Abstract
The metabolically inactive hyperpolarized agents HP001 and urea enable a new type of perfusion MRI based on a direct signal source that is background-free. The addition of perfusion information to metabolic information obtained by spectroscopic imaging of hyperpolarized [1-13C]pyruvate would be of great value in exploring the relationship between perfusion and metabolism in cancer. In preclinical normal murine and cancer model studies, we performed both dynamic multi-slice imaging of the specialized hyperpolarized perfusion compound HP001 (bis-1,1-(hydroxymethyl)-[1-13C]cyclopropane-d8, T1= 95 s ex vivo, 32 s in vivo at 3T) using a pulse sequence with balanced steady state free precession (bSSFP) and ramped flip angle over time for efficient utilization of the hyperpolarized magnetization, and 3D echo planar spectroscopic imaging (EPSI) of urea co-polarized with [1-13C]pyruvate, with compressed sensing for resolution enhancement. For the dynamic data, peak signal maps and blood flow maps derived from perfusion modeling were generated. The spatial heterogeneity of perfusion was increased 2.9-fold in tumor tissues (p= 0.05), and slower washout was observed in the dynamic data. The results of separate dynamic HP001 imaging and co-polarized pyruvate / urea imaging were compared. A strong and significant correlation (R= 0.73, p= 0.02) detected between the urea and HP001 data confirmed the value of co-polarizing urea with pyruvate for simultaneous assessment of perfusion and metabolism.
Keywords: hyperpolarized, C13, urea, HP001, perfusion, pyruvate
Introduction
The method of dynamic nuclear polarization (DNP) with rapid dissolution has allowed >10,000-fold signal enhancement of many 13C-labeled compounds in the liquid state (1). Preliminary studies of the metabolically inactive hyperpolarized 13C-labeled compounds [13C]urea and HP001 (or bis-1,1-(hydroxymethyl)-[1-13C]cyclopropane-d8, an exogenous compound with very long T1 of 95 s ex vivo, 32 s in vivo at 3T and good polarization by DNP) have demonstrated feasibility for a new form of perfusion MRI using hyperpolarized agents (2-4). As opposed to Gadolinium (Gd)-based studies, hyperpolarized studies of perfusion have a direct signal source that is background-free. The very long relaxation time and high polarization of HP001 make it an ideal perfusion agent, but urea is attractive because it is endogenous to humans with a known benign safety profile (5) and can be easily imaged simultaneously with pyruvate and its metabolic products using existing fast MR spectroscopic imaging (MRSI) methods (6,7) (unlike HP001, which at δ= 23 ppm has wide spectral separation from pyruvate of ~5 kHz at 3T).
Signal changes detected in perfusion imaging of cancer reflect spatially heterogeneous alterations to existing vasculature and neovascularization as tumors outstrip the normal blood supply, including microcirculatory disruption in some of the abnormal vessels (8). The addition of tumor perfusion data to the metabolic data available from spectroscopic imaging of [1-13C]pyruvate (9,10) would be of great value in exploring the complex relationship between perfusion and metabolism in cancer at the levels of both preclinical and clinical research (11). This study reports our initial data from dynamic perfusion imaging of HP001 in normal and cancerous murine models, interpreted in combination with metabolic data from spectroscopic imaging of pyruvate, with or without co-polarized urea (12) for simultaneous assessment of perfusion, in the same animals.
Methods
Sample preparation
Samples were initially prepared as follows: HP001-mixed with water in ratio of 2.78:1 by weight, [1-13C]pyruvic acidneat, and [13C]urea- dissolved in glycerol to 6.4M. The urea and HP001 solutions contained 18-23 mM of the trityl radical OX063 (GE Healthcare, Oslo, Norway) and 0.2-1.5 mM Dotarem (Guerbet, Roissy, France). The pyruvic acid preparation contained 16.5 mM of the trityl radical and 1.5 mM Dotarem. For each experiment, a sample was loaded into the 3.35T magnet of the HyperSense polarizer (Oxford Instruments Biotools, Oxford, UK), where it was cooled to 1.3 K and irradiated for ~1 hr, and then rapidly dissolved in ~4.5 mL heated buffer solution (HP001- 1X phosphate buffered saline, pyruvic acid- 80 mM NaOH / 40 mM Tris buffer), resulting in a 80-115 mM solution (HP001- 100 mM, pyruvate- 80 mM, urea- 115 mM) of pH ~7.5. For the co-polarization studies, urea and pyruvic acid were loaded into the sample cup separately in frozen layers to avoid mixing, by quickly immersing the cup in a liquid nitrogen bath after dispensing each layer, and the NaOH / Tris buffer solvent was used for dissolution. Animals were injected with 350 μL (mice) or 2.4 mL (rats) of the hyperpolarized solution over 12 s.
Animal experiments
Four rats and seven mice were imaged in a clinical GE 3T scanner, with custom dual-tuned 1H / 13C volume RF coils. Six of the mice were from transgenic cancer models: three from the transgenic adenocarcinoma of mouse prostate (TRAMP) model (13), and three from a liver tumor model (14). The rest of the animals were normal (male Sprague-Dawley rat or male FVB strain mouse). Non-localized, low flip angle spectroscopic imaging experiments were performed with HP001 to obtain the T1 values quoted above. Dynamic imaging of HP001 was performed for all animals, using a custom multi-slice pulse sequence employing balanced steady state free precession (bSSFP), with initial θ/2 pulse for signal stability (2-4,15). The flip angle was ramped with time to match T1 and T2 decay, based on a previously described signal model (15), at 10 time points every 6 s. Sequence parameters: flip angle= 4° (i.e. 2° - 4° - 4° - 4° - …) - 6° - 9° - 13° - 19° - 30° - 45° - 67° - 100° - 140°, acquisition matrix= 32×32, slices= 8, spatial resolution= 2.5 mm × 2.5 mm × 6 mm (0.038 cm3), TE / TR = 6ms / 12ms, BWread= ±10 kHz. Metabolic imaging of [1-13C]pyruvate was also performed in the mice, using 3D echo planar spectroscopic imaging (EPSI) with adiabatic double spin echo preparation and compressed sensing for fourfold resolution enhancement (16). Sequence parameters: matrix= 16 × 16 × 16, spatial resolution= 2.5mm × 2.5mm × 5.4mm (0.034 cm3), TE / TR = 140 ms / 215 ms, spectral bandwidth= 581 Hz, covering lactate to pyruvate at 3T, scan duration= 16 s, acquisition timing= 30 s post-injection in liver tumors, 35 s in others. In three of the mice, a multi-compound study was performed where [13C]urea was co-polarized and imaged using MRSI along with pyruvate and its metabolic products, folding into the spectral window between lactate and alanine.
Data analysis
For the dynamic HP001 data, blood flow maps were computed through deconvolution of the dynamic HP001 signal curves (with SVD thresholding at 10%), with arterial input functions (AIF’s) defined in the descending aorta (17). Peak signal maps were also created. For the spectroscopic data, images of pyruvate, lactate, and urea were generated from the spectral peak areas. All data was interpolated to 256×256 in-plane. Regions of interest (ROI’s) corresponding to the kidneys, liver, prostate, and any tumors were manually drawn on anatomic T2-weighted 1H FSE images (192×192, FOV= 10 cm, slice thickness= 2 mm, TE / TR = 79.7 ms / 628 ms, BWreadout= ±83.3 kHz, NEX=6), co-registered to the 13C imaging and spectroscopy data, and regional signals were tabulated. Raw signals were normalized by the percent polarization measured from an aliquot of the dissolved sample in a separate low-field spectrometer.
Results
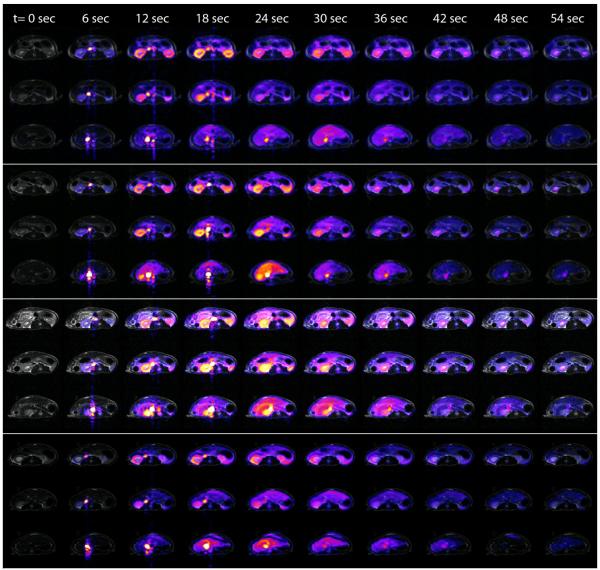
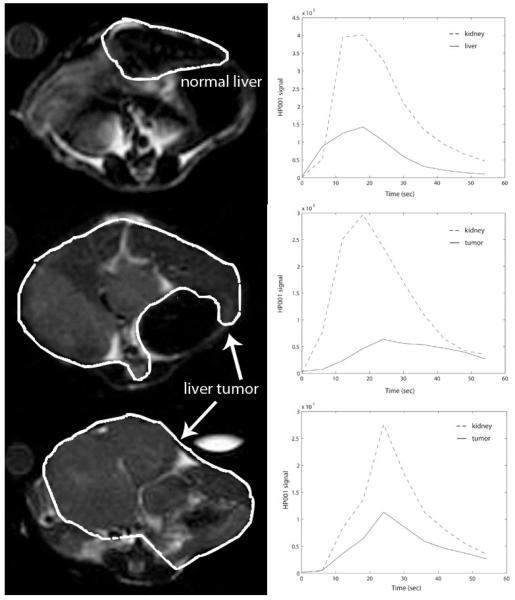
Among the rats, consistent mean peak HP001 signals and mean blood flows (BF) were measured in regions of the kidneys (pk: 138±7, BF: 156±29, both in a.u.) and liver (pk: 83±22, BF: 47±11). Liquid state polarizations ranged from 17-31% as measured by the low-field spectrometer, and the data was normalized by these values as described above. Images from the rats are shown in Figure 1. The most prominent feature of the perfusion data from murine tumor tissues was increased spatial heterogeneity in comparison to normal tissue, with typically high signal around the tumor periphery (e.g. Figure 2). As a quantitative example, the spatial variability for peak HP001 signals in liver tumor tissues was 2.9× greater than normal liver tissue, in terms of the coefficient of variation (p= 0.05). A slower washout of the hyperpolarized material, or extended mean transit time, was also observed in the tumors, as demonstrated in the dynamic signal curves in Figure 3.
Figure 1.
Dynamic bSSFP imaging of HP001 over 54 s in four normal rats, for three adjacent 6 mm axial slices extending from the kidneys (top) to the liver (bottom) in each animal. HP001 images are overlaid on T2-weighted 1H FSE images.
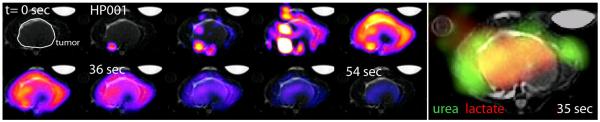
Figure 2.
Dynamic HP001 perfusion imaging data, and 3D MRSI data from co-polarized pyruvate / urea study (urea- green, lactate- red), for a single axial slice through mouse prostate tumor (HP001: 6 s increments from left to right, MRSI: acquisition started at 35 s after injection).
Figure 3.
Dynamic HP001 signal curves (right column) for normal mouse liver (top) and two transgenic liver tumors (below). Corresponding liver regions are outlined on axial T2-weighted 1H images (left column).
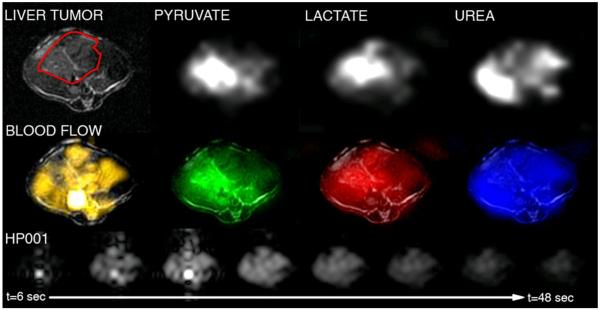
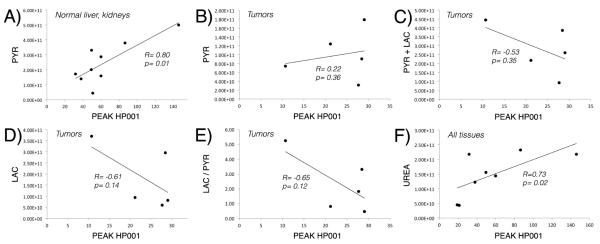
Similar to previous studies, high mean lactate-to-pyruvate ratios were detected in the tumors (liver tumors: 3.5, TRAMP’s: 0.65). Interestingly, while pyruvate levels were well correlated with peak HP001 signals in the kidneys and normal liver (correlation coefficient R= 0.80, p= 0.01), they were neither strongly nor significantly correlated within tumor tissues (R= 0.22, p= 0.36), without improvement by considering the sum of pyruvate and lactate. However, tumor lactate and lactate-to-pyruvate ratios were somewhat inversely correlated to peak HP001 signals, with correlation coefficients R= −0.61 (p= 0.14) and R= −0.65 (p= 0.12), respectively. In the animals with co-polarized urea data (e.g. Figure 5), urea was seen to have a similar distribution as HP001 (especially when comparing the closest time point in the dynamic data, e.g. see Figures 2&5), and a strong and significant correlation was detected between the urea and HP001 data (R= 0.73, p= 0.02). Graphical versions of these correlations are provided in Figure 4.
Figure 5.
Images of mouse liver tumor perfusion and metabolism probed with multiple hyperpolarized 13C compounds. Top row: Anatomic 1H image, and 13C 3D MRSI images from co-polarized pyruvate / urea study. Middle row: Color overlays for 13C MRSI data and blood flow image derived from dynamic HP001 data. Bottom row: Dynamic HP001 imaging data.
Figure 4.
Correlations between peak HP001 signal and pyruvate (A, B), pyruvate + lactate (C), lactate (D), lactate-to-pyruvate ratio (E), and urea (F) in normal and abnormal murine tissues. Strong and significant correlations were identified with pyruvate in normal tissues, and with urea in all tissues.
Discussion
Using an efficiently designed bSSFP pulse sequence, the specialized hyperpolarized perfusion agent HP001 allows dynamic multi-slice imaging of tissue perfusion over the time course of approximately one minute post injection, at sufficient spatial resolution (0.038 cm3) for preclinical murine perfusion imaging. Using fast MRSI methods, co-polarization of [13C]urea with [1-13C]pyruvate allows reliable assessment of perfusion in addition to metabolism. These methods enable imaging of the spatially heterogeneous perfusion and metabolism within tumors. The heterogeneity of tumor perfusion observed in this study is attributable to vascular changes, and formation of sub-regions of viable tumor, necrosis, and edema within the tumor region (8). The slow washouts, also frequently seen in Gd-based perfusion studies, are likely similarly explained by the hyperpermeability of angiogenic vessels (18). The extended imaging window allowed by the long T1 relaxation time of HP001 is critical for detecting the dynamics of the washout.
Comparison of the HP001 perfusion data to hyperpolarized MRSI data revealed interesting correlations. According to the lack of correlation between pyruvate and HP001 signals, pyruvate signal alone, or pyruvate plus lactate, does not accurately reflect tumor perfusion. However, the detected correlation between MRSI urea signals and separate dynamic HP001 imaging demonstrates the value of co-polarizing urea with pyruvate for a simultaneous assessment of perfusion and metabolism. This finding is significant because of the safety and ease of imaging hyperpolarized urea together with pyruvate. Inverse correlation of the lactate data with HP001 data may be a result of metabolic changes under hypoxic conditions induced by regionally poor perfusion (11).
HP001 and urea probably better reflect perfusion as compared to pyruvate or pyruvate plus lactate because there is no metabolic conversion, which may produce confusing results because of different T1’s of product compounds, and/or possible conversion to compounds not visible to the experiment. Another reason is the more intravascular distribution of these compounds, which is shown empirically by a large urea signal in the chest at 30 s / 35 s in all of the co-polarization studies, with very little pyruvate signal, indicating that a larger fraction of the injected urea remains in circulation.
Prior imaging studies of human cancers (using a variety of imaging techniques including dynamic contrast MRI, FDG-PET, H215O-PET, and perfusion CT) have shown that the relationship between tumor perfusion and metabolism is variable (19-27), reflecting the complexity of the underlying biology. However, several of these studies have reported that an uncoupling or mismatch of tumor perfusion and metabolism is associated with adverse tumor features (19,22-24). For example, Mankoff et al. found that a high ratio of metabolism to perfusion is associated with poor response to chemotherapy in locally advanced breast cancer (23), while Aronen et al. found that such a mismatch is associated with higher tumor grade in gliomas (19). Results of co-polarized studies with [13C]urea and [1-13C]pyruvate, which collect perfusion and metabolic data in a single safe imaging study, may ultimately produce novel sub-classifications of human cancers for improved individualized treatment.
Acknowledgements
We gratefully acknowledge grant support from NIH grants P41EB013598 and R01EB007588. We also acknowledge the assistance of Kristen Scott with the animal studies, in addition to help from Mark Van Criekinge, Galen Reed, Peter Shin, and Ilwoo Park. We also thank Kayvan Keshari and David Wilson for help with the copolarization procedure.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ardenkjaer-Larsen JH, Fridlund B, Gram A, Hansson G, Hansson L, Lerche MH, Servin R, Thaning M, Golman K. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc Natl Acad Sci USA. 2003;100(18):10158–10163. doi: 10.1073/pnas.1733835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Golman K, Ardenkjaer-Larsen JH, Petersson JS, Mansson S, Leunbach I. Molecular imaging with endogenous substances. Proc Natl Acad Sci USA. 2003;100(18):10435–10439. doi: 10.1073/pnas.1733836100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johansson E, Månsson S, Wirestam R, Svensson J, Petersson JS, Golman K, Ståhlberg F. Cerebral perfusion assessment by bolus tracking using hyperpolarized 13C. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2004;51(3):464–472. doi: 10.1002/mrm.20013. [DOI] [PubMed] [Google Scholar]
- 4.von Morze C, Larson PE, Hu S, Keshari K, Wilson DM, Ardenkjaer-Larsen JH, Goga A, Bok R, Kurhanewicz J, Vigneron DB. Imaging of Blood Flow Using Hyperpolarized [13C]Urea in Preclinical Cancer Models. J Magn Reson Imaging. 2011 doi: 10.1002/jmri.22484. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beks JW, Groen A, Huizinga T, Noordhoek KH, Smit JM, Walter WG. Effects of intravenously administered hypertonic urea solution. Acta Neurochir (Wien) 1965;13(1):1–10. doi: 10.1007/BF02074641. [DOI] [PubMed] [Google Scholar]
- 6.Cunningham CH, Chen AP, Albers MJ, Kurhanewicz J, Hurd RE, Yen YF, Pauly JM, Nelson SJ, Vigneron DB. Double spin-echo sequence for rapid spectroscopic imaging of hyperpolarized 13C. J Magn Reson. 2007;187(2):357–362. doi: 10.1016/j.jmr.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 7.Mayer D, Yen YF, Levin YS, Tropp J, Pfefferbaum A, Hurd RE, Spielman DM. In vivo application of sub-second spiral chemical shift imaging (CSI) to hyperpolarized 13C metabolic imaging: comparison with phase-encoded CSI. J Magn Reson. 204(2):340–345. doi: 10.1016/j.jmr.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gillies RJ, Schornack PA, Secomb TW, Raghunand N. Causes and effects of heterogeneous perfusion in tumors. Neoplasia. 1999;1(3):197–207. doi: 10.1038/sj.neo.7900037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen AP, Albers MJ, Cunningham CH, Kohler SJ, Yen YF, Hurd RE, Tropp J, Bok R, Pauly JM, Nelson SJ, Kurhanewicz J, Vigneron DB. Hyperpolarized C-13 spectroscopic imaging of the TRAMP mouse at 3T-initial experience. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2007;58(6):1099–1106. doi: 10.1002/mrm.21256. [DOI] [PubMed] [Google Scholar]
- 10.Park I, Larson PE, Zierhut ML, Hu S, Bok R, Ozawa T, Kurhanewicz J, Vigneron DB, Vandenberg SR, James CD, Nelson SJ. Hyperpolarized 13C magnetic resonance metabolic imaging: application to brain tumors. Neuro Oncol. 12(2):133–144. doi: 10.1093/neuonc/nop043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989;49(23):6449–6465. [PubMed] [Google Scholar]
- 12.Wilson DM, Keshari KR, Larson PE, Chen AP, Hu S, Criekinge MV, Bok R, Nelson SJ, Macdonald JM, Vigneron DB, Kurhanewicz J. Multi-compound polarization by DNP allows simultaneous assessment of multiple enzymatic activities in vivo. J Magn Reson. doi: 10.1016/j.jmr.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenberg NM, DeMayo F, Finegold MJ, Medina D, Tilley WD, Aspinall JO, Cunha GR, Donjacour AA, Matusik RJ, Rosen JM. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci U S A. 1995;92(8):3439–3443. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shachaf CM, Kopelman AM, Arvanitis C, Karlsson A, Beer S, Mandl S, Bachmann MH, Borowsky AD, Ruebner B, Cardiff RD, Yang Q, Bishop JM, Contag CH, Felsher DW. MYC inactivation uncovers pluripotent differentiation and tumour dormancy in hepatocellular cancer. Nature. 2004;431(7012):1112–1117. doi: 10.1038/nature03043. [DOI] [PubMed] [Google Scholar]
- 15.Svensson J, Månsson S, Johansson E, Petersson JS, Olsson LE. Hyperpolarized 13C MR angiography using trueFISP. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2003;50(2):256–262. doi: 10.1002/mrm.10530. [DOI] [PubMed] [Google Scholar]
- 16.Hu S, Lustig M, Balakrishnan A, Larson PE, Bok R, Kurhanewicz J, Nelson SJ, Goga A, Pauly JM, Vigneron DB. 3D compressed sensing for highly accelerated hyperpolarized (13)C MRSI with in vivo applications to transgenic mouse models of cancer. Magn Reson Med. 2010 Feb;63(2):312–21. doi: 10.1002/mrm.22233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ostergaard L, Weisskoff RM, Chesler DA, Gyldensted C, Rosen BR. High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part I: Mathematical approach and statistical analysis. Magn Reson Med. 1996;36(5):715–725. doi: 10.1002/mrm.1910360510. [DOI] [PubMed] [Google Scholar]
- 18.Barrett T, Brechbiel M, Bernardo M, Choyke PL. MRI of tumor angiogenesis. Journal of magnetic resonance imaging : JMRI. 2007;26(2):235–249. doi: 10.1002/jmri.20991. [DOI] [PubMed] [Google Scholar]
- 19.Aronen HJ, Pardo FS, Kennedy DN, Belliveau JW, Packard SD, Hsu DW, Hochberg FH, Fischman AJ, Rosen BR. High microvascular blood volume is associated with high glucose uptake and tumor angiogenesis in human gliomas. Clin Cancer Res. 2000;6(6):2189–2200. [PubMed] [Google Scholar]
- 20.Fukuda K, Taniguchi H, Koh T, Kunishima S, Yamagishi H. Relationships between oxygen and glucose metabolism in human liver tumours: positron emission tomography using (15)O and (18)F-deoxyglucose. Nucl Med Commun. 2004;25(6):577–583. doi: 10.1097/01.mnm.0000126627.01919.1d. [DOI] [PubMed] [Google Scholar]
- 21.Hirasawa S, Tsushima Y, Takei H, Hirasawa H, Taketomi-Takahashi A, Takano A, Oriuchi N, Endo K. Inverse correlation between tumor perfusion and glucose uptake in human head and neck tumors. Acad Radiol. 2007;14(3):312–318. doi: 10.1016/j.acra.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 22.Komar G, Kauhanen S, Liukko K, Seppanen M, Kajander S, Ovaska J, Nuutila P, Minn H. Decreased blood flow with increased metabolic activity: a novel sign of pancreatic tumor aggressiveness. Clin Cancer Res. 2009;15(17):5511–5517. doi: 10.1158/1078-0432.CCR-09-0414. [DOI] [PubMed] [Google Scholar]
- 23.Mankoff DA, Dunnwald LK, Gralow JR, Ellis GK, Charlop A, Lawton TJ, Schubert EK, Tseng J, Livingston RB. Blood flow and metabolism in locally advanced breast cancer: relationship to response to therapy. J Nucl Med. 2002;43(4):500–509. [PubMed] [Google Scholar]
- 24.Miles KA, Griffiths MR, Keith CJ. Blood flow-metabolic relationships are dependent on tumour size in non-small cell lung cancer: a study using quantitative contrast-enhanced computer tomography and positron emission tomography. Eur J Nucl Med Mol Imaging. 2006;33(1):22–28. doi: 10.1007/s00259-005-1932-7. [DOI] [PubMed] [Google Scholar]
- 25.Semple SI, Gilbert FJ, Redpath TW, Staff RT, Ahearn TS, Welch AE, Heys SD, Hutcheon AW, Smyth EH, Chaturvedi S. The relationship between vascular and metabolic characteristics of primary breast tumours. Eur Radiol. 2004;14(11):2038–2045. doi: 10.1007/s00330-004-2454-6. [DOI] [PubMed] [Google Scholar]
- 26.Stewart EE, Chen X, Hadway J, Lee TY. Correlation between hepatic tumor blood flow and glucose utilization in a rabbit liver tumor model. Radiology. 2006;239(3):740–750. doi: 10.1148/radiol.2393041382. [DOI] [PubMed] [Google Scholar]
- 27.Tateishi U, Nishihara H, Tsukamoto E, Morikawa T, Tamaki N, Miyasaka K. Lung tumors evaluated with FDG-PET and dynamic CT: the relationship between vascular density and glucose metabolism. J Comput Assist Tomogr. 2002;26(2):185–190. doi: 10.1097/00004728-200203000-00004. [DOI] [PubMed] [Google Scholar]