas.29 Coronal hippocampal sections 5 μm in thickness were Nissl-stained, and examined under a light microscope (Nikon ECLIPSE, 50i, Japan) to study the morphological changes of pyramidal neurones in the CA1 and CA3 regions of the hippocampus. We counted cells imaged from three sections per animal (n=3 for each group). Nissl-positive cells were counted only if the structures were of the appropriate size and shape, possessed a Nissl-positive nucleus and cytoplasmic Nissl-positive particles. The number of Nissl-positive neurones in the pyramidal cell layers of the bilateral CA1 regions was counted at ×400 magnification by two individuals in a blinded manner.30 Questionable structures were examined under ×1000 magnification and were not counted if identification remained uncertain.
Statistical analysis
Values are presented as mean (sem). The SPSS 13.0 software was used for statistical analysis. We tested for normality using the Shapiro–Wilk test and homogeneity of variance by Levene's test. Comparisons of means between two groups were performed using Student's t-test or the Wilcoxon W-test. Statistical significance was assessed using multivariate analysis of variance followed by the Bonferroni multiple comparison testing. When appropriate, 2×2 comparisons were made using a least significant difference test. P-values of ≤0.05 were considered statistically significant.
Results
Neurobehavioural development of neonatal rats
Physical development
Arterial blood analysis showed that pH, carbon dioxide tension, oxygen tension, and glucose levels were not significantly different between sevoflurane- and air-treated animal groups (Table 1). Pups treated with sevoflurane appeared pink during the 6 h of gas exposure. The appearance of eye opening or incisor eruption did not differ between sevoflurane- and air-treated animal groups (Table 2).
Table 1.
Arterial blood analysis. Neonatal exposure to sevoflurane does not induce significant cardiorespiratory dysfunction. Analysis of arterial blood gas revealed no significant differences in any of the measured parameters between mice exposed for 6 h in the sevoflurane and control groups to air (t-test, all P-values>0.05). 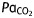 , arterial carbon dioxide tension;
, arterial carbon dioxide tension; 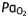 , arterial oxygen tension
, arterial oxygen tension
| Time (h) | n | Arterial blood analysis |
||||
|---|---|---|---|---|---|---|
| pH |
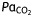 (kPa) (kPa) |
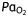 (kPa) (kPa) |
Glucose (mmol litre−1) | |||
| Control | 0 | 3 | 7.39 (0.08) | 3.59 (0.43) | 13.4 (0.52) | 5.5 ± 0.4 |
| 6 | 3 | 7.38 (0.07) | 3.56 (0.45) | 13.3 (0.60) | 5.3 ± 0.6 | |
| Sevoflurane | 0 | 3 | 7.39 (0.04) | 3.58 (0.39) | 13.4 (0.44) | 5.4 ± 0.7 |
| 6 | 3 | 7.34 (0.06) | 3.61 (0.46) | 13.3 (0.59) | 5.2 ± 0.5 | |
Table 2.
Appearance of physical and neurological signs in control or sevoflurane-exposed rats. The values represent the mean (sem) in days
| Days of appearance |
||
|---|---|---|
| Control (n=6) | Sevoflurane (n=6) | |
| Eye opening | 12.2 (0.09) | 12.2 (0.15) |
| Incisor eruption | 5.6 (0.90) | 5.6 (0.30) |
| Forelimb grasp | 2.4 (0.30) | 2.5 (0.34) |
| Hindlimb grasp | 10.8 (0.90) | 9.3 (0.30) |
| Crossed extensor reflex | 12.4 (0.40) | 11.3 (0.30) |
| Negative geotaxis 20° | 5.4 (0.10) | 5.4 (0.05) |
| Negative geotaxis 45° | 10.4 (1.00) | 10.0 (1.00) |
| Gait reflex | 11.1 (1.02) | 10.0 (0.83) |
Reflexes and motor development
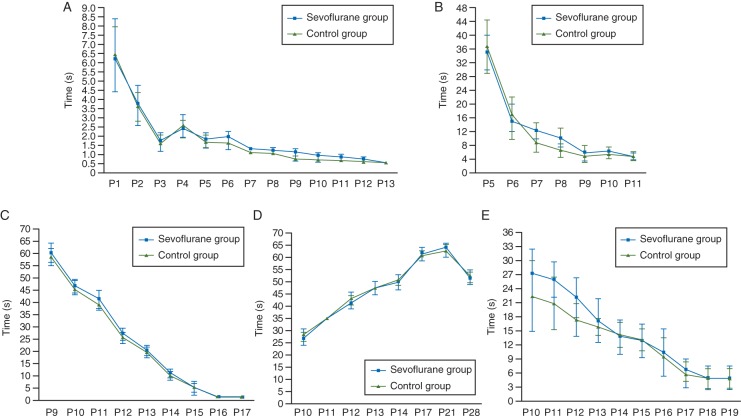
In the Fox battery test on P1–21 neonatal rats (Table 2), the appearance of hindlimb grasp and extensor reflexes was slightly delayed, but no significant differences were detected in the sevoflurane-treated group compared with the air-treated group. The appearance of the forelimb grasp reflex, negative geotaxis reflex, or the gait reflex was not altered in sevoflurane-treated rats (Table 2). From P1 to P21, the time required by the pups to achieve the righting reflex (Fig. 1a), negative taxis (Fig. 1b), and gait reflexes (Fig. 1c) decreased with age in both the air- and sevoflurane-treated groups. Although sevoflurane-treated rats at P7 and P9–12 displayed slightly lower performance in the righting reflex (Fig. 1a) and displayed a 20° negative geotaxis (Fig. 1b), these differences were not statistically significant compared with air-treated rats in their performances of the 40° negative geotaxis reflex (Fig. 1c), the gait reflex (Fig. 1d), or the inclined board test (Fig. 1e). The performance of sevoflurane-treated rats in the Fox battery test returned to the level of air-treated control rats at P13 (Fig. 1a–e).
Fig 1.
Effects of sevoflurane exposure at P7 on sensorimotor neurobehaviour of neonatal rats from P1 to P28 in the Fox battery test. Daily performance in the righting reflex (a); in the negative geotaxis on a 20°-tilted (b), and 45°-tilted board (c); and in the gait reflex (d). The time to achieve the righting reflex (in seconds) is expressed as mean (sem). (e) Daily performance on the inclined board test. The results are expressed as mean (sem) angle.
Spatial learning and memory development
During place navigation in the MWM test, P28–32 rats had an escape latency that was longer in the first trial (Fig. 2a) and shorter in the last trial (Fig. 2b) for every rat tested (n=15) in air- and sevoflurane-treated groups. This indicated the enhancement of escape acquisition (Fig. 2c). No significant differences were found between these two groups in escape latency at each time point (Fig. 2c). During the spatial bias of the MWM test, the average percentage of the swimming distance of sevoflurane-treated rats (Fig. 2d) compared with that of air-treated control rats (Fig. 2e) was 38.4% (13.2%) [mean (sem)] vs 44.5% (6.1%) in target, 21.3% (7.5%) vs 23.8% (5.5%) in right, 19.3% (9.6%) vs 12.8% (0.7%) in opposite, and 21.0% (6.9%) vs 19.0% (8.4%) in the left quadrants. Statistical analyses showed that the average percentage of the swimming distance in the target quadrant was significantly longer than in any of the other quadrants of either sevoflurane- or air-treated control groups (all P<0.05, Fig. 2f). The percentage of the swimming distance in sevoflurane-treated rats was not significantly different from that of air-treated control rats in every quadrant, including the target quadrant (all P>0.05, Fig. 2f). The swimming speed measured during the place navigation trial was not significantly different between sevoflurane-treated [13.1 (1.9) m min−1)] and air-treated control rats [12.5 (2.2) m min−1]. This ruled out the possibility that differences obtained during place navigation and spatial probe trials were due to physical impairments.
Fig 2.
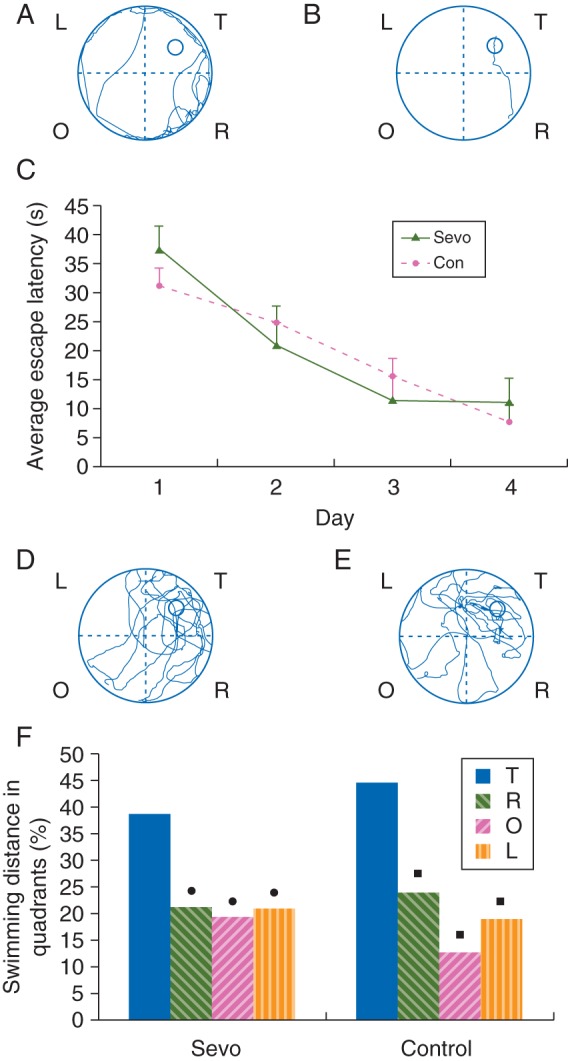
Effects of sevoflurane exposure at P7 on spatial learning and memory abilities of P28–32 rats in the MWM test. The learning and memory abilities of sevoflurane- (n=9) and air-treated (control, n=9) rats were assessed using place navigation (a–c) and spatial probe trials (d–f). A representative route of a control rat to reach the escape platform on day 1 (a) and day 4 (b). (c) The latencies to reach the escape platform in the 4 day place navigation trials, which show no significant differences between the control and sevoflurane groups. The results are expressed as the mean (sem) time in seconds. (d and e) A representative route of a sevoflurane-treated rat (d) and a control rat (e) in search of the escape platform on day 5. T, target quadrant; R, O, L, quadrants which were to the right, opposite, or left of the target quadrant. (f) A comparison of the average percentage of the swimming distance in each quadrant of the MWM for the sevoflurane and control groups. The target quadrant (T) in the spatial probe trial demonstrates the retention ability of spatial learning and memory in MWM. •P<0.05 compared with the T quadrant within the sevoflurane group; ▪P<0.05 compared with the T quadrant within the control group. There were no significant differences between the control and sevoflurane groups in any of the quadrants of the MWM.
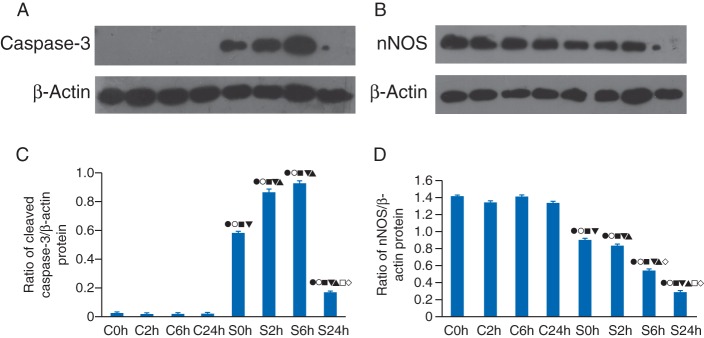
Sevoflurane-induced expression of cleaved caspase-3 and reduction in nNOS protein in neonatal rat hippocampus
Expression levels of cleaved caspase-3 (Fig. 3a) and nNOS (Fig. 3b) were examined by western blotting at four time points (0, 2, 6, and 24 h) after 6 h of sevoflurane or air exposure (Fig. 3c and d). Cleaved caspase-3 protein expression levels in the hippocampus of sevoflurane-treated rats significantly increased from 0 to 6 h, then declined, and was subsequently maintained at a level higher than that of control rats at 24 h (Fig. 3a). A quantitative study showed that expression levels of cleaved caspase-3 in the hippocampus of sevoflurane-treated rats were significantly higher than in air-treated control rats at each time point (all P<0.01, Fig. 3c). Expression of cleaved caspase-3 in sevoflurane-treated rats was significantly higher at 2 and 6 h, compared with any other time point (all P<0.01, Fig. 3c). There were no significant differences between the 2 and 6 h time points (all P>0.05, Fig. 3c).
Fig 3.
Western blot analysis showed that sevoflurane exposure induced an increase in cleaved caspase-3, but a reduction in the nNOS at P7. (a and b) Samples were obtained from the hippocampi of rats subjected to air (control, lanes 1–4) or sevoflurane treatment (lanes 2–5, respectively) at 0, 2, 6, and 24 h after treatment. Representative graphs of the effects of sevoflurane on cleaved caspase-3 and nNOS levels are shown. (c) Expression of cleaved caspase-3 in the sevoflurane group was significantly higher than that in the control group at each time point. The highest levels of cleaved caspase-3 expression occurred at 6 h, then 2, 0, and 24 h after sevoflurane exposure. There was no significant difference between each time point. (d) The expression of nNOS in the sevoflurane group at 24 h after exposure was significantly lower than that in the control group. The lowest level of nNOS occurred at 24 h, which was significantly lower than at other time points. There were no differences between each control group. (c and d) •P<0.05 vs control, 0 h; ○P<0.05 vs control, 2 h; ▪P<0.05 vs control, 6 h; ▾P<0.05 vs control, 24 h; and ▴P<0.05 vs sevoflurane, 0 h; □P<0.05 vs sevoflurane, 2 h; ◊P<0.05 vs sevoflurane, 6 h.
Sevoflurane treatment induced a higher level of cleaved caspase-3 and nNOS protein were lower in sevoflurane-treated rats from 0 to 24 h compared with control (Fig. 3b). Quantitative studies showed that levels of nNOS in the hippocampus of sevoflurane-treated rats were lower than in the control group at each time point (all P<0.01, Fig. 3d). In the sevoflurane-treated group, nNOS levels were the highest at 0 h but the lowest at 24 h (Fig. 3d). The difference in nNOS expression was significant between all time points of sevoflurane-treated subgroups (all P<0.05, Fig. 3d).
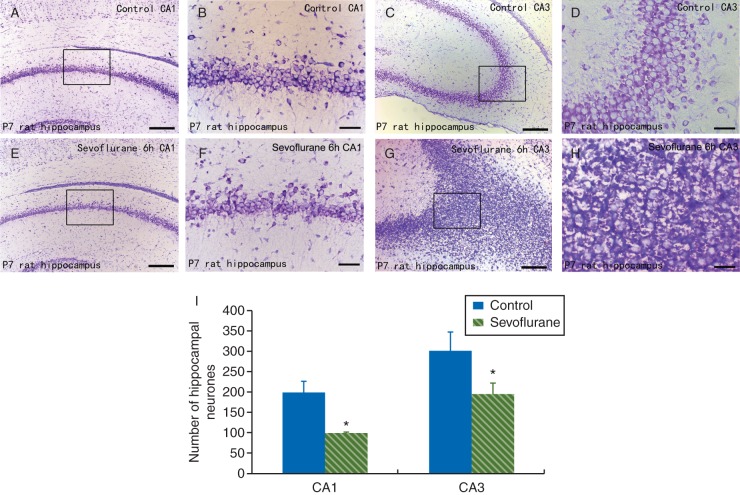
Sevoflurane results in histopathological changes in neonatal rat hippocampus
The Nissl staining revealed neuronal morphology changes that were apparent in the subfields of the hippocampus at 6 h after sevoflurane exposure. Compared with pyramidal neurones in the hippocampus of air-treated control rats (Fig. 4a–d), there were remarkable neuropathological changes including neuronal loss and nucleus shrinkage in the CA1 region (Fig. 4e and f). Oedema resulting from the neuronal cell bodies and cytoplasmic Nissl body loss were also found in the CA3 region (Fig. 4g and h) of the hippocampus of sevoflurane-treated rats. Statistical analysis showed a significant decrease in the density of healthy pyramidal neurones in CA1 and CA3 regions of the hippocampus in sevoflurane-treated rats compared with control rats (all P<0.05, Fig. 4i). These results indicate that sevoflurane exposure induced a reduction in the number of healthy pyramidal neurones in the hippocampus.
Fig 4.
Sevoflurane exposure-induced histopathological changes in the hippocampus of P7 neonatal rats at 6 h after the 6 h sevoflurane treatment as shown by the Nissl staining. (a–e) Representative photomicrographs of coronal sections of the hippocampus of P7 rats (magnification, ×100, scale bar, 50 μm in a, c, e, and g; magnification, ×400, scale bar, 200 μm in b, d, f, and h). The Nissl-stained normal pyramidal neurones in the CA1 (a and b) and CA3 (c and d) regions of air-treated rats. Histopathological changes of the Nissl-stained pyramidal neurones in the CA1 (e and f) and CA3 (g and h) regions of the hippocampus of sevoflurane-treated rats. (i) Comparison of the Nissl-positive pyramidal neurones in the CA1 and CA3 regions of the hippocampus between sevoflurane- and air-treated rats. Each value represents the mean (sem) (n=5 rats per group). *P<0.05 compared with air-treated controls in the same region.
Discussion
In this study, we used 2.3% sevoflurane as the highest concentration that did not inhibit respiration and circulation in rat pups under our experimental conditions, compared with 3.28%, which is the minimum alveolar concentration of sevoflurane in neonatal rats.31 Thus, 2.3% sevoflurane is comparable with that used in the clinical setting. The arterial blood analysis further confirmed that none of the rats suffered from apnoea or hypoxaemia during the 6 h sevoflurane exposure.
Neurones in the developing brain appear to be specifically vulnerable to neurotoxicity induced by general anaesthetics.1 Several studies have shown that sevoflurane exposure of neonatal animals significantly increases neurone apoptosis in several brain regions.3,32,33 Consistent with these findings, this study demonstrated that a single sevoflurane exposure to neonatal (P7) rats significantly increased the loss of pyramidal neurones in both the CA1 and CA3 subfields of the hippocampus. This study also showed that nNOS expression in hippocampal neurones was significantly decreased after sevoflurane exposure. We found that down-regulation of nNOS peaked at 24 h and up-regulation of activated caspase-3 peaked at 2–6 h after sevoflurane exposure. Because caspases are considered inducers of apoptotic cell death,3,32,33 our data suggest that the reduction in nNOS might result from sevoflurane-induced death of nNOS-positive neurones. Our results are consistent with results of a previous report demonstrating that prenatal stress reduces the density of nNOS-positive neurones in the fascia dentata and Ammon's horn of rats.21 However, Cattano and colleagues found that nitrous oxide induces a pro-apoptotic effect, which is associated with increased levels of intracellular free calcium, and followed by the up-regulation of nNOS and p53 in neonatal rat brain.34 35 These differences might be due to tissue-specificity of nNOS gene expression in response to anaesthetics. While Cattano and colleagues analysed nNOS mRNA expression levels in the forebrain, we investigated nNOS protein expression levels in the hippocampus. Importantly, when applied alone, nitrous oxide failed to induce robust neuronal cell death,36 37 whereas sevoflurane, which potentiates γ-aminobutyric acid type A (GABAA) receptors,38 exhibits deleterious effects including widespread degeneration of neurones and long-term abnormal social behaviour and cognitive dysfunction when applied to neonatal animals.1 3 39 Therefore, these two inhaled anaesthetics might affect cellular survival and cell death via different pathways, which could induce changes in nNOS expression.
Despite the increase in activated caspase-3 expression, the decrease in nNOS expression, and the neuronal loss in neonatal hippocampus around P7–8, there were no differences in behavioural performances in the MWM test at P28–32. This was consistent with findings obtained in P7–12 rats on the day of sevoflurane exposure, where sevoflurane-exposed pups did not show lower performance in sensorimotor reflexes. Our results are consistent with previous studies that showed that prenatal methylenedioxymethamphetamine-exposed pups displayed lower performances in the Fox battery test from P2 to P12 and were able to completely recover by P19 to P28.24 40 Recovery of neurobehavioural performance of neonatal pups indicates a high degree of plasticity of neonatal rat brain.24 40 This hypothesis was further supported by our present results from the MWM test, which confirmed that sevoflurane exposure at P7 does not affect spatial learning and memory activities at P28–32. Several previous reports also indicated that sevoflurane exposure induced only short-term dysfunctions of learning and memory.41 42 Previous studies showed that a reduction in nNOS expression contributes to deficits in hippocampal-dependent learning and long-term potentiation,34 35 and deletion of the nNOS gene in mice resulted in abnormal social behaviour and impaired remote spatial memory.43 Spatial learning and memory are complex, and involve different brain areas such as the cerebellum, striatum, cerebral cortex, amygdala, and hippocampus. The hippocampus has been extensively studied as a part of the brain system responsible for spatial learning and memory, the function of which can be specifically detected by the MWM test.25 Given that sevoflurane exposure can lead to persistent learning deficits in fear conditioning,3 which is thought to mainly depend upon amygdala function, we assume that spatial learning and memory functions are less vulnerable to sevoflurane-induced neurotoxicity. Finally and most importantly, our data show that sevoflurane-induced activation of caspase-3 was temporary (peaked at 6 h, but declined at 24 h). It is possible that nNOS protein expression in the hippocampus might have returned to levels that support learning and memory functions at P28–32. Taken together, the roles of these factors in learning, memory, and cognitive dysfunction are still unknown and warrant further study.
In summary, this study demonstrated that a single 6 h exposure of 2.3% sevoflurane in neonatal rats induced neuroapoptosis and a reduction in nNOS protein expression in the hippocampus within 24 h of sevoflurane exposure. However, sevoflurane exposure did not cause persistent changes in sensorimotor neurobehavioural performance. Moreover, sevoflurane did not affect learning and memory abilities of treated rats at juvenile ages. Given the serious implications for public health, further investigations, in particular, clinical trials, are needed to determine effects of the exposure of sevoflurane in early life on learning and memory abilities.
Declaration of interest
None declared.
Funding
This work was supported by research grants obtained from the National Science Foundation Council of China (81070995; 31171290; 31140050), the Guangdong Science Foundation (2010B031600037), National Institutes of Health, and the National Institute on Alcohol Abuse and Alcoholism, USA (AA016964, AA016618).
Acknowledgement
We appreciate the technical help of Dr Jing Li at the Department of Anaesthesiology, New Jersey Medical School, NJ, USA.
References
- 1.Jevtovic-Todorovic V, Hartman RE, Izumi Y, et al. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–82. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun L. Early childhood general anaesthesia exposure and neurocognitive development. Br J Anaesth. 2010;105(Suppl. 1)):i61–8. doi: 10.1093/bja/aeq302. doi:10.1093/bja/aeq302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Satomoto M, Satoh Y, Terui K, et al. Neonatal exposure to sevoflurane induces abnormal social behaviors and deficits in fear conditioning in mice. Anesthesiology. 2009;110:628–37. doi: 10.1097/ALN.0b013e3181974fa2. doi:10.1097/ALN.0b013e3181974fa2. [DOI] [PubMed] [Google Scholar]
- 4.Yeo ST, Holdcroft A, Yentis SM, Stewart A. Analgesia with sevoflurane during labour: I. Determination of the optimum concentration. Br J Anaesth. 2007;98:105–9. doi: 10.1093/bja/ael326. doi:10.1093/bja/ael326. [DOI] [PubMed] [Google Scholar]
- 5.Lerman J, Sikich N, Kleinman S, Yentis S. The pharmacology of sevoflurane in infants and children. Anesthesiology. 1994;80:814–24. doi: 10.1097/00000542-199404000-00014. doi:10.1097/00000542-199404000-00014. [DOI] [PubMed] [Google Scholar]
- 6.Conreux F, Best O, Preckel MP, et al. Electroencephalographic effects of sevoflurane in pediatric anesthesia: a prospective study of 20 cases. Ann Fr Anesth Reanim. 2001;20:438–45. doi: 10.1016/s0750-7658(01)00393-8. doi:10.1016/S0750-7658(01)00393-8. [DOI] [PubMed] [Google Scholar]
- 7.Vakkuri A, Yli-Hankala A, Sarkela M, et al. Sevoflurane mask induction of anaesthesia is associated with epileptiform EEG in children. Acta Anaesthesiol Scand. 2001;45:805–11. doi: 10.1034/j.1399-6576.2001.045007805.x. doi:10.1034/j.1399-6576.2001.045007805.x. [DOI] [PubMed] [Google Scholar]
- 8.Constant I, Seeman R, Murat I. Sevoflurane and epileptiform EEG changes. Paediatr Anaesth. 2005;15:266–74. doi: 10.1111/j.1460-9592.2004.01538.x. doi:10.1111/j.1460-9592.2004.01538.x. [DOI] [PubMed] [Google Scholar]
- 9.Wei H, Kang B, Wei W, et al. Isoflurane and sevoflurane affect cell survival and BCL-2/BAX ratio differently. Brain Res. 2005;1037:139–47. doi: 10.1016/j.brainres.2005.01.009. doi:10.1016/j.brainres.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 10.Alkire MT, Gruver R, Miller J, McReynolds JR, Hahn EL, Cahill L. Neuroimaging analysis of an anesthetic gas that blocks human emotional memory. Proc Natl Acad Sci USA. 2008;105:1722–7. doi: 10.1073/pnas.0711651105. doi:10.1073/pnas.0711651105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yun HY, Dawson VL, Dawson TM. Nitric oxide in health and disease of the nervous system. Mol Psychiatry. 1997;2:300–10. doi: 10.1038/sj.mp.4000272. doi:10.1038/sj.mp.4000272. [DOI] [PubMed] [Google Scholar]
- 12.Zhou QG, Hu Y, Hua Y, et al. Neuronal nitric oxide synthase contributes to chronic stress-induced depression by suppressing hippocampal neurogenesis. J Neurochem. 2007;103:1843–54. doi: 10.1111/j.1471-4159.2007.04914.x. doi:10.1111/j.1471-4159.2007.04914.x. [DOI] [PubMed] [Google Scholar]
- 13.Nobakht M, Gharavi MJ, Mousavizadeh K, Bakhshayesh M, Ghafourifar P. Alteration of rat hippocampal neurogenesis and neuronal nitric oxide synthase expression upon prenatal exposure to tamoxifen. Pathophysiology. 2011;18:263–72. doi: 10.1016/j.pathophys.2011.01.002. doi:10.1016/j.pathophys.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Keilhoff G, Seidel B, Noack H, Tischmeyer W, Stanek D, Wolf G. Patterns of nitric oxide synthase at the messenger RNA and protein levels during early rat brain development. Neuroscience. 1996;75:1193–201. doi: 10.1016/0306-4522(96)00330-2. doi:10.1016/0306-4522(96)00330-2. [DOI] [PubMed] [Google Scholar]
- 15.Muriach M, Lopez-Pedrajas R, Barcia JM, Sanchez-Villarejo MV, Almansa I, Romero FJ. Cocaine causes memory and learning impairments in rats: involvement of nuclear factor kappa B and oxidative stress, and prevention by topiramate. J Neurochem. 2010;114:675–84. doi: 10.1111/j.1471-4159.2010.06794.x. doi:10.1111/j.1471-4159.2010.06794.x. [DOI] [PubMed] [Google Scholar]
- 16.Bickler PE, Fahlman CS. The inhaled anesthetic, isoflurane, enhances Ca2+-dependent survival signaling in cortical neurons and modulates MAP kinases, apoptosis proteins and transcription factors during hypoxia. Anesth Analg. 2006;103:419–29. doi: 10.1213/01.ane.0000223671.49376.b2. table of contents doi:10.1213/01.ane.0000223671.49376.b2. [DOI] [PubMed] [Google Scholar]
- 17.Cattano D, Valleggi S, Abramo A, Forfori F, Maze M, Giunta F. Nitrous oxide discretely up-regulates nNOS and p53 in neonatal rat brain. Minerva Anestesiol. 2010;76:420–4. [PubMed] [Google Scholar]
- 18.Li Z JJ, Jia JM, Lo SC, et al. Caspase-3 activation via mitochondria is required for long-term depression and AMPA receptor internalization. Cell. 2010;141:859–71. doi: 10.1016/j.cell.2010.03.053. doi:10.1016/j.cell.2010.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lemkuil BP, Head B, Pearn ML, Patel HH, Drummond JC, Patel PM. Isoflurane neurotoxicity is mediated by p75NTR-RhoA activation and actin depolymerization. Anesthesiology. 2011;114:49–57. doi: 10.1097/ALN.0b013e318201dcb3. doi:10.1097/ALN.0b013e318201dcb3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee ST, Wu TT, Yu PY, Chen RM. Apoptotic insults to human HepG2 cells induced by S-(+)-ketamine occurs through activation of a Bax-mitochondria-caspase protease pathway. Br J Anaesth. 2009;102:80–9. doi: 10.1093/bja/aen322. doi:10.1093/bja/aen322. [DOI] [PubMed] [Google Scholar]
- 21.Young C, Jevtovic-Todorovic V, Qin YQ, et al. Potential of ketamine and midazolam, individually or in combination, to induce apoptotic neurodegeneration in the infant mouse brain. Br J Pharmacol. 2005;146:189–97. doi: 10.1038/sj.bjp.0706301. doi:10.1038/sj.bjp.0706301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu LX, Yon JH, Carter LB, Jevtovic-Todorovic V. General anesthesia activates BDNF-dependent neuroapoptosis in the developing rat brain. Apoptosis. 2006;11:1603–15. doi: 10.1007/s10495-006-8762-3. doi:10.1007/s10495-006-8762-3. [DOI] [PubMed] [Google Scholar]
- 23.Altman J, Sudarshan K. Postnatal development of locomotion in the laboratory rat. Anim Behav. 1975;23:896–920. doi: 10.1016/0003-3472(75)90114-1. doi:10.1016/0003-3472(75)90114-1. [DOI] [PubMed] [Google Scholar]
- 24.Heuland E, Germaux MA, Galineau L, Chalon S, Belzung C. Prenatal MDMA exposure delays postnatal development in the rat: a preliminary study. Neurotoxicol Teratol. 2010;32:425–31. doi: 10.1016/j.ntt.2010.03.006. doi:10.1016/j.ntt.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Liang G, Wang S, Meng Q, Wang Q, Wei H. Effects of fetal exposure to isoflurane on postnatal memory and learning in rats. Neuropharmacology. 2007;53:942–50. doi: 10.1016/j.neuropharm.2007.09.005. doi:10.1016/j.neuropharm.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li D, Wang J, Yew DT, Lucy Forster E, Yao Z. Age-related alterations of Nestin-immunoreactive neurons in rat basal forebrain with aged memory deficit. Neurochem Int. 2008;53:270–7. doi: 10.1016/j.neuint.2008.08.006. doi:10.1016/j.neuint.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 27.Uebing A, Arvanitis P, Li W, et al. Effect of pregnancy on clinical status and ventricular function in women with heart disease. Int J Cardiol. 2010;139:50–9. doi: 10.1016/j.ijcard.2008.09.001. doi:10.1016/j.ijcard.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Wang J, Yan L, Zhao X, Wu W, Zhou LH. The diversity of nNOS gene expression in avulsion-injured spinal motoneurons among laboratory rodents. Nitric Oxide. 2010;22:37–42. doi: 10.1016/j.niox.2009.11.005. doi:10.1016/j.niox.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 29.Ananth C, Dheen ST, Gopalakrishnakone P, Kaur C. Distribution of NADPH-diaphorase and expression of nNOS, N-methyl-d-aspartate receptor (NMDAR1) and non-NMDA glutamate receptor (GlutR2) genes in the neurons of the hippocampus after domoic acid-induced lesions in adult rats. Hippocampus. 2003;13:260–72. doi: 10.1002/hipo.10060. doi:10.1002/hipo.10060. [DOI] [PubMed] [Google Scholar]
- 30.He FQ, Qiu BY, Zhang XH, et al. Tetrandrine attenuates spatial memory impairment and hippocampal neuroinflammation via inhibiting NF-kappaB activation in a rat model of Alzheimer's disease induced by amyloid-beta(1–42) Brain Res. 2011;1384:89–96. doi: 10.1016/j.brainres.2011.01.103. doi:10.1016/j.brainres.2011.01.103. [DOI] [PubMed] [Google Scholar]
- 31.Orliaguet G, Vivien B, Langeron O, Bouhemad B, Coriat P, Riou B. Minimum alveolar concentration of volatile anesthetics in rats during postnatal maturation. Anesthesiology. 2001;95:734–9. doi: 10.1097/00000542-200109000-00028. doi:10.1097/00000542-200109000-00028. [DOI] [PubMed] [Google Scholar]
- 32.Liang G, Ward C, Peng J, Zhao Y, Huang B, Wei H. Isoflurane causes greater neurodegeneration than an equivalent exposure of sevoflurane in the developing brain of neonatal mice. Anesthesiology. 2010;112:1325–34. doi: 10.1097/ALN.0b013e3181d94da5. doi:10.1097/ALN.0b013e3181d94da5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang X, Xue Z, Sun A. Subclinical concentration of sevoflurane potentiates neuronal apoptosis in the developing C57BL/6 mouse brain. Neurosci Lett. 2008;447:109–14. doi: 10.1016/j.neulet.2008.09.083. doi:10.1016/j.neulet.2008.09.083. [DOI] [PubMed] [Google Scholar]
- 34.Reagan LP, McEwen BS. Diabetes, but not stress, reduces neuronal nitric oxide synthase expression in rat hippocampus: implications for hippocampal synaptic plasticity. Neuroreport. 2002;13:1801–4. doi: 10.1097/00001756-200210070-00022. doi:10.1097/00001756-200210070-00022. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Li N, Yang Z. Perinatal food restriction impaired spatial learning and memory behavior and decreased the density of nitric oxide synthase neurons in the hippocampus of adult male rat offspring. Toxicol Lett. 2010;193:167–72. doi: 10.1016/j.toxlet.2010.01.002. doi:10.1016/j.toxlet.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Ma D, Williamson P, Januszewski A, et al. Xenon mitigates isoflurane-induced neuronal apoptosis in the developing rodent brain. Anesthesiology. 2007;106:746–53. doi: 10.1097/01.anes.0000264762.48920.80. doi:10.1097/01.anes.0000264762.48920.80. [DOI] [PubMed] [Google Scholar]
- 37.Maze M, Fujinaga M. Recent advances in understanding the actions and toxicity of nitrous oxide. Anaesthesia. 2000;55:311–4. doi: 10.1046/j.1365-2044.2000.01463.x. doi:10.1046/j.1365-2044.2000.01463.x. [DOI] [PubMed] [Google Scholar]
- 38.Mihic SJ, Ye Q, Wick MJ, et al. Sites of alcohol and volatile anaesthetic action on GABA(A) and glycine receptors. Nature. 1997;389:385–9. doi: 10.1038/38738. doi:10.1038/38738. [DOI] [PubMed] [Google Scholar]
- 39.Mellon RD, Simone AF, Rappaport BA. Use of anesthetic agents in neonates and young children. Anesth Analg. 2007;104:509–20. doi: 10.1213/01.ane.0000255729.96438.b0. doi:10.1213/01.ane.0000255729.96438.b0. [DOI] [PubMed] [Google Scholar]
- 40.Vaccarino FM, Ment LR. Injury and repair in developing brain. Arch Dis Child Fetal Neonatal Ed. 2004;89:F190–2. doi: 10.1136/adc.2003.043661. doi:10.1136/adc.2003.043661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu XS, Xue QS, Zeng QW, et al. Sevoflurane impairs memory consolidation in rats, possibly through inhibiting phosphorylation of glycogen synthase kinase-3beta in the hippocampus. Neurobiol Learn Mem. 2010;94:461–7. doi: 10.1016/j.nlm.2010.08.011. doi:10.1016/j.nlm.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 42.Wiklund A, Granon S, Faure P, Sundman E, Changeux JP, Eriksson LI. Object memory in young and aged mice after sevoflurane anaesthesia. Neuroreport. 2009;20:1419–23. doi: 10.1097/WNR.0b013e328330cd2b. doi:10.1097/WNR.0b013e328330cd2b. [DOI] [PubMed] [Google Scholar]
- 43.Tanda K, Nishi A, Matsuo N, et al. Abnormal social behavior, hyperactivity, impaired remote spatial memory, and increased D1-mediated dopaminergic signaling in neuronal nitric oxide synthase knockout mice. Mol Brain. 2009;2:19. doi: 10.1186/1756-6606-2-19. doi:10.1186/1756-6606-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]