Abstract
BACKGROUND
Left atrial appendage (LAA) is implicated in maintenance of atrial fibrillation (AF) and atrial tachycardia (AT) associated with persistent AF (PsAF) ablation, although little is known about the incidence and mechanism of LAA AT.
OBJECTIVE
The purpose of this study was to characterize LAA ATs associated with PsAF ablation.
METHODS
In 74 consecutive patients undergoing stepwise PsAF ablation, 142 ATs were encountered during index and repeat procedures. Out of 78 focal-source ATs diagnosed by activation and entrainment mapping, 15 (19%) arose from the base of LAA. Using a 20-pole catheter, high-density maps were constructed (n = 10; age 57 ± 6 years) to characterize the mechanism of LAA-AT. The LAA orifice was divided into the posterior ridge and anterior-superior and inferior segments to characterize the location of AT.
RESULTS
Fifteen patients with LAA AT had symptomatic PsAF for 17 ± 15 months before ablation. LAA AT (cycle length [CL] 283 ± 30 ms) occurred during the index procedure in four and after 9 ± 7 months in 11 patients. We could map 89% ± 8% AT CLs locally with favorable entrainment from within the LAA, which is suggestive of localized reentry with centrifugal atrial activation. ATs were localized to inferior segment (n = 4), anterior-superior segment (n = 5), and posterior ridge (n = 6) with 1:1 conduction to the atria. Ablation targeting long fractionated or mid-diastolic electrogram within the LAA resulted in tachycardia termination. Postablation, selective contrast radiography demonstrated atrial synchronous LAA contraction in all but one patient. At 18 ± 7 months, 13/15 (87%) patients remained in sinus rhythm without antiarrhythmic drugs.
CONCLUSION
LAA is an important source of localized reentrant AT in patients with PsAF at index and repeat ablation procedures. Ablation targeting the site with long fractionated or mid-diastolic LAA electrogram is highly effective in acute and medium-term elimination of the arrhythmia.
Keywords: Left atrial appendage, Atrial tachycardia, Localized reentry, Persistent atrial fibrillation, Catheter ablation
Introduction
The role of left atrial appendage (LAA) has been increasingly recognized in the initiation and maintenance of atrial fibrillation (AF).1–3 Besides sporadic cases, systematically conducted studies have reported electrocardiographic and electrophysiologic characteristics of focal atrial arrhythmias arising from the LAA.4 –10 However, little is known about the mechanism and characteristics of organized atrial arrhythmia, atrial tachycardia (AT), arising from LAA in association with the ablation of persistent AF (PsAF).11 We present the incidence, electrophysiologic characteristics, and outcomes of LAA AT arising in the context of PsAF ablation in a consecutive series of patients.
Methods
Study population
Parent AF population
Seventy-four consecutive patients (ages 55 ± 13 years) who underwent ablation of PsAF (AF duration 17 ± 15 months) were included in the study. Among them, 44 patients had long-standing PsAF (AF duration 24 ± 14 months). The mean duration of PsAF in the remaining 30 patients was 7 ± 3 months. All patients provided written informed consent and were in spontaneous AF at the beginning of the procedure. The procedural target was termination of AF without antiarrhythmic drugs or electrical cardioversion. Ablation was undertaken in a stepwise manner as described elsewhere.3,12,13 Briefly, pulmonary vein isolation was performed with the endpoint of complete abolition or dissociation of potentials in all veins. Electrogram-guided ablation was performed at the sites featuring characteristic electrograms such as continuous electrical activity, disorganized, high-frequency, and multicomponent electrograms and a gradient of activation (a temporal gradient of at least 70 ms between the distal and proximal bipoles of the roving ablation catheter). These features may represent a local circuit. The endpoint was transformation of disorganized and fractionated electrograms into discrete electrograms associated with organization of local cycle length (CL). Linear ablation was performed if AF persisted at this stage. The left atrial (LA) roof and mitral isthmus were sequentially ablated with the endpoint of abolition of local electrograms or >80% reduction in their amplitude. After restoration of sinus rhythm, further ablation, if required, was performed at these sites to achieve bidirectional conduction block.14,15
Population with AT
Among 74 patients undergoing stepwise PsAF ablation, 50 ATs were encountered during the index procedure and 92 during the follow-up of 7 ± 5 months. Among 142 ATs, 64 were macroreentrant and 78 were diagnosed to have a focal source, including 15 (19%) wherein the focus was confined to the base of the LAA. High-density mapping was performed in 10 out of 15 LAA ATs using a multipolar catheter or three-dimensional electroanatomical mapping system.
Arrhythmia definition
AF was defined as persistent (sustained beyond 7 days or lasting less than 7 days but necessitating cardioversion) or long-lasting persistent (continuous AF of greater than 1 year duration) according to the Heart Rhythm Society/European Heart Rhythm Association/European Cardiac Arrhythmia Society 2007 Consensus Statement on Catheter and Surgical Ablation of AF.16
In accordance with the consensus statement from the Working Group of Arrhythmias of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, AT was defined as a rapid regular atrial rhythm with stable CL, surface ECG morphology, and endocardial activation pattern.17
Macroreentry was diagnosed and confirmed using the following criteria: (1) sequential activation mapping (with eight or more evenly distributed points in each atrium) demonstrating >50% of the tachycardia CL; and (2) post-pacing interval not longer than the tachycardia CL by ≥30 ms at two separate atrial sites. Typical macroreentrant circuits involved the mitral or the tricuspid annulus or the ipsilateral veins (indicating roof-dependent macroreentry).18
Focal AT was defined as AT with a discrete source of origin of activation spreading centrifugally to the rest of the atrium.
Electrophysiology study
Electrophysiological study was performed in the postabsorptive conscious state with minimal sedation. All antiarrhythmic drugs were discontinued for at least 5 half-lives before ablation, with the exception of amiodarone (n = 5). Oral anticoagulation was administered (target international normalized ratio 2–3) for at least 1 month before the procedure, and transesophageal echocardiography was performed within 5 days of the procedure to exclude atrial thrombus.
Surface electrocardiogram and bipolar endocardial electrograms were continuously monitored and stored on a computer-based digital amplifier/recorder system (Bard Electro-physiology, Lowell, MA). Intracardiac electrograms were filtered from 30 –500 Hz. After transseptal puncture, a single intravenous bolus of 50 IU/kg of heparin was administered. Heparin was repeated if the procedural duration exceeded 4 hours.
Conventional mapping
A steerable quadripolar/decapolar catheter (Xtrem; Ela Medical, Le Plessis Robinson, France) was placed in the coronary sinus, and a roving 3.5-mm externally irrigated tip ablation catheter (Thermocool, Biosense Webster, Diamond Bar, CA) was used for conventional mapping.
High-density mapping catheter and electroanatomical mapping
Mapping was performed using a high-density mapping catheter (20 pole PentaRay catheter, Biosense Webster) as described elsewhere.19 The atria were mapped to determine the location and mechanism of the AT.
Once the source of the AT had been determined using conventional mapping techniques, the high-density mapping catheter was applied to the endocardial surface around the source to identify the earliest endocardial activity relative to the surface tachycardia P wave and/or to a reference atrial electrogram on one of the coronary sinus electrodes. In patients in whom >70% of the tachycardia CL could be identified on the bipoles of PentaRay, entrainment maneuvers were used to confirm the reentrant mechanism. Finally, we evaluated the amplitude of electrograms during AT and in sinus rhythm at these sites.
Delineation of the LAA
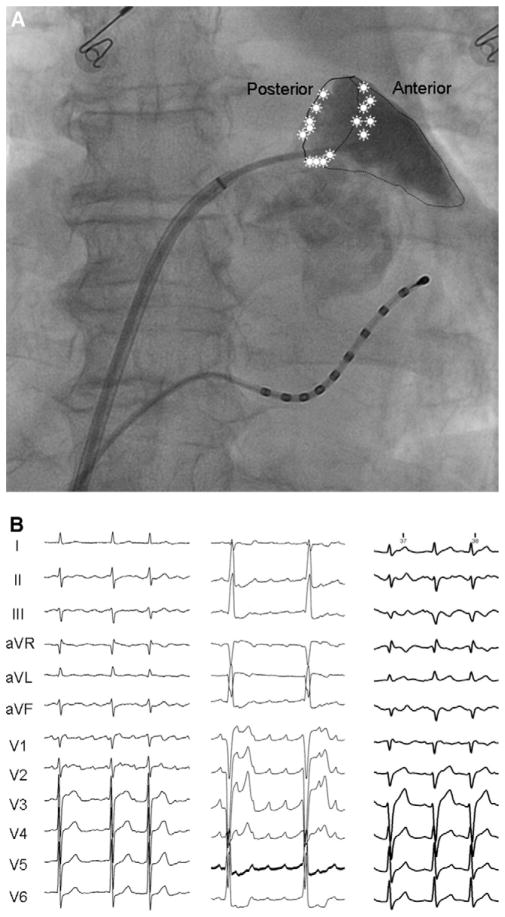
In patients with AT originating from the LAA, we anatomically delineated LAA by manually performing selective LAA contrast atriography using a 6-Fr National Institutes of Health catheter (Biosense Webster) through a long sheath. For precisely defining the location of LAA AT, the LAA was divided into three segments: posterior ridge, anterior-superior segment, and the inferior segment (Figure 1A). After restoration of sinus rhythm, the P-ALAA interval (on-set of P wave in lead II to atrial potential in the LAA) was measured to evaluate the effect of ablation on the conduction of sinus impulse to the LAA.
Figure 1.
A: Location of LAA AT. In this posteroanterior fluroscopic view of the LA with contrast opacified LAA, the location of each AT is marked with an asterisk on the segment of LAA involved. All the ATs are located at the base of the LAA: four at the inferior segment, six at the posterior ridge, and five at the anterior-superior segment. B: P-wave morphology of LAA AT. Typical P-wave morphology of LAA AT from three patients, showing positive polarity in lead V1 and an inferior axis in the frontal leads.
Radiofrequency (RF) ablation
During ablation, the LAA was targeted as a tachycardia source. If >70% of the tachycardia CL could be mapped in the LAA with the high-density mapping catheter, the site with the longest fractionated or mid-diastolic signal was targeted. Ablation was considered effective if tachycardia terminated or transformed into another AT. Transition to a different tachycardia was defined on the basis of any observed change in the P-wave morphology and/or the endocardial atrial activation sequence.
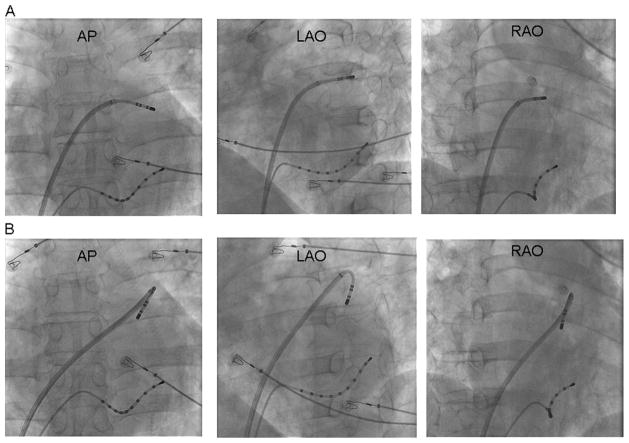
Different catheter positions facilitating ablation at the base of the LAA are illustrated in Figure 2. Posterior ridge ablation was typically commenced along the collar of the LAA either (1) by entering into the LAA along its long axis and pulling the catheter back with a clockwise rotation (Figure 2A) or (2) by looping the catheter inside the LAA and rotating both the sheath and the catheter posteriorly (perpendicular; Figure 2B). The anterior-superior and inferior aspects of the base of the LAA were targeted by dragging the catheter-sheath assembly back to the base of the LAA from inside while maintaining a constant counterclockwise torque.
Figure 2.
Ablation of LAA AT. Fluroscopic views of the ablation catheter and transseptal sheath to reach the posterior ridge are demonstrated. Ablation was typically commenced along the collar of the LAA either by (1) entering directly into the LAA along its long axis, turning the catheter with a clockwise rotation, and pulling back the catheter to the base (A); or (2) rotating both the sheath and the catheter posteriorly (perpendicular; B). PA: posteroanterior; LAO: left anterior oblique; RAO: right anterior oblique.
Ablation parameters
RF energy was delivered with power titrated to 30 W by adjusting the 0.9% normal saline flow rate to 17– 60 mL/min (Cool Flow pump; Biosense-Webster) to limit the tip temperature to ≤ 50°C. The energy delivery was limited to 28 W when the catheter tip was perpendicular to the ablation site to minimize the risk of perforation. If there was dramatic drop in impedance, we immediately withheld the application of RF energy for safety reasons.
The endpoint of ablation was termination of AT and restoration of sinus rhythm or transformation to another AT. The stability of the catheter was monitored during each RF application by assessing the beat-to-beat electrogram on the proximal bipole of the ablation catheter and its position on intermittent fluoroscopy and three-dimensional electroanatomical mapping system (whenever available) to exclude inadvertent catheter displacement into the left pulmonary veins. The duration of each RF application was noted.
Follow-up
Patients were hospitalized for 24 hours at 3, 6, and 12 months after the last procedure for transthoracic echocardiography, ambulatory monitoring, and stress test. Postprocedural anticoagulation was continued for 6 –9 months in the absence of arrhythmia recurrence or for a longer period otherwise.
Statistical analysis
Continuous variables are reported as mean ± standard deviation. Comparison between groups was performed with the Student’s t-test or the Wilcoxon rank sum test (nonparametric data). Categorical variables are reported as number and percentage and were compared using the Fisher’s exact test. P<.05 was considered statistically significant.
Results
The baseline characteristics of the study population and 15 patients with AT originating from the LAA are presented in Table 1. The AF CL determined in the LAA at baseline did not differ significantly between the LAA AT group and the study population (P = .112). Among 74 patients, AF got converted directly into sinus rhythm in 14% patients and into AT in 70%. Electrical cardioversion was required to terminate AF in the remaining 16% patients. AF terminated to AT in 15/15 patients in whom LAA AT was found. LAA AT occurred 9 ± 7 months after PsAF ablation in 11 patients. It was observed during the index procedure in the remaining four. Six patients experienced incessant, drug-refractory AT for 6 ± 5 months.
Table 1.
Baseline characteristics
| Characteristic | LAA AT cases (n = 15) | All AF cases (n = 74) |
|---|---|---|
| Age | 57 ± 6 | 55 ± 13 |
| Male | 13 | 61 |
| AF duration, months | 17 ± 15 | 20 ± 18 |
| LV ejection fraction, % | 61 ± 17 | 58 ± 16 |
| Structural heart disease, % | 40 | 21 |
| Failed antiarrhythmic drugs | 3 ± 1 | 2 ± 1 |
| LA parasternal size, mm | 57 ± 14 | 48 ± 8 |
Localization of AT
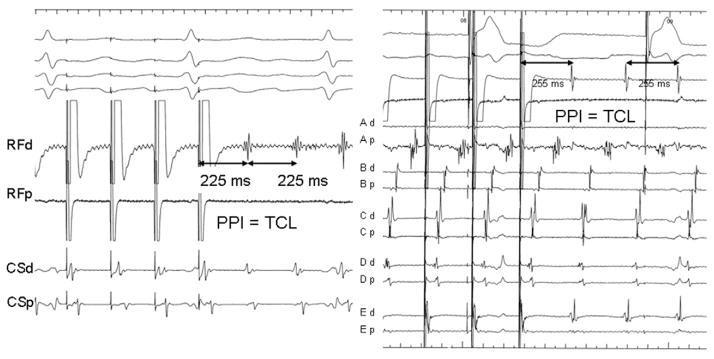
High-density mapping catheter was used in 10 patients. The CL of LAA AT was 283 ± 30 ms. Figure 1B shows the morphology of three representative P waves during LAA AT. In 12 patients, P wave was positive in all precordial leads, and in the remaining three, it was positive through V1–V3 and isoelectric in V4 –V6. In leads II, III, and aVF, P wave was positive in all but one patient. In lead I, P wave was positive in six patients and isoelectric or negative in others. LAA AT was confirmed in all cases with earliest activation at the LAA base followed by centrifugal impulse propagation toward the atria (Figure 3).
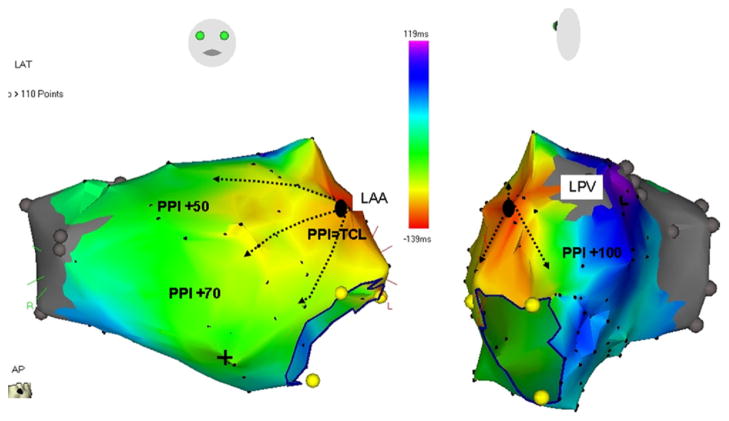
Figure 3.
Electroanatomical isochronal maps demonstrating centrifugal activation of the LA from the LAA AT. The numbers against PPI show the difference between PPI and TCL at the local site of entrainment mapping. PPI: postpacing interval; TCL: tachycardia CL; LPV: left superior pulmonary vein; LAT: local activation time.
LAA ATs originated from three distinct sites at the LAA base (Figure 1A): six from the posterior ridge, five from the anterior-superior aspect, and four from the inferior pole of the LAA. Location of AT source on the posterior ridge could be easily distinguished from the pulmonary venous source as prior mapping demonstrated no potentials inside the veins. In six patients, the origin of the AT was in close proximity to previous ablation sites. This was evidenced by marked conduction abnormality with low-amplitude, fractionated/double potentials in sinus rhythm (Figure 4). The mean amplitude and duration of electrograms at the site of successful ablation were 0.07 ± 0.04 mV and 140 ± 44 ms, respectively.
Figure 4.
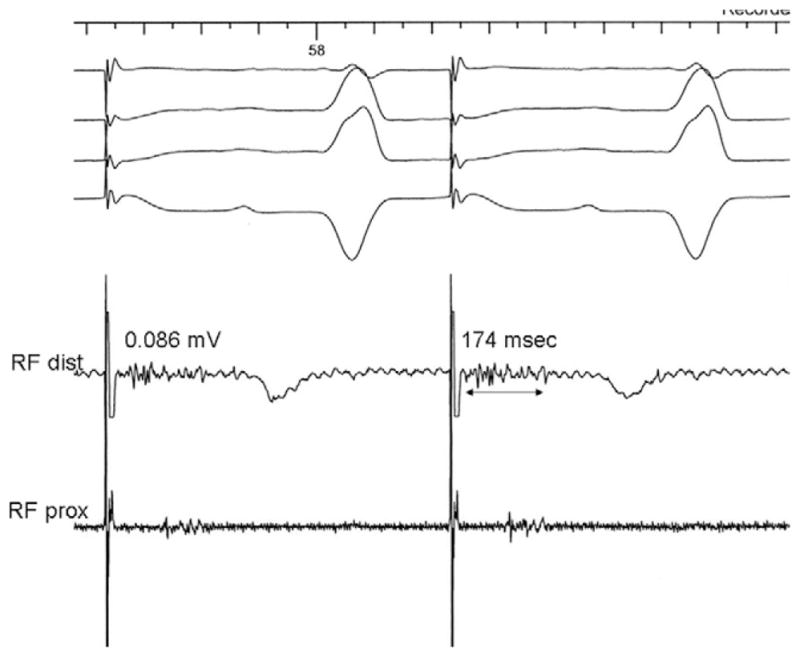
At the site of successful ablation of AT, marked conduction abnormality with low-amplitude, fractionated, and multicomponent electrograms was recorded during sinus rhythm (pacing).
Since there was no systematic record of previous ablation sites, the definite incidence of LAA AT arising owing to previous ablation could not be derived. Although there is some uncertainty about the numbers, previous ablation in the region of LAA could be responsible for LAA AT in about 75% cases. It occurred primarily owing to underlying substrate in 25% of cases.
Mechanism of AT
High-density mapping at the site of origin of LAA AT demonstrated 253 ± 42 ms of activation out of 283 ± 30 ms (89% ± 8%) of the tachycardia CL within the mapping field of the high-density mapping catheter (Figure 5). This suggested reentry that was localized to the mapping sites at the LAA base. In nine ATs, entrainment maneuvers could be performed within this region, demonstrating a postpacing interval <20 ms longer than the tachycardia CL (Figure 6), while the postpacing interval from the septal, posterior, and anterior LA was >40 ms longer than the tachycardia CL (Figure 3). In the remaining six ATs, while the entire CL of tachycardia was localized to the field of mapping, entrainment mapping could not be performed owing to failure of local capture at maximal output (10 V) in the area with very low signal amplitude (0.04 – 0.07 mV).
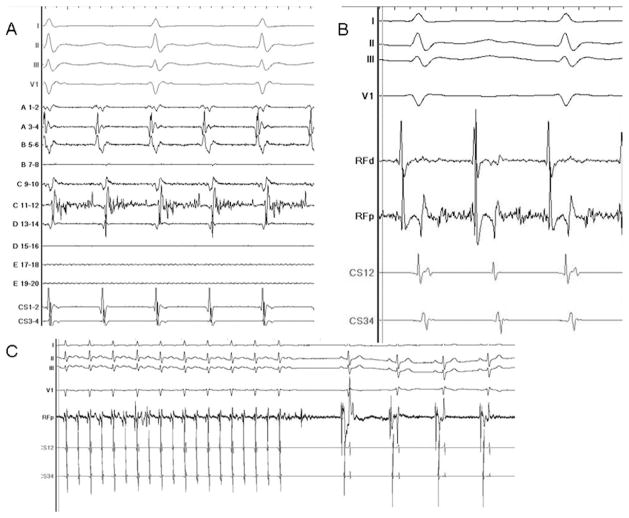
Figure 5.
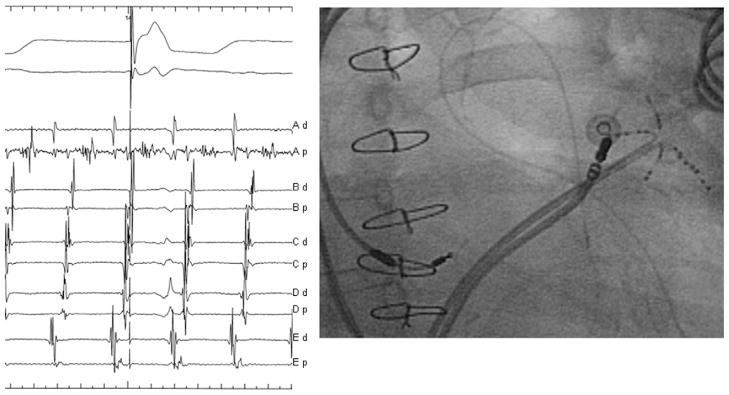
Mapping and ablation of localized reentry from the LAA. A: A long fractionated signal can be seen on bipole C11-12, representing >70% of the tachycardia CL. The ablation catheter was then moved to this area (B), and ablation terminated the tachycardia within few seconds (C).
Figure 6.
Two examples of entrainment from the LAA. Tachycardia entrainment from the LAA site demonstrates that the postpacing interval (PPI) = tachycardia CL (TCL), with the same activation sequence during pacing and tachycardia.
RF ablation
Ablation of LAA ATs was performed at the site of long fractionated signal or mid-diastolic electrograms (Figure 7). Tachycardia terminated within 30 seconds of RF application in all patients. ATs were successfully ablated using 2 ± 2 minutes of RF energy delivery, 46 ± 11minutes of fluoroscopic time, and 175 ± 45 minutes of procedural duration. After ablation, conduction between the LAA and the LA in sinus rhythm was preserved in all but one patient as demonstrated by the proximal-to-distal activation sequence on the ablation catheter positioned into the LAA.
Figure 7.
Left-hand panel intracardiac tracings on the PentaRay catheter show >70% of tachycardia CL recorded on bipole Ap. Right-hand panel: fluoroscopic image demonstrating the relative positions of the PentaRay catheter and the ablation catheter in the LAA. The roving ablation catheter is mapping the localized reentrant circuit to find the critical isthmus (long fractionated signal) located at the bipole Ap for ablation.
After successful ablation, LAA AT was not inducible in any of the 11/15 patients in whom it was encountered during the repeat AF ablation procedure. Induction was not attempted in the remaining four patients in whom LAA AT was encountered and successfully ablated during the index procedure.
LAA structure and function
Selective LAA contrast atriography did not demonstrate any structural anomaly including aneurysm. All patients underwent repeat echocardiography up to 1 year after the procedure for the assessment of LA/LAA function. During sinus rhythm, LAA contractile function was preserved in all but one patient as demonstrated by contrast ejection intraprocedurally and Doppler echocardiography (mitral A wave 45 ± 11 cm/s) 3 months postprocedurally. Life-long anticoagulation (currently, Previscan, vitamin K antagonist) was prescribed to this patient. Anticoagulation therapy was based on the CHADS2 score in the others.
Clinical follow-up
After medium-term follow-up of 18 ± 7 months, 13 out of 15 patients remained in sinus rhythm without antiarrhythmic drugs. One patient had recurrent AT around the right pulmonary venous ostia that was successfully ablated with roof linear ablation. Another one had focal right pulmonary venous AT. There were no periprocedural or delayed post-procedural (at follow-up visit) complications including thromboembolism in any patient.
Discussion
The role of LAA in organized atrial arrhythmias arising in the context of AF ablation has been systematically studied in the present report, thereby providing added information on the arrhythmogenic role of this structure. First, the study describes a cohort of patients with localized reentrant tachycardia harbored within the LAA post PsAF ablation. Second, it shows that catheter ablation targeting different segments of the LAA for termination of AT is feasible and safe. Third, the occurrence of AT during the index procedure and also during the follow-up suggests that the LAA may directly contribute to the maintenance of AF. Finally, these data provide insights into the potential mechanism of arrhythmogenesis from this structure.
LAA as a source of AT
Previous studies have reported ATs originating from the LAA.4 –10,20 In general, these have been de novo ATs unrelated to AF/ablation and have been mapped to a single site, as one would expect with a focal AT. However, these studies did not perform high-density mapping of the arrhythmia.
In the present study, the use of a multispline mapping catheter allowed mapping of both the origin of the tachycardia and the direction of wave front propagation. This strategy revealed novel insights into the electrophysiological mechanism of seemingly focal AT. Although the atrium was activated in a centrifugal manner from the LAA, which is one of the criteria for focal AT, the entire CL of the tachycardia was identified within the localized mapping field of the multispline catheter. Together with entrainment maneuvers, this suggests a localized, small (<2 cm) reentry as the underlying mechanism.
Localized reentry has been described for ATs with failed previous ablation or prior AF ablation using the PentaRay catheter to identify the entire tachycardia CL and undertake detailed entrainment mapping.19 In that study, all but one patient with localized reentry had undergone AF ablation previously. In the patient without prior AF ablation, localized reentrant AT originated from the area of scarring for unknown reasons.
The occurrence of localized reentry as a mechanism of LAA AT in patients with AF ablation raises the question of whether this observation is part of the primary disease process or secondary to ablation. Interestingly, in a small series of patients we have reported localized reentry at the anterior wall of LA in the area that was not ablated previously, supporting the notion that prior ablation is not a prerequisite for localized reentry.21
LAA and AF
In patients with paroxysmal AF undergoing pulmonary vein isolation, we have previously reported that a gradual increase in the AF CL often culminates in termination of AF.21,22 In patients with persistent or long-lasting AF, who often require more widespread ablation of thoracic veins and LA, prolongation of AF CL during ablation also presages AF termination.3,23 The increase in AF CL during ablation of the LAA was 8.87 ± 9.22 ms in 61% of patients with persistent/permanent AF in one of the previous reports.23 Notably, when ablation sites were randomized in sequence, the LAA emerged as an important region where AF CL not only slowed but AF terminated in many patients.3,23 Recently, Di Biase et al1 studied 987 patients undergoing redo catheter ablation for paroxysmal and PsAF. Two hundred sixty-six patients (27%) showed firing from the LAA, and in 86 patients (8.7%), the LAA was found to be the only source of arrhythmia without any evidence of pulmonary venous or extra pulmonary sources. In concurrence with these observations, intentional LAA isolation has been reported to improve the clinical outcomes in index and repeat ablation of long-lasting PsAF ablation.1,24
The present study adds further weight to the arrhythmogenic role of LAA, especially focusing on organized atrial arrhythmias associated with AF ablation. We observed LAA AT during the index procedure and on follow-up, potentially implicating arrhythmias at this location in the arrhythmogenic process leading to AF.
Anatomic characteristics of the LAA
The LAA is a structure anatomically appended to the main body of the LA with a wide perimeter that interfaces with the atrial musculature. The anisotropic junctional tissue with complex fiber orientation results in electrophysiological properties that may predispose this region to support anatomical reentry or anchor functional rotors. In this series, the posterior ridge of the LAA was the predominant site of origin of AT. The topography of the trabeculated LA is complex and dominated by extensive pectinate muscles.25,26 Heterogeneous fiber orientation and/or the presence of trabeculated muscle influence wave propagation and favor the formation of conduction block/slow conduction and initiation of reentry.27
Ablation at the LAA
Mapping and ablating in the trabeculated LAA can be challenging, as the presence of the pectinate muscles can impair catheter maneuverability and low blood flow in some segments of cul-de-sac can prevent adequate power delivery. In this series, an irrigated-tip catheter was used for ablation in all patients to enable desired power delivery and reduce the risk of thrombosis. AT terminated within seconds of RF delivery in all cases, and further consolidating applications were delivered to minimize the risk of recurrent arrhythmia.
Electrical isolation of the LAA may occur inadvertently during ablation of PsAF and AT even when the ablation site is remote from the LAA. This is due to disruption of the Bachmann bundle and its leftward extension, which courses along the anterior LA and bifurcates to surround the LAA.28,29 However, in the present study, ablation was not performed circumferentially and was targeted to the segment of interest only in an attempt to avoid severely delayed LAA activation or complete isolation. LAA is a contractile structure, and if it is activated with a substantial delay, sometimes simultaneously with or after the QRS, it could adversely impact LA function. LAA isolation leads to complete cessation of contraction in this contractile structure, putting the patients at potentially high risk of thromboembolism. Such an outcome would necessitate life-long anticoagulation or permanent exclusion of isolated LAA from the atrial circulation.30
Concerns have been raised about LAA perforation in patients undergoing ablation inside relatively thin-walled LAA.28 Importantly, despite performing ablation within the LAA, none of the patients in the current cohort had thromboembolic events or LAA perforation either acutely or during follow-up.
Clinical implications
The LAA can support organized atrial arrhythmias arising from localized reentry in patients with AF. Focal ablation can be safely performed in this region without the necessity for circumferential ablation or complete organelle isolation to achieve high immediate termination and medium-term freedom from AT. However, caution should be taken to limit the energy delivery to 30 W using an irrigated-tip catheter to avoid clot formation and perforation. Inability to recognize LAA as a critical source of AT in the context of AF ablation can lead to failure of arrhythmia elimination.
Conclusion
The LAA is an unreported source of localized reentrant AT. LAA AT occurs in 11% patients undergoing catheter ablation of PsAF. Focal ablation can be safely undertaken with a high degree of acute success and medium-term freedom from arrhythmia recurrence.
ABBREVIATIONS
- AF
atrial fibrillation
- AT
atrial tachycardia
- CL
cycle length
- LA
left atrium/atrial
- LAA
left atrial appendage
- PsAF
persistent atrial fibrillation
- RF
radiofrequency
- LV
left ventricular
References
- 1.Di Biase L, Burkhardt JD, Mohanty P, et al. Left atrial appendage: an under-recognized trigger site of atrial fibrillation. Circulation. 2010;122:109–118. doi: 10.1161/CIRCULATIONAHA.109.928903. [DOI] [PubMed] [Google Scholar]
- 2.Haïssaguerre M, Hocini M, Sanders P, et al. Localized sources maintaining atrial fibrillation organized by prior ablation. Circulation. 2006;113:616– 625. doi: 10.1161/CIRCULATIONAHA.105.546648. [DOI] [PubMed] [Google Scholar]
- 3.Haïssaguerre M, Sanders P, Hocini M, et al. Catheter ablation of long-lasting persistent atrial fibrillation: critical structures for termination. J Cardiovasc Electrophysiol. 2005;16:1125–1137. doi: 10.1111/j.1540-8167.2005.00307.x. [DOI] [PubMed] [Google Scholar]
- 4.Kato M, Adachi M, Yano A, et al. Radiofrequency catheter ablation for atrial tachycardia originating from the left atrial appendage. J Interv Card Electrophysiol. 2007;19:45– 48. doi: 10.1007/s10840-007-9131-z. [DOI] [PubMed] [Google Scholar]
- 5.Kistler PM, Roberts-Thomson KC, Haqqani HM, et al. P-wave morphology in focal atrial tachycardia: development of an algorithm to predict the anatomic site of origin. J Am Coll Cardiol. 2006;48:1010–1017. doi: 10.1016/j.jacc.2006.03.058. [DOI] [PubMed] [Google Scholar]
- 6.Wang YL, Li XB, Quan X, et al. Focal atrial tachycardia originating from the left atrial appendage: electrocardiographic and electrophysiologic characterization and long-term outcomes of radiofrequency ablation. J Cardiovasc Electrophysiol. 2007;18:459– 464. doi: 10.1111/j.1540-8167.2007.00808.x. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi Y, Sanders P, Rotter M, Haïssaguerre M. Disconnection of the left atrial appendage for elimination of foci maintaining atrial fibrillation. J Cardiovasc Electrophysiol. 2005;16:917–919. doi: 10.1046/j.1540-8167.2005.40804.x. [DOI] [PubMed] [Google Scholar]
- 8.Yamada T, McElderry HT, Allison JS, Kay GN. Focal atrial tachycardia originating from the epicardial left atrial appendage. Heart Rhythm. 2008;5:766–767. doi: 10.1016/j.hrthm.2007.12.025. [DOI] [PubMed] [Google Scholar]
- 9.Yamada T, Murakami Y, Yoshida Y, et al. Electrophysiologic and electrocardiographic characteristics and radiofrequency catheter ablation of focal atrial tachycardia originating from the left atrial appendage. Heart Rhythm. 2007;4:1284–1291. doi: 10.1016/j.hrthm.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Yamada Y, Ajiro Y, Shoda M, et al. Video-assisted thoracoscopy to treat atrial tachycardia arising from left atrial appendage. J Cardiovasc Electrophysiol. 2006;17:895– 898. doi: 10.1111/j.1540-8167.2006.00484.x. [DOI] [PubMed] [Google Scholar]
- 11.Miyazaki S, Nault I, Jaïs P, Haïssaguerre M. Atrial tachycardia confined within the left atrial appendage. J Cardiovasc Electrophysiol. 2010;21:933–935. doi: 10.1111/j.1540-8167.2009.01715.x. [DOI] [PubMed] [Google Scholar]
- 12.O’Neill MD, Jaïs P, Takahashi Y, et al. The stepwise ablation approach for chronic atrial fibrillation— evidence for a cumulative effect. J Interv Card Electrophysiol. 2006;16:153–167. doi: 10.1007/s10840-006-9045-1. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi Y, O’Neill MD, Hocini M, et al. Effects of stepwise ablation of chronic atrial fibrillation on atrial electrical and mechanical properties. J Am Coll Cardiol. 2007;49:1306–1314. doi: 10.1016/j.jacc.2006.11.033. [DOI] [PubMed] [Google Scholar]
- 14.Jaïs P, Hocini M, Hsu LF, et al. Technique and results of linear ablation at the mitral isthmus. Circulation. 2004;110:2996–3002. doi: 10.1161/01.CIR.0000146917.75041.58. [DOI] [PubMed] [Google Scholar]
- 15.Hocini M, Jaïs P, Sanders P, et al. Techniques, evaluation, and consequences of linear block at the left atrial roof in paroxysmal atrial fibrillation: a prospective randomized study. Circulation. 2005;112:3688–3696. doi: 10.1161/CIRCULATIONAHA.105.541052. [DOI] [PubMed] [Google Scholar]
- 16.Calkins H, Brugada J, Packer DL, et al. Heart Rhythm Society; European Heart Rhythm Association; European Cardiac Arrhythmia Society; American College of Cardiology; American Heart Association; Society of Thoracic Surgeons. HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation developed in partnership with the European Heart Rhythm Association (EHRA) and the European Cardiac Arrhythmia Society (ECAS); in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), and the Society of Thoracic Surgeons (STS). Endorsed and approved by the governing bodies of the American College of Cardiology, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, and the Heart Rhythm Society. Europace. 2007;9:335–379. doi: 10.1093/europace/eum120. [DOI] [PubMed] [Google Scholar]
- 17.Saoudi N, Cosio F, Waldo A, et al. Classification of atrial flutter and regular atrial tachycardia according to electrophysiologic mechanism and anatomic bases: a statement from a joint expert group from the Working Group of Arrhythmias of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. J Cardiovasc Electrophysiol. 2001;12:852– 866. doi: 10.1046/j.1540-8167.2001.00852.x. [DOI] [PubMed] [Google Scholar]
- 18.Jaïs P, Shah DC, Haïssaguerre M, et al. Mapping and ablation of left atrial flutters. Circulation. 2000;101:2928–2934. doi: 10.1161/01.cir.101.25.2928. [DOI] [PubMed] [Google Scholar]
- 19.Sanders P, Hocini M, Jaïs P, et al. Characterization of focal atrial tachycardia using high-density mapping. J Am Coll Cardiol. 2005;46:2088–2099. doi: 10.1016/j.jacc.2005.08.044. [DOI] [PubMed] [Google Scholar]
- 20.Wagshal AB, Applebaum A, Crystal P, et al. Atrial tachycardia as the presenting sign of a left atrial appendage aneurysm. Pacing Clin Electrophysiol. 2000;23:283–285. doi: 10.1111/j.1540-8159.2000.tb00815.x. [DOI] [PubMed] [Google Scholar]
- 21.Jaïs P, Sanders P, Hsu LF, et al. Flutter localized to the anterior left atrium after catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2006;17:279–285. doi: 10.1111/j.1540-8167.2005.00292.x. [DOI] [PubMed] [Google Scholar]
- 22.Haïssaguerre M, Sanders P, Hocini M, et al. Changes in atrial fibrillation cycle length and inducibility during catheter ablation and their relation to outcome. Circulation. 2004;109:3007–3013. doi: 10.1161/01.CIR.0000130645.95357.97. [DOI] [PubMed] [Google Scholar]
- 23.Haïssaguerre M, Hocini M, Sanders P, et al. Catheter ablation of long-lasting persistent atrial fibrillation: clinical outcome and mechanisms of subsequent arrhythmias. J Cardiovasc Electrophysiol. 2005;16:1138–1147. doi: 10.1111/j.1540-8167.2005.00308.x. [DOI] [PubMed] [Google Scholar]
- 24.Tilz RR, Chun KR, Schmidt B, et al. Catheter ablation of long-standing persistent atrial fibrillation: a lesson from circumferential pulmonary vein isolation. J Cardiovasc Electrophysiol. 2010;21:1085–1093. doi: 10.1111/j.1540-8167.2010.01799.x. [DOI] [PubMed] [Google Scholar]
- 25.Cabrera JA, Ho SY, Climent V, Sánchez-Quintana D. The architecture of the left lateral atrial wall: a particular anatomic region with implications for ablation of atrial fibrillation. Eur Heart J. 2008;29:356–362. doi: 10.1093/eurheartj/ehm606. [DOI] [PubMed] [Google Scholar]
- 26.Ho SY, Sanchez-Quintana D, Cabrera JA, Anderson RH. Anatomy of the left atrium: implications for radiofrequency ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 1999;10:1525–1533. doi: 10.1111/j.1540-8167.1999.tb00211.x. [DOI] [PubMed] [Google Scholar]
- 27.Berenfeld O, Zaitsev AV, Mironov SF, Pertsov AM, Jalife J. Frequency-dependent breakdown of wave propagation into fibrillatory conduction across the pectinate muscle network in the isolated sheep right atrium. Circ Res. 2002;90:1173–1180. doi: 10.1161/01.res.0000022854.95998.5c. [DOI] [PubMed] [Google Scholar]
- 28.Kuhne M, Ho SY, Morady F, Chugh A. Elimination of left atrial appendage potentials during radiofrequency ablation near the right superior pulmonary vein. Heart Rhythm. 2008;5:475– 478. doi: 10.1016/j.hrthm.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 29.Chan CP, Wong WS, Pumprueg S, et al. Inadvertent electrical isolation of the left atrial appendage during catheter ablation of persistent atrial fibrillation. Heart Rhythm. 2010;7:173–180. doi: 10.1016/j.hrthm.2009.10.036. [DOI] [PubMed] [Google Scholar]
- 30.Tilz R, Schmidt B, Menon S, et al. Left atrial appendage function and clinical outcome after electrical isolation of left atrial appendage in patients undergoing atrial fibrillation ablation. Circulation. 2008;118:S694–S695. [Google Scholar]