Abstract
Conventional models of G-protein coupled receptor (GPCR) signaling describe cell surface receptors binding to external ligands, such as hormones or circulating peptides, to induce intracellular signaling and a physiologic response. However, recent studies identify new paradigms indicating that GPCRs localize to and signal at the nucleus and that GPCRs oligomers can influence receptor function. Previously, we reported that endogenous α1-adrenergic receptors (α1-ARs) localize to and signal at the nuclei in adult cardiac myocytes. In this study, we examined the mechanisms behind α1-AR nuclear localization and how nuclear localization impacted receptor function. We verified that endogenous α1-ARs localized to the nuclear membrane of intact nuclei isolated from wild-type adult cardiac myocytes. Next, we identified and disrupted putative nuclear localization sequences in both the α1A- and α1B-adrenergic receptors, which led to mis-localization of α1-ARs in cultured adult cardiac myocytes. Using these mutants, we demonstrated that nuclear localization was required for α1-signaling in adult cardiac myocytes. We also found that the nuclear export inhibitor leptomycin B inhibited α1-AR signaling, indicating α1-AR signaling must arise in the nucleus in adult cardiac myocytes. Finally, we found that co-localization of the α1-subtypes at the nuclei in adult cardiac myocytes facilitated the formation of receptor oligomers that could affect receptor signaling. In summary, our data indicate that α1-AR nuclear localization can drive the formation of receptor oligomers and regulate signaling in adult cardiac myocytes.
Keywords: α1-adrenergic receptor, G protein-coupled receptor, cardiac myocyte, oligomer, ERK
1. INTRODUCTION
Conventional models of G-protein coupled receptor (GPCR) signaling describe receptor activation at the plasma membrane leading to initiation of downstream signaling within the cell commonly referred to as “outside-in” signaling. However, it has recently become clear that a number of GPCRs localize to and signal at the nuclear membrane in a variety of cell types including neurons, hepatocytes, and cardiac myocytes (reviews [1–3]). Endothelin receptors, angiotensin receptors, and β-adrenergic receptors (β-ARs) were all recently reported to localize to the nuclei in adult cardiac myocytes (cardiac myocytes in most species have two nuclei) [4–7]. However, each of these receptors also localizes to the plasma membrane, therefore the functional significance of localization to the nuclei in adult cardiac myocytes remains unclear. Yet, studies using nuclei isolated from adult cardiac myocytes indicated that endothelin receptors induced calcium transients [4], and β-ARs and angiotensin receptors increased gene transcription, suggesting a potential physiologic significance for nuclear GPCR signaling [6, 7].
Targeting of proteins to the nucleus involves nuclear localization sequences embedded in the protein, which consist of mono- or bi-partite basic residues, usually lysines and arginines, or glycine-arginine repeats [8–10]. A family of proteins called importins binds to nuclear localization sequences and facilitates localization to the nucleus. This importin-mediated nuclear localization occurs for proteins that target to the nucleoplasm as well as the inner nuclear membrane [11–13]. This method of nuclear localization was previously described for the type 1 parathyroid hormone receptor [14, 15] and more recently for the gonadotropin-releasing hormone type 1 receptor [16].
Another newly described function of GPCRs is the ability to form oligomers, and receptor hetero-oligomers have been proposed to affect receptor ligand binding, expression, internalization, and signaling. To date, several GPCRs have been shown to form homo- and hetero-oligomers that alter receptor function, including opioid receptors, dopamine receptors, adenosine receptors, angiotensin receptors, and β-ARs (reviews [17–20]). In HEK293 cells, α1-adrenergic receptors (α1-ARs) formed homo- and hetero-oligomers, demonstrating the capacity for α1-ARs to form oligomers [21–24]. Moreover, α1A- and α1B-subtype hetero-oligomers altered receptor internalization [23], and α1B- and α1D-subtype hetero-oligomers were required for cell surface expression of the α1D-subtype in HEK293 cells [22]. In cardiac myocytes, β1- and β2-ARs formed hetero-oligomers that altered β-AR mediated contractile function, suggesting the possibility that adrenergic receptor oligomers could affect cardiac myocyte function [25].
In general, the lack of validated α1-AR subtype-specific antibodies [26] has hindered studies attempting to define α1-AR subcellular localization. However, we overcame this obstacle by using radio-ligand binding assays on fractionated cardiac myocytes and a fluorescent α1-AR ligand to label receptors in cultured cardiac myocytes, and we localized endogenous α1-ARs to the nuclei in adult cardiac myocytes [27]. Further, we demonstrated that the downstream α1-AR signaling partners Gαq and phospholipase Cβ1 co-localized with α1-ARs only at the nuclei in adult cardiac myocytes [27]. We also developed a reconstitution system in α1A- and α1B-AR double knockout (α1ABKO) cardiac myocytes, which lack endogenous α1-ARs, using α1A- and α1B-GFP fusion proteins that recapitulated the localization of the two endogenous α1-subtypes at the nuclei [27]. Finally, we found that organic cation transperter-3 (OCT3) facilitated rapid uptake of catecholamines into adult cardiac myocytes and that this uptake was required for α1-AR signaling [27]. In short, our data indicated that endogenous α1-ARs localize to and initiate signaling at the nucleus in adult cardiac myocytes.
In this report, we examined α1-AR expression in nuclei isolated from adult cardiac myocytes and validated that endogenous α1-ARs localized to the nuclei in adult cardiac myocytes and that our α1-GFP constructs reproduced this localization. We identified putative nuclear localization sequences in both the α1A- and α1B-subtypes and demonstrated that α1-AR nuclear localization was required for signaling. Furthermore, we demonstrated that co-localization of the α1A- and α1B-subtypes at the nuclei in adult cardiac myocytes facilitated the formation of receptor oligomers that could regulate receptor signaling in adult cardiac myocytes. Together, our data demonstrate that α1-AR nuclear localization can drive the formation of receptor oligomers and regulate signaling in adult cardiac myocytes.
2. MATERIAL AND METHODS
2.1 Mice
The generation of α1A- (α1AKO) and α1B- (α1BKO) AR single knockout, as well as α1A-/α1B- (α1ABKO) AR double knockout mice, was previously described [28]. 12th-15th generation congenic C57BL/6J mice between 10–15 weeks of age were used in all experiments. The use of all animals in this study conformed to the PHS Guide for Care and Use of Laboratory Animals and was approved by Sanford Research/USD Institutional Animal Care and Use Committee.
2.2 Reagents
All reagents were made using chemicals purchased from Sigma-Aldrich (MO, USA) unless otherwise noted.
2.3 Culture of Adult Mouse Cardiac Myocytes
The procedures for the isolation and culture of adult mouse cardiac myocytes were previously described [29, 30].
2.4 Adenoviral Constructs
The generation of adenoviral constructs of the wild-type α1A- and α1B-ARs (α1A-WT, α1B-WT) with and without GFP have been previously described [31]. The following additional adenoviral constructs were made using the AdEasy System (Agilent, CA, USA). Adenoviral constructs of α1A-WT and α1B-WT tagged with CFP were made similarly to the GFP constructs. To generate nuclear localization sequence mutants in α1-ARs (α1-NLSmut), we used the α1-GFP constructs as templates. DNA mini-genes for mutating the putative nuclear localization sequences (Integrated DNA Technologies, IA, USA), flanked by unique FspAI and AscI restriction sites, were synthesized. For the α1A-subtype, arginines (R) or lysines (K) were replaced by alanine (A) at positions 334, 335, 342, 348, and 349 corresponding to the human sequence. Similarly, the α1B-NLSmut contains R/A exchanges at positions 368, 370, and 372 through 380 corresponding to the human sequence. All constructs were sequence verified.
2.5 Biochemical Fractionation of Adult Mouse Cardiac Myocytes
The procedures for biochemical fractionation of freshly isolated or cultured cardiac myocytes were described elsewhere [27]. The purity of each fraction was verified by Western blot using primary antibodies against Na+/Ca2+ exchanger (plasma membrane; Millipore, MA, USA), GAPDH (cytosol; Fitzgerald Industries International, MA, USA), and LAP2 (nuclear membrane marker; BD Biosciences, CA, USA).
2.6 Localization of α1-ARs by Confocal Microscopy
In all experiments, cultured cardiac myocytes were infected at a multiplicity of infection (MOI) 1000 for α1A-constructs or at MOI 3000 for α1B-constructs [31]. This results in approximately 2.5-fold overexpression of the α1A-constructs and 4-fold overexpression of the α1B-constructs relative to endogenous α1-ARs in WT adult mouse cardiac myocytes [31].
To detect endogenous α1-ARs in nuclei isolated from WT cardiac myocytes, 50 nM BODIPY-Prazosin (Molecular Probes, OR, USA) was added to the nuclei for 15 minutes, counterstained with the DNA marker Hoechst 33342, and mounted on coverslips. To detect α1-GFP constructs in nuclei isolated from cultured α1ABKO cardiac myocytes, cardiac myocytes were cultured in 150 mm laminin-coated dishes, infected with α1-GFP constructs as above, cultured for 40 hrs, fractionated as described above, and mounted on coverslips.
To localize α1-AR constructs in intact cardiac myocytes, α1AKO, α1BKO, or α1ABKO cardiac myocytes were cultured on coverslips, infected with α1-GFP constructs as above, cultured for 40 hrs, fixed with 4% paraformaldehyde, and mounted on slides with Fluoromount G (Electron Microscopy Sciences, PA, USA). To stain endoplasmic reticulum (ER), 2 µM ER Tracker (Molecular Probes, OR, USA) in Tyrode’s buffer (140 mM NaCl, 10 mM glucose, 10 mM HEPES, 4 mM KCl, 1 mM MgCl2, pH 7.45) was added one hour prior to capturing images. Where indicated, cardiac myocytes were counterstained with the DNA marker Hoechst 33342.
All fluorescent images were captured by confocal microscopy using Fluoview software (Olympus BX50 confocal microscope, Olympus, NY, USA). Images were processed for publication using Imaris software (Bitplane Scientific Solutions, MN, USA).
2.7 Sensitized Emission Förster Resonance Energy Transfer (FRET)
α1ABKO cardiac myocytes were cultured on coverslips as above. At plating, cardiac myocytes were infected with adenovirus expressing wild-type α1B-CFP. After 16 hours, cardiac myocytes were infected with adenovirus expressing wild-type α1A-GFP. After 24 hours (40 hours total), cardiac myocytes were fixed and mounted on slides for sensitized emission FRET analysis using an Olympus Fluoview 1000 confocal microscope and Olympus Fluoview 1000 software for FRET analysis. Images were collected for analysis on one scan plane where CFP and GFP were excited with the 405 and 488 lasers respectively. Emission detection for CFP was 470–485 nm and GFP 508–525 nm regardless of excitation source (laser or fluorophore). These narrow bands of detection reduced background auto-fluorescence from cardiac myocytes. Images of cardiac myocytes expressing only the donor (CFP) or acceptor (GFP) fluorophore excited by the donor and acceptor laser were incorporated into the sensitized emission FRET analysis to correct for channel crosstalk. Final image brightness was enhanced post-analysis to aid in visualizing the fluorescence in Figure 6A.
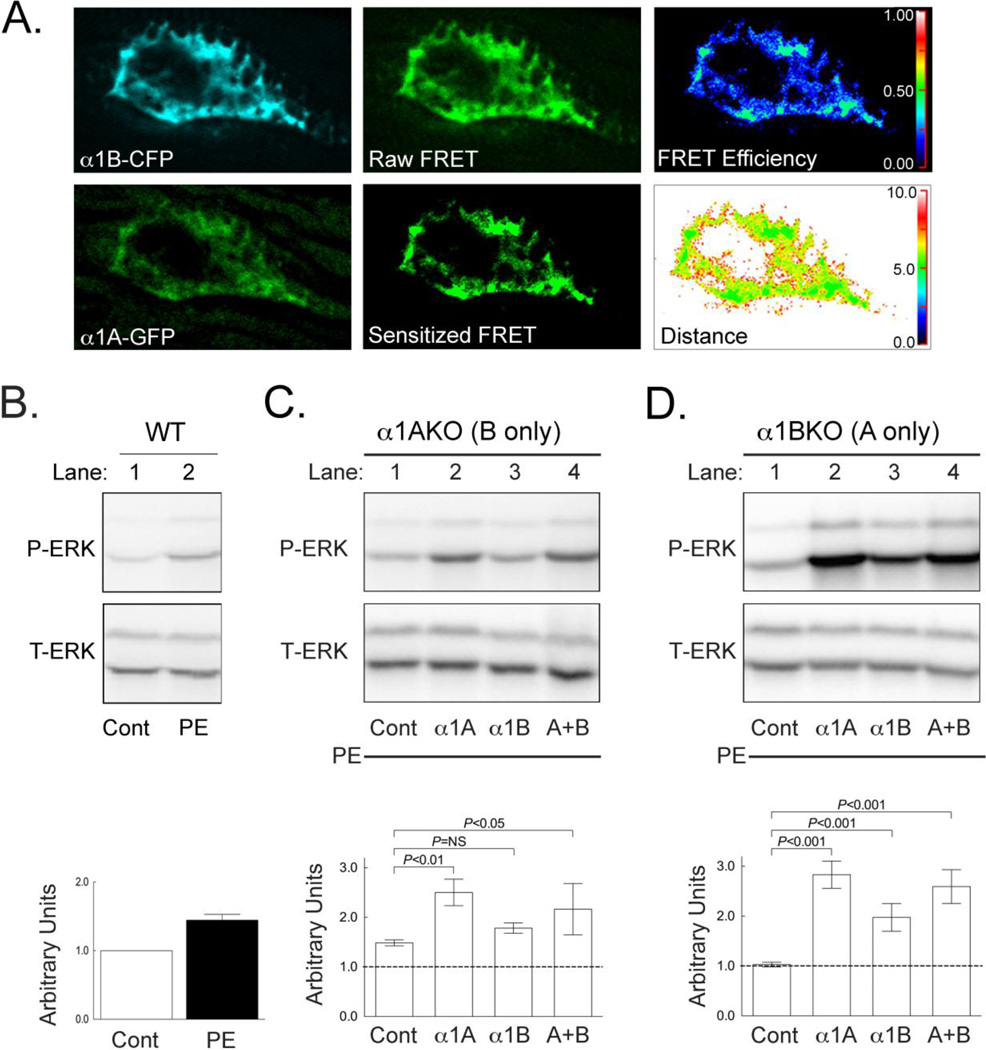
Figure 6. The α1A- and α1B-Subytpes Form Hetero-oligomers that Influence α1-AR Signaling in Adult Cardiac Myocytes.
(A) Representative confocal images of a nucleus from an α1ABKO cardiac myocyte expressing both the α1B-CFP (upper left) and α1A-GFP (lower left). The resulting raw FRET image (top center) and sensitized FRET image corrected for crosstalk between CFP and GFP emission (bottom center). FRET efficiency between α1B-CFP and α1A-GFP in the nucleus (upper right) and calculated FRET distances between α1B-CFP and α1A-GFP (bottom right) indicating hetero-oligomerization (n=3 separate cultures). The brightness of the fluorescent images was enhanced to aid visualization of color against the black background. (B) Representative Western blot for P-ERK and T-ERK in wild-type cardiac myocytes treated with 20 µM PE. (C) Representative Western blots for P-ERK and T-ERK in α1AKO cardiac myocytes infected with adenovirus expressing the α1A- alone (lane 2), the α1B- alone (lane 3) or both (lane 4) and treated with 20 µM PE. (D) Representative Western blots for P-ERK and T-ERK in α1BKO cardiac myocytes infected with adenovirus expressing the α1A- alone (lane 2), the α1B- alone (lane 3) or both (lane 4) and treated with 20 µM PE. Semi-quantitative analysis of Western Blots shown are shown in B, C and D. Replicate experiments (n=3 separate cultures) were quantified by densitometry and treatments were compared by ANOVA with a Bonferonni post-test. NS = not significant.
2.8 Western Blot Analysis of Phosphorylated ERK
WT, α1AKO, α1BKO, or α1ABKO cardiac myocytes were cultured as described above. Cardiac myocytes were infected with adenovirus expressing α1A-WT, α1A-NLSmut, α1B-WT, α1B-NLSmut, or β-galactosidase (βgal, control). After 40 hours, cardiac myocytes were treated with 20 µM phenylephrine (PE) for 15–20 minutes. In some experiments, cardiac myocytes were pre-treated with 18.5 nM leptomycin B for 20 minutes prior to PE treatment. After treatment, medium was aspirated, and lysates were made using ice-cold 1X RIPA buffer (50 mM Tris pH 7.4, 1% NP-40, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM phenylmethanesulfonylfluoride, 1 mM Na3VO4, 1 mM NaF, 1% Protease Inhibitor Cocktail). Total protein amounts were quantified by bicinchroninic acid protein assay (Pierce, IL, USA). Samples were prepared and resolved as previously described [27, 31, 32]. Blots were probed with primary antibodies for phosphorylated ERK or total ERK followed by a horseradish peroxidase conjugated rabbit secondary antibody (Cell Signaling Technology, MA, USA). Phosphorylated ERK and total ERK levels were detected by enhanced chemiluminescence (GE Healthcare, NJ, USA), and protein levels were quantified using Quantity One software (Bio-Rad, CA, USA).
2.9 Statistical Methods
Two-way ANOVA with Bonferroni post-test was used to determine differences in fold activation of ERK by densitometry. To test for differences in degree of nuclear localization, a repeated measures ANOVA was used to account for the correlation among technicians due to measuring the same animals. There was no interaction between technician and cell line (p=0.94).
3. RESULTS
3.1 Endogenous α1-ARs Localize to the Nuclei in Adult Cardiac Myocytes
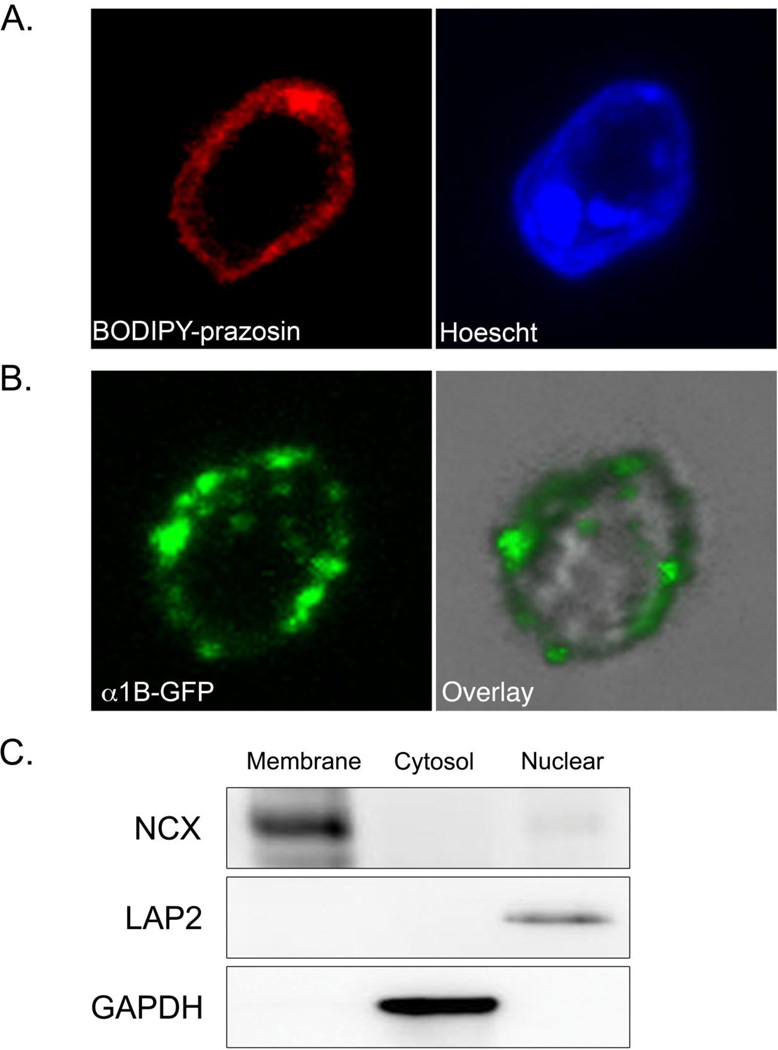
Here, we set out to validate and extend our previous result that endogenous α1-ARs localize to the nuclei in adult cardiac myocytes [27]. Intact nuclei were fractionated from freshly isolated wild-type adult mouse cardiac myocytes by sucrose density gradient ultracentrifugation. Endogenous α1-ARs were detected on the nuclear membrane with the fluorescently labeled α1-antagonist BODIPY-prazosin counterstained with the DNA stain Hoescht 33342 to identify nuclei (Figure 1A). We previously constructed α1-GFP fluorescent fusion proteins for both the α1A- and α1B-subtypes, the two α1-subtypes expressed in cardiac myocytes, and developed a reconstitution system in cultured α1ABKO cardiac myocytes [27]. Here, the α1B-GFP was detected on the nuclear membrane in nuclei isolated from cultured α1ABKO cardiac myocytes expressing the α1B-GFP (Figure 1B). The nuclear localization of the α1B-GFP recapitulated the localization of endogenous α1-ARs and again validated our reconstitution system [27]. In both experiments, the membrane, cytosolic, and nuclear fractions were validated by Western blots for Na+/Ca2+ exchanger (plasma membrane), GAPDH (cytosol), and LAP2 (nuclear membrane) respectively (Figure 1C).
Figure 1. Endogenous α1-ARs Localize to the Nuclei in Adult Cardiac Myocytes.
(A) Freshly isolated wild-type adult cardiac myocytes were fractionated by homogenization and sucrose density gradient ultracentrifugation. Isolated nuclei were stained with BODIPY-prazosin, counter-stained with Hoechst 33342, mounted on coverslips, and visualized by confocal microscopy. Original magnification = 1200x. (n=3 separate isolations) (B) Cultured α1ABKO adult cardiac myocytes were infected with adenovirus expressing α1B-GFP and, after 40 hrs, cardiac myocytes were fractionated as in (A). Isolated nuclei were mounted on coverslips and visualized by confocal microscopy. Original magnification = 1200x. (C) Membrane, cytosol, and nuclear fractions (30 µg) from freshly isolated adult cardiac myocytes were validated by Western blots for Na+/Ca2+ exchanger, GAPDH, or LAP2 respectively. Representative Western blots are shown. Similar results were obtained from cultured cardiac myocytes as we previously described [27].
3.2 The α1A and α1B-Subtypes Co-localize to the Nuclei in Adult Cardiac Myocytes
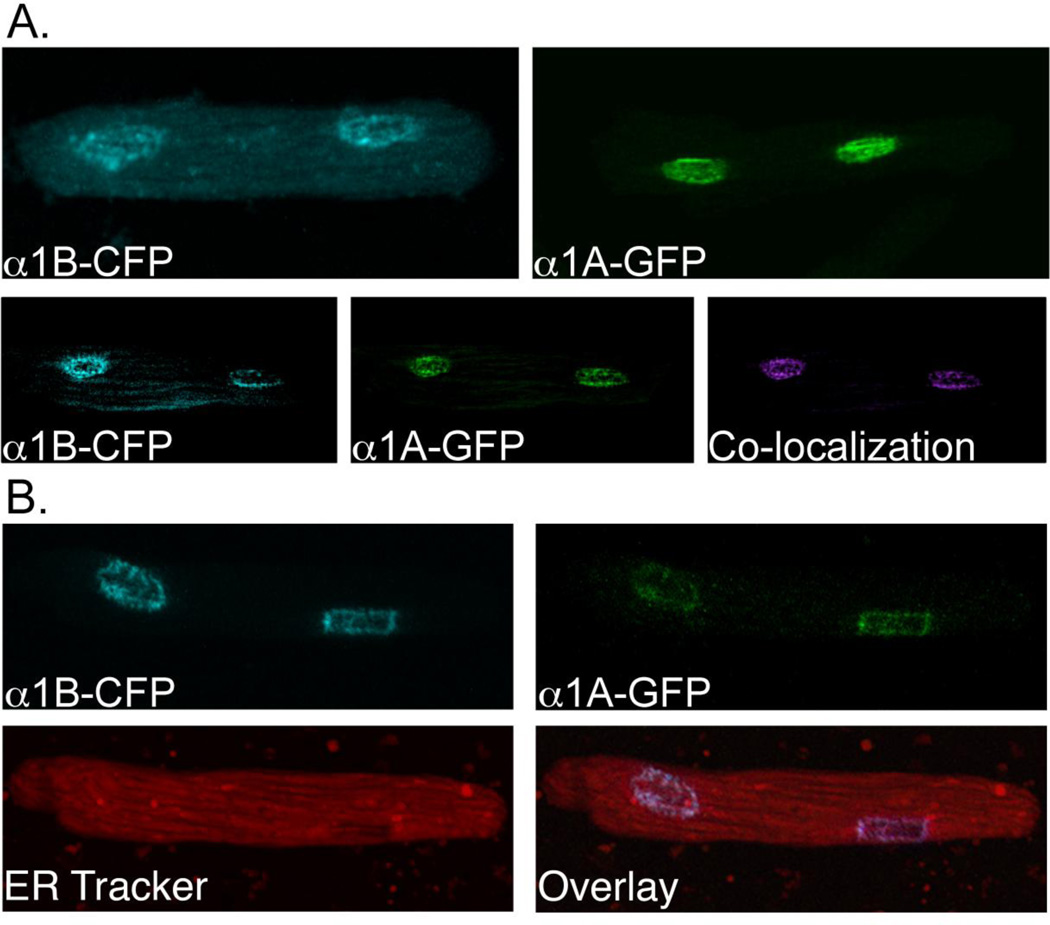
Using our reconstitution system in α1ABKO cardiac myocytes, we demonstrated that when expressed individually, either the α1A-GFP or α1B-GFP localized to the nuclei in adult cardiac myocytes (Figure 1B and 2A and [27, 31]). Here, we found that when we co-expressed the α1A- and α1B-subtypes, both receptors co-localized to the nuclei in adult cardiac myocytes (Figure 2A). This suggests the possibility that the receptors might interact at the nuclei to form oligomers that could regulate aspects of α1-AR biology in adult cardiac myocytes, as previously suggested in HEK293 cells [21–24]. To eliminate the possibility that the nuclear co-localization was due to a defect in synthesis, folding, or targeting causing the receptors to become trapped in the ER, we counterstained of the α1ABKO cardiac myocytes expressing both α1-AR subtypes with ER Tracker. Our results suggested that α1-AR localization to the nuclei was not the result of receptors becoming trapped in the endoplasmic reticulum (Figure 2B).
Figure 2. The α1A and α1B-Subtypes Co-localize to the Nuclei in Adult Cardiac Myocytes.
(A) Upper panel: representative confocal images of α1ABKO cardiac myocytes expressing only the α1B-CFP (upper left) or only α1A-GFP (upper right). Lower panel: representative confocal images of an α1ABKO cardiac myocyte expressing both the α1B-CFP (left) and the α1A-GFP (middle). Co-localization is pseudo-colored magenta (right). (B) Representative confocal images of an α1ABKO cardiac myocyte expressing both the α1B-CFP (upper left) and the α1A-GFP (upper right) and counterstained with 2 µM ER Tracker (lower left) and the overlay image (lower right). Final magnification = 600x (n=6 separate cultures).
3.3 Sequences in the C-terminus of the α1A- and α1B-Subtypes Drive Nuclear Localization in Adult Cardiac Myocytes
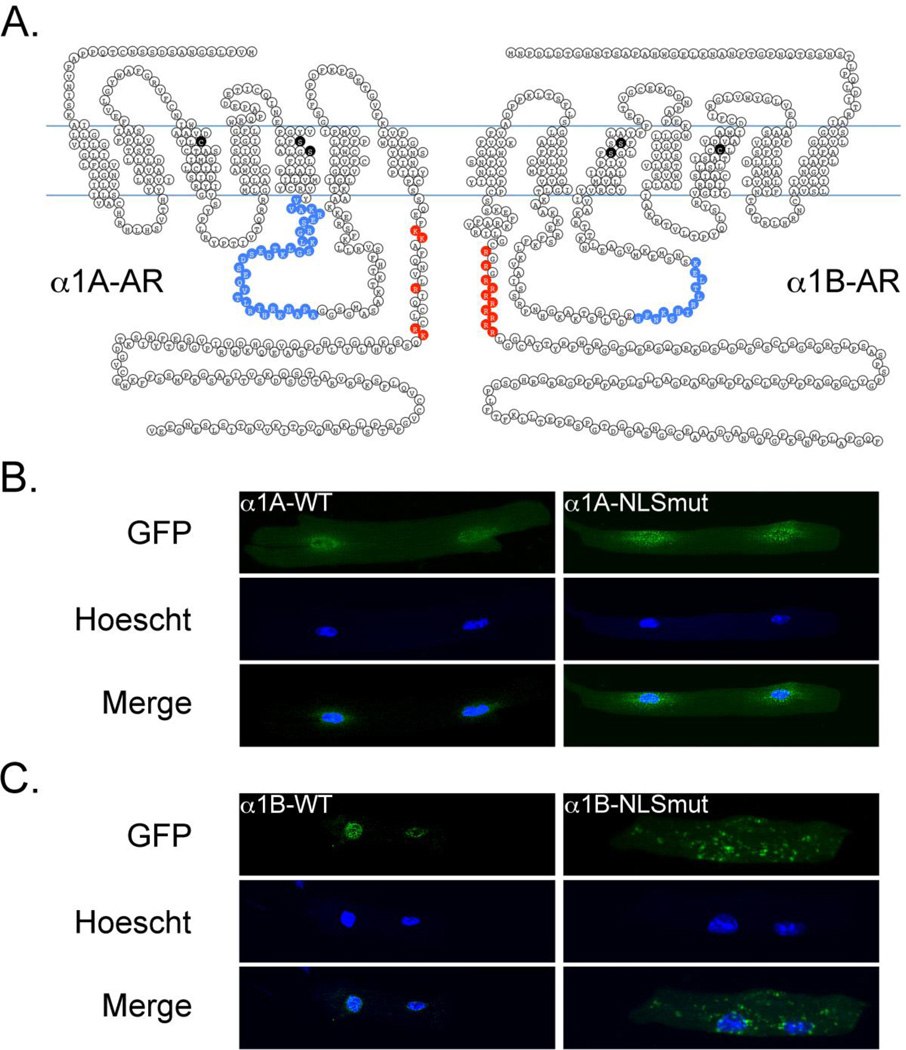
Based on our observation that endogenous α1-ARs localize to the nuclear membrane in adult cardiac myocytes (Figure 1A and [27]), we hypothesized that both the α1A- and the α1B-subtypes contained nuclear localization sequences. As depicted in Figure 3A, we identified a putative bi-partite nuclear localization sequence in the α1A-subtype and a glycine-arginine (GR) repeat/arginine-rich nuclear localization sequence in the α1B-AR, with both nuclear localization sequences in the carboxyl tail region of the respective receptors.
Figure 3. Sequences in the C-terminus of the α1A- and α1B-Subtypes Drive Nuclear Localization in Adult Cardiac Myocytes.
(A) Amino acid schematic diagram of the α1A- and α1B-ARs. Lysine (K) and arginine (R) residues mutated to alanine within the nuclear localization sequence for each receptor are indicated in red. Amino acid residues critical for ligand binding are indicated in black and regions for Gαq binding are indicated in blue [33–35]. (B) Representative confocal images of the α1A-WT (left) and the α1A-NLSmut (right) adrenergic receptors expressed in α1ABKO cardiac myocytes (n=6 separate cultures). (C) Representative confocal images of the α1B-WT (left) and α1B-NLSmut (right) adrenergic receptors express in α1ABKO cardiac myocytes (n=6 separate cultures).
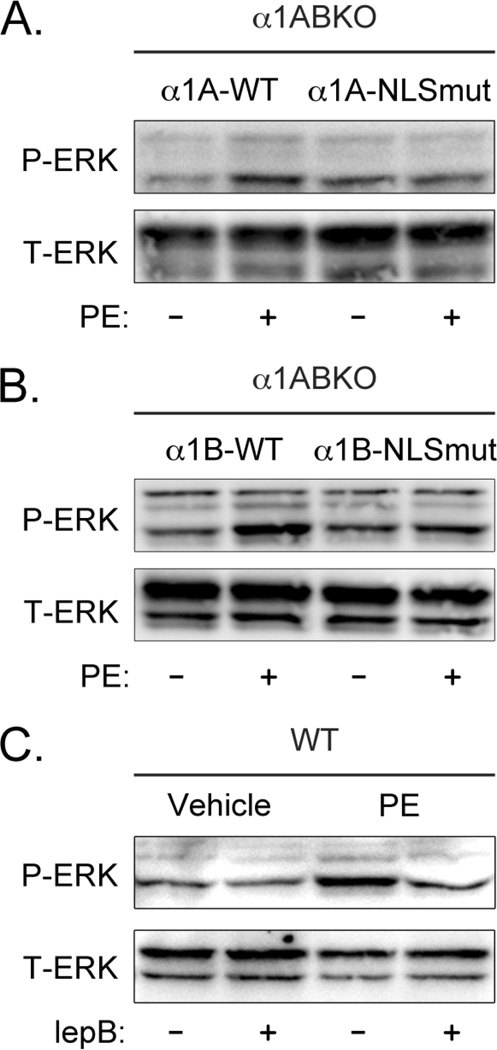
To test the functionality of each nuclear localization sequence, we mutated the arginines and lysines in these regions to alanines (Figure 3A). The localization of the α1-NLSmut constructs was compared to the localization of the α1-WT constructs expressed in α1ABKO cardiac myocytes. Using confocal microscopy, we found that mutation of the putative nuclear localization sequence in either the α1A-AR (Figure 3B) or the α1B-AR (Figure 3C) disrupted targeting of α1-ARs to the nucleus in α1ABKO cardiac myocytes. Interestingly, neither α1-subtype appeared to redistribute to the plasma membrane when the putative nuclear localization sequences were mutated. In summary, we identified functional nuclear localization sequences in both α1A-AR and α1B-AR, which provided a mechanism for the targeting of α1-ARs to the nuclei in adult cardiac myocytes.
3.4 Nuclear Localization is Required for α1-AR Signaling in Adult Cardiac Myocytes
Our previous studies indicated that α1-ARs co-localize with their downstream signaling partners Gαq and PLCβ1 only at the nucleus in adult cardiac myocytes, suggesting that α1-AR signaling must originate at the nucleus [27]. Although our previous results also suggested that α1-signaling was initiated intracellularly, we were neither able to definitively demonstrate nuclear α1-AR signaling nor exclude the possibility that a population of α1-ARs might initiate signaling at the plasma membrane. Therefore, to test the requirement for nuclear localization in α1-signaling in adult cardiac myocytes, we compared the ability of wild-type α1-ARs and α1-NLSmuts to induce the phosphorylation of ERK when receptor expression was reconstituted in α1ABKO cardiac myocytes. In our reconstitution system, both the α1A-WT and α1B-WT induced the phosphorylation of ERK upon treatment with the α1-agonist phenylephrine (PE), whereas neither α1-NLSmut could (Figure 4A and 4B). It is important to note that the sites mutated in the α1A- and α1B-NLSmut do not correspond to previously identified sites required for ligand binding (Figure 3A, residues highlighted in black) or for coupling to Gαq (Figure 3A, highlighted in blue) [33–35].
Figure 4. Nuclear Localization is Required for α1-AR Signaling in Adult Cardiac Myocytes.
Representative Western blot for phosphorylated ERK (P-ERK) and total ERK (T-ERK) from α1ABKO cardiac myocytes expressing (A) α1A-wild-type or α1A-NLSmut or (B) α1B-wild-type or α1B-NLSmut treated with 20 µM phenylephrine (PE) for 20 minutes (n=3). (C) Representative Western blot for P-ERK and T-ERK in wild-type cardiac myocytes treated with 20 µM PE for 20 minutes in the absence or presence of the nuclear export inhibitor leptomycin B (lepB) (n=6).
Our previous results also demonstrated that α1-AR signaling initiated intracellularly, presumably at the nuclei, induced the phosphorylation of ERK at the plasma membrane in adult cardiac myocytes [27]. This suggested an inside-out signaling mechanism, where α1-AR signaling initiated in the nuclei is transduced to the plasma membrane. Therefore, to confirm the finding that nuclear localization is required for α1-AR signaling in adult cardiac myocytes, we took the alternative approach of blocking nuclear export with leptomycin B to determine if endogenous α1-AR signaling in WT cardiac myocytes originated in the nucleus. Leptomycin B itself had no effect on the phosphorylation of ERK (Figure 4C). However, leptomycin B completely blocked PE-mediated phosphorylation of ERK in WT adult cardiac myocytes (Figure 4C). In summary, the data in Figure 4 indicated that nuclear localization was required for α1-AR signaling and suggested that endogenous α1-ARs activated signaling in the nuclei in adult cardiac myocytes.
3.5 The α1B-AR Forms Receptor Oligomers in Adult Cardiac Myocytes
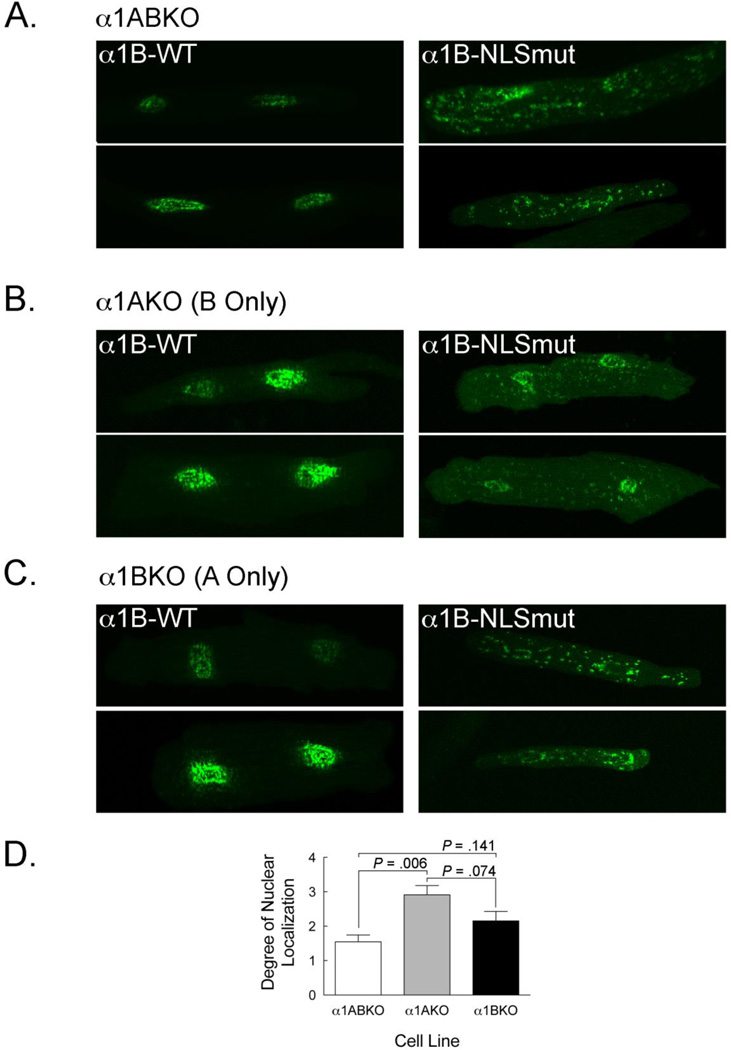
Prior reports suggested that α1-AR receptors form both homo- and hetero-oligomers and that α1-AR hetero-oligomers can dictate receptor localization in HEK293 cells [21–24]. To determine if α1-ARs could form receptor oligomers, we assessed the ability of endogenous α1-ARs to redirect expression of the α1-NLSmuts back to the nuclei in adult cardiac myocytes. In this case, we examined the α1B-NLSmut because it appeared to display a greater degree of redistribution away from the nuclei than that α1A-NLSmut. As before (Figure 3C), when expressed in α1ABKO cardiac myocytes, which lack both receptor subtypes, the α1B-NLSmut did not localize to the nuclei (Figure 5A). However, expression of the α1B-NLSmut in α1AKO cardiac myocytes, which only have the endogenous α1B-subtype, resulted in a significant degree of nuclear localization (P=0.006 vs. α1ABKO) (Figure 5B). In contrast, expression of the α1B-NLSmut in α1BKO cardiac myocytes, which only have the endogenous α1A-subtype, failed to result in a significant degree of nuclear localization, although a trend was detected (P=0.141 vs. α1ABKO) (Figure 5C). These data suggest that in α1AKO cardiac myocytes, the functional nuclear localization sequence in the endogenous α1B-subtype was sufficient to redirect expression of the α1B-NLSmut, presumably because of a direct interaction between the receptors. In a similar fashion, the endogenous α1A-subtype might interact with the α1B-NLSmut, although in this case only a trend was observed. In summary, these data suggest that the α1B-subtype and possibly the α1A-subtype can form oligomers in adult cardiac myocytes.
Figure 5. The α1B-AR Forms Receptor Oligomers in Adult Cardiac Myocytes.
(A) Representative confocal images of α1B-WT (left) and α1B-NLSmut (right) in α1ABKO cardiac myocytes. (B) Representative confocal images of α1B-WT (left) and α1B-NLSmut (right) in α1AKO cardiac myocytes which express only endogenous α1B-ARs. (C) Representative confocal images of α1B-WT (left) and α1B-NLSmut (right) in α1BKO cardiac myocytes which express only endogenous α1A-ARs. Final magnification = 600x. (D) Comparison of the degree of nuclear localization of the α1B-NLSmut in α1ABKO, α1AKO, and α1BKO myocytes. The degree of nuclear localization was determined by scoring each image on a scale from 0 to 5 with higher values representing increased nuclear fluorescence. Cells with nuclear and non-nuclear were scored 0 = no nuclear fluorescence, 1 = up to 25% of the nuclear membrane was identifiable, 2 = 26–50% of the nuclear membrane was identifiable, 3 = 51–75% of the nuclear membrane was identifiable, 4 = 76–100% of the nuclear membrane was identifiable. A score of 5 was given to cardiac myocytes with nuclear fluorescence and no non-nuclear fluorescence. Scores from three blinded technicians were included in the statistical analysis (n=6 for each genotype).
3.6 The α1A- and α1B-Subytpes Form Hetero-oligomers that Influence α1-AR Signaling in Adult Cardiac Myocytes
Our results in Figure 5 suggest the potential for the α1A- and α1B-subtypes to form hetero-oligomers, which has been demonstrated in HEK293 cells, but to our knowledge, not in cardiac myocytes. To test this possibility, we created adenoviruses expressing the α1A-GFP and α1B-CFP to constitute a Förster Resonance Energy Transfer (FRET) pair and measured receptor interaction using FRET [23]. We reconstituted this FRET pair in cultured α1ABKO cardiac myocytes, thereby avoiding the potential complications in measuring receptor interaction due to endogenous α1-ARs. Sensitized emission FRET analysis revealed that the α1A- and α1B-subtypes interacted on the nuclear membrane (FRET efficiency ranging from 0.25–0.50 and a FRET distance appropriate for this FRET pair, which is 4.88 nM, Figure 6A). In short, this provides biophysical evidence that α1-ARs form hetero-oligomers, which to our knowledge is the first such demonstration in adult cardiac myocytes.
The functional consequence of α1A- and α1B-subtype hetero-oligomers is unclear. Previous studies suggested that α1-AR hetero-oligomers could affect localization and internalization in other cell types [21–23]. However, to our knowledge, an effect of α1-subtype hetero-oligomers on α1-AR signaling has not been reported. To test how α1-AR hetero-oligomers might affect signaling, we measured PE-mediated phosphorylation of ERK, a common α1-AR signaling pathway [36]. Using our reconstitution system in α1ABKO cardiac myocytes, we previously reported that the α1A-subtype facilitated robust PE-mediated ERK phosphorylation, whereas the α1B-subtype had only a modest effect on PE-mediated ERK phosphorylation [27, 31].
Here, we employed cultured α1A- or α1B-single knockout (α1AKO or α1BKO) adult cardiac myocytes, reconstituting the missing α1-subtype to ascertain the potential effect of receptor hetero-oligomers. As a control, we found that PE induced the phosphorylation of ERK in wild-type cardiac myocytes (Figure 6B)
In α1AKO cardiac myocytes that express only the α1B-subtype at levels similar to wild type, PE induced modest ERK phosphorylation (Figure 6C). Reconstitution of the α1A-subtype resulted in robust PE-mediated ERK phosphorylation as expected based on results with the α1A-subtype in α1ABKO cardiac myocytes, but increasing α1B-subtype expression had no significant additive effect (Figure 6C).
In α1BKO cardiac myocytes that express only the α1A-subtype at levels similar to wild type, PE had no effect on ERK phosphorylation (Figure 6D). However, increasing α1A-subtype resulted in robust PE-mediated ERK phosphorylation, as expected, but so did reconstitution of the α1B-subtype, which was unexpected (Figure 6D). In this case, the ability of the α1B-subtype to facilitate robust PE-mediated ERK phosphorylation in α1BKO cardiac myocytes could suggest a functional interaction between the α1A- and α1B-subtypes. Combined with our results indicating the formation of α1A- and α1B-subtype hetero-oligomers at the nucleus in adult cardiac myocytes, these data suggest that α1-AR hetero-oligomers might facilitate the phosphorylation of ERK, which to our knowledge is the first demonstration of α1-AR oligomers regulating signaling in any cell type.
4. DISCUSSION
Here, we examined α1-AR localization to the nuclei in adult cardiac myocytes, the mechanism behind α1-AR nuclear localization, and how nuclear localization affected receptor function. In short, we validated our previous findings, clearly demonstrating that endogenous α1-ARs localized to the nuclear membrane in adult cardiac myocytes. Furthermore, we identified functional nuclear localization sequences in both the α1A- and α1B-subtypes that drove nuclear localization and demonstrated that nuclear localization was required for α1-AR signaling in adult cardiac myocytes. Finally, we found that co-localization of the α1-subtypes at the nuclei in adult cardiac myocytes facilitated the formation of receptor oligomers that could affect receptor signaling.
These conclusions are supported by the following observations. First, by using nuclei isolated from adult cardiac myocytes and stained with BODIPY-prazosin, we employed a third different technique from our previous work to demonstrate that endogenous α1-ARs localize to the nuclei in adult cardiac myocytes (Figure 1A). Second, we identified (Figure 3A) and disrupted putative nuclear localization sequences in both the α1A- and α1B-subtypes, which led to mis-localization of each subtype when expressed in α1ABKO adult cardiac myocytes (Figure 3B). Third, we found that disruption of the nuclear localization sequences in either the α1A- or α1B-subtypes prevented phosphorylation of ERK upon treatment with the α1-agonist phenylephrine (Figure 4A-B). Furthermore, based on our finding that α1-AR signaling activates ERK only at the plasma membrane in adult cardiac myocytes [27], we used leptomycin B to block nuclear export and demonstrate that signaling for endogenous α1-AR induced phosphorylation of ERK must arise in the nucleus (Figure 4C). Fourth, we showed that endogenous α1B-ARs can redirect expression of the α1B-NLSmut back to the nucleus, and observed a similar, although non-significant, trend for the α1A-AR, suggesting the formation of receptor oligomers. Fifth, we demonstrated direct biophysical interaction between the α1A- and α1B-subtypes at the nucleus using FRET (Figure 6A). Finally, we demonstrated that the α1B-subtype, which alone induces only modest PE-mediated phosphorylation of ERK, could facilitate robust PE-mediated phosphorylation of ERK in α1BKO cardiac myocytes, which express only the α1A-subtype that did not respond to PE (Figure 6B-D).
Convention suggests that GPCRs localize to and signal at the plasma membrane. However, several GPCRs have now been localized to the nuclear membrane in a variety of cell types [1–3]. In cardiac myocytes, endothelin receptors, angiotensin receptors, and β-ARs were all detected at the nuclei in adult cardiac myocytes [4–7], and our work indicates that α1-ARs localized to the nuclei in adult cardiac myocytes as well (Figure 1 and [27, 31]). Yet, the functional relevance of nuclear GPCRs in cardiac myocytes, or any cell, remains unclear. In nuclei isolated from adult cardiac myocytes, endothelin receptors induced calcium transients [4] and angiotensin receptors and β-ARs increased gene transcription [6, 7], but how this might impact overall cardiac myocyte function is uncertain. Here, we found that nuclear localization was required for α1-AR mediated phosphorylation (activation) of ERK in adult cardiac myocytes (Figure 4). We previously showed that α1-AR signaling induced phosphorylation of ERK only in caveolae at the plasma membrane in adult cardiac myocytes, describing an inside-out signaling mechanism [27]. Furthermore, we identified an α1A-subtype-ERK survival-signaling pathway in adult cardiac myocytes, and the absence of this signaling pathway might explain the development of heart failure and increased mortality in α1ABKO mice subjected to aortic constriction [28, 37]. Therefore, nuclear α1-AR mediated phosphorylation of ERK at the plasma membrane is an example of nuclear GPCR signaling with significant physiologic consequences.
Although endothelin receptors, angiotensin receptors, and β-ARs all localize to nuclear membrane in adult cardiac myocytes [4–7], our results indicated that unlike these other receptors the majority, if not all, α1-ARs localized to the nuclei in adult cardiac myocytes [27]. Using saturation radio-ligand binding analysis on nuclei, we demonstrated that at least 80% of α1-ARs were detected at the nuclei in adult cardiac myocytes, and we failed to detect a functional population of α1-ARs at the plasma membrane [27]. Subsequently, we performed a similar analysis to measure the distribution of endothelin and angiotensin receptors in adult cardiac myocytes. Our results indicated that 95% of endothelin receptors were detected at the plasma membrane, and only 5% were detected in the nuclei (data not shown), similar to previous studies [4]. However, angiotensin receptor expression levels were below the level of detection in this assay (data not shown). Interestingly, all three receptors signal through Gαq, but α1-AR signaling is cardio-protective (review [36]), whereas chronic endothelin and angiotensin receptor signaling is pathologic (reviews [38, 39]). This might suggest that receptor localization dictates function, or in other words, nuclear Gq-coupled receptor signaling is cardio-protective, whereas plasma membrane Gq-coupled receptor signaling is pathologic. However, this intriguing hypothesis remains to be proven.
Here, we demonstrated the capacity for the α1B-subtype to form homo-oligomers, and the α1A- and α1B-subtypes to form hetero-oligomers in adult cardiac myocytes (Figures 5–6), similar to previous reports in HEK293 cells [21–24]. The ability of the α1B-subtype to form homo-oligomers was inferred from studies where the α1B-NLSmut was redirected to the nucleus when expressed in α1AKO cardiac myocytes that express only endogenous levels of the α1B-subtype (Figure 5B). Interestingly, the formation of α1B-homo-oliogmers still occurred despite disruption of the nuclear localization sequence (Figure 5B), agreeing in part with previous reports indicating that the carboxyl tail domain of the α1B-subtype was not required for the formation of receptor oligomers [23]. However, in a similar experiment, endogenous levels of the α1A-subtype expressed in α1BKO cardiac myocytes only showed a trend towards redirecting the α1B-NLSmut to the nucleus (Figure 5C). Whether this is due to differences in expression levels between the receptors (exogenously expressed levels of the α1B-NLSmut were several fold higher than endogenous α1A-levels in α1BKO cardiac myocytes), or that the sequences mutated in the α1B-NLSmut were required for the formation of hetero-oligomers is unclear. The ability of the α1A- and α1B-subtypes to form hetero-oligomers was confirmed using FRET, and interestingly the formation of α1-AR hetero-oligomers affected α1-AR mediated phosphorylation of ERK. The ability of hetero-oligomers to affect α1-AR signaling was inferred from experiments where adding back the α1B-subtype to α1BKO cardiac myocytes facilitated robust phosphorylation of ERK. To our knowledge, this is the first demonstration that α1-AR hetero-oligomers affect signaling, which could have important implications for α1A-ERK mediated survival-signaling.
In summary, the data presented here demonstrated that α1-ARs localized to the nucleus, that nuclear localization was driven by nuclear localization sequences identified in both the α1A- and α1B-subtypes, that nuclear localization was required for α1-AR signaling, and that α1-ARs nuclear localization facilitated that formation of receptor oligomers that impacted receptor signaling. Combined with our previous studies identifying an α1A-ERK survival signaling pathway that protected the heart from pathologic stress [28, 31, 37], we can for the first time assign a significant physiologic role to nuclear GPCR signaling in cardiac myocytes that might influence the development of novel therapies for heart failure.
HIGHLIGHTS.
Endogenous α1-ARs localize to the nucleus in adult cardiac myocytes
Nuclear localization sequences drive α1-ARs to the nuclei in adult cardiac myocytes
α1-AR signaling is initiated in the nucleus in adult cardiac myocytes
α1-ARs form oligomers that alter receptor function in adult cardiac myocytes
ACKNOWLEDGEMENTS
This work was supported by grants from the National Institute of Health (P20 RR-017662, TDO; F32 HL085980-02, CDW). The authors would also like to thank Chastity L. Healy, Kelly Graber of the Sanford Research Imaging Core, Andy Cypher of the Sanford Research Cell Culture Core, and Yuan Huang, MD of the Sanford Research Molecular Biology Core for their excellent technical assistance. The Sanford Research Cores listed here were support by a grant from the National Institutes of Health (P20 RR-017662).
ABBREVIATIONS
- α1-AR
alpha1-adrenergic receptor
- α1A-WT
wild-type alpha1-adrenergic receptor A subtype
- α1AKO
alpha1-adrenergic receptor A subtype single knockout
- α1B-WT
wild-type alpha1-adrenergic receptor B subtype
- α1BKO
alpha1-adrenergic receptor B subtype single knockout
- α1ABKO
alpha1-adrenergic receptor A and B subtype double knockout
- α1A- or α1B-CFP
GFP, cyan or green fluorescent protein for alpha1-adrenergic receptor subtypes
- β-AR
β-adrenergic receptor
- βgal
beta-galactosidase
- BODIPY
boron-dipyrromethene fluorescent dye
- ER
endoplasmic reticulum
- ERK
extracellular-regulated kinase
- FRET
Förster Resonance Energy Transfer
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GPCR
G-protein coupled receptor
- LAP2
lamin-associated protein 2
- lepB
leptomycin B
- MOI
multiplicity of infection
- NCX
Na+/Ca2+ exchanger
- NLSmut
nuclear localization sequence mutant
- PE
phenylephrine
- P-ERK
phosphorylated extracellular-regulated kinase
- T-ERK
total extracellular-regulated kinase
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Bkaily G, Avedanian L, Jacques D. Can J Physiol Pharmacol. 2009;87:108–119. doi: 10.1139/Y08-115. [DOI] [PubMed] [Google Scholar]
- 2.Boivin B, Vaniotis G, Allen BG, Hebert TE. J Recept Signal Transduct Res. 2008;28:15–28. doi: 10.1080/10799890801941889. [DOI] [PubMed] [Google Scholar]
- 3.Gobeil F, Fortier A, Zhu T, Bossolasco M, Leduc M, Grandbois M, Heveker N, Bkaily G, Chemtob S, Barbaz D. Can J Physiol Pharmacol. 2006;84:287–297. doi: 10.1139/y05-127. [DOI] [PubMed] [Google Scholar]
- 4.Boivin B, Chevalier D, Villeneuve LR, Rousseau E, Allen BG. J Biol Chem. 2003;278:29153–29163. doi: 10.1074/jbc.M301738200. [DOI] [PubMed] [Google Scholar]
- 5.Boivin B, Lavoie C, Vaniotis G, Baragli A, Villeneuve LR, Ethier N, Trieu P, Allen BG, Hebert TE. Cardiovasc Res. 2006;71:69–78. doi: 10.1016/j.cardiores.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 6.Tadevosyan A, Maguy A, Villeneuve LR, Babin J, Bonnefoy A, Allen BG, Nattel S. J Biol Chem. 2010;285:22338–22349. doi: 10.1074/jbc.M110.121749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaniotis G, Del Duca D, Trieu P, Rohlicek CV, Hebert TE, Allen BG. Cellular signalling. 2011;23:89–98. doi: 10.1016/j.cellsig.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu D, Yang H, Shaw G, Raizada MK. Endocrinology. 1998;139:365–375. doi: 10.1210/endo.139.1.5679. [DOI] [PubMed] [Google Scholar]
- 9.Hock R, Scheer U, Bustin M. J Cell Biol. 1998;143:1427–1436. doi: 10.1083/jcb.143.6.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dono R, James D, Zeller R. Oncogene. 1998;16:2151–2158. doi: 10.1038/sj.onc.1201746. [DOI] [PubMed] [Google Scholar]
- 11.Cook A, Bono F, Jinek M, Conti E. Annu Rev Biochem. 2007;76:647–671. doi: 10.1146/annurev.biochem.76.052705.161529. [DOI] [PubMed] [Google Scholar]
- 12.King MC, Lusk CP, Blobel G. Nature. 2006;442:1003–1007. doi: 10.1038/nature05075. [DOI] [PubMed] [Google Scholar]
- 13.Lusk CP, Blobel G, King MC. Nat Rev Mol Cell Biol. 2007;8:414–420. doi: 10.1038/nrm2165. [DOI] [PubMed] [Google Scholar]
- 14.Pickard BW, Hodsman AB, Fraher LJ, Watson PH. Endocrinology. 2006;147:3326–3332. doi: 10.1210/en.2005-1408. [DOI] [PubMed] [Google Scholar]
- 15.Pickard BW, Hodsman AB, Fraher LJ, Watson PH. Endocrinology. 2007;148:2282–2289. doi: 10.1210/en.2007-0157. [DOI] [PubMed] [Google Scholar]
- 16.Re M, Pampillo M, Savard M, Dubuc C, McArdle CA, Millar RP, Conn PM, Gobeil F, Jr, Bhattacharya M, Babwah AV. PLoS One. 2010;5:e11489. doi: 10.1371/journal.pone.0011489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Breitwieser GE. Circ Res. 2004;94:17–27. doi: 10.1161/01.RES.0000110420.68526.19. [DOI] [PubMed] [Google Scholar]
- 18.Milligan G. Mol Pharmacol. 2004;66:1–7. doi: 10.1124/mol.104.000497.. [DOI] [PubMed] [Google Scholar]
- 19.Prinster SC, Hague C, Hall RA. Pharmacol Rev. 2005;57:289–298. doi: 10.1124/pr.57.3.1. [DOI] [PubMed] [Google Scholar]
- 20.Smith NJ, Milligan G. Pharmacological reviews. 2010;62:701–725. doi: 10.1124/pr.110.002667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hague C, Lee SE, Chen Z, Prinster SC, Hall RA, Minneman KP. Mol Pharmacol. 2006;69:45–55. doi: 10.1124/mol.105.014985. [DOI] [PubMed] [Google Scholar]
- 22.Hague C, Uberti MA, Chen Z, Hall RA, Minneman KP. J Biol Chem. 2004;279:15541–15549. doi: 10.1074/jbc.M314014200. [DOI] [PubMed] [Google Scholar]
- 23.Stanasila L, Perez JB, Vogel H, Cotecchia S. J Biol Chem. 2003;278:40239–40251. doi: 10.1074/jbc.M306085200. [DOI] [PubMed] [Google Scholar]
- 24.Uberti MA, Hall RA, Minneman KP. Mol Pharmacol. 2003;64:1379–1390. doi: 10.1124/mol.64.6.1379. [DOI] [PubMed] [Google Scholar]
- 25.Zhu WZ, Chakir K, Zhang S, Yang D, Lavoie C, Bouvier M, Hebert TE, Lakatta EG, Cheng H, Xiao RP. Circ Res. 2005;97:244–251. doi: 10.1161/01.RES.0000176764.38934.86. [DOI] [PubMed] [Google Scholar]
- 26.Jensen BC, Swigart PM, Simpson PC. Naunyn Schmiedebergs Arch Pharmacol. 2009;379:409–412. doi: 10.1007/s00210-008-0368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wright CD, Chen Q, Baye NL, Huang Y, Healy CL, Kasinathan S, O'Connell TD. Circ Res. 2008;103:992–1000. doi: 10.1161/CIRCRESAHA.108.176024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Connell TD, Ishizaka S, Nakamura A, Swigart PM, Rodrigo MC, Simpson GL, Cotecchia S, Rokosh DG, Grossman W, Foster E, Simpson PC. J Clin Invest. 2003;111:1783–1791. doi: 10.1172/JCI16100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Connell TD, Ni Y, Lin KM, Han H, Yan Z. AfCS Research Reports. 2003;1 [Google Scholar]
- 30.O'Connell TD, Rodrigo MC, Simpson PC. Methods Mol Biol. 2007;357:271–296. doi: 10.1385/1-59745-214-9:271. [DOI] [PubMed] [Google Scholar]
- 31.Huang Y, Wright CD, Merkwan CL, Baye NL, Liang Q, Simpson PC, O'Connell TD. Circulation. 2007;115:763–772. doi: 10.1161/CIRCULATIONAHA.106.664862. [DOI] [PubMed] [Google Scholar]
- 32.Huang Y, Wright CD, Kobayashi S, Healy CL, Elgethun M, Cypher A, Liang Q, O'Connell TD. Am J Physiol Heart Circ Physiol. 2008;295:H699–H707. doi: 10.1152/ajpheart.01204.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cotecchia S, Stanasila L, Diviani D, Bjorklof K, Rossier O, Fanelli F. Biol Cell. 2004;96:327–333. doi: 10.1016/j.biolcel.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 34.Hwa J, Graham RM, Perez DM. J. Biol. Chem. 1995;270:23189–23195. doi: 10.1074/jbc.270.39.23189. [DOI] [PubMed] [Google Scholar]
- 35.Wu D, Jiang H, Simon MI. J. Biol. Chem. 1995;270:9828–9832. doi: 10.1074/jbc.270.17.9828. [DOI] [PubMed] [Google Scholar]
- 36.Jensen BC, O'Connell TD, Simpson PC. J Mol Cell Cardiol. 2011 doi: 10.1016/j.yjmcc.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Connell TD, Swigart PM, Rodrigo MC, Ishizaka S, Joho S, Turnbull L, Tecott LH, Baker AJ, Foster E, Grossman W, Simpson PC. J Clin Invest. 2006;116:1005–1015. doi: 10.1172/JCI22811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chrysant SG. Clin Ther. 2008;30 Pt 2:2181–2190. doi: 10.1016/j.clinthera.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 39.Kedzierski RM, Yanagisawa M. Annu Rev Pharmacol Toxicol. 2001;41:851–876. doi: 10.1146/annurev.pharmtox.41.1.851. [DOI] [PubMed] [Google Scholar]