Abstract
Background
Although acupuncture analgesia is well documented, its mechanisms have not been thoroughly clarified. We previously showed that electroacupuncture (EA) activates supraspinal serotonin- and norepinephrine-containing neurones that project to the spinal cord. This study investigates the involvement of spinal alpha(2)-adrenoceptors (α2-ARs) and 5-hydroxytryptamine (serotonin) receptors (5-HTRs) in EA effects on an inflammatory pain rat model.
Methods
Inflammatory hyperalgesia was induced by injecting complete Freund's adjuvant (CFA, 0.08 ml) into the plantar surface of one hind paw and assessed by paw withdrawal latency (PWL) to a noxious thermal stimulus. The selective α2a-AR antagonist BRL-44408, α2b-AR antagonist imiloxan hydrochloride, 5-HT2B receptor (5-HT2BR) antagonist SB204741, 5-HT3R antagonist LY278584, or 5-HT1AR antagonists NAN-190 hydrobromide, or WAY-100635 were intrathecally administered 20 min before EA or sham EA, which was given 2 h post-CFA at acupoint GB30.
Results
EA significantly increased PWL compared with sham [7.20 (0.46) vs 5.20 (0.43) s]. Pretreatment with α2a-AR [5.35 (0.45) s] or 5-HT1AR [5.22 (0.38) s] antagonists blocked EA-produced anti-hyperalgesia; α2b-AR, 5-HT2BR, and 5-HT3R antagonist pretreatment did not. Sham plus these antagonists did not significantly change PWL compared with sham plus vehicle, indicating that the antagonists had little effect on PWL. Immunohistochemical staining demonstrated that α2a-ARs are on primary afferents and 5-HT1ARs are localized in N-methyl-d-aspartic acid (NMDA) subunit NR1-containing neurones in the spinal dorsal horn.
Conclusions
The data show that α2a-ARs and 5-HT1ARs are involved in the EA inhibition of inflammatory pain and that the NMDA receptors are involved in EA action.
Keywords: acupuncture, norepinephrine, pain, serotonin, spinal cord
Editor's key points.
The analgesic effects of acupuncture are well known, but the underlying mechanisms are not well understood.
The effects of electroacupuncture (EA) are studied in an inflammatory pain model.
Subtype selective agents were used to study the role of serotonin and norepinephrine in EA analgesia.
EA analgesia involves the α2a adrenoreceptor and the 5-HT1a receptor in inflammatory pain.
Electroacupuncture (EA) studies, including our own,1 have shown that EA produces anti-hyperalgesia in animal models of inflammatory pain.2 However, the mechanisms of acupuncture have not been thoroughly clarified.
Previous studies in naive animals suggest that EA may induce analgesia by inhibiting the release of norepinephrine in areas of the brain.3,4 In a recent study, the alpha(2)-adrenoceptor (α2-AR) antagonist yohimbine (i.t.) significantly blocked EA analgesia in a neuropathic pain model, while the α1-AR antagonist prazosin did not.5
Serotonin is also involved in acupuncture analgesia in naive animals.6 Systemic 5-HT1A receptor (5-HT1AR) and 5-HT3 receptor (5-HT3R) antagonists prevented EA-produced inhibitory effects in a collagen-induced arthritis pain model.7 Another study showed that spinal 5-HT1AR and 5-HT3R antagonists blocked EA alleviation of cold allodynia in a rat model of neuropathic pain.5 However, spinal norepinephrine and serotonin involvement in the EA inhibition of thermal hyperalgesia has not been previously explored in an inflammatory pain model.
It has been well demonstrated that spinal α2-ARs play a crucial role in spinal cord anti-nociception8 and that 5-HT1ARs,9 5-HT2BRs,10 and 5-HT3Rs11 in the spinal cord are involved in pain modulation. Thus, we used the corresponding antagonists to investigate our hypothesis that these norepinephrine and serotonin receptor subtypes are involved in EA anti-hyperalgesia.
Methods
Animal
Male Sprague–Dawley rats weighing 280–320 g (Harlan, Indianapolis, IN, USA) were kept under controlled conditions (22°C, relative humidity 40–60%, a 12 h alternative light–dark cycle, and food and water ad libitum). The Institutional Animal Care and Use Committee (IACUC) of the University of Maryland, School of Medicine approved the experimental procedures, licence reference number: IACUC 0506010.
Experimental design
Five sets of experiments were conducted to investigate the involvement of specific serotonin and norepinephrine receptors in EA anti-hyperalgesia: (1) EA plus norepinephrine receptor antagonists, (2) EA plus a 5-HT2BR antagonist, (3) EA plus 5-HT3R antagonist, (4) EA plus 5-HT1AR antagonists, and (5) immunostaining of spinal serotonin and norepinephrine receptors which are involved in EA action. Drugs were administered intrathecally (see below).
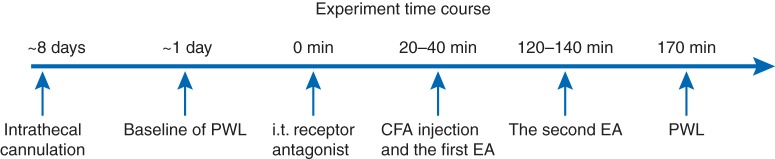
In experiment 1, cannulated rats were injected with complete Freund's adjuvant (CFA; 40 μg of Mycobacterium tuberculosis suspended in a 1:1 oil/saline emulsion; Sigma, St Louis, MO, USA) and randomly divided into eight groups (n=6–8 per group): (i) EA plus 14 nmol of BRL-44408, a selective α2a-AR antagonist, in 10 μl of saline; (ii) EA plus 70 nmol of BRL-44408; (iii) sham EA plus 70 nmol of BRL-44408 solution; (iv) EA plus 14 nmol of imiloxan hydrochloride, a selective α2b-AR antagonist in 10 μl of saline; (v) EA plus 70 nmol of imiloxan hydrochloride; (vi) sham EA plus 70 nmol of the α2b-AR antagonist solution; (vii) EA plus 10 μl of saline; and (viii) sham EA plus the saline. BRL-44408 or imiloxan hydrochloride solutions were administered i.t. Twenty minutes later, CFA at 0.08 ml was injected s.c. into one hind paw and, EA or sham EA was given, followed 30 min later by a paw withdrawal latency (PWL) test that was conducted by an investigator blinded to the treatment assignments (Fig. 1).
Fig 1.
Outline of the experimental protocol.
In experiment 2, cannulated rats with CFA-induced inflammation were randomly divided into five groups (n=6–8 per group): (i) EA+14 nmol of SB204741, a selective 5-HT2BR antagonist in 5 μl of dimethyl sulphoxide, DMSO; (ii) EA+70 nmol of SB204741; (iii) sham EA+70 nmol of the 5-HT2BR antagonist; (iv) EA+DMSO; and (v) sham EA+DMSO. The SB204741 was dissolved in DMSO and was administered (i.t.) 20 min before EA or sham treatment; PWL was tested 30 min after the treatment (Fig. 1).
In experiment 3, cannulated rats with CFA-induced inflammation were randomly divided into five groups (n=6–8 per group): (i) EA+48 nmol of LY278584, a selective 5-HT3R antagonist, in 10 μl of saline; (ii) EA+10 nmol of LY278584; (iii) sham EA+48 nmol of the 5-HT3R antagonist; (iv) EA+saline (10 μl); and (v) sham EA+saline. The antagonist was dissolved in saline and administered (i.t.) 20 min before EA or sham treatment; PWL was tested 30 min after the treatment (Fig. 1).
In experiment 4, cannulated rats with CFA-induced inflammation were randomly divided into six groups (n=6–8 per group): (i) EA+8 nmol of NAN-190 hydrobromide, a selective 5-HT1AR antagonist in 5 μl of DMSO; (ii) EA+42 nmol of NAN-190 hydrobromide; (iii) sham EA+42 nmol of NAN-190 hydrobromide; (iv) EA+8 nmol of WAY-100635 maleate salt, another selective 5-HT1AR antagonist in 10 μl of saline; (v) EA+42 nmol of WAY-100635 maleate salt; and (vi) sham EA+42 nmol of WAY-100635 maleate salt. This experiment shared the EA or sham EA plus DMSO or saline control groups of experiments 2 and 3. The antagonists were dissolved in DMSO or saline and administered (i.t.) 20 min before EA or sham treatment; PWL was tested 30 min after the treatment.
In experiment 5, double immunostaining of α2a-AR and calcitonin gene-related peptides (CGRP) and also 5-HT1A and the N-methyl-d-aspartate (NMDA) NR1 subunit was performed in four rats 2.5 h post-CFA injection to determine the location of serotonin and norepinephrine receptors in the spinal cord.
Intrathecal cannulation
Rats were prepared for i.t. injection under pentobarbital sodium anaesthesia (50 mg kg–1, i.p.).12 The atlanto-occipital membrane between the head and the neck (i.e. approximately the obex level) was exposed. A slit was cut, into which a 7 cm length of PE-10 tubing was inserted into the subarachnoid space. This catheter was advanced to the level of the lumbar spinal cord, filled with 7–10 μl of saline, and plugged at its outer end. The animals were allowed to recover for 7 days after the operation before experimentation; those with gross signs of motor impairment were excluded from the study. After the experiments, the location of the distal end of the catheter was verified when the spinal cord was removed.
Hyperalgesia testing
Inflammatory hyperalgesia, induced by injecting CFA s.c. into the plantar surface of one hind paw of the rat,1 was defined as a decrease in PWL to a noxious thermal stimulus. PWL was assessed as reported previously.1 Each rat was acclimatized for 30 min before the test under a clear inverted plastic chamber on the glass surface of a Paw Thermal Stimulator System (UCSD, San Diego, CA, USA). The glass surface was set at 30°C, then the plantar surface of each hind paw was stimulated with a thermal stimulus set at 5.0 A of current. PWL was automatically recorded when the rat withdrew its paw from the stimulus. In all cases, a cut-off of 20 s was used to avoid tissue injury. To establish the mean PWL, four tests were conducted and averaged. A previous study demonstrated that after 20 s of heating at 4.9, 5.3, and 6.0 A at a glass setting of 30°C, paw temperatures return to baseline 5 min after stimulus.13 To avoid possible thermal stimulus-induced sensitization, a 5 min interval was used between each test to allow the paw tissue to return to baseline temperature, thus excluding possible sensitization.
Acupuncture treatment procedures
To maximize the anti-inflammatory effect and to treat animals prophylactically, EA treatment was given twice for 20 min each, once immediately after the administration of CFA and again 2 h post-CFA. EA parameters of 10 Hz, 3 mA, 0.1 ms pulse width, which showed significant anti-inflammatory and anti-hyperalgesic effects in the rat inflammation model in our previous studies,1 were used in the present study.
Based on traditional Chinese medicine meridian theory,14 on its successful use in our previous studies, and on studies by others,1,15 the equivalent of the human acupoint Huantiao (GB30, the 30th acupoint on the gall bladder meridian) was chosen for EA. In our previous point-specificity study,1 EA produced better anti-hyperalgesia at GB30 than at Waiguan (TE5, the fifth acupoint on the triple energizer meridian) on the forepaw or at two non-specific points, an abdominal point and a point on the quadriceps opposite to GB30. In humans, GB30 is located at the junction of the lateral one-third and medial two-thirds of the distance between the sacral hiatus and the greater trochanter; underneath are the sciatic nerve, inferior gluteal nerve, and gluteal muscles.14 GB30 was located on the rat's hind limbs using the comparable anatomical landmarks, the sacral hiatus and the greater trochanter. The identification of acupoint in rats has been successfully used in previous studies.1,15
GB30 was needled bilaterally.1 The animals were handled gently for 30 min a day for 2–3 days and habituated to the acupuncture treatment before the experiment. After the skin was cleaned with alcohol swabs, a disposable acupuncture needle, 0.25 mm thick×12.5 mm long, was inserted obliquely at each of the animal's hind limbs ∼12 mm so that the needle tip reached GB30, and a pair of electrodes was attached to the handles of the needles. The needles and electrodes were stabilized with an adhesive tape. The procedure typically lasted <20 s and caused little distress to the animal.
The needles were electrically stimulated with an A300 Pulsemaster (World Precision Instruments, Sarasota, FL, USA) via an A360D Stimulus Isolator (World Precision Instruments) that converts electrical voltage into constant electrical current, the level of which is shown on the panel of the device. The current was measured with an oscilloscope that converts current to voltage with a dummy load resistor (World Precision Instruments). This bilateral, cross-limb connection has been previously used by our team and others with no adverse effects.1,16 Similar connections are frequently used in clinic, and no adverse effects have been reported.17
While EA frequency was held constant, intensity was adjusted slowly over the period of ∼2 min to the designated level of 3 mA, which is the maximum EA current intensity that a conscious animal can tolerate. The stimulation was administered to the muscles, not directly to the peripheral nerve, and produced a mild muscle twitching. Each rat was placed under a clear, inverted plastic chamber (∼13×20×28 cm) during EA treatment. The animals remained awake and still; they were neither restrained nor given anaesthetic and showed no signs of distress. For sham treatment control, acupuncture needles were inserted bilaterally into GB30 without electrical stimulation or manual manipulation. This procedure produced no anti-hyperalgesia in this animal model in our previous study.1 Since it is comparable with the treatment procedure but lacks therapeutic effect, we used it as sham control in this study.
Immunofluorescence
Rats were deeply anaesthetized with sodium pentobarbital (60 mg kg−1, i.p.) and immediately perfused transcardially with 4% paraformaldehyde (Sigma) in 0.1 M phosphate buffer (PB) at pH 7.4. The lumbar 4–5 spinal cord was removed, immersed in the same fixative for 2 h at 4°C, and transferred to 30% sucrose (w/v) in PB saline (PBS) for overnight cryoprotection. Thirty-micrometre-thick sections were cut on a cryostat, rinsed in PBS, blocked in PBS with 10% normal donkey serum for 60 min and incubated overnight at room temperature with a mixture of rabbit polyclonal CGRP (1:200, Peninsula, Torrance, CA, USA) and goat polyclonal α2-AR (1:100, Santa Cruz Biotechnology, Santa Cruz, CA, USA). Another set of sections was incubated with a mixture of guinea pig polyclonal 5-HT1AR (1:50, Cat# AB5406, Millipore, Billerica, MA, USA) and goat polyclonal NR1 (1:100, Santa Cruz). After three 10 min washings in PBS, the first set of sections was incubated in a mixture of CY2-conjugated donkey anti-rabbit (1:200, Jackson ImmunoResearch Laboratories, West Grove, PA, USA) and CY3-conjugated donkey anti-goat (1:200) for 1 h at room temperature. The second set of sections was incubated with a mixture of CY2-conjugated donkey anti-guinea pig (1:200) and CY3-conjugated donkey anti-goat (1:200). After another three 10 min washings, the sections were mounted on slides, coverslipped with aqueous mounting medium (Biomeda Corp., CA, USA), and observed with a fluorescence microscope. Control sections were similarly processed, but with no primary antisera, which yielded no staining. The specificity of the primary antibodies has been confirmed in previous studies.18
Statistical analyses
Data from the behavioural tests were presented as mean (se) and analysed using repeated-measure analysis of variance followed by the Bonferroni multiple comparisons (Graphpad Prism). P<0.05 was set as the level of statistical significance.
Results
Effect of α2a- and α2b-AR antagonists on EA-produced anti-hyperalgesia
As shown in Table 1, baseline hind paw PWL was similar before the CFA injection in rats given saline and those given antagonists. A 0.08 ml injection of CFA induced a significant (F1,119=411, P<0.05) decrease in PWL. Contralateral PWLs were unchanged from baseline. EA plus vehicle (i.t.) significantly increased PWL compared with sham EA plus vehicle, indicating that EA inhibits hyperalgesia. EA did not change PWL of contralateral hind paws. EA plus 14 nmol of the α2a-AR antagonist BRL-44408 partially but not significantly increased PWL compared with sham EA plus saline. EA plus 70 nmol of BRL-44408 produced the same PWL as that produced by sham EA plus saline. These data suggest that the α2a-AR antagonist blocks EA anti-hyperalgesia. In contrast, EA plus the α2b-AR antagonist imiloxan hydrochloride at 14–70 nmol significantly increased PWL compared with sham EA plus saline, indicating that this antagonist did not block EA anti-hyperalgesia. Sham EA plus BRL-44408 or imiloxan hydrochloride did not alter PWL compared with sham EA plus vehicle, indicating that these antagonists had no effect on PWL.
Table 1.
Effects of α2a-AR antagonist BRL-44408 and α2b-AR antagonist imiloxan hydrochloride on 10 Hz EA anti-hyperalgesia in rats with CFA-induced hind paw inflammation (n=6–8). *P<0.05 vs sham+saline
| Groups | Baseline (sec) |
After EA or sham (sec) |
||
|---|---|---|---|---|
| Ipsilateral | Contralateral | Ipsi ateral | Contralateral | |
| EA+14 nmol of BRL-44408 | 9.46 (0.32) | 10.02 (0.32) | 6.18 (0.32) | 10.55 (0.59) |
| EA+70 nmol of BRL-44408 | 9.90 (0.28) | 9.82 (0.50) | 5.35 (0.45) | 10.23 (0.34) |
| Sham EA+70 nmol of BRL-44408 | 10.04 (0.46) | 10.33 (0.45) | 5.25 (0.44) | 10.26 (0.49) |
| EA+14 nmol of imiloxan hydrochloride | 9.85 (0.21) | 9.67 (0.23) | 6.91 (0.19)* | 9.68 (0.46) |
| EA+70 nmol of imiloxan hydrochloride | 10.18 (0.41) | 10.25 (0.54) | 7.55 (0.92)* | 10.32 (0.47) |
| Sham EA+70 nmol of imiloxan hydrochloride | 10.23 (0.49) | 9.89 (0.35) | 5.08 (0.16) | 10.16 (0.35) |
| EA+saline | 9.94 (0.48) | 10.41 (0.35) | 6.89 (0.26)* | 10.35 (0.41) |
| Sham+saline | 10.11 (0.32) | 10.38 (0.26) | 5.13 (0.39) | 10.15 (0.51) |
Effect of 5-HT1A, 2B, and 3 receptor antagonists on EA-produced anti-hyperalgesia
Table 2 shows that EA plus vehicle (i.t.) significantly lengthened PWL compared with sham EA plus vehicle 2.5 h post-CFA, suggesting that EA inhibits hyperalgesia. Like EA plus vehicle, EA plus the 5-HT2BR antagonist SB204741 lengthened PWL, indicating that the antagonist had no effect on EA anti-hyperalgesia. Sham EA plus SB204741 did not significantly alter PWL compared with sham EA plus vehicle, indicating that the antagonists did not affect PWL (Table 2). Table 3 shows that pretreatment with the 5-HT3R antagonist LY278584 at 10–48 nmol did not block EA anti-hyperalgesia.
Table 2.
Effects of 5-HT2B receptor antagonist SB204741 on 10 Hz EA anti-hyperalgesia in rats with CFA-induced hind paw inflammation (n=6–8). *P<0.05 vs sham+DMSO
| Groups | Baseline (s) |
After EA or sham (s) |
||
|---|---|---|---|---|
| Ipsilateral | Contralateral | Ipsilateral | Contralateral | |
| EA+14 nmol of SB204741 | 10.14 (0.19) | 9.59 (0.24) | 6.86 (0.31)* | 10.01 (0.29) |
| EA+70 nmol of SB204741 | 9.64 (0.67) | 9.89 (0.45) | 7.89 (0.89)* | 10.14 (0.54) |
| Sham EA+70 nmol of SB204741 | 9.87 (0.42) | 10.05 (0.45) | 5.20 (0.42) | 9.89 (0.56) |
| EA+DMSO | 9.87 (0.35) | 10.23 (0.46) | 7.20 (0.46)* | 10.34 (0.52) |
| Sham+DMSO | 9.82 (0.38) | 9.97 (0.35) | 5.20 (0.43) | 10.43 (0.60) |
Table 3.
Effects of 5-HT3 receptor antagonist LY278584 on 10 Hz EA anti-hyperalgesia in rats with CFA-induced hind paw inflammation (n=6–8). *P<0.05 vs sham +saline
| Groups | Baseline |
After EA or sham (s) |
||
|---|---|---|---|---|
| Ipsilateral | Contralateral | Ipsilateral | Contralateral | |
| EA+10 nmol of LY278584 | 9.74 (0.35) | 9.84 (0.29) | 6.79 (0.22)* | 10.29 (0.55) |
| EA+48 nmol of LY278584 | 9.70 (0.47) | 10.20 (0.42) | 7.9 (0.83)* | 10.25 (0.38) |
| Sham EA+48 nmol of LY278584 | 9.60 (0.58) | 9.87 (0.41) | 5.23 (0.54) | 9.95 (0.43) |
| EA+saline | 9.95 (0.60) | 10.16 (0.43) | 7.06 (0.48)* | 10.51 (0.60) |
| Sham+saline | 10.20 (0.50) | 9.84 (0.38) | 5.30 (0.40) | 10.23 (0.50) |
In contrast, EA plus 8 nmol of the 5-HT1AR antagonist NAN-190 hydrobromide lengthened PWL at 2.5 h compared with sham EA plus DMSO, while EA plus 42 nmol of the 5-HT1AR antagonist NAN-190 hydrobromide did not. It is known that WAY-100635 maleate salt is a more potent antagonist than NAN-190 hydrobromide.19 EA plus 8–42 nmol of WAY-100635 maleate salt did not increase PWL compared with sham EA plus DMSO. This indicates that the 5-HT1AR antagonist blocked EA anti-hyperalgesia. Sham EA plus the two antagonists did not significantly alter PWL compared with sham EA plus vehicle, indicating that these antagonists had no effect on PWL (Table 4).
Table 4.
Effects of 5-HT1AR antagonists NAN-190 hydrobromide and WAY-100635 maleate salt on 10 Hz EA anti-hyperalgesia in rats with CFA-induced hind paw inflammation (n=6–8). *P<0.05 vs sham + DMSO
| Groups | Baseline (s) |
After EA or sham (s) |
||
|---|---|---|---|---|
| Ipsilateral | Contralateral | Ipsilateral | Contralateral | |
| EA+8 nmol of NAN-190 hydrobromide | 9.51 (0.33) | 10.29 (0.35) | 6.96 (0.23)* | 10.37 (0.38) |
| EA+42 nmol of NAN-190 hydrobromide | 10.28 (0.60) | 10.03 (0.50) | 5.52 (0.54) | 9.95 (0.47) |
| Sham EA+42 nmol of NAN-190 hydrobromide | 10.20 (0.35) | 10.32 (0.52) | 5.30 (0.34) | 9.98 (0.49) |
| EA+DMSO | 9.87 (0.35) | 10.23 (0.46) | 7.20 (0.46)* | 10.34 (0.52) |
| Sham+DMSO | 9.82 (0.38) | 9.97 (0.35) | 5.20 (0.43) | 10.43 (0.60) |
| EA+8 nmol of WAY-100635 maleate salt | 9.66 (0.38) | 9.69 (0.31) | 5.75 (0.28) | 9.70 (0.20) |
| EA+42 nmol of WAY-100635 maleate salt | 9. 49 (0.37) | 9.70 (0.41) | 5.22 (0.38) | 10.17 (0.49) |
| Sham EA+42 nmol of WAY-100635 maleate salt | 9.85 (0.41) | 10.05 (0.52) | 5.60 (0.35) | 9.98 (0.43) |
Expression of 5-HT1AR and α2-AR in the spinal cord
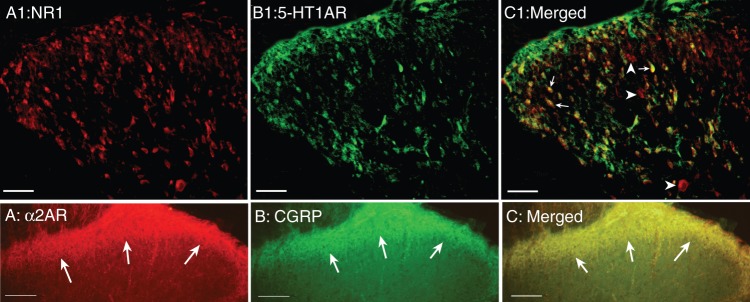
Double immunofluorescence labelling demonstrated that almost all 5-HT1AR immunoreactivity was co-localized with NR1 in the spinal dorsal horn neurones (Fig. 2). It also demonstrated that the α2-AR-positive profile is CGRP-immunoreactive fibres, likely to be primary afferent neurone terminations in spinal superficial laminae (Fig. 2).
Fig 2.
(a1–c1) Immunostaining microphotographs show that 5-HT1ARs are located in NR-containing neurones in the spinal dorsal horn. Arrows point to double-stained neurones; arrow heads point to NR1-containing neurones. Scale is 50 μm. (a–c) Immunostaining microphotographs show that α2a-ARs are located in CGRP-containing primary afferents in the spinal cord. Arrows point to superficial laminae. Scale is 100 μm.
Discussion
Previous studies showed that i.t. administration of yohimbine, an α2 adrenergic antagonist, reduced EA-induced analgesia in a rat model of ankle sprain20 and a neuropathic pain model.5 In the present study, an α2a-AR blocked EA anti-hyperalgesia in an inflammatory pain rat model, while an α2b-AR antagonist did not. We previously reported that EA activates norepinephrine-containing locus coeruleus neurones that project to the spinal cord.21 These data suggest that EA activated supraspinal norepinephrine-containing neurones and increased the spinal release of norepinephrine to alleviate hyperalgesia in inflammatory pain rat model. This is consistent with previous studies in uninjured rats, showing that EA is potentiated by spinal norepinephrine.22 Our data are also consistent with previous electrophysiological studies which show that norepinephrine significantly reduces spinal dorsal horn neurone excitability in a neuropathic pain model.23 In a clinical study, a highly selective norepinephrine reuptake inhibitor significantly reduced pain scores in patients with fibromyalgia.24 Collectively, EA may act on acute and persistent pain by enhancing noradrenergic descending inhibitory pathways. A similar enhancement was seen in neurotropin-caused pain inhibition in an adjuvant-induced rat model of arthritis.25 The data suggest that augmenting spinal norepinephrine, either pharmacologically or otherwise, inhibits pain.
Prior studies demonstrate that α2a-AR stimulation decreases glutamate release from the spinal cord26 and that group I metabotropic glutamate receptors up-regulate NMDA receptor subunit NR1 phosphorylation,27 which is known to modulate NMDAR activity and facilitate the transmission of nociceptive inputs in inflammatory and neuropathic pain models.28 It has also been reported that an i.t. injection of the α2-AR agonist clonidine significantly inhibits the phosphorylation of NR1 in a neuropathic pain model.29 Our immunohistochemistry data and a previous report30 show that α2a-ARs are located in the primary afferents in the spinal cord. We hypothesize that EA may increase spinal norepinephrine to pre-synaptically decrease glutamate release, thus leading to the inhibition of NR1 phosphorylation and pain. This warrants further study.
As reported before, a 5-HT1AR antagonist blocked EA anti-hyperalgesia.31 Previous studies showed that 5-HT is involved in EA analgesia in a neuropathic pain model.22 Our data further demonstrated that 5-HT1ARs are involved in EA-anti-hyperalgesia in an inflammatory pain rat model, but 5-HT2BRs and 5-HT3Rs are not. We previously reported that EA activated nucleus raphe magnus (NRM)-spinal cord serotonergic neurones.21 Those data indicate that EA may increase the spinal release of 5-HT that acts on 5-HT1AR to alleviate hyperalgesia. The previous study also shows that 5-HT1AR activation prevents the phosphorylation of the NMDA receptor NR1 subunit32 and that serotonin depletion increases nociception-evoked trigeminal NMDA receptor phosphorylation.33 Our immunohistochemistry data show that 5-HT1ARs are localized in NR1-containing neurones in the spinal cord. These data lead us to hypothesize that EA may enhance spinal 5-HT1AR activation to post-synaptically inhibit NR1 phosphorylation to alleviate pain.
Although we did not measure spinal serotonin and norepinephrine after EA, in our previous study, EA activated NRM-spinal cord serotonergic and locus coeruleus-spinal cord noragrenergic neurones.21 A previous study also demonstrated that EA increases the content of 5-HT in the NRM and the spinal cord22 and increases the spinal release of norepinephrine.34 These studies show that EA induces the release of both serotonin and norepinephrine in the spinal cord.
Spinal opioids are involved in EA analgesia in uninjured animal models,35 and EA anti-hyperalgesia in inflammatory12 and neuropathic36 pain and postoperative pain.37 The present study demonstrates that i.t. α2a adrenergic and 5-HT1AR antagonists block EA anti-hyperalgesia. Our unpublished data show that µ-receptor antagonist pretreatment block i.t. serotonin or clonidine-produced inhibition of inflammation-caused hyperalgesia. Consistent with these results, naloxone inhibited i.t. 5-HT anti-nociception,38 and selective norepinephrine reuptake inhibitors significantly increased the intensity and duration of morphine anti-nociceptive activity via both α2 adrenergic and opioid receptors.39 Taken together, these data suggest that spinal 5-HT, norepinephrine, and opioids are, together, involved in EA action.
Declaration of interest
None declared.
Funding
This work was supported by the National Center for Complementary and Alternative Medicine (NCCAM) at the National Institutes of Health (NIH), grants R21AT004113 and P01 AT002605.
Acknowledgement
We would like to thank Dr Lyn Lowry for her editorial support.
References
- 1.Lao L, Zhang R-X, Zhang G, Wang X, Berman BM, Ren K. A parametric study of electroacupuncture on persistent hyperalgesia and Fos protein expression in rats. Brain Res. 2004;1020:18–29. doi: 10.1016/j.brainres.2004.01.092. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y-Q, Ji G-C, Wu G-C, Zhao Z-Q. Excitatory amino acid receptor antagonists and electroacupuncture synergetically inhibit carrageenan-induced behavioral hyperalgesia and spinal fos expression in rats. Pain. 2002;99:525–35. doi: 10.1016/S0304-3959(02)00268-3. [DOI] [PubMed] [Google Scholar]
- 3.Wang H, Jiang J, Can X. Changes of norepinephrine release in rat's nucleus reticularis paragigantocellularis lateralis in acupuncture analgesia. Acupuncture Res. 1994;19:20–5. [PubMed] [Google Scholar]
- 4.Murotani T, Ishizuka T, Nakazawa H, et al. Possible involvement of histamine, dopamine, and noradrenalin in the periaqueductal gray in electroacupuncture pain relief. Brain Res. 2010;1306:62–8. doi: 10.1016/j.brainres.2009.09.117. [DOI] [PubMed] [Google Scholar]
- 5.Kim SK, Park JH, Bae SJ, et al. Effects of electroacupuncture on cold allodynia in a rat model of neuropathic pain: mediation by spinal adrenergic and serotonergic receptors. Exp Neurol. 2005;195:430–6. doi: 10.1016/j.expneurol.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 6.Chang FC, Tsai HY, Yu MC, Yi PL, Lin JG. The central serotonergic system mediates the analgesic effect of electroacupuncture on ZUSANLI (ST36) acupoints. J Biomed Sci. 2004;11:179–85. doi: 10.1007/BF02256561. [DOI] [PubMed] [Google Scholar]
- 7.Baek YH, Choi DY, Yang HI, Park DS. Analgesic effect of electroacupuncture on inflammatory pain in the rat model of collagen-induced arthritis: mediation by cholinergic and serotonergic receptors. Brain Res. 2005;1057:181–5. doi: 10.1016/j.brainres.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 8.Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 9.You H-J, Colpaert FC, Arendt-Nielsen L. The novel analgesic and high-efficacy 5-HT1A receptor agonist F 13640 inhibits nociceptive responses, wind-up, and after-discharges in spinal neurons and withdrawal reflexes. Exp Neurol. 2005;191:174–83. doi: 10.1016/j.expneurol.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 10.Lin SY, Chang WJ, Lin CS, Huang CY, Wang HF, Sun WH. Serotonin receptor 5-HT2B mediates serotonin-induced mechanical hyperalgesia. J Neurosci. 2011;31:1410–8. doi: 10.1523/JNEUROSCI.4682-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dogrul A, Ossipov MH, Porreca F. Differential mediation of descending pain facilitation and inhibition by spinal 5HT-3 and 5HT-7 receptors. Brain Res. 2009;1280:52–9. doi: 10.1016/j.brainres.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Zhang R-X, Lao L, Wang L, et al. Involvement of opioid receptors in electroacupuncture-produced anti-hyperalgesia in rats with peripheral inflammation. Brain Res. 2004;1020:12–7. doi: 10.1016/j.brainres.2004.05.067. [DOI] [PubMed] [Google Scholar]
- 13.Dirig DM, Salami A, Rathbun ML, Ozaki GT, Yaksh TL. Characterization of variables defining hindpaw withdrawal latency evoked by radiant thermal stimuli. J Neurosci Methods. 1997;76:183–91. doi: 10.1016/s0165-0270(97)00097-6. [DOI] [PubMed] [Google Scholar]
- 14.Cheng X. Chinese Acupuncture and Moxibustion. Beijing: Foreign Languages Press; 1999. [Google Scholar]
- 15.Xu R, Guan X, Wang C. Influence of capsaicin treating sciatic nerve on the pain threshold and the effect of acupuncture analgesia of rats. Acupunct Res. 1993;18:280–4. [PubMed] [Google Scholar]
- 16.Iwa M, Matsushima M, Nakade Y, Pappas TN, Fujimiya M, Takahashi T. Electroacupuncture at ST-36 accelerates colonic motility and transit in freely moving conscious rats. Am J Physiol Gastrointest Liver Physiol. 2006;290:G285–92. doi: 10.1152/ajpgi.00068.2005. [DOI] [PubMed] [Google Scholar]
- 17.Lao L, Hamilton GR, Fu J, Berman BM. Is acupuncture safe? A systematic review of case reports. Altern Ther Health Med. 2003;9:72–83. [PubMed] [Google Scholar]
- 18.Xiao Z, Deng PY, Rojanathammanee L, et al. Noradrenergic depression of neuronal excitability in the entorhinal cortex via activation of TREK-2 K+ channels. J Biol Chem. 2009;284:10980–91. doi: 10.1074/jbc.M806760200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schiapparelli L, Del Río J, Frechilla D. Serotonin 5-HT1A receptor blockade enhances Ca2+/calmodulin-dependent protein kinase II function and membrane expression of AMPA receptor subunits in the rat hippocampus: implications for memory formation. J Neurochem. 2005;94:884–95. doi: 10.1111/j.1471-4159.2005.03193.x. [DOI] [PubMed] [Google Scholar]
- 20.Koo ST, Lim KS, Chung K, Ju H, Chung JM. Electroacupuncture-induced analgesia in a rat model of ankle sprain pain is mediated by spinal alpha-adrenoceptors. Pain. 2008;135:11–9. doi: 10.1016/j.pain.2007.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li A, Wang Y, Xin J, et al. Electroacupuncture suppresses hyperalgesia and spinal Fos expression by activating the descending inhibitory system. Brain Res. 2007;1186:171–9. doi: 10.1016/j.brainres.2007.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao Z. Neural mechanism underlying acupuncture analgesia. Prog Neurobiol. 2008;85:355–75. doi: 10.1016/j.pneurobio.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Jiang LY, Li SR, Zhao FY, Spanswick D, Lin MT. Norepinephrine can act via alpha(2)-adrenoceptors to reduce the hyper-excitability of spinal dorsal horn neurons following chronic nerve injury. J Formos Med Assoc. 2010;109:438–45. doi: 10.1016/S0929-6646(10)60075-7. [DOI] [PubMed] [Google Scholar]
- 24.Arnold LM, Chatamra K, Hirsch I, Stoker M. Safety and efficacy of esreboxetine in patients with fibromyalgia: an 8-week, multicenter, randomized, double-blind, placebo-controlled study. Clin Ther. 2010;32:1618–32. doi: 10.1016/j.clinthera.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Miura T, Okazaki R, Yoshida H, Namba H, Okai H, Kawamura M. Mechanisms of analgesic action of neurotropin on chronic pain in adjuvant-induced arthritic rat: roles of descending noradrenergic and serotonergic systems. J Pharmacol Sci. 2005;97:429–36. doi: 10.1254/jphs.fpj04050x. [DOI] [PubMed] [Google Scholar]
- 26.Li X, Eisenach JC. alpha2A-adrenoceptor stimulation reduces capsaicin-induced glutamate release from spinal cord synaptosomes. J Pharmacol Exp Ther. 2001;299:939–44. [PubMed] [Google Scholar]
- 27.Choe ES, Shin EH, Wang JQ. Regulation of phosphorylation of NMDA receptor NR1 subunits in the rat neostriatum by group I metabotropic glutamate receptors in vivo. Neurosci Lett. 2006;394:246–51. doi: 10.1016/j.neulet.2005.10.072. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X, Wu J, Lei Y, Fang L, Willis WD. Protein phosphatase modulates the phosphorylation of spinal cord NMDA receptors in rats following intradermal injection of capsaicin. Mol Brain Res. 2005;138:264–72. doi: 10.1016/j.molbrainres.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 29.Roh DH, Kim HW, Yoon SY, et al. Intrathecal clonidine suppresses phosphorylation of the N-methyl-d-aspartate receptor NR1 subunit in spinal dorsal horn neurons of rats with neuropathic pain. Anesth Analg. 2008;107:693–700. doi: 10.1213/ane.0b013e31817e7319. [DOI] [PubMed] [Google Scholar]
- 30.Stone LS, Vulchanova L, Riedl MS, et al. Effects of peripheral nerve injury on alpha-2A and alpha-2C adrenergic receptor immunoreactivity in the rat spinal cord. Neuroscience. 1999;93:1399–407. doi: 10.1016/s0306-4522(99)00209-2. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Li A, Xin J, et al. Involvement of spinal serotonin receptors in electroacupuncture anti-hyperalgesia in an inflammatory pain rat model. Neurochem Res. 2011;36:1785–92. doi: 10.1007/s11064-011-0495-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salazar-Colocho P, Del Rio J, Frechilla D. Serotonin 5-hT1A receptor activation prevents phosphorylation of NMDA receptor NR1 subunit in cerebral ischemia. J Physiol Biochem. 2007;63:203–11. doi: 10.1007/BF03165783. [DOI] [PubMed] [Google Scholar]
- 33.Maneepak M, le Grand S, Srikiatkhachorn A. Serotonin depletion increases nociception-evoked trigeminal NMDA receptor phosphorylation. Headache. 2009;49:375–82. doi: 10.1111/j.1526-4610.2009.01341.x. [DOI] [PubMed] [Google Scholar]
- 34.Liu BH, Zhu SL, Chen QZ, et al. The changes in noradrenaline content in CSF of patients under electroacupuncture. Zhen Ci Yan Jiu. 1988;13:243–6. [PubMed] [Google Scholar]
- 35.Han J-S. Acupuncture: neuropeptide release produced by electrical stimulation of different frequencies. Trends Neurosci. 2003;26:17–22. doi: 10.1016/s0166-2236(02)00006-1. [DOI] [PubMed] [Google Scholar]
- 36.Kim JH, Min B-I, Na HS, Park DS. Relieving effects of electroacupuncture on mechanical allodynia in neuropathic pain model of inferior caudal trunk injury in rat: mediation by spinal opioid receptors. Brain Res. 2004;998:230–6. doi: 10.1016/j.brainres.2003.11.045. [DOI] [PubMed] [Google Scholar]
- 37.Groppetti D, Pecile AM, Sacerdote P, Bronzo V, Ravasio G. Effectiveness of electroacupuncture analgesia compared with opioid administration in a dog model: a pilot study. Br J Anaesth. 2011;107:612–8. doi: 10.1093/bja/aer199. [DOI] [PubMed] [Google Scholar]
- 38.Kellstein DE, Malseed RT, Goldstein FJ. Opioid-monoamine interactions in spinal antinociception: evidence for serotonin but not norepinephrine reciprocity. Pain. 1988;34:85–92. doi: 10.1016/0304-3959(88)90185-6. [DOI] [PubMed] [Google Scholar]
- 39.Pettersen VLA, Zapata-Sudo G, Raimundo JM, Trachez MM, Sudo RT. The synergistic interaction between morphine and maprotiline after intrathecal injection in rats. Anesth Analg. 2009;109:1312–7. doi: 10.1213/ane.0b013e3181b16ff5. [DOI] [PubMed] [Google Scholar]