Summary
Background
Adeno-associated viral vector (AAV)-mediated and muscle-directed gene therapy is a safe and noninvasive approach to treat hemophilia B and other genetic diseases. However, low efficiency of transduction, inhibitor formation and high prevalence of pre-existing immunity to the AAV capsid in humans remain as main challenges for AAV2-based vectors using this strategy. Vectors packaged with AAV7, 8, and 9 serotypes have improved gene transfer efficiencies and may provide potential alternatives to overcome these problems.
Objective
To compare the long-term expression of canine factor IX (cFIX) levels and anti-cFIX antibody responses following intramuscular injection of vectors packaged with AAV1, 2, 5, 7, 8, and 9 capsid in immunocompetent hemophilia B mice.
Methods and results
Highest expression was detected in mice injected with AAV2/8 vector (28% of normal), followed by AAV2/9 (15%) and AAV2/7 (10%). cFIX expression by AAV2/1 only ranged from 0–5% of normal levels. High incidences of anti-cFIX inhibitor (IgG) were detected in mice injected with AAV2 and 2/5 vectors, followed by AAV2/1. None of the mice treated with AAV2/7, 2/8, and 2/9 developed inhibitors or capsid T cells.
Conclusions
AAV7, 8, and 9 are more efficient and safer vectors for muscle-directed gene therapy with high levels of transgene expression and absence of inhibitor formation. The absence of antibody response to transgene by AAV7, 8, and 9 is independent of vector dose but may be due to the fact that these three serotypes are associated with high level distribution to, and transduction of, hepatocytes following i.m. injection.
Keywords: Hemophilia B, gene therapy, adeno-associated viruses (AAV), factor IX, muscle
Introduction
The goal of gene therapy for hemophilia B, an X-linked severe bleeding disorder caused by a deficiency in blood clotting factor IX (FIX), is to generate long-term therapeutic levels of clotting factor following vector administration without generating harmful immune responses. Long-term correction of the bleeding disorder has been demonstrated in the murine and dog models of hemophilia B using AAV vectors [1–8]. However, such success has not been realized in human clinical trials using vectors based on AAV serotype 2 [9–12].
Muscle and liver are the two main tissues for targeting AAV-mediated gene therapy for hemophilia B. Skeletal muscle-directed gene transfer strategies have the advantage that the vector delivery is relatively noninvasive, and the performance and status of vector delivered in the muscle is unlikely to be influenced by the high prevalence of hepatitis in the adult hemophilia population which could be a concern for liver-directed approaches. In addition, pre-existing neutralizing antibodies have less impact on muscle-directed gene transfer compared to systemic vector delivery [10, 12]. However, the major concerns with muscle-directed AAV-FIX gene therapy are anti-FIX inhibitor formation and relatively low transduction efficiency compared to liver-directed approaches. Long-term therapeutic levels of hFIX was achieved in immunodeficient mice following intramuscular (i.m.) injection of high titer AAV2 vectors, but in immunocompetent mice, no circulating hFIX could be detected due to the development of anti-hFIX antibodies [13, 14]. Hemophilia B dogs with a null mutation invariably developed anti-cFIX antibodies following i.m. delivery of AAV vector, unless they received transient immunosuppression [15]. In most hemophilia B dogs with a missense mutation, anti-cFIX antibodies were absent or transient and therefore did not prevent sustained systemic expression of FIX, albeit at subtherapeutic levels [16, 17]. The risk of inhibitor development correlated with increasing vector doses and especially dose per injection site [18]. A phase I clinical trial on AAV mediated, muscle-targeted gene transfer in severe hemophilia B patients showed evidence of gene transfer in biopsied tissues but modest changes in clinical end points without measurable level of hFIX in the circulation [9–11].
Improved transduction efficiencies have been achieved with the second generation of vectors represented by novel AAV serotypes in many different model systems [19, 20]. AAV1 resulted in robust transduction of skeletal muscle [21, 22], leading to phenotypic correction of hemophilia B mice after intramuscular injection [5]. However, discrepancies exist regarding levels of transgene expression levels and the development of inhibitor following i.m. injection [5, 22–24]. Novel serotypes including AAV7, 8, and 9 appear to be promising candidates for hemophilia gene therapy, especially by systemic delivery [7, 25–29]. Muscle-directed applications for hemophilia gene therapy using these novel serotypes are less well explored [30], although AAV8 has been extensively studied for muscle-directed gene therapy vector for other diseases, such as Duchenne muscular dystrophy [31].
In this study, we compared the long-term cFIX expression levels and anti-cFIX antibody responses following i.m. injection of six AAV pseudotypes (AAV2/1, 2, 2/5, 2/7, 2/8, and 2/9) in immunocompetent hemophilia B mice. We demonstrate that AAV7, 8, and 9 are more efficient and safer vectors for muscle-directed gene therapy with high levels of transgene expression and absence of inhibitor formation or capsid T cell responses. The absence of antibody response to transgene by AAV7, 8, and 9 is conferred by the uniqueness of these serotypes regardless of vector dose.
Materials and Methods
Vector production
All AAV vectors used in this study were made by the Vector Core of the University of Pennsylvania as described previously [32]. pAAV-CMV-cFIX-WPRE or pAAV-CMV-ffLuciferase was co-transfected with a trans plasmid expressing AAV rep and cap and an adenovirus helper plasmid (pAdΔF6). AAV2 vectors were purified by a single-step gravity-flow heparin column method as described [33]. AAV vectors with other serotypes were purified by three rounds of cesium chloride gradient centrifugation. Vector genome titers were determined by Real-time PCR using linearized plasmid as standard.
Animal procedures
All animal procedures were performed in accordance with protocols approved by the Institute Animal Care and Use Committees (IACUC) at the University of Pennsylvania and The Wistar Institute. FIX knock-out mice [34] have been backcrossed to C57BL/6 background for over ten generations, and male mice at 6–8 weeks old were used in this study. Wild type (WT) C57BL/6, BALB/c, and BALB/c nude mice were purchased from Charles River Laboratories (Wilmington, MA, USA). To prevent bleeding from the surgery, 5 international units (IU) of purified human FIX (Enzyme Research Laboratories, South Bend, IN, USA) diluted in PBS were injected intravenously into hemophilic mice 1–2 hour before the surgery. Small skin incisions were made in the targeted muscle areas after the mouse was anesthetized, and 25µl of vector was injected into the Biceps Femoris and the Gastrocnemius of each leg using a Hamilton syringe. Blood was collected from the animals by retro-orbital plexus bleed into 1/10 volume of 3.2% sodium citrate.
Assays for antigen levels and biological activity of canine FIX
Canine FIX antigen levels in mouse plasma were determined by ELISA as described [32]. Activated partial thromboplastin time (aPTT) was performed as previously reported [34]. Purified canine factor IX protein (Enzyme Research Laboratories) was spiked in and serially diluted in pooled hemophilia B mouse plasma to serve as references.
Assays for circulating antibody against cFIX
Anti-cFIX IgG1 levels in mouse plasma were determined by ELISA as described [35] but using purified cFIX (1µg/ml, Enzyme Research Laboratories) to coat the plate. For each assay, a mouse reference serum (Sigma Chemical, St. Louis, MO, USA) with 150ng/ml or less mouse IgG1 was included as standards and used to calculate the relative amount of antibody in microgram per milliliter. Negative controls included PBS and AAV2/8 CMV-LacZ injected mice. Anti-cFIX IgG2a and IgG2b levels in mouse plasma were assayed similarly by using the corresponding secondary antibodies. Bethesda assay for cFIX inhibitors were performed as described [36].
Immunofluorescence staining of canine FIX
Muscles were harvested and immediately frozen in OCT freezing compound in isopentane cooled in liquid nitrogen. Serial cryostat sections (10 µm) were fixed first with 4% paraformaldehyde in PBS, washed twice with PBS, then blocked with 1% goat serum, 0.5% BSA, and 0.2% Triton X-100 in PBS for 30 minutes. Sections were then incubated with primary antibody (rabbit anti-canine FIX antibody, 1:500, Enzyme Research Laboratories), then with FITC-conjugated goat anti-rabbit IgG (1:200, Sigma, MO, USA). PBS- injected muscles were included as controls.
Whole-body bioluminescence imaging
Luciferase expression levels in muscle and liver were measured by whole-body bioluminescence imaging using Xenogen IVIS Lumina (Caliper Life Sciences, Hopkinton, MA). Anesthetized mice were imaged in a light-tight chamber 15 minutes after intraperitoneal injection of D-luciferin (Caliper Life Sciences, 150 mg/kg). Images were analyzed using the Caliper software and luciferase expression levels within identical regions of interest for each organ were presented as photons/sec/cm2/steradian (p/s).
IFN-γ ELISPOT assay
Splenocytes were isolated and pooled from each group of mice (n=3) 7 days after i.m. of AAV vectors. IFN-γ ELISPOT assays were performed as previously described [37]. Peptide-specific cells were represented as spot forming cells (SFC) per 106 splenocytes.
Statistical analysis
Statistical differences between different serotype groups were determined using analysis of variance (ANOVA). Values of P < 0.05 were considered statistically significant.
Results
AAV serotype comparison in the muscle of two strains of wild type mice
We first compared the in vivo performance of six AAV vectors (AAV2/1, 2, 2/5, 2/7, 2/8, and 2/9) carrying CMV-cFIX-WPRE in the muscle of WT mice from two strains: BALB/c nude and C57BL/6. BALB/c nude is immunodeficient, thus it should eliminate the complications of inhibitor that may arise from certain serotypes and directly reflect gene transfer efficiency by different serotypes in mouse muscle. cFIX expression levels in C57BL/6 could be influenced by inhibitor formation; the final levels of cFIX antigen in these animals are the function of both transduction efficiency and transgene immunogenicity. Results at weeks 2 and 4 are shown in Fig. 1 Greater than therapeutic levels of cFIX were generated by AAV2/1, 2/7, 2/8 and 2/9 vectors in both strain backgrounds. BALB/c nude mice injected with AAV2 and 2/5 vectors showed low but detectable levels of cFIX, while only baseline levels were detected in C57BL/6 mice treated with AAV2 and 2/5 vectors, suggesting AAV2 and 2/5 vectors are less efficient in transducing muscles and/or may elicit inhibitor formation in immune-competent mice. Differences between the two strains of mice could also reflect their different genetic backgrounds or gender (Table 1).
Fig. 1. cFIX expression levels in WT mice from different AAV pseudotyped vectors after intramuscular injection.
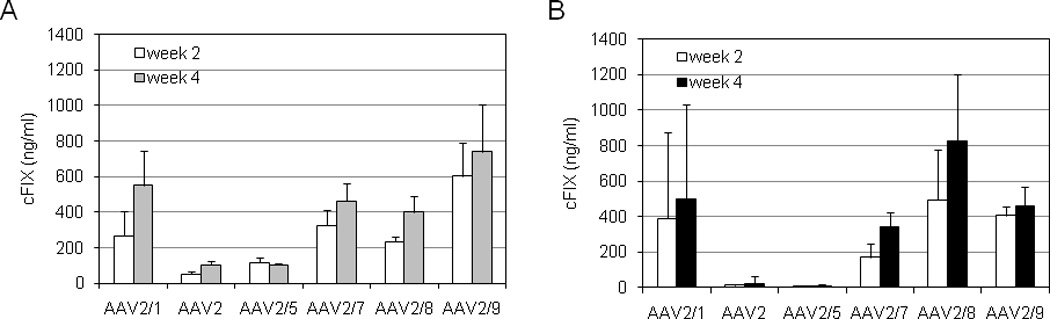
Female BALB/c nude (A) and C57BL/6 (B) mice (n=5) were injected with AAV2/1, 2, 2/5, 2/7, 2/8 and 2/9-CMV-cFIX-WPRE vectors at the dose of 1×1011 GC intramuscularly. The cFIX expression levels in the mouse plasma 2 and 4 weeks after injection are shown (mean ± SD).
Table 1.
Summary of mean cFIX expression levels in different strains of mice 4 weeks after i.m. injection of 1×1011 GC of different pseudotyped AAV vectors.
| cFIX (ng/ml) at week 4 (mean ± S.D.) | ||||
|---|---|---|---|---|
| Serotype | BALB/c nude (F) | WT BALB/c (M) | WT C57BL/6 (F) | Hemophilia B (M) |
| AAV2/1 | 551.7 ± 196.4 | 22.9 ± 11.2 | 498.9 ± 530.9 | 54.4 ± 63.1 |
| AAV2 | 102.3 ± 21.5 | 4.8 ± 5.7 | 24.4 ± 40.3 | 21.7 ± 35.9 |
| AAV2/5 | 102.9 ± 9.0 | n.t. | 12.3 ± 8.6 | 20.6 ± 25.7 |
| AAV2/7 | 462.2 ± 99.6 | n.t. | 345.2 ± 76.6 | 419.7 ± 90.8 |
| AAV2/8 | 401.6 ± 88.1 | 139.9 ± 59.5 | 823.1 ± 375.8 | 1330.8 ± 356.6 |
| AAV2/9 | 739.6 ± 266.6 | n.t. | 458.1 ± 106.4 | 824.1 ± 237.2 |
Abbreviations: cFIX, canine factor IX; F, female; M, male
Superior cFIX expression by AAV2/8, 2/9 and 2/7 vectors in the muscle of immunocompetent hemophilia B mice
To further assess the utility of these six AAV vectors for hemophilia B gene therapy, we compared them in adult immunocompetent hemophilia B mice (C57BL/6 background) by monitoring cFIX gene expression and anti-cFIX inhibitor formation. Vectors of different serotypes were injected into two sites (biceps femoris and gastrocnemius) of each leg of adult male hemophilic mice at the dose of 1×1011 GC per mouse. Two weeks after injection, increased plasma levels of cFIX were detected in mice treated with AAV2/7, 2/8, and 2/9 vectors. The increased levels were sustained above therapeutic levels throughout the length of the experiment (5 months) (Fig. 2A). Highest levels of expression were observed for the mice treated with AAV2/8 vector, followed by AAV2/9 and AAV2/7 vectors. Statistical analyses showed that expression in AAV2/8-treated mice were significantly higher than all other serotypes at all time points except AAV2/9 at 12 weeks. cFIX expression levels in AAV2 and 2/5 injected mice were close to baseline. To our surprise, cFIX expression by AAV2/1 vector only ranged from 0–127 ng/ml in different animals at 6 weeks after injection (mean: 31 ± 45 ng/ml, n=14), contrasting with the cFIX levels achieved by AAV2/1 in WT C57BL/6 and BALB/c nude mice.
Fig. 2. Antigen levels and activity of cFIX in immunocopetent hemophilia B mice from different AAV vectors after intramuscular injection.
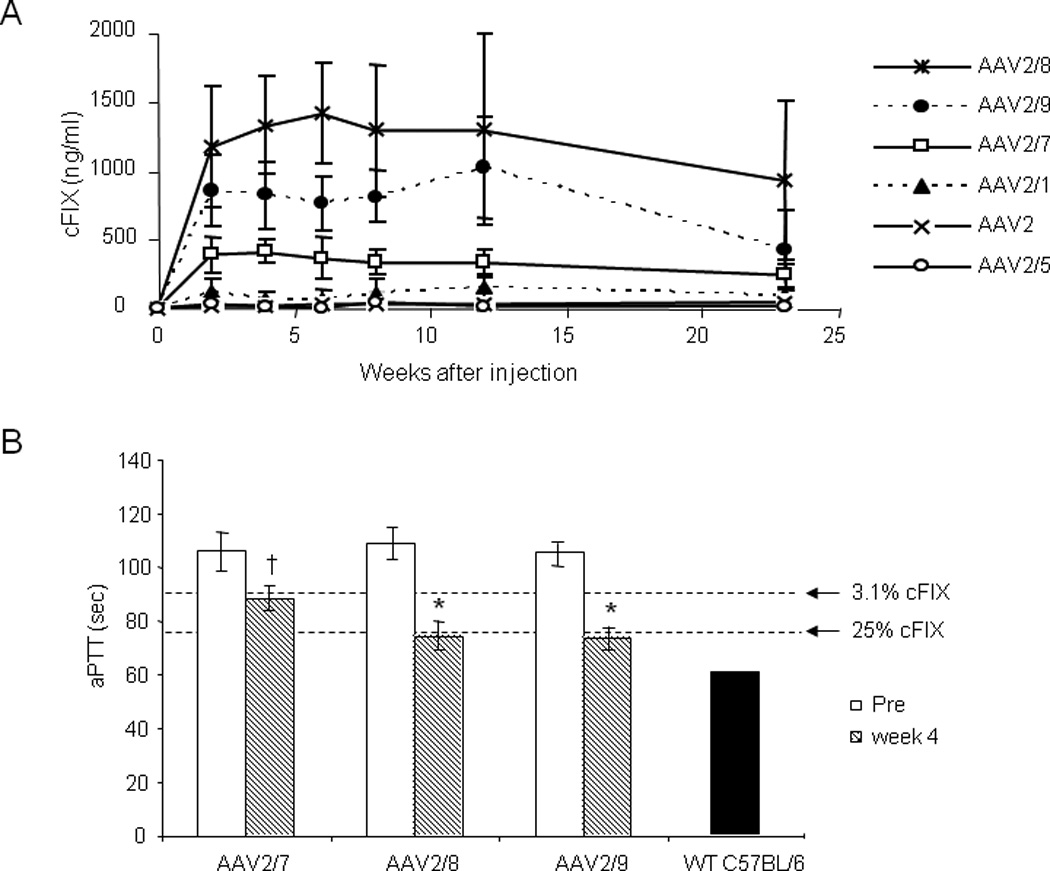
A. Time course of cFIX expression. Male C57BL/6 hemophilia B mice were injected with AAV2/1 (n=14), 2 (n=7), 2/5 (n=7), 2/7 (n=9), 2/8 (n=7), and 2/9-CMV-cFIX-WPRE (n=6) at the dose of 1×1011 GC intramuscularly. Levels of cFIX expression in plasma were followed for over 23 weeks after injection (mean ± SD). AAV2/8 vector generated statistically higher cFIX levels than all other serotype vectors at all time points, with one exception (AAV2/9, week 12). ANOVA, p<0.05. B. aPTT assays were performed on plasma samples from hemophilia B mice before and 4 weeks after i.m. injection with 1×1011 GC of AAV2/7, 2/8, and 2/9 vectors. aPTT for hemophilia B mouse plasma spiked in with different amount of purified cFIX protein (3.1% or 25%) and pooled mouse plasma from wild type male C57BL/6 mice were included as references (5µg/ml is presumed as 100%). † p<0.005; * p<0.001, two-tailed t-test.
Activated partial thromboplastin time (aPTT) assay, an in vitro functional assay for FIX, demonstrated that the cFIX expressed in AAV2/7, 2/8, and 2/9 injected animals was biologically active. Four weeks after the injection, clotting time was significantly shortened compared to the values before treatment for all three groups. aPTT was shortened close to the level of 25% of normal cFIX level in AAV2/8- and 2/9-treated mice, while clotting time was shortened to that of 3% of normal cFIX level in AAV2/7 treated mice (Fig. 2B).
cFIX immunostaining in mouse muscles transduced by different serotypes of AAV vectors
Transduction efficiency by different AAV serotypes in mouse muscle was also assessed by cFIX immunostaining five months after injection. High percentage of transgene positive fibers and strong cFIX staining was observed in muscles transduced with AAV2/1, 2/7, 2/8 and AAV2/9 vectors (Fig. 3). cFIX-positive fibers were also observed in mice injected with AAV2 and 2/5 vectors, but with lower frequency and intensity. cFIX-staining was seen both in the muscle fibers and in the interstitial spaces between the fibers. Mosaic pattern of staining was observed in muscle sections injected with all six different serotypes. There was no significant difference between the frequency of cFIX-positive fibers in the Biceps Femoris and Gastrocnemius muscles for all tested serotypes. No cFIX-staining was observed in the muscles of the PBS control animals.
Fig. 3. Immunofluorescence staining for cFIX in the muscles of C57BL/6 hemophilia B mice 5 months after intramuscular injection with different AAV pseudotyped vectors.
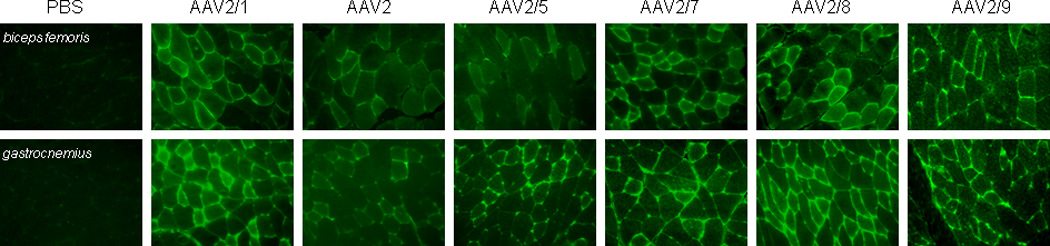
Hemophilia B mice were injected with different AAV pseudotyped vectors at the dose of 1×1011 GC at 4 intramuscular sites. The upper panels show immunofluerence staining of the biceps femoris muscles and the lower panels show staining of the gastrocnemius muscles. (X200).
Absence of immune response to cFIX by AAV2/7, 2/8, and 2/9 vectors
B cell responses to the transgene product were evaluated by anti-cFIX-specific IgG1 levels in the hemophilic mice treated with different AAV vectors (Fig. 4), since it has been previously demonstrated that inhibitors developed after AAV2-FIX gene transfer were primarily IgG1 immunoglobulins representing a Th2-driven humoral immune response [38, 39]. In this study, highest levels and incidence of antibodies were observed in mice injected with AAV2/5 and AAV2 vectors, followed by AAV2/1 vectors. Anti-cFIX-specific IgG1 levels in AAV2/1 (4 out of 6) and AAV2 (4 out of 5) treated mice peaked 4 weeks after injection. In the majority of the animals, antibody levels gradually decreased to baseline while in one mouse from each group, the levels remained high by the end of the experiment (23 weeks). AAV2/5 treated mice (5 out of 5) had peak antibody levels at 6 weeks after injection, and 3 out of 5 still had antibody levels beyond 1 µg/ml at the end of the experiment. Furthermore, inhibitory antibodies were detected in 2 out of 5 AAV2/5-treated mice (1–2 BU/ml at week 10). In contrast, no anti-cFIX specific IgG1 was observed in mice injected with AAV2/7, 2/8, and 2/9 vectors (0 out of 5) or PBS control (0 out of 4), suggesting that these novel AAV serotypes are less immunogenic in eliciting antibody response to the transgene product. To rule out the possibility that there might be Th1-driven humoral immune response to cFIX in mice treated with novel AAV serotypes, we also tested those samples for anti-cFIX specific IgG2a and IgG2b antibodies. None of those samples showed detectable anti-cFIX specific IgG2a and IgG2b antibodies (data not shown). We did not test for antibody response to human FIX which was infused at one time before surgery to prevent bleeding during surgery. It has been shown in the literature that to induce antibody response to human FIX in mice, human FIX has to be formulated in adjuvant or repeatedly injected if formulated in PBS [35, 40].
Fig. 4. Anti-cFIX IgG1 in hemophilia B mice after intramuscular injection of different AAV pseudotyped vectors.
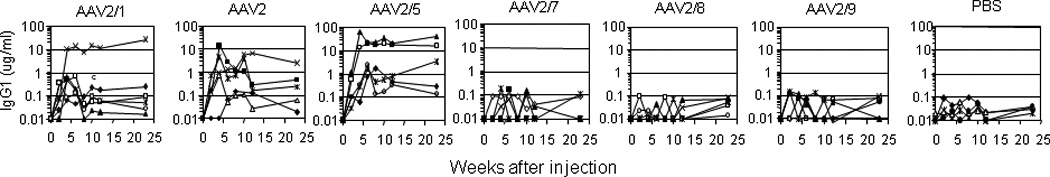
C57BL/6 hemophilia B mice were intramuscularly injected with different AAV pseudotyped vectors at the dose of 1×1011 GC. In AAV2/1, 2, and 2/5 injected mice, anti-cFIX-specific IgG1 peaked at 4 to 6 weeks after injection. None of the mice treated with AAV2/7 (n=5), AAV2/8 (n=5), AAV2/9 (n=6), or PBS (n=4) had detectable anti-cFIX-specific IgG1. Each line represents an individual mouse.
Dose response and its effect on host immune response
The absence of immune response to transgene by the novel AAV serotypes could be due to the high gene transfer efficiency leading to immune tolerance, or it could be attributed to the unique immunological properties of these novel serotypes. If it was only due to the transgene expression levels, lowering the vector dose would decrease the transgene expression levels leading to immune responses. Thus, dose response studies using AAV2/8 (4×109 to 5×1011 GC per mouse, 5-fold increase) and AAV2/1 vectors (1×1011 and 5×1011 GC per mouse) were carried out in the C57BL/6 hemophilia B mice. cFIX expression levels correlated well with the vector dose injected (Fig. 5A). Reducing the dose of AAV2/8 to 4×109 or 2×1010 GC decreased the cFIX expression levels, however, none of the animals developed cFIX-specific IgG1 response (Fig. 5B). Meanwhile, increasing the dose of AAV2/8 by 5-fold to 5×1011 GC increased the cFIX expression levels by 2-fold to 55% of normal levels six weeks after injection, and none of the animals developed anti-cFIX-specific IgG1. Thus, absence of immune response to transgene by AAV2/8 vector is due to the unique immunological aspects of the capsid protein, regardless of the vector dose or transgene expression levels.
Fig. 5. Dose response of AAV2/8 and AAV2/1 on transgene expression levels and anti-cFIX antibody levels in immunocompetent hemophilia B mice.
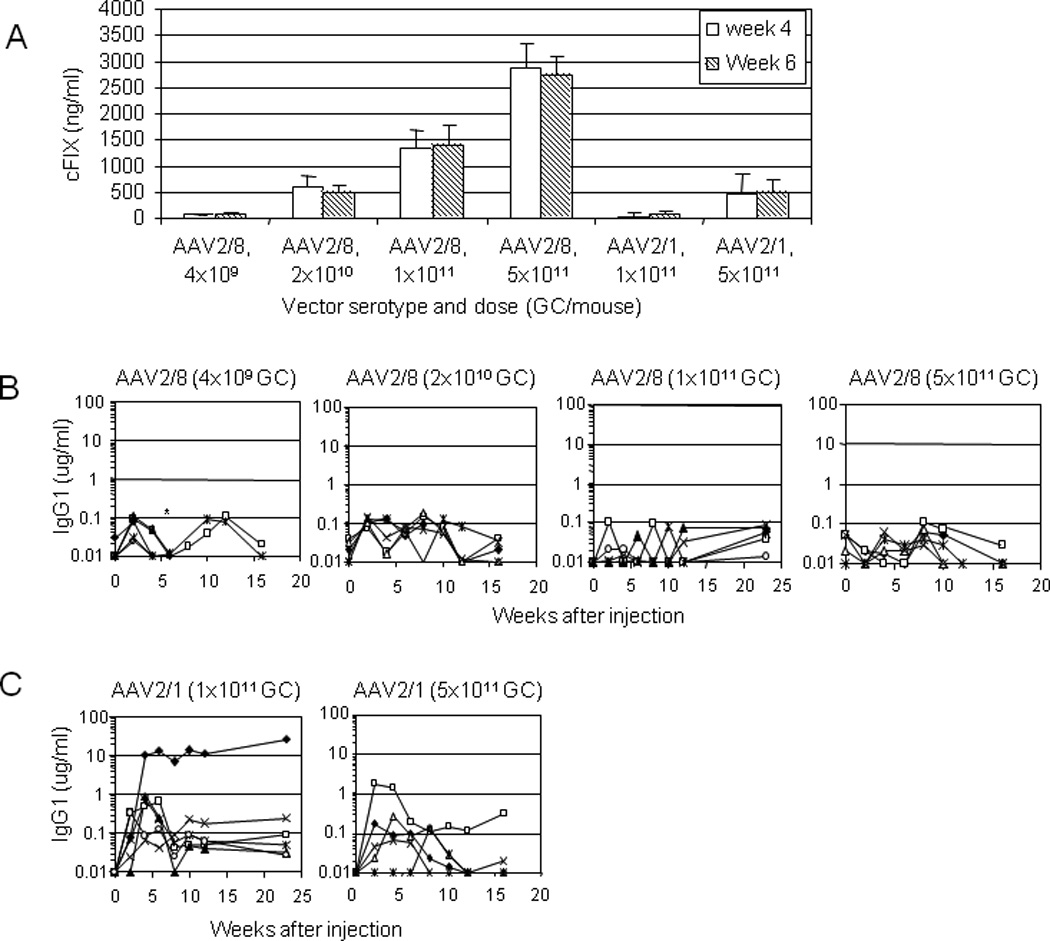
C57BL/6 hemophilia B mice were intramuscularly injected with AAV2/8 vector at a very low (4×109 GC/mouse), low (2×1010 GC), medium (1×1011 GC), or high dose (5×1011 GC), or AAV2/1 vector at a medium (1×1011 GC) or high dose (5×1011 GC; n=5 for each group). A. cFIX expression levels at 4 and 6 weeks after injection (mean ± SD). B. Anti-cFIX-specific IgG1 in animals injected with AAV2/8 vector at 4 different doses. None of the animals developed anti-cFIX-specific IgG1 regardless of the AAV2/8 vector doses. Mice injected with 1×1011 GC of AAV2/1 or 2/8 vectors were the same as presented in Fig. 4. C. Anti-cFIX-specific IgG1 in animals injected with AAV2/1 vector at medium and high doses. Incidence and titer of anti-cFIX-specific IgG1 were reduced in mice received high dose of AAV2/1 vector. Each line represents an individual mouse. Mice injected with 1×1011 GC of AAV2/1 vector were the same as presented in Fig. 4. *Denote the death of one animal after week 4 and two animals after week 6 in the group of mice treated with 4×109 GC of AAV2/8 vector.
For AAV2/1 vector, increasing the vector dose 5-fold to 5×1011 GC improved the cFIX expression to levels that were similar to that achieved by animals injected with 2×1010 GC of AAV2/8 vector (Fig. 5A). This indicated that AAV2/8 vector is about 25 fold more efficient than AAV2/1 vector in immunocompetent hemophilia B mice by intramuscular delivery. Increased dose of AAV2/1 vector also reduced the incidence and titer of anti-cFIX-specific IgG1 (Fig. 5C). This suggests that host immune response to cFIX delivered by AAV2/1 vector could be influenced by vector dose and transgene expression level. For AAV2/1 vector, the higher the dose, the less likely the antibody response to transgene product.
Absence of immune response to cFIX by AAV2/8 vector in BALB/c mice
So far, all the observations concerning the absence of immune response to cFIX by novel AAV serotypes were done in WT or hemophilic mice in C57BL/6 background. To rule out the possibility that such observation was strain-specific, we also tested AAV2/1, 2, and 2/8 vectors in wild type BALB/c mice (H-2d) which have a different MHC haplotype than C57BL/6 (H-2b). It has been shown that i.m. injection of AAV2.CMV.hA1AT (1×1011 GC/mouse) in BALB/c mice generated humoral response to human A1AT but not in C57BL/6 mice [41]. Thus the BALB/c mouse appears to be a more challenging model for muscle-directed gene transfer. We wanted to use this stringent model to test whether AAV2/8 has advantages in evading host immune response to transgene, since this is one of the main concerns for muscle-directed gene transfer for hemophilia B and applications of transgenes encoding secreted proteins.
BALB/c mice that received 1×1011 GC of AAV2/1, 2, and 2/8 vectors i.m. were followed for 10 weeks for cFIX antigen levels and cFIX inhibitors (Fig. 6). None of the mice that received AAV2 vector had detectable cFIX antigen in the circulation for the duration of the experiment, while 4 of 5 AAV2-injected mice had high and persistent levels of cFIX-specific IgG1. A Bethesda assay confirmed the presence of inhibitory antibodies (1–3 BU/ml) in 4 of 5 AAV2-injected BALB/c mice at week 8. The antibody response in BALB/c mice was stronger than it was in C57BL/6 mice receiving the same dose of AAV2 vector. In AAV2/1-injected mice, cFIX level slowly reached peak expression at 4% of normal levels 8 to 10 weeks after injection, 2 of 5 mice had intermediate levels of cFIX-specific IgG1 but these antibodies were not inhibitory based on Bethesda assay (data not shown). In AAV2/8-injected mice, cFIX antigen levels gradually reached 16% of normal levels eight weeks after injection without the presence of cFIX-specific IgG1. The kinetics of transgene expression in BALB/c mice injected with AAV2/8 is slower than it is in C57BL/6 mice, and the peak level of expression in BALB/c is about 60% of it is in C57BL/6, suggesting the influence of strain difference on gene transfer efficiency (Table 1). Nevertheless, the overall patterns are similar that AAV2/8 vector transduces muscle most efficiently without eliciting harmful inhibitor response to FIX, in contrast to AAV2 vector.
Fig. 6. cFIX antigen levels and anti-cFIX antibodies in BALB/c mice after intramuscular injection with AAV2/1, 2, or 2/8-CMV-cFIX-WPRE vectors at the dose of 1×1011 GC.
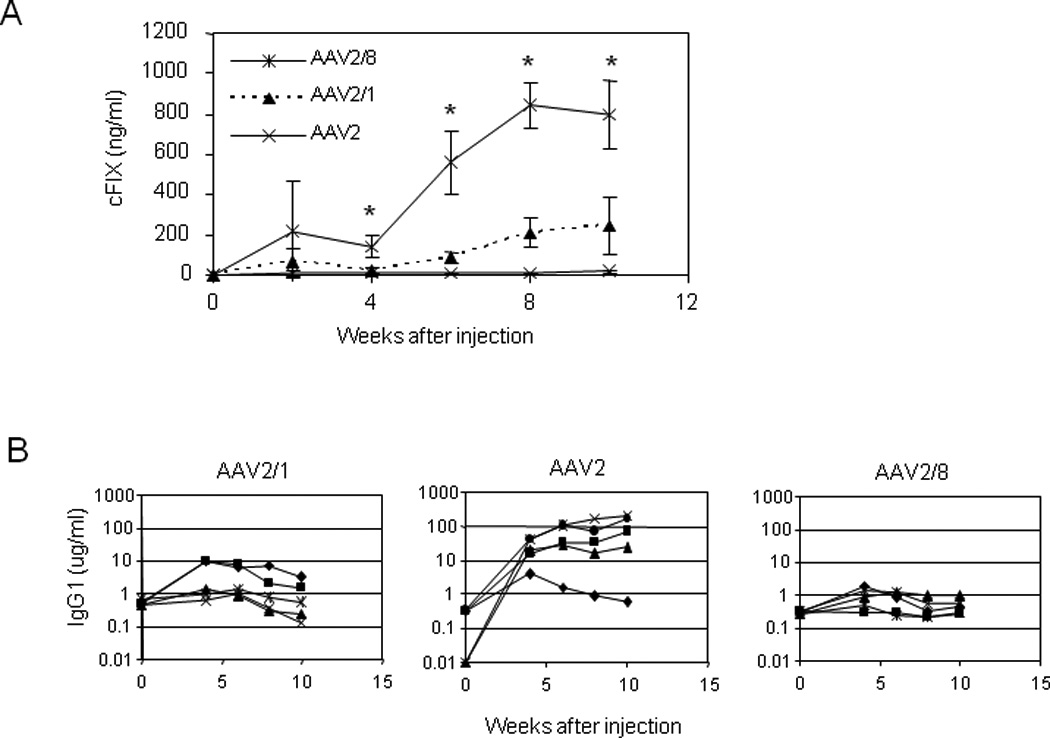
A. Time course of cFIX expression levels in the plasma of mice following i.m. injection with different pseudotyped AAV vectors (mean ± SD, n=5). cFIX in AAV2/8-injected mice were statistically higher than that in AAV2/1 or AAV2-injected mice. * p<0.001, ANOVA, Dunnett test. B. Anti-cFIX-specific IgG1. Each line represents an individual mouse.
Vector dissemination in liver after i.m. injection
It has been well established in mouse models that hepatic gene transfer by AAV vectors can induce transgene product-specific immune tolerance [42]. If there are vectors disseminated to liver after i.m. injection which would lead to hepatic transgene expression, such expression may lead to induction of immune tolerance. To investigate this, we replaced cFIX in the vector by the firefly luciferase (fluc) which allowed us to evaluate regional distribution of transgene expression (i.e., muscle vs. liver). WT C57BL/6 male mice were injected i.m. with AAV.CMV.fluc vectors at the same dose and in the same fashion as the cFIX vectors. Luciferase expression levels in muscle and liver were measured by Xenogen at days 7 and 28 after injection (Fig. 7). Mice were sacrificed 28 days after injection and vector genome copies in liver were quantified by real-time PCR (Fig. 7B). AAV2/1, 2/7, 2/8, and 2/9 had higher expression in muscle than AAV2 and 2/5, similar to what have been observed in BALB/c nude and WT C57BL/6 mice (Fig. 1). Vector dissemination to liver following i.m. injection was detected, and vector GC and luciferase expression in liver were highest in AAV2/8-, 2/9, and 2/7-injected mice (Fig. 7B). Luciferase expression in the liver of AAV2/8-injected mice was 55-fold higher than AAV1-injected mice at day 7. Liver expression levels in AAV2/7-, 2/8-, and 2/9-injected mice decreased over time likely due to CMV promoter shutoff in liver, although similar decreases were not observed in AAV2/1-, 2-, and 2/5-injected mice which had acutely low expression levels. In AAV2/8-injected mice, expression from liver represented 11% and 0.2% of the overall transgene expression levels at days 7 and 28, respectively, while in AAV1-injected mice, expression from liver only accounted for 0.06% and 0.03% of the overall transgene expression levels at days 7 and 28, respectively. The initial relatively high expression in liver by AAV2/8, 2/9, and 2/7 vectors may have induced immune tolerance, consistent with the lack of inhibitor response to cFIX by the novel AAV serotypes.
Fig. 7. Luciferase expression from muscle and liver following i.m. injection of different AAV serotypes.
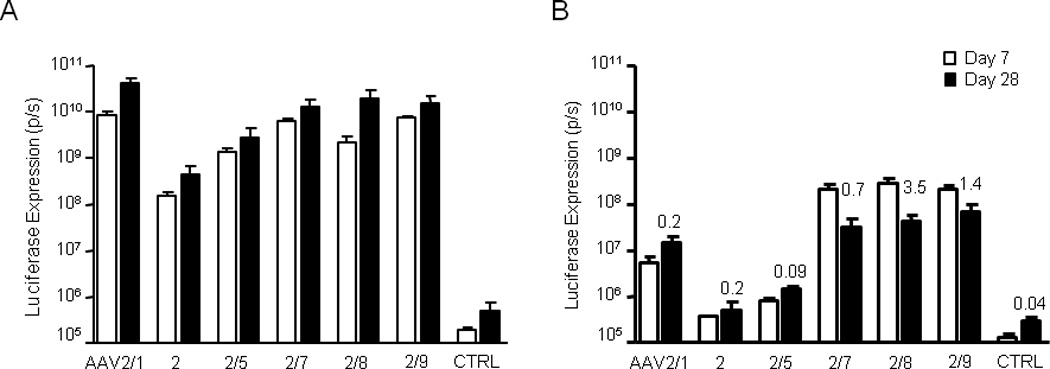
Luciferase expression visualized by whole-body bioluminescence imaging 7 and 28 days post-i.m. injection of 1×1011 GC of AAV.CMV.ffLuciferase per mouse (n = 4). AAV vector was delivered in two 25 µl i.m. injections into both legs (gastrocnemius and biceps femoris and of each leg). (A) Muscle and (B) liver luciferase expression was quantified separately for each mouse. Numbers presented above bars indicate GC per diploid genome in liver at day 28 (n = 2).
T cell response to different AAV capsids
Because of the implication of capsid T cell response in the loss of transgene expression in the AAV2 hemophilia B liver trial and more recently the AAV1-LPL muscle trial [12, 43], we compared the host T cell response to different AAV serotype capsids following muscle-directed vector delivery. BALB/c mice were injected i.m. with 1×1012 GC of AAV vectors packaged with different serotypes, and T cells were isolated from the spleen 7 days later and stimulated with H-2d-restricted dominant epitopes (AAV2: VPQYGYLTL; other serotypes: IPQYGYLTL) for IFN-γ ELISPOT assay. As shown in Fig. 8, AAV2 elicited the highest capsid-specific T cell response, followed by AAV2/1 and AAV2/5. AAV2/7 and 2/8 did not induce any detectable capsid T cell response, consistent with previous findings [37], while AAV2/9-injected mice had a very minor T response to the capsid.
Figure 8. T cell response to the capsid by different AAV serotypes.
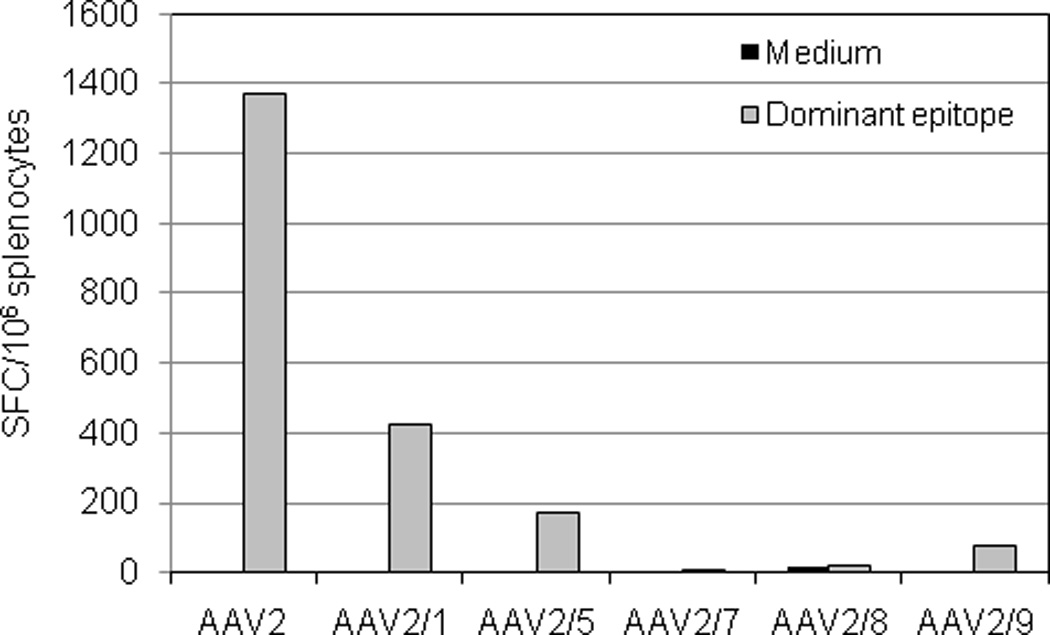
BALB/c mice were intramuscularly injected with AAV vectors at 1×1012 GC/mouse (n=3 per vector). Seven days after injection, splenocytes were isolated, pooled, and stimulated with H-2d-restricted dominant epitopes (AAV2: VPQYGYLTL; other serotypes: IPQYGYLTL) or medium alone for IFN-γ ELISPOT assay. Peptide-specific cells are presented as spot forming cells (SFC) per 106 splenocytes.
Discussion
The isolates of novel AAV serotypes from tissues of nonhuman and human primates quickly expanded the AAV family from six to over a hundred [19, 44]. Many of the novel serotypes have demonstrated improved in vivo gene transfer efficiency in various tissues compared to the existing AAV serotypes, including AAV2 which has been used in phase I/II human clinical trials of a number of genetic diseases. Neutralizing antibody screening on human samples has shown significantly lower frequency and titer to many of the novel AAV serotypes than to AAV2 [45]. Furthermore, studies done in mice and nonhuman primates showed that AAV7 and 8 were inefficient in inducing host T cell response to capsid [37, 46]. Thus, the novel AAV serotypes have created opportunities to improve the outcomes of AAV-mediated gene therapy.
Previously, we compared transduction efficiency in skeletal muscle by AAV1, 2, 5, 7, and 8 using LacZ as a reporter gene and showed AAV1, 7 and 8 are more efficient than AAV2 and 5 [47]. This study compared the efficacy of six AAV serotypes for muscle-directed gene therapy of hemophilia B in immunocompetent C57BL/6 hemophilia B mice. We demonstrated that AAV8, 9, and 7 are more efficient and safer vectors for muscle-directed gene therapy with long-lasting high levels of transgene expression and absence of inhibitor formation. In hemophilia B mice treated with 1×1011 GC of vectors, AAV2/8 was the most efficient vector with peak cFIX antigen levels at 1.4 µg/ml (28% of normal levels), 2- to 3-fold the levels found in animals injected with AAV2/9 and 2/7 vectors; while AAV2 and 2/5 vectors were ineffective in expressing cFIX and AAV2/1 vector only generated very low levels of cFIX. The aPTT assay confirmed that the expressed cFIX by AAV2/7, 2/8, and 2/9 vectors was biologically active.
Immune response to transgene product is one of the main concerns in gene replacement therapies. AAV vectors have shown long-term transgene expression in many studies and have been generally considered as a less immunogenic vector compared to adenoviral or lentiviral vectors, possibly due to the lack of inflammatory response induced by the AAV vector backbone and inefficient transduction of antigen presenting cells (APCs) by AAV vectors [48, 49]. However, many other factors, such as serotype, route of administration, host strain, target tissue, vector dose, promoter, and the nature of transgene product could influence the outcome of a gene therapy regimen. For hemophilia B gene therapy, studies have demonstrated that immune tolerance mediated by regulatory T cells can be induced by the hepatic gene transfer approach using AAV2 vector containing a liver-specific promoter [50], and more efficiently, by AAV8 vectors [51, 52]. While AAV2-mediated, muscle-directed gene transfer in hemophilia B models had high incidence of FIX inhibitor formation resulting in no detectable antigen in the circulation [13, 23], discrepancies exist in the literature regarding the efficacy of AAV2/1 vector for muscle-directed gene transfer for hemophilia B. Chao et al reported 100- to 1000-fold higher levels of cFIX (up to 30 µg/ml) in mice injected i.m. with AAV1 versus AAV2, and absence of anti-cFIX inhibitor formation in these mice [5, 22]. Further studies by this group suggested tolerance induction by AAV1 muscle gene transfer [24, 53]. However, Arruda et al reported only 10–20 fold of hFIX increase with AAV1 and anti-hFIX inhibitor formation following i.m. injection of AAV1-FIX in immunocompetent mice [23]. The level of increase by AAV1 vector reported by the latter paper is more consistent with the original findings by Xiao el al which showed 2- to 10-fold increase of gene expression by AAV1 versus AAV2 vectors [21]. In our hands, AAV2/1 is about 5-fold more efficient than AAV2 in BALB/c nude mice. In wild type C57BL/6 mice, AAV2/1 vector was able to generate up to 500 ng/ml of cFIX antigen, however, in hemophilia B mice, cFIX antigen levels only ranged from 0–127 ng/ml in different animals at 6 weeks after injection and remained at this low level throughout the course of the experiment (5 months). The endogenous expression of factor IX is substantially eliminated in the knockout mice, potentially leading to the formation of B cells to factor IX epitopes that would not be present in wild type mice. Anti-cFIX-specific IgG1 assays confirmed this. In wild type C57BL/6 mice, none of the 5 mice developed anti-cFIX IgG1 (data not shown), while in C57BL/6 hemophilia B mice, anti-cFIX IgG1 was detected in 4 out of 6 animals at 4 weeks after injection of 1×1011 GC of vector. Increasing the dose to 5×1011 GC decreased the incidence of immune response (only one out of 5 mice developed antibody) and improved the cFIX expression levels to 500 ng/ml, similar to what was achieved in mice injected with 2×1010 GC of AAV2/8.
In this study, we show that three novel AAV serotypes, AAV7, 8, and 9, not only have higher gene transfer efficiency in murine muscle, but also have the ability to evade host immune response to the transgene product as measured by antibodies. While most of the mice that received intramuscular injection of AAV2, 2/5, and 2/1-cFIX vectors developed anti-cFIX-specific IgG1, none of the animals injected with AAV2/7, 2/8, and 2/9 vectors developed antibodies to cFIX. Interestingly, these serotypes also were less likely to activate T cells to capsid epitopes. These vectors could be used as a new generation of safer and efficient vectors for muscle-directed gene transfer. However, it is important to note that the propensity of different serotypes to elicit transgene-specific immune responses is transgene-dependent and/or species-dependent, and could also be promoter-related. These novel serotypes have also been explored as vectors for vaccines and shown to generate high frequency of antigen (HIV Gag)-specific CD8+ T cells, although those T cells appeared to be functionally impaired [54, 55]. It should also be noted that unlike the other serotypes which were purified by three rounds of cesium chloride gradient centrifugation, AAV2 vectors used in this study were purified by the gravity-flow heparin column method which generates more pure and potent AAV2 vector [33], however, the column purified AAV2 likely contained more empty capsids. Our previous studies in mouse have shown that AAV2 elicits host T cell response to capsid independent of the vector preparation, purification method, or the nature of the transgene [37].
Previous studies in hemophilia B dogs treated with AAV2 vectors intramuscularly showed that the risk of inhibitory antibody formation to FIX was roughly proportional to the dose administered, and that the closest correlation was to the dose per injection site [18]. Further study in hemophilia B mice and dogs treated with AAV2/1 vectors indicated that the amount of transgene product synthesized locally is the major factor promoting the development of a harmful immune response [23]. In the current study, the absence of antibody response to transgene by AAV2/7, 2/8, and 2/9 is conferred by the unique properties of the capsids regardless of the dose of the vector and transgene expression level as shown here with AAV2/8. In the dose response experiment with AAV2/8 vector, doses per animal ranged from 4×109 to 5×1011 GC by 5-fold increments. Antigen levels in the circulation correlated well with the vector doses and ranged from 1% to 57% of normal levels of cFIX. Importantly, none of the animals in any dose groups developed anti-cFIX IgG1 during the course of the experiments. It is possible that due to higher gene transfer efficiency, antigen levels in the medium and high dose groups were able to induce immune tolerance. In the low dose group, either due to insufficient antigen levels or insufficient “danger signals” caused by the low vector dose, it was not enough to mount an immune response to transgene. Whether the immune tolerance would be broken or sustained following immune challenges in these mice remains to be studied. Further studies using luciferase as a reporter gene showed dissemination of vector to liver after i.m. injection, especially for AAV7, 8 and 9. Initial expression from the novel AAV serotypes accounted for over one tenth of the total expression, but gradually decreased to about 0.2%, likely due to CMV promoter shutoff in liver [56]. The initial relatively high expression in liver may play an important role in inducing immune tolerance from these capsids. Whether this can be extended to large animal models is yet to be determined.
In summary, AAV8, 9, and 7 are superior vectors for muscle-directed gene therapy in hemophilia B mice, compared to AAV2/1, 2, and 2/5 vectors. These novel vectors are not only more efficient in transducing muscle fibers, they are also less immunogenic in eliciting B cell responses to the cFIX transgene product and T cell response to the capsid protein. These principles should be considered in other applications of AAV gene transfer to express secreted proteins such as therapeutic antibodies. Examples include gene transfer into the retina to express angiogenic inhibitors for the treatment of age-related macular degeneration and intramuscular gene transfer to express systemic levels of antibodies against HIV.
Acknowledgements
We appreciate the support from the Vector Core and Animal Model Core of the Gene Therapy Program at the University of Pennsylvania. Supported by National Institute of Health grants 2-P01-HL-059407-06A1 (J.M.W.) and 5-P30-DK-47757-12 (J.M.W.), and grants from GlaxoSmithKline Pharmaceuticals, Inc. (J.M.W.). J-P.L is currently at Department of Pathology, Thomas Jefferson University, Philadelphia, PA, USA. Y.L. is currently at Department of Surgery, University of Miami, Miami, FL, USA.
Footnotes
Addendum
L. Wang designed the study, performed the experiments, analyzed the data and wrote the manuscript. J.P. Louboutin and P. Bell performed the experiments and edited the manuscript. J. Greig, Y. Li, and D. Wu performed the experiments. J. M. Wilson supervised the study and edited the manuscript.
Disclosure of Conflict of Interest
J.M.W. is a consultant to ReGenX Holdings, and is a founder of, holds equity in, and receives a grant from affiliates of ReGenX Holdings; in addition, he is an inventor on patents licensed to various biopharmaceutical companies, including affiliates of ReGenX Holdings.
References
- 1.Snyder RO, Miao C, Meuse L, Tubb J, Donahue BA, Lin HF, Stafford DW, Patel S, Thompson AR, Nichols T, Read MS, Bellinger DA, Brinkhous KM, Kay MA. Correction of hemophilia B in canine and murine models using recombinant adeno-associated viral vectors. Nat Med. 1999;5:64–70. doi: 10.1038/4751. [DOI] [PubMed] [Google Scholar]
- 2.Wang L, Takabe K, Bidlingmaier SM, Ill CR, Verma IM. Sustained correction of bleeding disorder in hemophilia B mice by gene therapy. Proc Natl Acad Sci U S A. 1999;96:3906–3910. doi: 10.1073/pnas.96.7.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hagstrom JN, Couto LB, Scallan C, Burton M, McCleland ML, Fields PA, Arruda VR, Herzog RW, High KA. Improved muscle-derived expression of human coagulation factor IX from a skeletal actin/CMV hybrid enhancer/promoter. Blood. 2000;95:2536–2542. [PubMed] [Google Scholar]
- 4.Wang L, Nichols TC, Read MS, Bellinger DA, Verma IM. Sustained expression of therapeutic level of factor IX in hemophilia B dogs by AAV-mediated gene therapy in liver. Mol Ther. 2000;1:154–158. doi: 10.1006/mthe.2000.0031. [DOI] [PubMed] [Google Scholar]
- 5.Chao H, Monahan PE, Liu Y, Samulski RJ, Walsh CE. Sustained and complete phenotype correction of hemophilia B mice following intramuscular injection of AAV1 serotype vectors. Mol Ther. 2001;4:217–222. doi: 10.1006/mthe.2001.0449. [DOI] [PubMed] [Google Scholar]
- 6.Mount JD, Herzog RW, Tillson DM, Goodman SA, Robinson N, McCleland ML, Bellinger D, Nichols TC, Arruda VR, Lothrop CD, Jr, High KA. Sustained phenotypic correction of hemophilia B dogs with a factor IX null mutation by liver-directed gene therapy. Blood. 2002;99:2670–2676. doi: 10.1182/blood.v99.8.2670. [DOI] [PubMed] [Google Scholar]
- 7.Wang L, Calcedo R, Nichols TC, Bellinger DA, Dillow A, Verma IM, Wilson JM. Sustained correction of disease in naive and AAV2-pretreated hemophilia B dogs: AAV2/8-mediated, liver-directed gene therapy. Blood. 2005;105:3079–3086. doi: 10.1182/blood-2004-10-3867. [DOI] [PubMed] [Google Scholar]
- 8.Arruda VR, Stedman HH, Nichols TC, Haskins ME, Nicholson M, Herzog RW, Couto LB, High KA. Regional intravascular delivery of AAV-2-F.IX to skeletal muscle achieves long-term correction of hemophilia B in a large animal model. Blood. 2005;105:3458–3464. doi: 10.1182/blood-2004-07-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kay MA, Manno CS, Ragni MV, Larson PJ, Couto LB, McClelland A, Glader B, Chew AJ, Tai SJ, Herzog RW, Arruda V, Johnson F, Scallan C, Skarsgard E, Flake AW, High KA. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nat Genet. 2000;24:257–261. doi: 10.1038/73464. [DOI] [PubMed] [Google Scholar]
- 10.Manno CS, Chew AJ, Hutchison S, Larson PJ, Herzog RW, Arruda VR, Tai SJ, Ragni MV, Thompson A, Ozelo M, Couto LB, Leonard DG, Johnson FA, McClelland A, Scallan C, Skarsgard E, Flake AW, Kay MA, High KA, Glader B. AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood. 2003;101:2963–2972. doi: 10.1182/blood-2002-10-3296. [DOI] [PubMed] [Google Scholar]
- 11.Jiang H, Pierce GF, Ozelo MC, de Paula EV, Vargas JA, Smith P, Sommer J, Luk A, Manno CS, High KA, Arruda VR. Evidence of multiyear factor IX expression by AAV-mediated gene transfer to skeletal muscle in an individual with severe hemophilia B. Mol Ther. 2006;14:452–455. doi: 10.1016/j.ymthe.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, Ozelo MC, Hoots K, Blatt P, Konkle B, Dake M, Kaye R, Razavi M, Zajko A, Zehnder J, Rustagi PK, Nakai H, Chew A, Leonard D, Wright JF, Lessard RR, Sommer JM, Tigges M, Sabatino D, Luk A, Jiang H, Mingozzi F, Couto L, Ertl HC, High KA, Kay MA. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 13.Herzog RW, Hagstrom JN, Kung SH, Tai SJ, Wilson JM, Fisher KJ, High KA. Stable gene transfer and expression of human blood coagulation factor IX after intramuscular injection of recombinant adeno-associated virus. Proc Natl Acad Sci U S A. 1997;94:5804–5809. doi: 10.1073/pnas.94.11.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nathwani AC, Davidoff A, Hanawa H, Zhou JF, Vanin EF, Nienhuis AW. Factors influencing in vivo transduction by recombinant adeno-associated viral vectors expressing the human factor IX cDNA. Blood. 2001;97:1258–1265. doi: 10.1182/blood.v97.5.1258. [DOI] [PubMed] [Google Scholar]
- 15.Herzog RW, Mount JD, Arruda VR, High KA, Lothrop CD., Jr Muscle-directed gene transfer and transient immune suppression result in sustained partial correction of canine hemophilia B caused by a null mutation. Mol Ther. 2001;4:192–200. doi: 10.1006/mthe.2001.0442. [DOI] [PubMed] [Google Scholar]
- 16.Herzog RW, Yang EY, Couto LB, Hagstrom JN, Elwell D, Fields PA, Burton M, Bellinger DA, Read MS, Brinkhous KM, Podsakoff GM, Nichols TC, Kurtzman GJ, High KA. Long-term correction of canine hemophilia B by gene transfer of blood coagulation factor IX mediated by adeno-associated viral vector. Nat Med. 1999;5:56–63. doi: 10.1038/4743. [DOI] [PubMed] [Google Scholar]
- 17.Chao H, Samulski R, Bellinger D, Monahan P, Nichols T, Walsh C. Persistent expression of canine factor IX in hemophilia B canines. Gene Ther. 1999;6:1695–1704. doi: 10.1038/sj.gt.3301024. [DOI] [PubMed] [Google Scholar]
- 18.Herzog RW, Fields PA, Arruda VR, Brubaker JO, Armstrong E, McClintock D, Bellinger DA, Couto LB, Nichols TC, High KA. Influence of vector dose on factor IX-specific T and B cell responses in muscle-directed gene therapy. Hum Gene Ther. 2002;13:1281–1291. doi: 10.1089/104303402760128513. [DOI] [PubMed] [Google Scholar]
- 19.Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci U S A. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao G, Vandenberghe LH, Wilson JM. New recombinant serotypes of AAV vectors. Curr Gene Ther. 2005;5:285–297. doi: 10.2174/1566523054065057. [DOI] [PubMed] [Google Scholar]
- 21.Xiao W, Chirmule N, Berta SC, McCullough B, Gao G, Wilson JM. Gene therapy vectors based on adeno-associated virus type 1. J Virol. 1999;73:3994–4003. doi: 10.1128/jvi.73.5.3994-4003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chao H, Liu Y, Rabinowitz J, Li C, Samulski RJ, Walsh CE. Several log increase in therapeutic transgene delivery by distinct adeno-associated viral serotype vectors. Mol Ther. 2000;2:619–623. doi: 10.1006/mthe.2000.0219. [DOI] [PubMed] [Google Scholar]
- 23.Arruda VR, Schuettrumpf J, Herzog RW, Nichols TC, Robinson N, Lotfi Y, Mingozzi F, Xiao W, Couto LB, High KA. Safety and efficacy of factor IX gene transfer to skeletal muscle in murine and canine hemophilia B models by adeno-associated viral vector serotype 1. Blood. 2004;103:85–92. doi: 10.1182/blood-2003-05-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohn EF, Zhuo J, Kelly ME, Chao HJ. Efficient induction of immune tolerance to coagulation factor IX following direct intramuscular gene transfer. J Thromb Haemost. 2007;5:1227–1236. doi: 10.1111/j.1538-7836.2007.02522.x. [DOI] [PubMed] [Google Scholar]
- 25.Sarkar R, Tetreault R, Gao G, Wang L, Bell P, Chandler R, Wilson JM, Kazazian HH., Jr Total correction of hemophilia A mice with canine FVIII using an AAV 8 serotype. Blood. 2004;103:1253–1260. doi: 10.1182/blood-2003-08-2954. [DOI] [PubMed] [Google Scholar]
- 26.Sarkar R, Mucci M, Addya S, Tetreault R, Bellinger DA, Nichols TC, Kazazian HH., Jr Long-term efficacy of adeno-associated virus serotypes 8 and 9 in hemophilia a dogs and mice. Hum Gene Ther. 2006;17:427–439. doi: 10.1089/hum.2006.17.427. [DOI] [PubMed] [Google Scholar]
- 27.Nathwani AC, Gray JT, Ng CY, Zhou J, Spence Y, Waddington SN, Tuddenham EG, Kemball-Cook G, McIntosh J, Boon-Spijker M, Mertens K, Davidoff AM. Self-complementary adeno-associated virus vectors containing a novel liver-specific human factor IX expression cassette enable highly efficient transduction of murine and nonhuman primate liver. Blood. 2006;107:2653–2661. doi: 10.1182/blood-2005-10-4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nathwani AC, Gray JT, McIntosh J, Ng CY, Zhou J, Spence Y, Cochrane M, Gray E, Tuddenham EG, Davidoff AM. Safe and efficient transduction of the liver after peripheral vein infusion of self-complementary AAV vector results in stable therapeutic expression of human FIX in nonhuman primates. Blood. 2007;109:1414–1421. doi: 10.1182/blood-2006-03-010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nathwani AC, Cochrane M, McIntosh J, Ng CY, Zhou J, Gray JT, Davidoff AM. Enhancing transduction of the liver by adeno-associated viral vectors. Gene Ther. 2009;16:60–69. doi: 10.1038/gt.2008.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishiwata A, Mimuro J, Kashiwakura Y, Niimura M, Takano K, Ohmori T, Madoiwa S, Mizukami H, Okada T, Naka H, Yoshioka A, Ozawa K, Sakata Y. Phenotype correction of hemophilia A mice with adeno-associated virus vectors carrying the B domain-deleted canine factor VIII gene. Thromb Res. 2006;118:627–635. doi: 10.1016/j.thromres.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 31.Ohshima S, Shin JH, Yuasa K, Nishiyama A, Kira J, Okada T, Takeda S. Transduction efficiency and immune response associated with the administration of AAV8 vector into dog skeletal muscle. Mol Ther. 2009;17:73–80. doi: 10.1038/mt.2008.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang L, Cao O, Swalm B, Dobrzynski E, Mingozzi F, Herzog RW. Major role of local immune responses in antibody formation to factor IX in AAV gene transfer. Gene Ther. 2005;12:1453–1464. doi: 10.1038/sj.gt.3302539. [DOI] [PubMed] [Google Scholar]
- 33.Auricchio A, Hildinger M, O'Connor E, Gao GP, Wilson JM. Isolation of highly infectious and pure adeno-associated virus type 2 vectors with a single-step gravity-flow column. Hum Gene Ther. 2001;12:71–76. doi: 10.1089/104303401450988. [DOI] [PubMed] [Google Scholar]
- 34.Wang L, Zoppe M, Hackeng TM, Griffin JH, Lee KF, Verma IM. A factor IX-deficient mouse model for hemophilia B gene therapy. Proc Natl Acad Sci U S A. 1997;94:11563–11566. doi: 10.1073/pnas.94.21.11563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang J, Xu L, Haskins ME, Parker Ponder K. Neonatal gene transfer with a retroviral vector results in tolerance to human factor IX in mice and dogs. Blood. 2004;103:143–151. doi: 10.1182/blood-2003-06-2181. [DOI] [PubMed] [Google Scholar]
- 36.Kung SH, Hagstrom JN, Cass D, Tai SJ, Lin HF, Stafford DW, High KA. Human factor IX corrects the bleeding diathesis of mice with hemophilia B. Blood. 1998;91:784–790. [PubMed] [Google Scholar]
- 37.Wang L, Figueredo J, Calcedo R, Lin J, Wilson JM. Cross-presentation of adeno-associated virus serotype 2 capsids activates cytotoxic T cells but does not render hepatocytes effective cytolytic targets. Hum Gene Ther. 2007;18:185–194. doi: 10.1089/hum.2007.001. [DOI] [PubMed] [Google Scholar]
- 38.Fields PA, Kowalczyk DW, Arruda VR, Armstrong E, McCleland ML, Hagstrom JN, Pasi KJ, Ertl HC, Herzog RW, High KA. Role of vector in activation of T cell subsets in immune responses against the secreted transgene product factor IX. Mol Ther. 2000;1:225–235. doi: 10.1006/mthe.2000.0032. [DOI] [PubMed] [Google Scholar]
- 39.Fields PA, Arruda VR, Armstrong E, Chu K, Mingozzi F, Hagstrom JN, Herzog RW, High KA. Risk and prevention of anti-factor IX formation in AAV-mediated gene transfer in the context of a large deletion of F9. Mol Ther. 2001;4:201–210. doi: 10.1006/mthe.2001.0441. [DOI] [PubMed] [Google Scholar]
- 40.Waddington SN, Buckley SM, Nivsarkar M, Jezzard S, Schneider H, Dahse T, Kemball-Cook G, Miah M, Tucker N, Dallman MJ, Themis M, Coutelle C. In utero gene transfer of human factor IX to fetal mice can induce postnatal tolerance of the exogenous clotting factor. Blood. 2003;101:1359–1366. doi: 10.1182/blood-2002-03-0779. [DOI] [PubMed] [Google Scholar]
- 41.Song S, Morgan M, Ellis T, Poirier A, Chesnut K, Wang J, Brantly M, Muzyczka N, Byrne BJ, Atkinson M, Flotte TR. Sustained secretion of human alpha-1-antitrypsin from murine muscle transduced with adeno-associated virus vectors. Proc Natl Acad Sci U S A. 1998;95:14384–14388. doi: 10.1073/pnas.95.24.14384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.LoDuca PA, Hoffman BE, Herzog RW. Hepatic gene transfer as a means of tolerance induction to transgene products. Curr Gene Ther. 2009;9:104–114. doi: 10.2174/156652309787909490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mingozzi F, Meulenberg JJ, Hui DJ, Basner-Tschakarjan E, Hasbrouck NC, Edmonson SA, Hutnick NA, Betts MR, Kastelein JJ, Stroes ES, High KA. AAV-1-mediated gene transfer to skeletal muscle in humans results in dose-dependent activation of capsid-specific T cells. Blood. 2009;114:2077–2086. doi: 10.1182/blood-2008-07-167510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao G, Vandenberghe LH, Alvira MR, Lu Y, Calcedo R, Zhou X, Wilson JM. Clades of Adeno-associated viruses are widely disseminated in human tissues. J Virol. 2004;78:6381–6388. doi: 10.1128/JVI.78.12.6381-6388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Calcedo R, Vandenberghe LH, Gao G, Lin J, Wilson JM. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J Infect Dis. 2009;199:381–390. doi: 10.1086/595830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vandenberghe LH, Wang L, Somanathan S, Zhi Y, Figueredo J, Calcedo R, Sanmiguel J, Desai RA, Chen CS, Johnston J, Grant RL, Gao G, Wilson JM. Heparin binding directs activation of T cells against adeno-associated virus serotype 2 capsid. Nat Med. 2006;12:967–971. doi: 10.1038/nm1445. [DOI] [PubMed] [Google Scholar]
- 47.Louboutin JP, Wang L, Wilson JM. Gene transfer into skeletal muscle using novel AAV serotypes. J Gene Med. 2005;7:442–451. doi: 10.1002/jgm.686. [DOI] [PubMed] [Google Scholar]
- 48.Jooss K, Chirmule N. Immunity to adenovirus and adeno-associated viral vectors: implications for gene therapy. Gene Ther. 2003;10:955–963. doi: 10.1038/sj.gt.3302037. [DOI] [PubMed] [Google Scholar]
- 49.Vandendriessche T, Thorrez L, Acosta-Sanchez A, Petrus I, Wang L, Ma L, L DEW, Iwasaki Y, Gillijns V, Wilson JM, Collen D, Chuah MK. Efficacy and safety of adeno-associated viral vectors based on serotype 8 and 9 vs. lentiviral vectors for hemophilia B gene therapy. J Thromb Haemost. 2007;5:16–24. doi: 10.1111/j.1538-7836.2006.02220.x. [DOI] [PubMed] [Google Scholar]
- 50.Mingozzi F, Liu YL, Dobrzynski E, Kaufhold A, Liu JH, Wang Y, Arruda VR, High KA, Herzog RW. Induction of immune tolerance to coagulation factor IX antigen by in vivo hepatic gene transfer. J Clin Invest. 2003;111:1347–1356. doi: 10.1172/JCI16887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cooper M, Nayak S, Hoffman BE, Terhorst C, Cao O, Herzog RW. Improved induction of immune tolerance to factor IX by hepatic AAV-8 gene transfer. Hum Gene Ther. 2009;20:767–776. doi: 10.1089/hum.2008.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cao O, Loduca PA, Herzog RW. Role of regulatory T cells in tolerance to coagulation factors. J Thromb Haemost. 2009;7(Suppl 1):88–91. doi: 10.1111/j.1538-7836.2009.03417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kelly ME, Zhuo J, Bharadwaj AS, Chao H. Induction of immune tolerance to FIX following muscular AAV gene transfer is AAV-dose/FIX-level dependent. Mol Ther. 2009;17:857–863. doi: 10.1038/mt.2009.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin J, Zhi Y, Mays L, Wilson JM. Vaccines based on novel adeno-associated virus vectors elicit aberrant CD8+ T-cell responses in mice. J Virol. 2007;81:11840–11849. doi: 10.1128/JVI.01253-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lin SW, Hensley SE, Tatsis N, Lasaro MO, Ertl HC. Recombinant adeno-associated virus vectors induce functionally impaired transgene product-specific CD8+ T cells in mice. J Clin Invest. 2007;117:3958–3970. doi: 10.1172/JCI33138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Snyder RO, Miao CH, Patijn GA, Spratt SK, Danos O, Nagy D, Gown AM, Winther B, Meuse L, Cohen LK, Thompson AR, Kay MA. Persistent and therapeutic concentrations of human factor IX in mice after hepatic gene transfer of recombinant AAV vectors. Nat Genet. 1997;16:270–276. doi: 10.1038/ng0797-270. [DOI] [PubMed] [Google Scholar]


