Abstract
Emerging evidence suggests that motivation enhances episodic memory formation through interactions between medial temporal lobe (MTL) structures and dopaminergic midbrain. In addition, recent theories propose that motivation specifically facilitates hippocampal associative binding processes, resulting in more detailed memories that are readily reinstated from partial input. Here, we used high-resolution functional magnetic resonance imaging to determine how motivation influences associative encoding and retrieval processes within human MTL subregions and dopaminergic midbrain. Participants intentionally encoded object associations under varying conditions of reward and performed a retrieval task during which studied associations were cued from partial input. Behaviorally, cued recall performance was superior for high-value relative to low-value associations; however, participants differed in the degree to which rewards influenced memory. The magnitude of behavioral reward modulation was associated with reward-related activation changes in dentate gyrus/CA2,3 during encoding and enhanced functional connectivity between dentate gyrus/CA2,3 and dopaminergic midbrain during both the encoding and retrieval phases of the task. These findings suggests that within the hippocampus, reward-based motivation specifically enhances dentate gyrus/CA2,3 associative encoding mechanisms through interactions with dopaminergic midbrain. Furthermore, within parahippocampal cortex and dopaminergic midbrain regions, activation associated with successful memory formation was modulated by reward across the group. During the retrieval phase, we also observed enhanced activation in hippocampus and dopaminergic midbrain for high-value associations that occurred in the absence of any explicit cues to reward. Collectively, these findings shed light on fundamental mechanisms through which reward impacts associative memory formation and retrieval through facilitation of MTL and VTA/SN processing.
INTRODUCTION
Only a small fraction of experiences are remembered. A primary challenge for theories of episodic memory is to understand the psychological processes and neural mechanisms that determine which experiences will be stored in memory. Motivational goals, such as rewards, are a likely driving force in determining whether a particular event will be remembered (Adcock, Thangavel, Whitfield-Gabrieli, Knutson, & Gabrieli, 2006; Gruber & Otten, 2010). According to this view, encoding and retrieval processes in medial temporal lobe (MTL) regions critical to episodic memory (Eichenbaum & Cohen, 2001; Gabrieli, 1998; Squire, Stark, & Clark, 2004) should be subject to motivational influence and reflect enhanced processing of motivationally significant events.
Emerging evidence suggests that interactions between MTL regions and reward-sensitive dopaminergic midbrain (Akil & Lewis, 1993; Gasbarri, Packard, Campana, & Pacitti, 1994b; Luo, Tahsili-Fahadan, Wise, Lupica, & Aston-Jones, 2011; Swanson, 1982) play an important role in episodic memory formation. The midbrain regions that release dopamine—ventral tegmental area (VTA) and substantia nigra (SN)—target the MTL (Akil & Lewis, 1993; Gasbarri, Packard, Campana, & Pacitti, 1994a; Gasbarri, Sulli, & Packard, 1997; Gasbarri, Verney, Innocenzi, Campana, & Pacitti, 1994) and receive indirect input from MTL regions (Blaha, Yang, Floresco, Barr, & Phillips, 1997; Floresco, Todd, & Grace, 2001; Floresco, West, Ash, Moore, & Grace, 2003; Luo, et al., 2011; Taepavarapruk, Floresco, & Phillips, 2000). Neurons within these midbrain regions release dopamine in response to the receipt of a reward as well as to cues that predict reward (Schultz, 1998; Schultz, Apicella, & Ljungberg, 1993). Dopaminergic stimulation of MTL enhances plasticity (Li, Cullen, Anwyl, & Rowan, 2003; Otmakhova & Lisman, 1996), resulting in superior MTL-dependent learning (Bernabeu, et al., 1997; Granado, et al., 2008; Lemon & Manahan-Vaughan, 2006). In humans, activation of MTL and dopaminergic midbrain is associated with an encoding advantage for individual novel (Bunzeck & Duzel, 2006; Wittmann, Bunzeck, Dolan, & Duzel, 2007) or reward-predicting stimuli (Adcock, et al., 2006; Wittmann, et al., 2005).
Based on such evidence, recent theories have proposed an MTL–midbrain loop whereby dopaminergic signals enhance episodic encoding for salient or rewarding events (Lisman & Grace, 2005; Shohamy & Adcock, 2010). According to these theories, increased dopaminergic drive associated with rewarding events may enhance plasticity at CA3–CA1 synapses to promote memory formation (Lisman & Otmakhova, 2001; Otmakhova & Lisman, 1999). By specifically impacting hippocampal processing, dopamine release may facilitate associative encoding processes that bind event elements together into coherent memory representations (Shohamy & Adcock, 2010); such a mechanism would confer adaptive memory benefits by increasing the likelihood of memory formation and by incorporating motivational information into stored representations. Moreover, reinstatement of motivational salience may play an important role at retrieval by providing additional cues to enable the reconstruction of detailed event information from partial input (Kennedy & Shapiro, 2004, 2009) or by signaling what rewards should be expected during the current experience (Schultz, 1998; Schultz, et al., 1993). Recent rodent research suggests that a functional circuit from CA3 to the VTA (via the lateral septum) plays an important role in reinstating the motivational salience of specific events to guide behavioral choice (Luo, et al., 2011).
Despite the importance of this topic for memory research, the neural mechanisms that mediate motivational influences on memory in the human brain are only beginning to be explored. No studies to date have addressed how reward impacts associative memory in human hippocampal subfields. Instead, existing research has focused on motivational influences that impact encoding of individual items using neuroimaging techniques that limit the ability to localize activation to specific hippocampal subregions (Adcock, et al., 2006; Gruber & Otten, 2010; Wittmann, et al., 2005). These studies thus leave key hypotheses about motivation’s influence on associative binding and retrieval processes in the hippocampus untested. Here, we used high-resolution functional magnetic resonance imaging (fMRI) of the MTL (Carr, Rissman, & Wagner, 2010) to provide a first look at how reward-based motivation influences encoding and retrieval of associative memory through differential engagement of hippocampal subregions. We hypothesized that reward would improve associative binding of event details, by specifically enhancing CA3 encoding processes previously implicated in successful associative binding (e.g., Eldridge, Engel, Zeineh, Bookheimer, & Knowlton, 2005; Zeineh, Engel, Thompson, & Bookheimer, 2003). In line with recent evidence documenting hippocampally-mediated reactivation of value information for highly rewarding events (Kuhl, Shah, DuBrow, & Wagner, 2010), we additionally predicted that enhanced recall of associative information for high-value events would be reflected in hippocampal and midbrain cued recall responses.
It is important to note that sensitivity to reward varies greatly among individuals (Gray, 1987). Individual differences in reward sensitivity have been associated with reward-related neural activation in dopaminergic midbrain regions (Krebs, Schott, & Duzel, 2009), with differences in mesolimbic dopamine function correlating with the degree of learning in reinforcement tasks (Cools, et al., 2009; Schonberg, Daw, Joel, & O’Doherty, 2007). Additionally, individual differences in recognition memory success for highly rewarded stimuli have been shown to correlate with subsequent memory effects (i.e., greater activation for remembered compared to forgotten stimuli) in dopaminergic midbrain (Adcock, et al., 2006). Understanding how reward impacts MTL subregional function during associative encoding and retrieval may similarly rely on a characterization of individual differences in behavioral sensitivity to reward. Here, we assessed how individual differences in the degree of reward-based memory modulation (i.e., the relative memory benefit for high-value vs. low-value associations) were related to reward-based modulation of hippocampal subfields and dopaminergic midbrain as well as the functional connectivity between these regions. Based on the proposed MTL–midbrain loop, we hypothesized that individual differences in reward modulation of episodic memory would be reflected in enhanced connectivity between dopaminergic midbrain and hippocampus.
MATERIALS AND METHODS
Participants
Thirty-seven healthy, English-speaking individuals (16 females, ages 18-29, mean age = 20) were recruited for participation in the fMRI study. All participants were right-handed with normal or corrected to normal vision. Prior to beginning the experiment, participants gave informed consent in accordance with a protocol approved by the Institutional Review Boards of Stanford University and The University of Texas at Austin. Participants received $20/hr for their involvement, and received additional bonus money based on task performance (up to $34). Data from nine participants were excluded from analysis due to excessive head motion (4 participants), an equipment malfunction that resulted in loss of behavioral responses (1 participant), and poor performance (4 participants). Poor performance was defined as performance 2.5 standard deviations below the group mean, which corresponded to a d-prime of less than 0.75 and a corrected hit rate of less than 0.25 for all trials. All participants included in analysis had overall task corrected hit rates above 0.38 (mean ± standard error: 0.65 ± 0.03). Thus, data from 28 participants (11 female, mean age = 21) were included in the fMRI analyses.
Materials
Stimuli consisted of 480 color photographs of common objects organized into 240 object pairs. Object pairs were further organized into high-value and low-value trials (120 pairs in each condition). The presentation of object stimuli across reward conditions and the order of presentation were randomized across participants by assigning each participant to one of eight randomization groups.
Procedures
Encoding
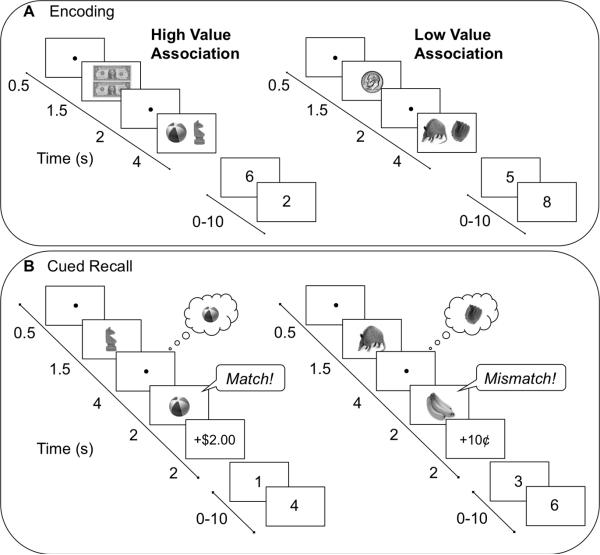
Across eight event-related functional runs, participants intentionally encoded object pairs under varying conditions of reward using a modified version of the monetary incentive encoding task (Adcock, et al., 2006). At the beginning of each encoding trial, a fixation dot (0.5 s) preceded presentation of a monetary cue (1.5 s) indicating how much money a participant could earn for successfully recalling the association at test (Figure 1A). High-value object pairs were worth two dollars if judged correctly at test, while low-value object pairs were worth ten cents. Participants were informed that they would be paid 20% of what they earned in the experiment in addition to the base pay of $20/hour. After each monetary cue, a delay period (2 s) preceded presentation of an object pair (4 s). During presentation of the object pairs, participants provided a judgment of learning (Kao, Davis, & Gabrieli, 2005), indicating how well they learned each association. These judgments were collected to ensure participants’ attention during the encoding phase and were not considered in the analysis of fMRI data.
Figure 1.
Encoding and cued recall tasks. (A) During each encoding trial, participants viewed monetary cues indicating the possible reward for successfully recalling the association at test, followed by a pair of objects. Associative encoding trials were jittered with an odd-even baseline task. (B) During cued recall, a studied object was presented as a cue followed by a delay period during which participants were instructed to recall the learned associate. At the end of the trial, a probe object was presented that was either the correct association (a match) or a studied object that was paired with another object during encoding (a mismatch). After providing a match or mismatch response, participants received feedback regarding their performance. As with encoding, cued recall trials were jittered with an odd-even baseline task.
Each functional encoding run consisted of 15 high-value and 15 low-value trials. A third of the encoding pairs within each run (5 high-value and 5 low-value) served as mismatch probes for the cued recall phase. Within each run, associative encoding trials were intermixed with an odd/even digit baseline task (Stark & Squire, 2001) with a total baseline time equal to 25% of total task time. During each 2 s baseline trial, a single digit between one and eight was presented on the screen and participants indicated whether the digit was odd or even. The order of conditions, including baseline trials, was determined by a sequencing algorithm to optimize the efficiency of the event-related fMRI design (Dale, 1999). The optimization procedure thus ensured that 1) event onsets were not periodic, which is critical for estimation efficiency in fast event-related designs with multiple event types (Liu, Frank, Wong, & Buxton, 2001), and 2) events were jittered by 2 second intervals so that events either occurred at TR onset or 2 s after TR onset.
Cued recall
The experiment alternated between encoding and cued recall phases. An initial set of four encoding runs was followed by four cued recall test runs; a second set of four encoding runs was followed by the final four cued recall runs. On each cued recall trial, a fixation dot (0.5 s) preceded presentation of a previously studied object cue (1.5 s; Figure 1B). During a delay period (4 s), participants were instructed to recall and imagine the object associated with the cue. The delay period was followed by a decision probe (2 s), and participants were asked to judge if the probe was the object originally paired with the cue at encoding (a “match”) or another object viewed at encoding, but as part of a different object pair (a “mismatch”). A correct judgment resulted in the receipt of a monetary reward, while an incorrect judgment resulted in a corresponding monetary loss. At the end of the trial, participants received feedback (2 s) about the amount gained or lost on that trial.
The order of object pair presentations within each test run was organized pseudo-randomly with the restriction that within each run, 10 high-value and 10 low-value object pairs were tested. Half of these trials (5 high-value, 5 low-value) contained match probes, and the other half contained mismatch probes. After an object from a studied pair was presented either as a retrieval cue or a probe, neither object appeared in subsequent trials. Similar to encoding, 2 s odd/even digit trials (Stark & Squire, 2001) were intermixed with cued recall trials so that baseline represented 25% of total task time. The order of conditions was determined by a sequencing program to optimize design efficiency (Dale, 1999) and allow for subsampling of the TR.
Stimuli were generated using Matlab (The MathWorks, Inc., Natick, MA, USA) on a Macbook laptop computer and back-projected via a magnet-compatible projector onto a screen that could be viewed through a mirror mounted above the participant’s head. Participants responded with a button pad held in their right hand. Prior to scanning, participants practiced the encoding and cued recall tasks using stimuli distinct from those presented during functional scanning.
fMRI Acquisition
Imaging data were acquired on a 3.0 T GE Signa whole-body MRI system (GE Medical Systems, Milwaukee, WI, USA) with an 8-channel head coil array. Prior to functional scanning, high-resolution, T2-weighted, flow-compensated spin-echo structural images (TR = 3 s, TE = 68 ms, 0.43 × 0.43 inplane resolution) were acquired with 20 3-mm thick slices perpendicular to the main axis of hippocampus to enable visualization of hippocampal subfields, MTL cortical subregions, and midbrain. Functional images were acquired using a high-resolution T2*-sensitive gradient echo spiral in/out pulse sequence (Glover & Law, 2001), with the same slice locations as the structural images (TR = 4s, TE = 34ms, flip angle = 80°, FOV = 22 cm, 1.7 × 1.7 × 3.0 mm resolution). Before functional scanning, a high-order shimming procedure, based on spiral acquisitions, was utilized to reduce B0 heterogeneity (Kim, Adalsteinsson, Glover, & Spielman, 2002). Critically, spiral in/out methods are optimized to increase SNR and BOLD contrast-to-noise ratio in uniform brain regions while reducing signal loss in regions compromised by susceptibility-induced field gradients (SFG) (Glover & Law, 2001), including the anterior MTL. Compared to other imaging techniques (Glover & Lai, 1998), spiral in/out methods result in less signal dropout and greater task-related activation in MTL (Preston, Thomason, Ochsner, Cooper, & Glover, 2004), allowing targeting of structures that have previously proven difficult to image due to SFG.
A total of 1184 volumes were acquired for each participant (640 during encoding runs and 544 volumes during cued recall). To obtain a field map for correction of magnetic field heterogeneity, the first time frame of the functional timeseries was collected with an echo time 2 ms longer than all subsequent frames. For each slice, the map was calculated from the phase of the first two time frames and applied as a first order correction during reconstruction of the functional images. In this way, blurring and geometric distortion were minimized on a per-slice basis. In addition, correction for off-resonance due to breathing was applied on a per-time-frame basis using phase navigation (Pfeuffer, Van de Moortele, Ugurbil, Hu, & Glover, 2002). This initial volume was then discarded as well as the following two volumes of each scan (a total of 12 s) to allow for T1 stabilization.
fMRI Analyses
fMRI data were analyzed using SPM5 (Wellcome Department of Cognitive Neurology, London, UK) and custom MATLAB routines. T2*-weighted functional images were corrected to account for the differences in slice acquisition times by interpolating the voxel time series using sinc interpolation and resampling the time series using the center slice as a reference point. Images were then realigned to the first volume of the time series to correct for motion. A mean T2*-weighted functional image was computed during realignment, and the T2-weighted anatomical volume was coregistered to this mean functional volume.
Voxel-based statistical analyses were first conducted at the individual participant level according to the general linear model (Worsley & Friston, 1995). Each phase (encoding and cued recall) was analyzed separately. Regressor functions for each phase were constructed using a finite impulse response (FIR) basis set (2 s temporal resolution) that began at trial onset and continued 20 s post-trial onset for encoding trials and 24 s for cued recall trials. Because the events were intermixed with 2 s odd/even baseline trials, events either occurred at TR onset or 2 s after TR onset. Thus, our design was optimized for sampling of the event-related hemodynamic response function with a 2 s resolution.
To implement voxel-level group analyses for our high-resolution data, we used a non-linear diffeomorphic transformation method (Vercauteren, Pennec, Perchant, & Ayache, 2009) implemented in the software package MedINRIA (version 1.8.0, ASCLEPIOS Research Team, France). Specifically, each participant’s anatomically defined MTL regions-of-interest (ROIs) were aligned with those of a representative “target” subject using a diffeomorphic deformation algorithm that implements a biologically plausible transformation that respects the boundaries dictated by the anatomical ROIs. As a first step, anatomically defined ROIs were demarcated on the T2-weighted, high-resolution inplane structural images for each individual participant, using techniques adapted for analysis and visualization of MTL subregions (Amaral & Insausti, 1990; Insausti, et al., 1998; Preston, et al., 2010; Pruessner, et al., 2002; Pruessner, et al., 2000; Zeineh, Engel, & Bookheimer, 2000; Zeineh, et al., 2003). Eight MTL subregions were defined in each hemisphere: the hippocampal subfields (dentate gyrus/CA2/3, CA1, and subiculum) within the body of the hippocampus and surrounding MTL cortices, including perirhinal cortex (PRc), parahippocampal cortex (PHc), and entorhinal cortex (ERc). Because the hippocampal subfields cannot be delineated in the most anterior and posterior extents of the hippocampus at the resolution employed, anterior hippocampal and posterior hippocampal ROIs (inclusive of all subfields) were also demarcated on the most rostral and caudal 1-2 slices of the hippocampus, respectively (Olsen, et al., 2009; Preston, et al., 2010; Zeineh, et al., 2003). These regions roughly correspond to MNI coordinates of y = 0 to y = −6 for the anterior hippocampus and y = −33 to y = −40 for the posterior hippocampus (Preston, et al., 2010).
A single participant’s structural images were chosen as the target, and accordingly, all other participants’ images were warped into a common space in a manner that maintained the between-region boundaries. To select the target participant, we measured the anterior-posterior length (number of slices) of the MTL for each participant and selected the participant with a length closest to the group average for each hemisphere. This selection process helped to minimize distortion caused by variability in the length of the MTL across subjects. To maximize the accuracy of registration within local regions and minimize distortion, separate registrations were performed for left hippocampus, right hippocampus, left MTL cortex, and right MTL cortex. Compared to standard whole-brain normalization techniques, this ROI-alignment or “ROI-AL-Demons” approach results in more accurate correspondence of MTL subregions across subjects and higher statistical sensitivity (e.g., Kirwan, Jones, Miller, & Stark, 2007; Yassa & Stark, 2009)
In addition to performing this procedure for the MTL ROIs, a separate normalization was performed for midbrain. Anatomical landmarks, including the red nucleus and superior colliculus (D’Ardenne, McClure, Nystrom, & Cohen, 2008; Oades & Halliday, 1987) were used to align each individual participant’s midbrain region to the model subject’s midbrain region. The aligned structural images were then normalized using non-linear diffeomorphic demons. Our regions of interest within midbrain included the dopaminergic midbrain regions SN and VTA. As no clear anatomical boundaries delineating SN or VTA can be used to draw a precise ROI, an anterior midbrain mask was drawn on the target structural image using identifiable landmarks on the T2-weighted structural images (see inset in Figure 3A). These landmarks included the red nucleus, identified as a hypointense region near the center of the midbrain, and the anterior boundary of the midbrain. VTA is located medial to and immediately anterior to the red nucleus, while SN extends laterally along the anterolateral boundary of the red nucleus (D’Ardenne, et al., 2008). Based on this anatomical knowledge, the anterior midbrain mask was defined as the region between the posterior end of the red nucleus and the anterior boundary of the midbrain, between the superior and inferior end of the red nucleus.
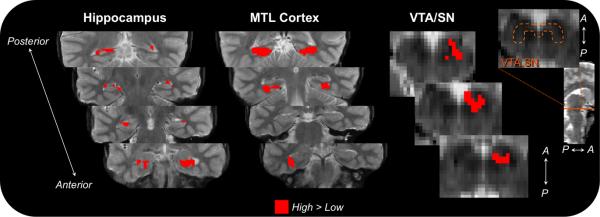
Figure 3.
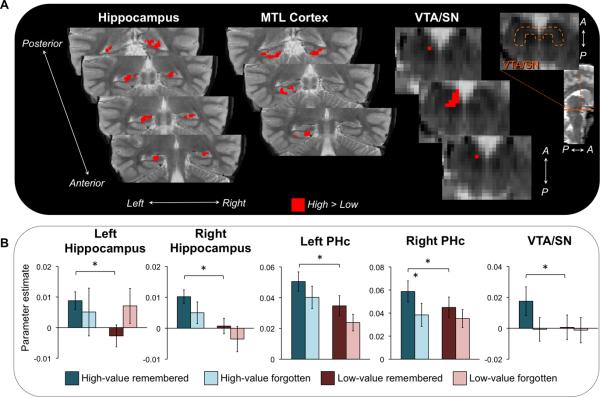
Regions differentially sensitive to reward value during the encoding phase. Encoding activation in VTA/SN and several MTL subregions (shown in red) showed greater activation for high-value relative to low-value associations. An orange dotted outline indicates the location of VTA/SN on a representative slice.
The transformation matrix generated from the anatomical data for each region-of-interest was then applied to the first-level statistical contrast maps, which enabled second-level, group statistical analyses. For all comparisons, group-level statistical maps were first created using an uncorrected voxel-wise threshold of p < 0.025. To correct for multiple comparisons, a small-volume correction was employed to establish a cluster-level corrected threshold of p < 0.05. Small volume correction was determined using Monte Carlo simulations implemented in the AlphaSim tool in AFNI, which takes into account the size and shape of each region, as well as the height threshold p-value and smoothness of actual data. Simulations were performed for each region bilaterally (hippocampus, MTL cortex, and VTA/SN). Cluster sizes that occurred with probability less than 0.05 across 5000 simulations were considered significant. This yielded a minimum cluster size of 32 voxels (108 mm3) for hippocampus, 37 (125 mm3) voxels for MTL cortex, and 20 voxels (68 mm3) for VTA/SN. The resulting group-level results were then localized to specific ROIs to examine condition-specific responses in MTL subregions and VTA/SN.
Functional ROIs were determined by linear contrasts during the stimulus encoding time period (8-12 s post trial onset) and during the combined cue and delay period at test (2-6 s post trial onset). Four sets of functional ROIs were generated: two sets each from the encoding and test phases. First, to confirm that participants were sensitive to the reward manipulation, we identified regions showing greater activation for high-value compared to low-value associations at encoding. We constructed an FIR general linear model containing regressors for high-value and low-value events irrespective of memory status and performed a linear contrast comparing these conditions (high > low) during encoding. The same comparison of high-value and low-value trials was performed for the cue and delay period of the retrieval phase; however, the goal of this contrast at test was to isolate reactivation of reward-related information that occurs in the absence of explicit cues to reward. Next, to test how reward impacts memory processing in MTL subregions during encoding and cued recall, we isolated regions showing memory success effects (greater activation for remembered compared to forgotten associations) for both the encoding and retrieval phases of the experiment. To do so, we constructed an FIR general linear model containing regressors for remembered and forgotten associations, irrespective of reward status; a linear contrast then compared these event types (remembered > forgotten). Together, these contrasts yielded four sets of functionally defined ROIs: regions that differentiated between 1) high > low during stimulus encoding, 2) remembered > forgotten during stimulus encoding, 3) high > low during cued recall, and 4) remembered > forgotten during cued recall.
For each of the functionally defined ROIs generated from the four contrasts of interest, we then extracted mean beta values for the conditions from the corresponding task phase (encoding, retrieval) using a general linear model that contained regressors for high-value remembered, high-value forgotten, low-value remembered, and low-value forgotten associations. Activation associated with each condition of interest was computed as the average of beta values for the time points corresponding to each event type in the FIR models. Group-level repeated-measures ANOVA with reward (high-value, low-value) and memory status (remembered, forgotten) as factors was used to test for differences in BOLD activity between conditions in each of the ROIs. One participant was excluded from these analyses due to a lack of forgotten associations in the high-value condition.
Additionally, we considered how activation in functionally defined ROIs tracked individual differences in behavior. We were particularly interested in whether behavioral sensitivity to reward (the difference in corrected hit rate for high-value and low-value associations) was related to reward-related changes in brain activation. For each set of functionally defined ROIs, we conducted a multiple regression analysis with the difference in subsequent memory effect (remembered – forgotten) between high-value and low-value pairs (the memory x reward interaction) as regressors and behavioral reward modulation as the outcome measure. Because this analysis did not reveal a relationship between the memory x reward interaction and behavioral reward modulation of memory in any region, we conducted a similar analysis that was restricted to remembered associations from both reward conditions. Specifically, this analysis assessed how the activation differences for high-value remembered associations relative to low-value remembered associations were related to the degree of behavioral reward modulation across participants. The participant demonstrating ceiling performance on high-value trials was again excluded from the individual differences analyses due to a value for behavioral reward modulation that was greater than three standard deviations from the group mean.
Finally, we were interested in whether individual differences in VTA/SN-MTL connectivity are related to individual differences in behavioral reward modulation of memory. We hypothesized that differences in sustained VTA/SN-MTL connectivity independent of task-based fluctuations would be related to across-participant differences in the degree of behavioral reward modulation of memory, with greater VTA/SN-MTL connectivity for those participants with greater behavioral sensitivity to reward. To test this hypothesis, we examined connectivity between VTA/SN and MTL regions during the encoding and cued recall phases, while controlling for common co-activation of these regions due to task-related rewards. We conducted functional connectivity analyses at the individual participant level using a general linear model (Worsley & Friston, 1995) that included the mean activation timecourse in a seed region (anatomical VTA/SN), regressors respresenting motion parameters, and FIR regressors for each of the four task conditions (high-value remembered, high-value forgotten, low-value remembered, low-value forgotten). Including regressors for the task conditions allowed us to assess how residual intrinsic connectivity between VTA/SN and MTL regions during the encoding and retrieval phases was related to behavioral reward modulation, above and beyond covariation due to task-related differences between individual reward-predicting cues. Separately for encoding and cued recall, we contrasted the VTA/SN seed regressor with baseline to isolate MTL regions that showed a significant sustained connectivity with VTA/SN. The contrast maps from the individual subjects were then submitted to a second-level group analysis to determine how connectivity between VTA/SN and MTL varies as a function of behavioral sensitivity to reward. For this analysis, the individual participant functional connectivity maps from the encoding and retrieval phases were weighted by behavioral reward modulation to identify MTL regions for which sustained connectivity between VTA/SN was positively related to the degree of behavioral reward modulation.
RESULTS
Influence of Reward on Memory Performance
The hit rate (correct responses to associative match trials) during cued recall was significantly greater than the false alarm rate (incorrect responses to associative mismatch trials) for both high-value (t(27) = 20.1, p < 0.001) and low-value (t(27) = 16.1, p < 0.001) associations (Figure 2A). Critically, participants showed better memory for high-value associations than low-value associations, as indexed by corrected hit rate (hit rate – false alarm rate; mean ± standard error: high-value = 0.70 ± 0.03, low-value = 0.60 ± 0.04; t(27) = 2.28, p < 0.05). This difference in memory was not due to failure to learn the low-value associations, as participants showed above chance performance on the low-value associations across the group (t(27) = 16.1, p < 0.001). At the level of individual participants, a binomial test revealed that all participants but one showed above chance performance on low-value associations (all p < 0.01); performance for the remaining participant was only marginally above chance on low-value associations (p = 0.07).
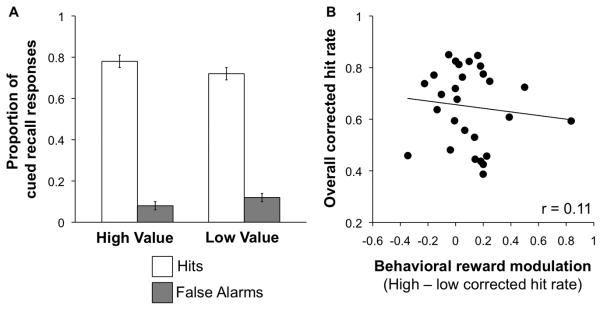
Figure 2.
Behavioral results. (A) Percentage of hits (white bars) and false alarms (gray bars) for high-value and low-value associations. Error bars represent standard error of the mean. (B) Cued recall performance (as measured by overall corrected hit rate) as a function of behavioral reward modulation (the difference in corrected hit rate for high and low-value associations). Overall corrected hit rate was not correlated with the degree of behavioral reward modulation (p > 0.5).
While we observed significantly better memory for high-value relative to low-value associations across the group, there were large individual differences in the degree of reward modulation of memory across participants. To capture these individual differences, we calculated the difference in corrected hit rate between high-value and low-value associations for each participant as an index of behavioral reward modulation of memory. The observed behavioral differences in reward modulation enabled us to explore how individual differences in performance are related to the degree of neural reward modulation within MTL regions and VTA/SN. Importantly, those participants who demonstrated the greatest reward modulation of cued recall performance did not demonstrate the greatest overall memory performance, as the overall corrected hit rate was not correlated with the degree of behavioral reward modulation (r = 0.11, p = 0.59; Figure 2B). Median reaction times during cued recall did not differ between high-value (1031 ms ± 26 ms) and low-value (1064 ms ± 28 ms) associations (t(27) = 1.6, p = 0.13).
Regions Differentially Sensitive to Reward during the Encoding Phase
First, to confirm that participants demonstrated neural sensitivity to the reward manipulation, we compared responses for high-value and low-value associations (high > low) during the encoding phase, irrespective of memory performance. This contrast revealed several regions that were differentially sensitive to the reward conditions, including VTA/SN, right PRc, bilateral PHc, and hippocampus, including left CA1, left subiculum, and bilateral DG/CA2,3 (Figure 3). While these regions demonstrated greater activation for high-value relative to low-value trials, only PHc responses were related to successful associative encoding, as revealed by a repeated-measures ANOVA with memory (remembered, forgotten) and reward condition (high-value, low-value) as factors. PHc activation showed a main effect of memory in addition to the main effect of reward (all other F(1,26) < 3.10, p > 0.09); left PHc activation showed a main effect of memory (F(1,26) = 4.72, p = 0.04), and right PHc activation showed a main effect of memory F(1,26) = 7.42, p = 0.01) as well as a memory × reward interaction (F(1,26) = 6.02, p = 0.02).
Reward Modulation of Successful Memory Formation
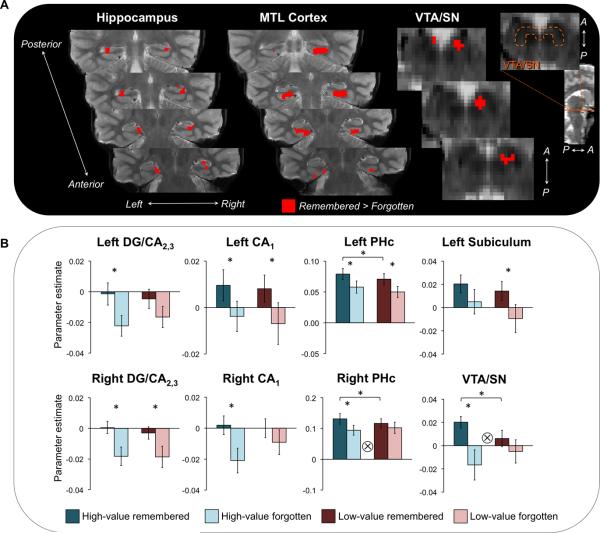
The central focus of the present study was to understand how reward influences associative encoding processes in MTL subregions. To do so, we isolated those regions that were related to subsequent memory performance (Paller & Wagner, 2002) by performing a linear contrast comparing activation for remembered and forgotten associations (remembered > forgotten) regardless of reward status during the encoding phase. This contrast revealed a spatially restricted set of MTL regions associated with successful associative encoding, including bilateral CA1, bilateral dentate gyrus/CA2,3 (DG/CA2,3), left subiculum, and bilateral parahippocampal cortex (PHc), as well as VTA/SN (Figure 4A). To investigate how monetary incentives influence associative encoding in these memory sensitive regions, we examined whether subsequent memory effects in these regions were modulated by reward status at encoding, using repeated-measures ANOVA with memory (remembered, forgotten) and reward (high-value, low-value) as factors. Encoding activation in right PHc (F(1,26) = 5.34, p = 0.03) and VTA/SN (F(1,26) = 4.89, p = 0.04) showed a significant memory × reward interaction, with greater subsequent memory effects for high-value relative to low-value associations (Figure 4B). No other region showed a memory × reward interaction (all F(1,26) < 2.44, p > 0.13) or a main effect of reward (all F(1,26) < 3.59, p > 0.07).
Figure 4.
Reward modulation of successful memory formation. (A) Encoding activation in VTA/SN and several MTL subregions (shown in red) was associated with subsequent cued recall performance, with greater activation for remembered relative to forgotten associations. An orange dotted outline indicates the boundaries of the anatomical VTA/SN region on a representative slice (see Methods for full description of localization procedures). (B) Subsequent memory effects (remembered > forgotten associations) in MTL and VTA/SN plotted as a function of reward value: high-value remembered (dark blue), high-value forgotten (light blue), low-value remembered (red), and low-value forgotten (pink). A significant reward x memory interaction was observed in right PHc and VTA/SN (denoted by a ⊗). Error bars represent standard error of the mean. Asterisks indicate significant pairwise differences (p < 0.05).
Notably, we observed large differences in the degree of behavioral reward modulation of memory (the difference in corrected hit rate between high-value and low-value associations) between individuals. Given this behavioral finding, the effect of reward on encoding processes may be best reflected in individual differences in the relationship between subsequent memory effects for high-value relative to low-value associations and the degree of behavioral reward modulation of memory. To test this hypothesis, we assessed how the difference in subsequent memory effects between high-value and low-value pairs (the memory x reward interaction) was associated with the degree of behavioral reward modulation of memory across participants. We conducted a multiple regression analysis with the memory x reward interaction term for each of the eight subsequent memory regions as regressors and behavioral reward modulation across participants as the outcome measure. We did not observe a positive relationship between the memory x reward interaction term and the behavioral reward effect in any region (all t(18) < 0.80, p > 0.43). However, when we limited our analysis to remembered trials only, we observed a positive relationship between the difference in reward-related activity during successful encoding (high-value remembered – low-value remembered activation) and behavioral reward modulation in right DG/CA2,3 (t(18) = 2.45, p = 0.03). Those participants who showed greater activation for high-value remembered associations relative to low-value remembered associations in right DG/CA2,3 demonstrated the greatest behavioral sensitivity to reward (Pearson’s r = 0.41; Figure 5). The multiple regression analysis did not reveal a positive relationship between activation differences for high-value and low-value remembered associations and the amount of behavioral reward modulation in any other region (all other t(18) < 1.51, p > 0.14).
Figure 5.
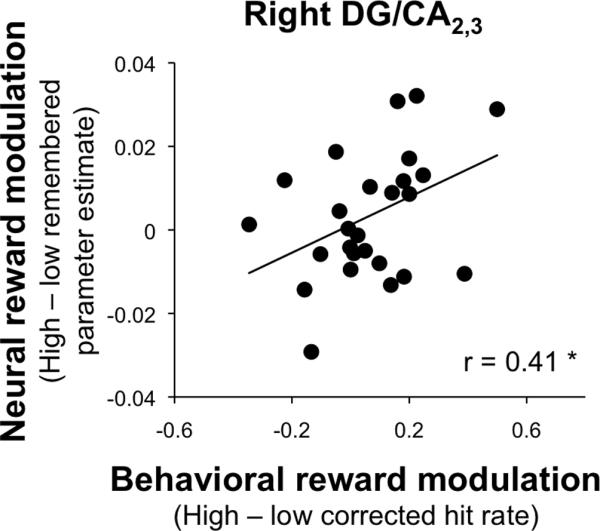
Brain-behavior correlations during associative encoding. We observed a positive relationship between the reward effect for successfully remembered events (high-value remembered parameter estimate – low-value remembered parameter estimate) in DG/CA2,3 and behavioral reward modulation of memory (corrected hit rate for high-value trials – corrected hit rate for low-value trials).
VTA/SN-MTL Functional Interactions during the Encoding Phase
We hypothesized that behavioral sensitivity to reward would be reflected in across-participant differences in sustained VTA/SN-MTL functional connectivity that are independent of task-based connectivity changes. To examine this hypothesis, we derived individual participant functional connectivity maps from the encoding phase that represented the residual connectivity between VTA/SN and MTL above and beyond task-based covariation in activation. We then submitted these maps to a second-level regression analysis to determine how sustained connectivity varied as a function of behavioral reward modulation. This analysis revealed activation in bilateral DG/CA2,3 for which sustained functional connectivity with VTA/SN was positively correlated with behavioral reward modulation (Figure 6A). This relationship between VTA/SN connectivity and performance was unique to the DG/CA2,3, as no other region was identified by this analysis. These results indicate that individuals who demonstrate higher levels of sustained connectivity between VTA/SN and DG/CA2,3 throughout the encoding phase of the task are more likely to demonstrate behavioral effects of reward on memory performance.
Figure 6.
Sustained functional connectivity between VTA/SN and MTL is associated with behavioral sensitivity to reward during associative encoding and cued recall task phases. (A) Activation in DG/CA2,3 (shown in red) for which functional connectivity with VTA/SN was positively correlated with behavioral reward modulation during associative encoding. (B) Activation in DG/CA2,3 (shown in red) for which functional connectivity with VTA/SN was positively correlated with behavioral reward modulation during cued recall.
Regions Differentially Sensitive to Reward during the Retrieval Phase
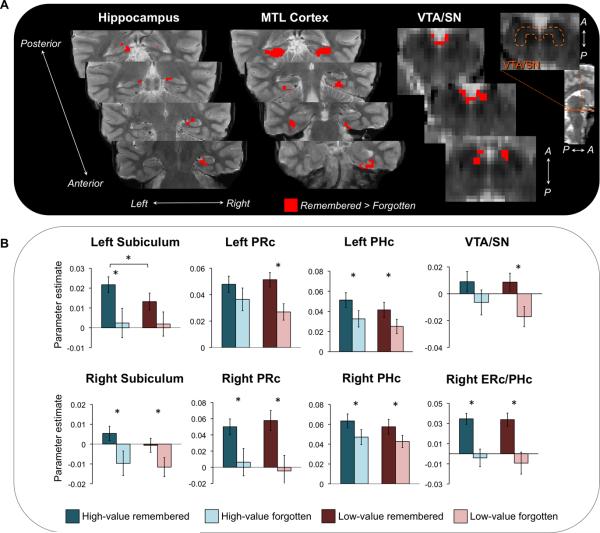
Although participants did not receive explicit cues to reward during cue and delay period of the cued recall task, we hypothesized that monetary incentives presented during encoding would be reflected in later cued recall activation. To examine this hypothesis, we identified regions showing reward effects (high > low) during the combined cue and delay period at test. This analysis revealed activation in bilateral hippocampus (inclusive of all subfields), bilateral PHc, and VTA/SN (Figure 7A). Repeated measures ANOVA further revealed that within these regions sensitive to reward status, PHc activation further differentiated trials based on memory success, with greater activation for remembered relative to forgotten associations (main effect of memory: left: F(1,26) = 5.66, p = 0.03; right: F(1,26) = 6.55, p = 0.02; Figure 7B). No other region showed a main effect of memory (all F(1,26) < 2.74, p > 0.11), and no region showed a memory × reward interaction (all F(1,26) < 2.24, p > 0.14). These results indicate that reward cues presented during encoding impact later cued recall responses, even in the absence of explicit cues to reward.
Figure 7.
Regions differentially sensitive to reward value during the retrieval phase. (A) Direct comparison of high-value and low-value cued recall trials revealed enhanced activation for high-value information in VTA/SN and MTL subregions (shown in red). An orange dotted outline indicates the boundaries of the anatomical VTA/SN region on a representative slice (B) Cue and delay period activation as a function of memory (remembered and forgotten associations) and reward (high- and low-value associations) in MTL and VTA/SN: high-value remembered (dark blue), high-value forgotten (light blue), low-value remembered (red), and low-value forgotten (pink) within regions showing a reward effect (high-value > low-value). Error bars represent standard error of the mean. Asterisks indicate significant pairwise differences (p < 0.05).
Regions Sensitive to Retrieval Success during Cued Recall
To investigate whether monetary incentives presented during associative encoding modulate retrieval success effects during cued recall, we identified regions showing retrieval success effects (remembered > forgotten) during the combined cue and delay period at test. This comparison revealed activation in bilateral regions of subiculum, PHc, and PRc, as well as right ERc and VTA/SN (Figure 8A) that differentiated between successfully remembered and forgotten associations. None of these retrieval success regions demonstrated a main effect of reward (all F(1,26) < 3.41, p > 0.07) or a memory × reward interaction (all F(1,26) < 1.16, p > 0.29) (Figure 8B). Multiple regression analysis also revealed that neither the memory x reward interaction (t(18) < 1.20, p > 0.24) nor the reward effect for successfully remembered events (t(18) < 1.56, p > 0.13) in these retrieval success regions was related to the degree of behavioral reward modulation across participants. Thus, although the direct comparison of high-value and low-value associations in the previous section revealed reward sensitive regions at retrieval, activation in regions sensitive to cued recall success was not modulated by reward. This pattern differs from the encoding phase results and suggests that brain processes and regions that support reinstatement of reward value and retrieval of correct paired associations may be distinct.
Figure 8.
Regions sensitive to retrieval success during cued recall. (A) Cue and delay period activation at test was associated with cued recall success in MTL and VTA/SN (shown in red), with greater activation for successfully recalled relative to forgotten associations. An orange dotted outline indicates the boundaries of the anatomical VTA/SN region on a representative slice. (B) Retrieval success effects (remembered > forgotten associations) in MTL and VTA/SN plotted as a function of reward value: high-value remembered (dark blue), high-value forgotten (light blue), low-value remembered (red), and low-value forgotten (pink). A significant reward effect for successfully remembered events (high-value remembered parameter estimate – low-value remembered parameter estimate) was observed in left subiculum and left PHc. Error bars represent standard error of the mean. Asterisks indicate significant pairwise differences (p < 0.05).
VTA/SN-MTL Functional Interactions during the Retrieval Phase
We were also interested in whether behavioral sensitivity to reward was related to individual differences in sustained VTA/SN-MTL connectivity throughout the retrieval phase. Utilizing the same approach applied to the encoding phase data, we derived maps of sustained VTA/SN-MTL functional connectivity for each individual during the retrieval phase and conducted regression analysis to examine whether individual differences in sustained functional connectivity varied as a function of behavioral reward modulation. This analysis revealed activation in right DG/CA2,3 for which sustained functional connectivity with VTA/SN was positively correlated with behavioral reward modulation (Figure 6B). This relationship between VTA/SN connectivity and performance was unique to the DG/CA2,3, as no other region was identified by this analysis.
DISCUSSION
Several leading theories propose that motivation plays a key role in the formation of detailed episodic memories through modulation of MTL processing. The current study used the potential of future monetary rewards to examine how motivation impacts associative encoding and retrieval processes in hippocampal and surrounding MTL cortical subregions. Participants were more likely to remember object pairs linked to high-value compared to low-value monetary incentives. Within PHc and VTA/SN, subsequent memory effects were modulated by reward, with greater activation for remembered compared to forgotten associations for high-value but not low-value pairs. Individuals differed in the degree to which reward influenced memory performance; these differences were associated with reward-related changes in DG/CA2,3 activation during encoding and greater sustained functional connectivity between DG/CA2,3 and VTA/SN during both encoding and retrieval phases of the task. Furthermore, at retrieval, we observed enhanced activation in hippocampus, PHc, and VTA/SN during the combined cue and delay period for high-value relative to low-value associations that occurred in the absence of explicit reward cues. In the present study, the use of high-resolution fMRI affords a more detailed view into the neural mechanisms by which motivation impacts associative memory formation in the human brain and characterizes how those mechanisms differ across individuals.
Reward Modulation of Subsequent Memory Effects in PHc and VTA/SN
Several MTL subregions as well as VTA/SN demonstrated sensitivity to the reward manipulation during the encoding phase, with greater activation for high-value relative to low-value associations. However, a more spatially restricted set of regions was associated with successful associative encoding across the group, including hippocampal subfields, PHc, and VTA/SN. Within these regions, only right PHc and VTA/SN showed reward modulation of encoding activation, with subsequent memory effects for high-value but not low-value pairs. This interaction pattern suggests that right PHc and VTA/SN were selectively engaged during high-value trials to promote memory formation.
The present finding that VTA/SN is exclusively engaged during successful encoding of high-value associations is consistent with prior research demonstrating that VTA/SN responses to high-value reward cues prior to individual item encoding are related to successful subsequent memory (Adcock, et al., 2006). This increased activation may reflect enhanced dopamine release from VTA/SN in response to high-value cues when participants are successfully motivated to learn. During high-value forgotten trials, VTA/SN activation was not above baseline, possibly reflecting a lack of motivation on these trials. However, in contrast to Adcock and colleagues, VTA/SN responses observed here were not associated with individual differences in behavior. As discussed below, the reward-related activation in VTA/SN may relate to individual differences in hippocampal activation due to differences in functional connectivity with MTL structures.
Several prior studies have implicated PHc in the binding of associative information (Davachi, Mitchell, & Wagner, 2003; Dobbins, Rice, Wagner, & Schacter, 2003; Duzel, et al., 2003; Kirwan & Stark, 2004; Ranganath, et al., 2004). The present data extend this work by demonstrating that engagement of PHc associative encoding processes are influenced by reward, leading to superior memory for motivationally significant events. Here, enhanced subsequent memory effects for high-value associations in right PHc could reflect the selective engagement of PHc encoding processes during high-value trials to promote binding of the objects themselves. Alternatively, recent theoretical accounts of MTL function propose that PHc plays an important role in the representation of spatial and non-spatial contextual information surrounding individual events (Aminoff, Gronau, & Bar, 2007; Bar & Aminoff, 2003; Davachi, 2006; Diana, Yonelinas, & Ranganath, 2007). According to this view, enhanced right PHc encoding activation during high-value events may reflect facilitated binding of a specific paired associate to the reward context in which it was presented. Future work that separately indexes memory for item associations and memory for reward context would help to clarify which of these potential PHc binding mechanisms (or both) are reflected in the present findings.
Relationship between DG/CA2,3 Activation and Individual Differences in Reward Modulation of Memory
Few studies to date have examined how individual differences in reward sensitivity impact episodic memory through modulation of MTL processing. The present findings demonstrate a high-degree of behavioral variability in the influence of motivational cues on subsequent memory performance. These individual differences in behavioral reward modulation of memory were related to across participant differences in the relative engagement of DG/CA2,3 during the encoding phase, with larger reward-based enhancements in memory performance associated with greater DG/CA2,3 activation for high-value remembered relative to low-value remembered associations.
Prior high-resolution fMRI studies in humans have implicated DG/CA2,3 in successful associative memory formation (Eldridge, et al., 2005; Zeineh, et al., 2003), and the present data extend these findings by demonstrating that associative memory processing in this region is subject to motivational influences. One mechanism through which reward impacts memory may be by enhancing learning-related CA3 plasticity (Lisman & Otmakhova, 2001; Otmakhova & Lisman, 1999) that is hypothesized to support the rapid binding of events into integrated memory representations (Marr, 1971; McClelland, McNaughton, & O’Reilly, 1995; O’Reilly & Rudy, 2001). This interpretation is supported by a recent animal study demonstrating reward-related increases in CA3 activity that were enhanced during new associative learning (Singer & Frank, 2009). The present findings suggest that reward exerts a similar influence in human hippocampus, whereby reward-related engagement of CA3 serves to enhance memory for events that lead to future reward.
It is important to note that the relationship between reward-related activation in DG/CA2,3 during encoding and behavioral reward modulation of memory was only observed when we restricted our analysis to remembered associations. We did not observe an interaction between reward and subsequent memory effects in DG/CA2,3, nor did we observe a correlation between behavioral reward modulation and the degree of reward modulation of subsequent memory effects (the memory x reward interaction) in this (or any other) region. Thus, the present findings contrast to some degree with previous results demonstrating reward-related increases in hippocampal subsequent memory effects during item encoding (Adcock, et al., 2006; Wittmann, et al., 2005). In the present study, several possibilities could account for the observation that the relationship between DG/CA2,3 activation and individual differences in reward modulation of memory was limited to remembered trials.
One possibility is that signal reductions in the hippocampus relative to PHc in this high-resolution fMRI study (see Liang, Wagner, & Preston, 2012, in press) may limit the ability to observe interaction effects in hippocampal subregions. In addition, performance in the present study was well-above chance for high-value and low-value associations, leading to a reduced number of forgotten associations relative to prior studies of individual item encoding (Adcock, et al., 2006), which when combined with hippocampal signal reductions would further reduce statistical power. An additional possibility is that the relationship between DG/CA2,3 activation and behavioral reward modulation may be biased by the asymmetry in the number of remembered trials between those participants who showed large behavioral effects of reward relative to those who showed no reward modulation of memory. However, this bias applies to the neural reward effect in all regions, yet only activation in DG/CA2,3—a region hypothesized to play a key role in associative encoding—was found to correlate with reward modulation of memory and demonstrate a behaviorally-relevant pattern of functional connectivity with VTA (see below).
Aspects of the present task design that differ from prior related studies may also underlie differences in the observed pattern of hippocampal activation across studies. In particular, Adcock et al. (2006) examined the relationship between reward-related hippocampal activation and subsequent memory performance during an anticipatory phase prior to individual item encoding. Here, we examined associative encoding, and the present design did not afford the ability to differentiate between the cue period and presentation of the associations.
In addition, and as noted above, participants performed well on both high-value and low-value associations in contrast to prior research. Because DG/CA2,3 regions are implicated in successful associative binding, significant DG/CA2,3 subsequent memory effects should be expected for high-value and low-value associations, as observed here. Thus, greater DG/CA2,3 encoding activation for high-value remembered events relative to low-value remembered events may reflect enhancement of associative encoding processes that are engaged during both event types, but enhanced for high-value trials. One possibility is that enhanced DG/CA2,3 processing specific to remembered associations may reflect the formation of stronger, or more detailed, memory traces for high-value associations than those formed for low-value associations. Follow-up high-resolution fMRI studies could help resolve differences between the present findings and existing research by separately examining cue-related and stimulus-related responses in MTL subregions and by providing more detailed assessments of the quality of successfully formed memories under different motivational conditions.
VTA/SN—DG/CA2,3 Functional Connectivity and Individual Differences
The pattern of functional connectivity between DG/CA2,3 and VTA/SN further supports the link between DG/CA2,3 processing and individual differences in reward modulation of memory. During both the encoding and cued recall phases, greater sustained functional connectivity between VTA/SN and DG/CA2,3 was associated with enhanced memory for high-value relative to low-value associations across individuals. Thus, while VTA/SN responses themselves demonstrated sensitivity to reward across the entire group, only those individuals with greater sustained VTA/SN-DG/CA2,3 functional connectivity showed behavioral evidence for reward modulation of memory.
Notably, DG/CA2,3 was the only MTL region whose connectivity with VTA/SN had a functional relationship to performance. The localization of the functional connectivity results to DG/CA2,3 is consistent with theoretical models and empirical findings that suggest dopaminergic VTA/SN signals facilitate hippocampal processing (Lisman & Grace, 2005; Shohamy & Adcock, 2010) by enhancing CA3 plasticity (Lisman & Otmakhova, 2001; Ortiz, et al., 2010; Otmakhova & Lisman, 1999). The present findings provide the first evidence for this proposed mechanism of motivation’s influence on memory in the human brain.
It should be emphasized that our specific measure of functional connectivity assessed how sustained VTA/SN connectivity with DG/CA2,3 throughout the encoding and retrieval phases was related to performance and was not specific to one particular task condition (e.g., high-value trials). One possible explanation for the present findings is that high-value events yield greater dopamine release in VTA/SN than low-value events in all participants, leading to enhanced VTA/SN activation for high-value associations across the group. However, the impact of VTA/SN activation on MTL responses may differ between individuals. Accordingly, individual differences in sustained VTA/SN-DG/CA2,3 functional connectivity may reflect intrinsic differences in the degree of communication between these regions throughout the encoding and cued recall phases. Those participants with greater intrinsic VTA/SN-DG/CA2,3 connectivity (as measured by the sustained effects in the present study) would be more likely to demonstrate behavioral reward modulation of memory, presumably because of greater reward modulation of DG/CA2,3 responses. While intrinsic connectivity is typically measured during rest, we used a residual connectivity measure that accounted for covariation due to the different task conditions to examine intrinsic differences, as no rest scans were collected in the present study. Future work may help to better establish how our measure of sustained connectivity relates to intrinsic functional connectivity measured during rest.
An alternative, but not mutually exclusive, possibility is that associative binding processes in CA3 serve to link particular events with their reward value for both high- and low-value events during encoding, and thus different levels of VTA/SN-DG/CA2,3 functional connectivity across participants may reflect the influence of downstream projections from CA3 to VTA/SN via the lateral septum (Luo, et al., 2011). Correspondingly, differences in VTA/SN-DG/CA2,3 functional connectivity at retrieval may reflect reinstatement of the encoded reward context through CA3 pattern completion processes that target VTA/SN neurons (Kennedy & Shapiro, 2009; Luo, et al., 2011). Consistent with this interpretation, the retrieval phase data indicate that monetary incentives presented at encoding are reflected in hippocampal, PHc, and VTA/SN cued recall responses even in the absence of explicit reward cues. Notably, reward sensitive regions at retrieval were distinct from those demonstrating retrieval success effects, suggesting that reinstatement of reward value and retrieval of item associations are supported by distinct MTL processes. Reactivation of value information in MTL and VTA/SN and enhanced connectivity between regions could establish an expectation of trial outcome (Schultz, 1998; Schultz, et al., 1993), such as what probe stimulus should be expected or how much money is at stake, to guide behavioral choice. Alternatively, such enhancements for high-value associations at retrieval could also reflect enhanced re-encoding of retrieved value-pair associations during test (Stark & Okado, 2003).
Conclusions
The present findings indicate that reward-based motivation serves to enhance episodic encoding and retrieval through facilitation of MTL and VTA/SN processing. By specifically influencing associative binding processes in PHc and DG/CA2,3, motivational goals may serve to make memories more detailed and flexible, thus better adapted for future use (Shohamy & Adcock, 2010). In particular, this high-resolution fMRI study provides novel insights into the specific mechanisms by which motivation influences episodic memory by linking them to the differential engagement of specific hippocampal and MTL cortical subregions. In doing so, our findings allow for greater convergence with electrophysiological and pharmacological research in animals and provide a foundation for future research aimed at understanding how specific hippocampal–midbrain interactions guide adaptive learning processes that promote the flexible encoding and use of experience.
Acknowledgments
This work was supported by a National Science Foundation CAREER Award (A.R.P.), the National Alliance for Research on Schizophrenia and Depression (A.R.P.), Army Research Office Grant 55830-LS-YIP (A.R.P.), NIMH National Research Service Award F31MH092032 (S.M.W.), and an American Psychological Association Diversity Program in Neuroscience Fellowship (S.M.W.). The authors thank Nicolaus Schmandt, April Dominick, and Manoj Doss for assistance with data collection and analysis.
REFERENCES
- Adcock RA, Thangavel A, Whitfield-Gabrieli S, Knutson B, Gabrieli JD. Reward-motivated learning: mesolimbic activation precedes memory formation. Neuron. 2006;50(3):507–517. doi: 10.1016/j.neuron.2006.03.036. [DOI] [PubMed] [Google Scholar]
- Akil M, Lewis DA. The dopaminergic innervation of monkey entorhinal cortex. Cereb Cortex. 1993;3(6):533–550. doi: 10.1093/cercor/3.6.533. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Insausti R. Hippocampal formation. In: Paxinos G, editor. The Human Nervous System. Academic Press; San Diego: 1990. pp. 711–755. [Google Scholar]
- Aminoff E, Gronau N, Bar M. The parahippocampal cortex mediates spatial and nonspatial associations. Cereb Cortex. 2007;17(7):1493–1503. doi: 10.1093/cercor/bhl078. [DOI] [PubMed] [Google Scholar]
- Bar M, Aminoff E. Cortical analysis of visual context. Neuron. 2003;38(2):347–358. doi: 10.1016/s0896-6273(03)00167-3. [DOI] [PubMed] [Google Scholar]
- Bernabeu R, Bevilaqua L, Ardenghi P, Bromberg E, Schmitz P, Bianchin M, et al. Involvement of hippocampal cAMP/cAMP-dependent protein kinase signaling pathways in a late memory consolidation phase of aversively motivated learning in rats. Proc Natl Acad Sci U S A. 1997;94(13):7041–7046. doi: 10.1073/pnas.94.13.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaha CD, Yang CR, Floresco SB, Barr AM, Phillips AG. Stimulation of the ventral subiculum of the hippocampus evokes glutamate receptor-mediated changes in dopamine efflux in the rat nucleus accumbens. Eur J Neurosci. 1997;9(5):902–911. doi: 10.1111/j.1460-9568.1997.tb01441.x. [DOI] [PubMed] [Google Scholar]
- Bunzeck N, Duzel E. Absolute coding of stimulus novelty in the human substantia nigra/VTA. Neuron. 2006;51(3):369–379. doi: 10.1016/j.neuron.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Carr VA, Rissman J, Wagner AD. Imaging the human medial temporal lobe with high-resolution fMRI. Neuron. 2010;65(3):298–308. doi: 10.1016/j.neuron.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Frank MJ, Gibbs SE, Miyakawa A, Jagust W, D’Esposito M. Striatal dopamine predicts outcome-specific reversal learning and its sensitivity to dopaminergic drug administration. J Neurosci. 2009;29(5):1538–1543. doi: 10.1523/JNEUROSCI.4467-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ardenne K, McClure SM, Nystrom LE, Cohen JD. BOLD responses reflecting dopaminergic signals in the human ventral tegmental area. Science. 2008;319(5867):1264–1267. doi: 10.1126/science.1150605. [DOI] [PubMed] [Google Scholar]
- Dale AM. Optimal experimental design for event-related fMRI. Hum Brain Mapp. 1999;8(2-3):109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L. Item, context and relational episodic encoding in humans. Curr Opin Neurobiol. 2006;16(6):693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Davachi L, Mitchell JP, Wagner AD. Multiple routes to memory: distinct medial temporal lobe processes build item and source memories. Proc Natl Acad Sci U S A. 2003;100(4):2157–2162. doi: 10.1073/pnas.0337195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends Cogn Sci. 2007;11(9):379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Rice HJ, Wagner AD, Schacter DL. Memory orientation and success: separable neurocognitive components underlying episodic recognition. Neuropsychologia. 2003;41(3):318–333. doi: 10.1016/s0028-3932(02)00164-1. [DOI] [PubMed] [Google Scholar]
- Duzel E, Habib R, Rotte M, Guderian S, Tulving E, Heinze HJ. Human hippocampal and parahippocampal activity during visual associative recognition memory for spatial and nonspatial stimulus configurations. J Neurosci. 2003;23(28):9439–9444. doi: 10.1523/JNEUROSCI.23-28-09439.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ. From Conditioning to Conscious Recollection: Memory Systems of the Brain. Oxford University Press; New York: 2001. [Google Scholar]
- Eldridge LL, Engel SA, Zeineh MM, Bookheimer SY, Knowlton BJ. A dissociation of encoding and retrieval processes in the human hippocampus. J Neurosci. 2005;25(13):3280–3286. doi: 10.1523/JNEUROSCI.3420-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Todd CL, Grace AA. Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. J Neurosci. 2001;21(13):4915–4922. doi: 10.1523/JNEUROSCI.21-13-04915.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6(9):968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- Gabrieli JD. Cognitive neuroscience of human memory. Annu Rev Psychol. 1998;49:87–115. doi: 10.1146/annurev.psych.49.1.87. [DOI] [PubMed] [Google Scholar]
- Gasbarri A, Packard MG, Campana E, Pacitti C. Anterograde and retrograde tracing of projections from the ventral tegmental area to the hippocampal formation in the rat. Brain Res Bull. 1994a;33(4):445–452. doi: 10.1016/0361-9230(94)90288-7. [DOI] [PubMed] [Google Scholar]
- Gasbarri A, Packard MG, Campana E, Pacitti C. Anterograde and retrograde tracing of projections from the ventral tegmental area to the hippocampal formation in the rat. Brain Research Bulletin. 1994b;33:445–452. doi: 10.1016/0361-9230(94)90288-7. [DOI] [PubMed] [Google Scholar]
- Gasbarri A, Sulli A, Packard MG. The dopaminergic mesencephalic projections to the hippocampal formation in the rat. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21(1):1–22. doi: 10.1016/s0278-5846(96)00157-1. [DOI] [PubMed] [Google Scholar]
- Gasbarri A, Verney C, Innocenzi R, Campana E, Pacitti C. Mesolimbic dopaminergic neurons innervating the hippocampal formation in the rat: a combined retrograde tracing and immunohistochemical study. Brain Res. 1994;668(1-2):71–79. doi: 10.1016/0006-8993(94)90512-6. [DOI] [PubMed] [Google Scholar]
- Glover GH, Lai S. Self-navigated spiral fMRI: Interleaved versus single-shot. Magnetic Resonance In Medicine. 1998;39:361–368. doi: 10.1002/mrm.1910390305. [DOI] [PubMed] [Google Scholar]
- Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magnetic Resonance in Medicine. 2001;46:515–522. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- Granado N, Ortiz O, Suarez LM, Martin ED, Cena V, Solis JM, et al. D1 but not D5 dopamine receptors are critical for LTP, spatial learning, and LTP-Induced arc and zif268 expression in the hippocampus. Cereb Cortex. 2008;18(1):1–12. doi: 10.1093/cercor/bhm026. [DOI] [PubMed] [Google Scholar]
- Gray JA. The psychology of fear and stress. 2nd ed Cambridge University Press; Cambridge ; New York: 1987. [Google Scholar]
- Gruber MJ, Otten LJ. Voluntary control over prestimulus activity related to encoding. J Neurosci. 2010;30(29):9793–9800. doi: 10.1523/JNEUROSCI.0915-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insausti R, Juottonen K, Soininen H, Insausti AM, Partanen K, Vainio P, et al. MR volumetric analysis of the human entorhinal, perirhinal, and temporopolar cortices. AJNR Am J Neuroradiol. 1998;19(4):659–671. [PMC free article] [PubMed] [Google Scholar]
- Kao YC, Davis ES, Gabrieli JD. Neural correlates of actual and predicted memory formation. Nat Neurosci. 2005;8(12):1776–1783. doi: 10.1038/nn1595. [DOI] [PubMed] [Google Scholar]
- Kennedy PJ, Shapiro ML. Retrieving memories via internal context requires the hippocampus. J Neurosci. 2004;24(31):6979–6985. doi: 10.1523/JNEUROSCI.1388-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy PJ, Shapiro ML. Motivational states activate distinct hippocampal representations to guide goal-directed behaviors. Proc Natl Acad Sci U S A. 2009;106(26):10805–10810. doi: 10.1073/pnas.0903259106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Adalsteinsson E, Glover GH, Spielman DM. Regularized higher-order in vivo shimming. Magn Reson Med. 2002;48(4):715–722. doi: 10.1002/mrm.10267. [DOI] [PubMed] [Google Scholar]
- Kirwan CB, Jones CK, Miller MI, Stark CE. High-resolution fMRI investigation of the medial temporal lobe. Hum Brain Mapp. 2007;28(10):959–966. doi: 10.1002/hbm.20331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwan CB, Stark CE. Medial temporal lobe activation during encoding and retrieval of novel face-name pairs. Hippocampus. 2004;14(7):919–930. doi: 10.1002/hipo.20014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs RM, Schott BH, Duzel E. Personality traits are differentially associated with patterns of reward and novelty processing in the human substantia nigra/ventral tegmental area. Biol Psychiatry. 2009;65(2):103–110. doi: 10.1016/j.biopsych.2008.08.019. [DOI] [PubMed] [Google Scholar]
- Kuhl BA, Shah AT, DuBrow S, Wagner AD. Resistance to forgetting associated with hippocampus-mediated reactivation during new learning. Nat Neurosci. 2010;13(4):501–506. doi: 10.1038/nn.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon N, Manahan-Vaughan D. Dopamine D1/D5 receptors gate the acquisition of novel information through hippocampal long-term potentiation and long-term depression. J Neurosci. 2006;26(29):7723–7729. doi: 10.1523/JNEUROSCI.1454-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Cullen WK, Anwyl R, Rowan MJ. Dopamine-dependent facilitation of LTP induction in hippocampal CA1 by exposure to spatial novelty. Nat Neurosci. 2003;6(5):526–531. doi: 10.1038/nn1049. [DOI] [PubMed] [Google Scholar]
- Liang JC, Wagner AD, Preston AR. Content Representation in the Human Medial Temporal Lobe. Cereb Cortex. 2012 doi: 10.1093/cercor/bhr379. EPub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46(5):703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Otmakhova NA. Storage, recall, and novelty detection of sequences by the hippocampus: elaborating on the SOCRATIC model to account for normal and aberrant effects of dopamine. Hippocampus. 2001;11(5):551–568. doi: 10.1002/hipo.1071. [DOI] [PubMed] [Google Scholar]
- Liu TT, Frank LR, Wong EC, Buxton RB. Detection power, estimation efficiency, and predictability in event-related fMRI. Neuroimage. 2001;13(4):759–773. doi: 10.1006/nimg.2000.0728. [DOI] [PubMed] [Google Scholar]
- Luo AH, Tahsili-Fahadan P, Wise RA, Lupica CR, Aston-Jones G. Linking context with reward: a functional circuit from hippocampal CA3 to ventral tegmental area. Science. 2011;333(6040):353–357. doi: 10.1126/science.1204622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr D. Simple memory: a theory for archicortex. Philos Trans R Soc Lond B Biol Sci. 1971;262(841):23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, O’Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: Insights from the successes and failures of connectionist models of learning and memory. Psychological Review. 1995;102:419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- O’Reilly RC, Rudy JW. Conjunctive representations in learning and memory: principles of cortical and hippocampal function. Psychol Rev. 2001;108(2):311–345. doi: 10.1037/0033-295x.108.2.311. [DOI] [PubMed] [Google Scholar]
- Oades RD, Halliday GM. Ventral tegmental (A10) system: neurobiology. 1. Anatomy and connectivity. Brain Res. 1987;434(2):117–165. doi: 10.1016/0165-0173(87)90011-7. [DOI] [PubMed] [Google Scholar]
- Olsen RK, Nichols EA, Chen J, Hunt JF, Glover GH, Gabrieli JD, et al. Performance-related sustained and anticipatory activity in human medial temporal lobe during delayed match-to-sample. J Neurosci. 2009;29(38):11880–11890. doi: 10.1523/JNEUROSCI.2245-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz O, Delgado-Garcia JM, Espadas I, Bahi A, Trullas R, Dreyer JL, et al. Associative learning and CA3-CA1 synaptic plasticity are impaired in D1R null, Drd1a-/- mice and in hippocampal siRNA silenced Drd1a mice. J Neurosci. 2010;30(37):12288–12300. doi: 10.1523/JNEUROSCI.2655-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otmakhova NA, Lisman JE. D1/D5 dopamine receptor activation increases the magnitude of early long-term potentiation at CA1 hippocampal synapses. J Neurosci. 1996;16(23):7478–7486. doi: 10.1523/JNEUROSCI.16-23-07478.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otmakhova NA, Lisman JE. Dopamine selectively inhibits the direct cortical pathway to the CA1 hippocampal region. J Neurosci. 1999;19(4):1437–1445. doi: 10.1523/JNEUROSCI.19-04-01437.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paller KA, Wagner AD. Observing the transformation of experience into memory. Trends Cogn Sci. 2002;6(2):93–102. doi: 10.1016/s1364-6613(00)01845-3. [DOI] [PubMed] [Google Scholar]
- Pfeuffer J, Van de Moortele PF, Ugurbil K, Hu X, Glover GH. Correction of physiologically induced global off-resonance effects in dynamic echo-planar and spiral functional imaging. Magn Reson Med. 2002;47(2):344–353. doi: 10.1002/mrm.10065. [DOI] [PubMed] [Google Scholar]
- Preston AR, Bornstein AM, Hutchinson JB, Gaare ME, Glover GH, Wagner AD. High-resolution fMRI of Content-sensitive Subsequent Memory Responses in Human Medial Temporal Lobe. J Cogn Neurosci. 2010;22(1):156–173. doi: 10.1162/jocn.2009.21195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston AR, Thomason ME, Ochsner KN, Cooper JC, Glover GH. Comparison of spiral-in/out and spiral-out BOLD fMRI at 1.5 and 3 T. Neuroimage. 2004;21(1):291–301. doi: 10.1016/j.neuroimage.2003.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Kohler S, Crane J, Pruessner M, Lord C, Byrne A, et al. Volumetry of temporopolar, perirhinal, entorhinal and parahippocampal cortex from high-resolution MR images: considering the variability of the collateral sulcus. Cereb Cortex. 2002;12(12):1342–1353. doi: 10.1093/cercor/12.12.1342. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Li LM, Serles W, Pruessner M, Collins DL, Kabani N, et al. Volumetry of hippocampus and amygdala with high-resolution MRI and three-dimensional analysis software: minimizing the discrepancies between laboratories. Cereb Cortex. 2000;10(4):433–442. doi: 10.1093/cercor/10.4.433. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Yonelinas AP, Cohen MX, Dy CJ, Tom SM, D’Esposito M. Dissociable correlates of recollection and familiarity within the medial temporal lobes. Neuropsychologia. 2004;42(1):2–13. doi: 10.1016/j.neuropsychologia.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Schonberg T, Daw ND, Joel D, O’Doherty JP. Reinforcement learning signals in the human striatum distinguish learners from nonlearners during reward-based decision making. J Neurosci. 2007;27(47):12860–12867. doi: 10.1523/JNEUROSCI.2496-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80(1):1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Schultz W, Apicella P, Ljungberg T. Responses of monkey dopamine neurons to reward and conditioned stimuli during successive steps of learning a delayed response task. J Neurosci. 1993;13(3):900–913. doi: 10.1523/JNEUROSCI.13-03-00900.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohamy D, Adcock RA. Dopamine and adaptive memory. Trends Cogn Sci. 2010;14(10):464–472. doi: 10.1016/j.tics.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Singer AC, Frank LM. Rewarded outcomes enhance reactivation of experience in the hippocampus. Neuron. 2009;64(6):910–921. doi: 10.1016/j.neuron.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Stark CE, Okado Y. Making memories without trying: medial temporal lobe activity associated with incidental memory formation during recognition. J Neurosci. 2003;23(17):6748–6753. doi: 10.1523/JNEUROSCI.23-17-06748.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark CE, Squire LR. When zero is not zero: the problem of ambiguous baseline conditions in fMRI. Proc Natl Acad Sci U S A. 2001;98(22):12760–12766. doi: 10.1073/pnas.221462998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9(1-6):321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Taepavarapruk P, Floresco SB, Phillips AG. Hyperlocomotion and increased dopamine efflux in the rat nucleus accumbens evoked by electrical stimulation of the ventral subiculum: role of ionotropic glutamate and dopamine D1 receptors. Psychopharmacology (Berl) 2000;151(2-3):242–251. doi: 10.1007/s002130000376. [DOI] [PubMed] [Google Scholar]
- Vercauteren T, Pennec X, Perchant A, Ayache N. Diffeomorphic demons: efficient non-parametric image registration. Neuroimage. 2009;45(1 Suppl):S61–72. doi: 10.1016/j.neuroimage.2008.10.040. [DOI] [PubMed] [Google Scholar]
- Wittmann BC, Bunzeck N, Dolan RJ, Duzel E. Anticipation of novelty recruits reward system and hippocampus while promoting recollection. Neuroimage. 2007;38(1):194–202. doi: 10.1016/j.neuroimage.2007.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann BC, Schott BH, Guderian S, Frey JU, Heinze HJ, Duzel E. Reward-related FMRI activation of dopaminergic midbrain is associated with enhanced hippocampus-dependent long-term memory formation. Neuron. 2005;45(3):459–467. doi: 10.1016/j.neuron.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Friston KJ. Analysis of fMRI time-series revisited--again. Neuroimage. 1995;2(3):173–181. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- Yassa MA, Stark CE. A quantitative evaluation of cross-participant registration techniques for MRI studies of the medial temporal lobe. Neuroimage. 2009;44(2):319–327. doi: 10.1016/j.neuroimage.2008.09.016. [DOI] [PubMed] [Google Scholar]
- Zeineh MM, Engel SA, Bookheimer SY. Application of cortical unfolding techniques to functional MRI of the human hippocampal region. Neuroimage. 2000;11(6 Pt 1):668–683. doi: 10.1006/nimg.2000.0561. [DOI] [PubMed] [Google Scholar]
- Zeineh MM, Engel SA, Thompson PM, Bookheimer SY. Dynamics of the hippocampus during encoding and retrieval of face-name pairs. Science. 2003;299(5606):577–580. doi: 10.1126/science.1077775. [DOI] [PubMed] [Google Scholar]