Summary
Three types of motors, myosins, kinesins and cytoplasmic dynein, cooperate to transport intracellular membrane organelles. Transport of each cargo is determined by recruitment of specific sets of motors and their regulation. Targeting of motors to membranes often depends on the formation of large multiprotein assemblies and can be influenced by membrane lipid composition. Motor activity can be regulated by cargo-induced conformational changes such as unfolding or dimerization. The architecture and function of motor: cargo complexes can also be controlled by phosphorylation, calcium signalling, and proteolysis. The complexity of transport systems is further increased by mechanical and functional cross-talk between different types of motors on the same cargo and by participation of the same motor in the movement of different organelles.
Introduction
Two types of cytoskeletal fibers, microtubules and actin filaments, serve as tracks for intracellular transport. These tracks possess intrinsic polarity, as each of them has two structurally and functionally distinct ends, the plus end and the minus end (called the barbed and pointed ends, respectively, in actin filaments). Microtubule-based motors include kinesins, which with a few exceptions move towards the microtubule plus end, and cytoplasmic dynein that moves to the minus end. Actin-based motors, myosins, predominantly walk to the barbed end of the actin filament; the only exception to date is the pointed end-directed motor myosin VI. Eukaryotic genomes typically encode tens of kinesins and myosins with similar motor domains but completely divergent, class-specific, cargo-binding regions while cytoplasmic dynein, when present, is usually represented by a small set of closely related isoforms. Here we describe the emerging principles of motor recruitment and regulation on membrane cargo. For the discussion of the actin-based motors, we focus exclusively on type V and VI unconventional myosins, because much of our current knowledge regarding myosin-dependent organelle transport has come from the study of just these two motors.
Motor recruitment by multiprotein assemblies
The simplest mode of motor recruitment is through direct binding of the motor to the cytoplasmic portion of transmembrane cargo molecules on transport vesicles/organelles. For example, the yeast type V myosin Myo2 binds to the peroxisome through a direct interaction of its cargo binding domain (CBD) with the transmembrane protein Inp2 [1]. Similarly, kinesin-1 has been suggested to interact directly with the transmembrane amyloid precursor protein (APP) on axonal vesicles [2], while dynein light chains LC8 and TcTex1 were reported to recruit dynein to certain transmembrane receptors [3]. We note, however, that these latter two simple targeting schemes have been disputed, as binding of kinesin-1 to APP transport vesicles might require the c-Jun N-terminal kinase interacting protein (JIP) and/or the Rab GTPase Rab3 [4], while recent structural evidence indicates that LC8 and TcTex1 cannot interact with cargo and dynein at the same time, bringing into question their role as motor-cargo bridging factors [5].
A large body of evidence now points to the importance of multiprotein complexes in the recruitment of motors (Table 1). These can include components of cargo sorting coats such as retromer on endosome-to-TGN carriers [6] which binds to dynactin, as well as the coatomer subunit Ret2, which is required along with the Rab GTPase Ypt11 to recruit Myo2 to Golgi membranes [7]. Similarly, clathrin adaptors can contribute to motor attachment, as AP-1 might link kinesin-3 KIF13A to TGN and endosome-derived carriers [8], while AP-2, together with the endosomal adaptor Dab2, is a part of the complex that links myosin VI to LDL receptor-containing clathrin coated pits and vesicles [9 ‴″]. Many motor complexes include compartment-specific Rab GTPases, which frequently associate with their cognate motor through a specialized adaptor protein (Table 1). These adaptors, such as melanophilin (Rab27a) [10–12], optineurin (Rab8) [13], Bicaudal D (Rab6) [14–16], and RILP (Rab7) [17,18], usually recognise their cognate GTPase in its GTP-bound state and thus serve as Rab effectors. By binding simultaneously to the Rab and the motor through non-overlapping domains, these adaptors form a bridge between the activated Rab and the motor. Other tripartite receptor complexes that do not involve a Rab GTPase also exist, such as the complex of Vac8 and Vac17, where the adaptor Vac17 recruits Myo2 to the yeast vacuole by forming a bridge between Vac8 in the membrane and Myo2 [19]. Another type of adaptor is exemplified by GRIP1 and GIPC that interact with kinesin-1 and myosin VI, respectively, and through their PDZ domains associate with the C-termini of transmembrane cargo proteins [20,21]. In addition, multifunctional scaffolding proteins, such as liprins [22] may also be involved in organizing motor complexes and linking them to membranes.
Table 1. Composition of multiprotein motor recruitment complexes on different organelles.
The table shows examples of motor complexes with at least two different components in addition to the motor. The table is meant to serve as an illustration and is not comprehensive; the reference list is not complete and is mostly confined to the recent literature that can be used as a source of older references. Cargo proteins are included in the list if they are known to form a part of the motor attachment complex on the membrane. Where known, dynein or dynactin subunits known to be involved in the interaction are indicated. For dynein/dynactin, only a few examples involving Rab proteins are shown, as a multisubunit complex including dynein and dynactin as well as their multiple accessory factors is expected to participate in transport in most if not all cases. Proteins acting as adaptors between small GTPases and motors (Rab effectors) are underlined. Such proteins typically contain separate Rab-binding and motor-interacting domains. Many adaptors are dimers, presumably to support interactions with dimeric, processive motors, and encompass sequences that can form extended coiled coils, probably to facilitate their linker function. In addition to protein-protein interaction domains, some adaptors (FIP2, Slp1) also contain regions potentially involved in lipid binding, such as C2 domains.
| Motor | Organelle, cargo | Species | Accessory factors | Ref | |
|---|---|---|---|---|---|
| Small GTPase | Adaptors, scaffolds and other proteins | ||||
| Myosins | |||||
| Myosin Va | Melanosomes | mamm. | Rab27a | melanophilin | [10–12] |
| Myosin Vb | Recycling endosomes AMPA rec. | mamm | Rab11 | FIP2 | [24″,25] |
| Myosin V | Secretory vesicles (rhodopsin) | fly | Rab11 | dRip11 | [78] |
| Myosin V Myo2 | Vacuole | yeast | Vac8, Vac 17 | [19] | |
| Myosin V Myo2 | Golgi | yeast | Ypt11 | Ret2 | [7] |
| Myosin VI | Post-Golgi vesicles | mamm. | Rab8 | Optineurin, Huntingtin | [13,59] |
| Myosin VI | Clathrin coated structures | mamm. | LDL rec., Dab2 AP2 | [9″‴,31] | |
| Myosin VI | Clathrin coated structures | mamm. | AMPA rec., AP-2, SAP97 | [79] | |
| Myosin VI | Uncoated endocytic vesicles | mamm. | Membrane receptors with PDZ ligand sites (e.g. GLUT1) GIPC | [21] | |
| Kinesins | |||||
| Kinesin-1 | Mitochondria | mamm. | Miro | Milton/TRAK | [34,70″,71″] |
| Kinesin-1 | TrkB carriers | mamm. | Rab27b | TrkB, Slp1 CRMP-2 | [23″] |
| Kinesin-1 | APP carriers | mamm. | Rab3? | APP, JIP1? | [2,4] |
| Kinesin-1 | Neuronal vesicles | mamm. | Membrane receptors with PDZ ligand sites (e.g. AMPA rec) GRIP | [20] | |
| Kinesin-1 | GABAA rec. carriers | mamm. | GABAA rec., HAP1 Huntingtin | [51] | |
| Kinesin-2 KIF17 | NMDA rec. carriers | mamm. | NMDA rec.. Mint1, CASK, MALS | [66″″] | |
| Kinesin-2 | Recycling endosomes | mamm. | Rab11 | Rab11-FIP5 | [55] |
| Kinesin-3 KIF1A/1Bβ | Synaptic vesicles | mamm. | Rab3 | DENN/MADD | [80] |
| Cytoplasmic dynein/dynactin | |||||
| Dynein and dynactin | Exocytotic vesicles | mamm. | Rab6 | Bicaudal D1/2 | [14–16] |
| p150Glued | Late endosomes | mamm. | Rab7 | RILP, ORP1L | [17,18] |
| Dynein LIC1 | Recycling endosomes | mamm. | Rab11a | Rab11-FIP3 | [56] |
The emerging view of motor: cargo interactions is, therefore, of complex sets of proteins that assemble on the membrane cargo by interacting with each other. For example, the association of kinesin-1 with Trk receptor carriers requires participation of Rab27b, melanophilin-like adaptor Slp1, and multifunctional protein CRMP-2 [23 ″], while targeting of dynein-dynactin to late endosomes depends on Rab7, its effector RILP, lipid-binding protein ORP1L, and βIII spectrin [17] (Table 1). The architecture of such complexes is generally poorly understood and might be complicated by the presence of seemingly redundant interactions. For example, recruitment of myosin Vb to recycling endosomes involves the association of its CBD directly with Rab11 and also with the Rab11 effector FIP2 [24 ″,25]. Similarly, dynein-dynactin can bind to Rab6-positive exocytotic vesicles by interacting directly with Rab6 as well as with the Rab6 effector Bicaudal D [14,15]. In the case of dynein/dynactin, complexity is further enhanced by the important albeit poorly understood roles of Lissencephaly 1 and NUDE/NUDEL proteins, which participate in most dynein-mediated transport processes and might contribute to its membrane targeting [26].
Importantly, in cases where it has been investigated in detail, a relatively small numbers of motors might be involved in pulling cargo at each particular moment [27 ″″], and the overall number of attached motors might be small (see for review [28]). In contrast, Rabs, adaptors and scaffolds often abundantly decorate the cargo, suggesting that motor recruitment by these molecules might be relatively inefficient.
Control of motor recruitment by the lipids
Membrane compartments can differ in their lipid composition, and these differences appear to influence motor recruitment. Among the lipids that show significant degrees of compartment specificity are phosphoinositides, which can be recognised by various domains in motors or adaptors. For example, the pleckstrin homology (PH) domains of kinesins-3 KIF1A/KIF1Bβ/Unc-104 can interact with phospatidylinositol-4,5-bisphosphate (PIP2) on synaptic vesicles [29], while the PX domain of kinesin-3 KIF16B can bind to phospatidylinositol-3-phosphate (PI(3)P) on early endosomes [30]. A polybasic region, which represents another type of lipid binding domain, is present in the PIP2-interacting CBD of myosin VI [31]. In the case of the myosin Vb-FIP2-Rab11 complex, FIP2’s C2A domain might associate with PIP3 in the recycling endosome membrane [32]. Motor recruitment can also be affected by cholesterol, the enrichment of which in late endosomes can regulate the architecture of the dynein-dynactin binding complex through the cholesterol sensor ORP1L [18]. Similarly, the Myo2-Vac17-Vac8 transport complex on the vacuole binds specifically to ergosterol and sphingolipid-rich membrane domains after the palmitoylation of Vac8 [19]. While lipids are unlikely to act as sole targeting factors, they probably contribute to motor recruitment specificity by coincidence detection mechanisms.
Motor “multitasking”
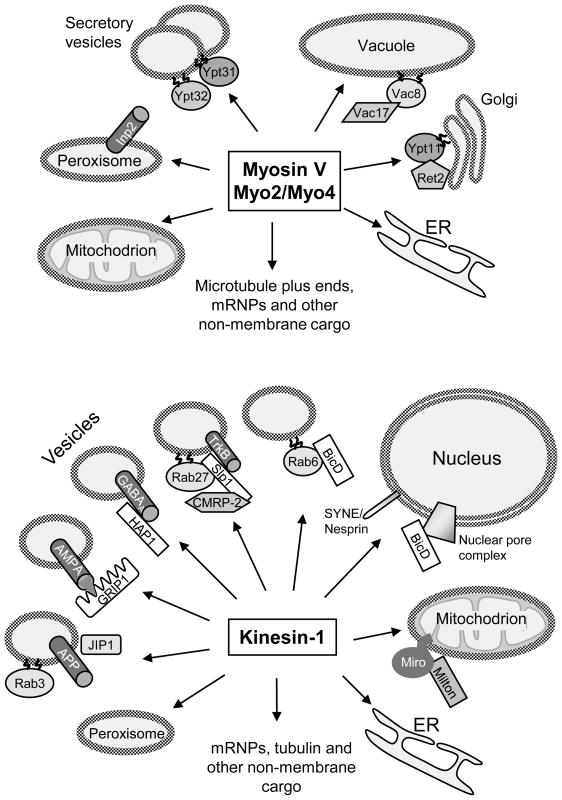
The job of transporting numerous cellular cargos is not distributed evenly among the members of the three motor families. For example, in mammals and flies the majority of microtubule minus end-directed transport processes are carried out by cytoplasmic dynein [26]. Since most known cargos can move along microtubules in both directions, this means that virtually every organelle in animal cells has some kind of dynein receptor. Myosin V is the main “workhorse” in yeast, an actin-centric organism, as it transports at least six distinct membrane compartments (Figure 1). Among animal kinesins, the most ubiquitous and versatile motor is kinesin-1 [33] (Figure 1). Some of these transport pathways require separate adaptors: for example, a complex of the Rho-like GTPase Miro and Milton1/TRAK1 adaptor attaches kinesin-1 to mitochondria [34]. Other adaptors are shared between different pathways: for example, Bicaudal D participates in kinesin-1 and dynein-mediated movements of exocytotic vesicles, nuclei and mRNPs [35–38]. Further, the versatility of a particular motor can be enhanced by alternative splicing: for example, Exon F is required for myosin Va to interact stably with melanophilin [10], while the “long insert” in the tail domain of myosin VI augments its interaction with Dab2 [39].
Figure 1. “Multitasking” motors.
The scheme illustrates the multiplicity of cargos transported by the two type V myosins (Myo2 and Myo4) in budding yeast and kinesin-1 in mammals. Where known, components of membrane attachment protein complexes and their modes of membrane interaction are indicated. Membrane attachment often depends on lipid anchors, such as geranylgeranyl groups in the case of Rabs (Ypt11, Ypt31/32 and Rab3, Rab6 and Rab27a), and myristoyl and palmitoyl groups for the yeast vacuole protein Vac8 [19]. Transmembrane proteins, such as Inp2 [1], the small GTPase Miro [34], APP [2], and various receptors such as AMPAR [20], GABAA [51] and TrkB [23 ″] can also serve as a part of the motor attachment complex, often in conjunction with adaptors. Animal nuclei can be linked to microtubule motors through proteins of the Syne/nesprin family, large cytoskeletal linkers that pierce the outer nuclear membrane [52 ″], or through nuclear pore complexes [35]. Compartment-specific motor receptors can attach to unique regions on the surface of the motor’s CBD, as has been described for Myo2 [76]. Kinesin-1 also uses different binding sites for different cargo: it is a heterotetramer of two heavy and two light chains, and some adaptors such as Milton, HAP1, and GRIP1 interact with the heavy chains [20,34,51], while others, such as JIP1 and CMRP-2 [23 ″,40], bind to the light chains. Possible competition for the same binding site on the motor has also been described [1], underscoring the need for regulation and coordination in multitasking. For some organelles, such as the ER, motor receptors are still elusive. In mammalian cells, the transmembrane ER protein kinectin was proposed to act as a kinesin receptor, but its importance was later disputed [33].
Coordination of motor activity with cargo binding
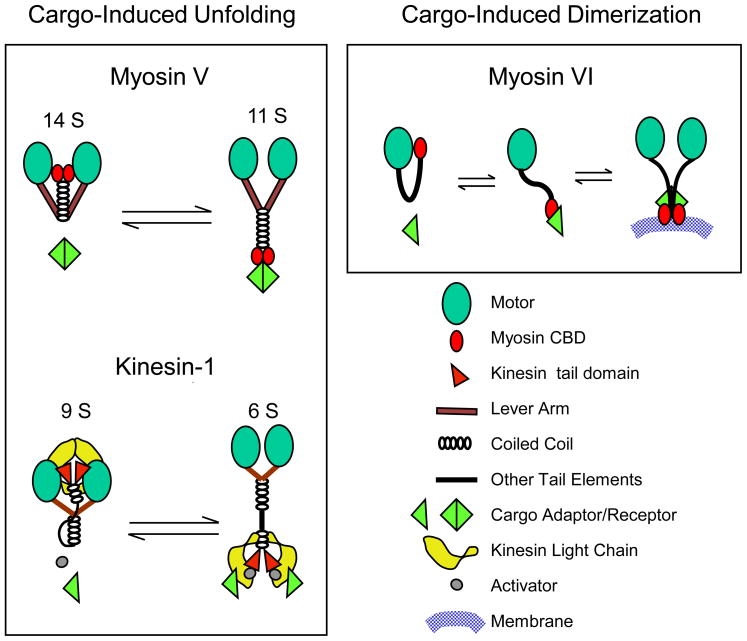
To avoid the useless expenditure of energy, many motors may exist in an enzymatically and mechanically inactive form when they are not bound to cargo [40,41]. A classic example is represented by the self-folding of kinesin-1, where in the absence of cargo the tail domain folds back to interact with the motor domain and inhibit its ATPase activity [42 ″]. This autoinhibition is released by the binding of kinesin-1 to two partners: the cargo protein JIP1 (via the kinesin’s light chain) and the activator protein FEZ1 (via the kinesin’s heavy chain) (Figure 2) [40]. Myosin Va represents another clear example of cargo-driven unfolding/activation, as the binding of melanophilin to the myosin’s CBD drives the myosin from its inactive, cargo-free, folded (14S) conformation to its processive, cargo-bound, extended (11S) conformation (Figure 2) [41].
Figure 2. Cargo-dependent regulation of motors.
The schemes illustrate the two main mechanisms identified to date where cargo can regulate motor mechanochemistry: cargo-driven unfolding (myosin V and kinesin-1) and cargo-driven dimerization (myosin VI). In the case of myosin V [41] and kinesin-1 [40], these intrinsically dimeric molecules exist in a folded, enzymatically and mechanically quiescent state in the absence of cargo, and in an extended, active state in the presence of cargo. In the case of myosin V, one such unfolding/activating cargo is melanophilin [77], while the unfolding/activation of kinesin-I requires an activator (FEZ1) in addition to a cargo (JIP1) [40]. Whether cargos simply trap the motor kinetically in its extended state or can allosterically induce the extended state remains an important unanswered question. Interestingly, fluorescence resonance energy transfer (FRET) studies showed that in folded kinesin-I, the motor’s light chains push the heads far apart, presumably to inhibit motility [40]. Other kinesin family members (e.g. kinesin-2, kinesin-7) are also subject to cargo-dependent unfolding/activation [40]. In the case of myosin VI, a quiescent, folded monomer can be converted to an extended processive dimer through interaction with dimeric cargo [9 ″″]. Dimerization may be facilitated by subsequent self association of the myosin VI heavy chains through weak coiled coil interactions, as well as by the sensing of membrane lipids by the CBD. Note that the properties of the medial tail of myosin VI (e.g. contribution to dimerization, lever arm length, reverse movement, etc) are currently areas of intense debate (see [43] for review). The cargo-unfolded, monomeric, non-processive version of myosin VI might also support certain cellular functions.
In addition to cargo-dependent unfolding, the activity of some motors is regulated by cargo-dependent dimerization. Motor processivity is generally thought to require the presence of two motor heads working in a coordinated fashion. However, some motors exist as monomers and only dimerize when attached to cargo. One clear example of this is myosin VI: the binding of cargo like Dab2 to myosin VI’s CBD converts the soluble monomer to a membrane-associated processive dimer [9 ″″], although dimerization may be further enhanced by subsequent interactions between the two myosin VI heavy chains [43] (Figure 2). Cargo-dependent dimerization via PH domain-dependent interaction with lipids/rafts was also proposed for the kinesin-3 KIF1A [44], although this has recently been disputed [45]. Nevertheless, cargo-dependent control of motor mechanochemistry as a general biological mechanism just makes great sense - it avoids energy waste, prevents free motors from piling up at the ends of cytoskeletal tracks, and promotes motor recycling by diffusion.
Motor cross-talk
It is generally accepted that different motors coexist on the same cargo. Moreover, in microtubule-based transport the ability of organelles to switch their direction of movement is the rule rather than the exception [28,46]. Interestingly, the knockdown of just the plus (or minus) end-directed motor on an organelle can result in a complete block in its bidirectional motility [47 ″″]. There is mounting evidence that different motors on the same cargo can either undergo a tug-of-war or be switched on and off in a coordinated manner [46,48]. Although bidirectional transport can be explained by purely mechanical motor interactions without invoking their physical association [48], detailed studies of motor complexes indicate that certain adaptor molecules can bind to motors of opposite polarity, placing these adaptors in an ideal position to coordinate bidirectional organelle movement. Most of the known examples involve accessory factors for dynein: for example, dynactin can interact with kinesin-2 and affects its processivity [49]. Similarly, Bicaudal D and NUDC, known primarily as dynein co-factors, interact with kinesin-1 and are required for certain kinesin-mediated processes [35,36,50]. HAP-1, an adaptor involved in GABAA receptor delivery to the synapses [51], interacts with both dynactin and kinesin-1. Syne/nesprin family members, scaffolding proteins in outer nuclear membrane, serve as attachment sites for both dynein and kinesin-1 [38,52 ″]. Physical connections between dynein and kinesins probably reflect the importance of dynein in virtually every transport pathway in animal cells. While the mechanistic reasons for bidirectional transport are not yet entirely clear, it is possible that opposite polarity motors might help each other to avoid roadblocks or activate each other through the generation of mechanical strain [47 ″″]. Furthermore, by linking to various anterograde motors, dynein might be able to hitch a ride to distal cell areas where it cannot be synthesized but needs to function (e.g. the end of an axon or the tip of the cilium).
Motors of the same polarity can also be linked together. A clear example occurs in intraflagellar transport in worms, where two different kinesin-2 motors with distinct intrinsic velocities drive together the same cargo particles, resulting in an intermediate speed of movement [53]. In fact, many organelles such as mitochondria, exocytotic vesicles, and synaptic cargo are probably transported by several redundant kinesins whose cooperation makes transport more robust. Finally, transport of certain cargo, such as pigment granules or recycling endosomes, can be driven by the action of both microtubule and actin-based motors [10,25,54 ″″–56]. Therefore, components of the motor recruitment machinery might facilitate switching between the two cytoskeletal systems. For example, in mouse pigment cells the myosin V adaptor melanophilin accumulates at growing microtubule ends through its interaction with the microtubule plus end-binding protein EB1 [57], while in zebrafish pigment cells it facilitates granule dispersion by regulating dynein [58]. Huntingtin, the protein affected in the neurodegenerative disorder Huntington’s disease, interacts not only with dynein, kinesin-1 and dynactin through HAP1, but also with myosin VI through optineurin, suggesting that it might control switching between different motors and filament systems [59]. In addition, microtubule and actin motors can positively or negatively affect the processivity of each other [60,61].
Control of motor association and function
Motor: cargo interaction must be reversible and its regulation can determine the cargo’s final destination. In those instances where a Rab GTPase participates in motor recruitment, the Rab’s nucleotide state will be a critical regulatory site. Indeed, manipulation of the expression levels of a Rab27a-specific GAP or GEF dramatically affects myosin V-dependent melanosome distribution in melanocytes [62,63]. Other regulatory mechanisms might involve control of the abundance of the motor and/or cargo adaptor. The clearest example to date is the temporally and spatially controlled degradation of the Myo2 adaptor Vac17 that is required to deposit the vacuole in its correct location during budding [64]. Similarly, the levels of Myo2 receptor Inp2 on peroxisomes are regulated by cell cycle progression and organelle position in the cell [1].
Factors involved in signaling can be an integral part of the motor recruitment complex. For example, motors on frog melanosomes associate with a regulatory subunit of protein kinase A that regulates their movement [65]. Similarly, JIP1, a cargo adaptor of kinesin-1, not only links it to vesicular cargo but also recruits MAPKKK, MAPKK and JNK signaling pathway components that can induce kinesin release (see [40] for review). Another kinase that can exert both local and temporal control of motor dissociation is CAMKII. In neurons, CAMKII activated by elevated cytosolic calcium at post-synaptic sites phosphorylates the CBD of the kinesin-2 KIF17, causing it to dissociate from its adaptor on NMDA receptor vesicles [66 ″″]. CAMKII can also affect synaptic cargo trafficking by inducing the degradation of the scaffolding protein liprin-α1 [67]. During mitosis, CAMKII phosphorylates the CBD of myosin V causing its dissociation from melanosomes [68]. Kinase activity can also potentiate motor: cargo interactions; for example, the Cdk1-dependent phosphorylation of Vac17 increases its affinity for Myo2 [69 ″].
Calcium can also regulate motor complexes directly. For example, calcium influx arrests mitochondria movement in neurons. The key player in this process is the Rho-like GTPase Miro, which participates in kinesin-1 recruitment to mitochondria, and which also contains EF-hand motifs that sense calcium levels. A calcium-induced conformational change in Miro is thought to result in the arrest of mitochondrial movement by causing either the dissociation of kinesin-1 from mitochondria or an inhibition of kinesin-1’s interaction with microtubules [70 ″,71 ″]. Calcium influx can also activate organelle movement, as elevated calcium levels in dendritic spines following strong presynaptic input are thought to trigger the movement of AMPA receptor-containing recycling endosomes into the spine by driving the unfolding and activation of myosin Vb [24 ″]. A similar calcium-dependent activation of myosin V might underlie the light-induced movement of pigment granules that drives “pupil” constriction in the fly eye [72].
Finally, a plethora of factors ranging from very small proteins such as Halo [73] to very large proteins such as Huntingtin [59] can affect motor activity, processivity and switching.
Conclusions
Intracellular transport systems face the daunting task of differentially localizing a large number of very diverse cellular structures by using the same set of cytoskeletal tracks in a common cytoplasm. Some organelles have to be distributed evenly, while others must be concentrated in certain regions of the cell or relocated rapidly in response to different stimuli. This complexity in transport requirements places a great number of demands on motor: cargo interaction - demands for specificity, temporal and spatial regulation, cooperation with other motors, and communication with the machineries that drive the formation and eventual fate of organelles and vesicular carriers. It is becoming increasingly clear that cells deal with these demands by recruiting motors to cargo through large protein complexes that contain small GTPases, specific adaptors, multifunctional scaffolds, and regulatory factors. As more information is becoming available, it appears that in addition to some generic elements, almost every cargo utilizes some specific molecules that participate in motor recruitment. While the catalogue of motor-binding proteins on different organelles is rapidly expanding, understanding how they work together represents a major challenge. In vitro reconstitution experiments using purified components at physiological concentrations will be needed to dissect which components and protein interactions are sufficient as well as necessary for all transport steps [74]. For example, the reconstitution of a Rab- and SNARE-dependent vesicle fusion complex involving 17 recombinant proteins has recently been achieved [75 ″″]. It is likely that similar efforts will be needed to fully understand the complexities of motor recruitment and regulation.
Acknowledgments
We thank Xufeng Wu for assistance in preparing Figure 2. We apologize for not citing many important references due to space limitations. A.A. is supported by the Netherlands Organisation for Scientific Research grants ALW-VICI and ZonMW-TOP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fagarasanu A, Mast FD, Knoblach B, Jin Y, Brunner MJ, Logan MR, Glover JN, Eitzen GA, Aitchison JD, Weisman LS, et al. Myosin-driven peroxisome partitioning in S. cerevisiae. J Cell Biol. 2009;186:541–554. doi: 10.1083/jcb.200904050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kamal A, Stokin GB, Yang Z, Xia CH, Goldstein LS. Axonal transport of amyloid precursor protein is mediated by direct binding to the kinesin light chain subunit of kinesin-I. Neuron. 2000;28:449–459. doi: 10.1016/s0896-6273(00)00124-0. [DOI] [PubMed] [Google Scholar]
- 3.Vallee RB, Williams JC, Varma D, Barnhart LE. Dynein: An ancient motor protein involved in multiple modes of transport. J Neurobiol. 2004;58:189–200. doi: 10.1002/neu.10314. [DOI] [PubMed] [Google Scholar]
- 4.Szodorai A, Kuan YH, Hunzelmann S, Engel U, Sakane A, Sasaki T, Takai Y, Kirsch J, Muller U, Beyreuther K, et al. APP anterograde transport requires Rab3A GTPase activity for assembly of the transport vesicle. J Neurosci. 2009;29:14534–14544. doi: 10.1523/JNEUROSCI.1546-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams JC, Roulhac PL, Roy AG, Vallee RB, Fitzgerald MC, Hendrickson WA. Structural and thermodynamic characterization of a cytoplasmic dynein light chain-intermediate chain complex. Proc Natl Acad Sci U S A. 2007;104:10028–10033. doi: 10.1073/pnas.0703614104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wassmer T, Attar N, Harterink M, van Weering JR, Traer CJ, Oakley J, Goud B, Stephens DJ, Verkade P, Korswagen HC, et al. The retromer coat complex coordinates endosomal sorting and dynein-mediated transport, with carrier recognition by the trans-Golgi network. Dev Cell. 2009;17:110–122. doi: 10.1016/j.devcel.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arai S, Noda Y, Kainuma S, Wada I, Yoda K. Ypt11 functions in bud-directed transport of the Golgi by linking Myo2 to the coatomer subunit Ret2. Curr Biol. 2008;18:987–991. doi: 10.1016/j.cub.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 8.Delevoye C, Hurbain I, Tenza D, Sibarita JB, Uzan-Gafsou S, Ohno H, Geerts WJ, Verkleij AJ, Salamero J, Marks MS, et al. AP-1 and KIF13A coordinate endosomal sorting and positioning during melanosome biogenesis. J Cell Biol. 2009;187:247–264. doi: 10.1083/jcb.200907122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9″″.Yu C, Feng W, Wei Z, Miyanoiri Y, Wen W, Zhao Y, Zhang M. Myosin VI undergoes cargo-mediated dimerization. Cell. 2009;138:537–548. doi: 10.1016/j.cell.2009.05.030. This paper combines NMR and crystallographic studies to demonstrate that myosin VI is a stable monomer in solution but undergoes dimerization after binding to the clathrin vesicle adaptor Dab2. The 3D structure of the complex between myosin VI’s CBD and the myosin VI interaction region (MIR) of Dab2 shows how two MIR peptides drive the dimerization of the CBD by having the two ends of each individual MIR cross over to interact with the two CBDs in the complex. [DOI] [PubMed] [Google Scholar]
- 10.Wu XS, Rao K, Zhang H, Wang F, Sellers JR, Matesic LE, Copeland NG, Jenkins NA, Hammer JA., 3rd Identification of an organelle receptor for myosin-Va. Nat Cell Biol. 2002;4:271–278. doi: 10.1038/ncb760. [DOI] [PubMed] [Google Scholar]
- 11.Strom M, Hume AN, Tarafder AK, Barkagianni E, Seabra MC. A family of Rab27-binding proteins. Melanophilin links Rab27a and myosin Va function in melanosome transport. J Biol Chem. 2002;277:25423–25430. doi: 10.1074/jbc.M202574200. [DOI] [PubMed] [Google Scholar]
- 12.Fukuda M, Kuroda TS, Mikoshiba K. Slac2-a/melanophilin, the missing link between Rab27 and myosin Va: implications of a tripartite protein complex for melanosome transport. J Biol Chem. 2002;277:12432–12436. doi: 10.1074/jbc.C200005200. [DOI] [PubMed] [Google Scholar]
- 13.Sahlender DA, Roberts RC, Arden SD, Spudich G, Taylor MJ, Luzio JP, Kendrick-Jones J, Buss F. Optineurin links myosin VI to the Golgi complex and is involved in Golgi organization and exocytosis. J Cell Biol. 2005;169:285–295. doi: 10.1083/jcb.200501162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Short B, Preisinger C, Schaletzky J, Kopajtich R, Barr FA. The Rab6 GTPase regulates recruitment of the dynactin complex to Golgi membranes. Curr Biol. 2002;12:1792–1795. doi: 10.1016/s0960-9822(02)01221-6. [DOI] [PubMed] [Google Scholar]
- 15.Matanis T, Akhmanova A, Wulf P, Del Nery E, Weide T, Stepanova T, Galjart N, Grosveld F, Goud B, De Zeeuw CI, et al. Bicaudal-D regulates COPI-independent Golgi-ER transport by recruiting the dynein-dynactin motor complex. Nat Cell Biol. 2002;4:986–992. doi: 10.1038/ncb891. [DOI] [PubMed] [Google Scholar]
- 16.Hoogenraad CC, Wulf P, Schiefermeier N, Stepanova T, Galjart N, Small JV, Grosveld F, de Zeeuw CI, Akhmanova A. Bicaudal D induces selective dynein-mediated microtubule minus end-directed transport. Embo J. 2003;22:6004–6015. doi: 10.1093/emboj/cdg592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johansson M, Rocha N, Zwart W, Jordens I, Janssen L, Kuijl C, Olkkonen VM, Neefjes J. Activation of endosomal dynein motors by stepwise assembly of Rab7-RILP-p150Glued, ORP1L, and the receptor betalll spectrin. J Cell Biol. 2007;176:459–471. doi: 10.1083/jcb.200606077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rocha N, Kuijl C, van der Kant R, Janssen L, Houben D, Janssen H, Zwart W, Neefjes J. Cholesterol sensor ORP1L contacts the ER protein VAP to control Rab7-RILP-p150 Glued and late endosome positioning. J Cell Biol. 2009;185:1209–1225. doi: 10.1083/jcb.200811005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishikawa K, Catlett NL, Novak JL, Tang F, Nau JJ, Weisman LS. Identification of an organelle-specific myosin V receptor. J Cell Biol. 2003;160:887–897. doi: 10.1083/jcb.200210139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Setou M, Seog DH, Tanaka Y, Kanai Y, Takei Y, Kawagishi M, Hirokawa N. Glutamate-receptor-interacting protein GRIP1 directly steers kinesin to dendrites. Nature. 2002;417:83–87. doi: 10.1038/nature743. [DOI] [PubMed] [Google Scholar]
- 21.Arden SD, Puri C, Au JS, Kendrick-Jones J, Buss F. Myosin VI is required for targeted membrane transport during cytokinesis. Mol Biol Cell. 2007;18:4750–4761. doi: 10.1091/mbc.E07-02-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spangler SA, Hoogenraad CC. Liprin-alpha proteins: scaffold molecules for synapse maturation. Biochem Soc Trans. 2007;35:1278–1282. doi: 10.1042/BST0351278. [DOI] [PubMed] [Google Scholar]
- 23″.Arimura N, Kimura T, Nakamuta S, Taya S, Funahashi Y, Hattori A, Shimada A, Menager C, Kawabata S, Fujii K, et al. Anterograde transport of TrkB in axons is mediated by direct interaction with Slp1 and Rab27. Dev Cell. 2009;16:675–686. doi: 10.1016/j.devcel.2009.03.005. By combining detailed biochemical analyses with imaging in cultured neurons, this study identifies the multicomponent protein complex that is responsible for linking axonal carriers of the neurotrophin receptor TrkB to kinesin-1. [DOI] [PubMed] [Google Scholar]
- 24″.Wang Z, Edwards JG, Riley N, Provance DW, Jr, Karcher R, Li XD, Davison IG, Ikebe M, Mercer JA, Kauer JA, et al. Myosin Vb mobilizes recycling endosomes and AMPA receptors for postsynaptic plasticity. Cell. 2008;135:535–548. doi: 10.1016/j.cell.2008.09.057. Previous work from this lab had demonstrated that strong, long-term potentiation (LTP) -inducing stimulation causes AMPA receptor-containing recycling endosomes to move into dendritic spines, where they then provide AMPA receptors for insertion into the post-synaptic density to drive LTP. Using a plethora of approaches, these authors show in this paper that the translocation of these endosomes is driven by myosin Vb. Moreover, evidence is presented that elevation of calcium in dendritic spines triggers the movement by driving the unfolding and activation of the myosin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hales CM, Vaerman JP, Goldenring JR. Rab11 family interacting protein 2 associates with Myosin Vb and regulates plasma membrane recycling. J Biol Chem. 2002;277:50415–50421. doi: 10.1074/jbc.M209270200. [DOI] [PubMed] [Google Scholar]
- 26.Kardon JR, Vale RD. Regulators of the cytoplasmic dynein motor. Nat Rev Mol Cell Biol. 2009;10:854–865. doi: 10.1038/nrm2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27″″.Shubeita GT, Tran SL, Xu J, Vershinin M, Cermelli S, Cotton SL, Welte MA, Gross SP. Consequences of motor copy number on the intracellular transport of kinesin-1-driven lipid droplets. Cell. 2008;135:1098–1107. doi: 10.1016/j.cell.2008.10.021. By using advanced biophysical methods, the authors measure the force that kinesin-1 exerts on lipid droplets in fly embryos, and thus provide an estimate of the number of active motors on an individual droplet in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gross SP, Vershinin M, Shubeita GT. Cargo transport: two motors are sometimes better than one. Curr Biol. 2007;17:R478–486. doi: 10.1016/j.cub.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 29.Klopfenstein DR, Tomishige M, Stuurman N, Vale RD. Role of phosphatidylinositol(4,5)bisphosphate organization in membrane transport by the Unc104 kinesin motor. Cell. 2002;109:347–358. doi: 10.1016/s0092-8674(02)00708-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoepfner S, Severin F, Cabezas A, Habermann B, Runge A, Gillooly D, Stenmark H, Zerial M. Modulation of receptor recycling and degradation by the endosomal kinesin KIF16B. Cell. 2005;121:437–450. doi: 10.1016/j.cell.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 31.Spudich G, Chibalina MV, Au JS, Arden SD, Buss F, Kendrick-Jones J. Myosin VI targeting to clathrin-coated structures and dimerization is mediated by binding to Disabled-2 and PtdIns(4,5)P2. Nat Cell Biol. 2007;9:176–183. doi: 10.1038/ncb1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lindsay AJ, McCaffrey MW. The C2 domains of the class I Rab11 family of interacting proteins target recycling vesicles to the plasma membrane. J Cell Sci. 2004;117:4365–4375. doi: 10.1242/jcs.01280. [DOI] [PubMed] [Google Scholar]
- 33.Hirokawa N, Noda Y, Tanaka Y, Niwa S. Kinesin superfamily motor proteins and intracellular transport. Nat Rev Mol Cell Biol. 2009;10:682–696. doi: 10.1038/nrm2774. [DOI] [PubMed] [Google Scholar]
- 34.Glater EE, Megeath LJ, Stowers RS, Schwarz TL. Axonal transport of mitochondria requires milton to recruit kinesin heavy chain and is light chain independent. J Cell Biol. 2006;173:545–557. doi: 10.1083/jcb.200601067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Splinter D, Tanenbaum ME, Lindqvist A, Jaarsma D, Flotho A, Yu KL, Grigoriev I, Engelsma D, Haasdijk ED, Keijzer N, et al. Bicaudal D2, dynein and kinesin-1 associate with nuclear pore complexes and regulate centrosome and nuclear positioning during mitotic entry. PLoS Biol. 2010 doi: 10.1371/journal.pbio.1000350. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grigoriev I, Splinter D, Keijzer N, Wulf PS, Demmers J, Ohtsuka T, Modesti M, Maly IV, Grosveld F, Hoogenraad CC, et al. Rab6 regulates transport and targeting of exocytotic carriers. Dev Cell. 2007;13:305–314. doi: 10.1016/j.devcel.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 37.Dienstbier M, Boehl F, Li X, Bullock SL. Egalitarian is a selective RNA-binding protein linking mRNA localization signals to the dynein motor. Genes Dev. 2009;23:1546–1558. doi: 10.1101/gad.531009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fridolfsson HN, Ly N, Meyerzon M, Starr DA. UNC-83 coordinates kinesin-1 and dynein activities at the nuclear envelope during nuclear migration. Dev Biol. 2010;338:237–250. doi: 10.1016/j.ydbio.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buss F, Kendrick-Jones J. How are the cellular functions of myosin VI regulated within the cell? Biochem Biophys Res Commun. 2008;369:165–175. doi: 10.1016/j.bbrc.2007.11.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verhey KJ, Hammond JW. Traffic control: regulation of kinesin motors. Nat Rev Mol Cell Biol. 2009;10:765–777. doi: 10.1038/nrm2782. [DOI] [PubMed] [Google Scholar]
- 41.Sellers JR, Thirumurugan K, Sakamoto T, Hammer JA, 3rd, Knight PJ. Calcium and cargoes as regulators of myosin 5a activity. Biochem Biophys Res Commun. 2008;369:176–181. doi: 10.1016/j.bbrc.2007.11.109. [DOI] [PubMed] [Google Scholar]
- 42″.Dietrich KA, Sindelar CV, Brewer PD, Downing KH, Cremo CR, Rice SE. The kinesin-1 motor protein is regulated by a direct interaction of its head and tail. Proc Natl Acad Sci U S A. 2008;105:8938–8943. doi: 10.1073/pnas.0803575105. By using cryoelectron microscopy-based reconstruction of the kinesin-1 head-tail complex, the authors provide important insights into the molecular basis of kinesin-1 autoinhibition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spudich JA, Sivaramakrishnan S. Myosin VI: an innovative motor that challenged the swinging lever arm hypothesis. Nat Rev Mol Cell Biol. 2010;11:128–137. doi: 10.1038/nrm2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tomishige M, Klopfenstein DR, Vale RD. Conversion of Unc104/KIF1A kinesin into a processive motor after dimerization. Science. 2002;297:2263–2267. doi: 10.1126/science.1073386. [DOI] [PubMed] [Google Scholar]
- 45.Hammond JW, Cai D, Blasius TL, Li Z, Jiang Y, Jih GT, Meyhofer E, Verhey KJ. Mammalian Kinesin-3 motors are dimeric in vivo and move by processive motility upon release of autoinhibition. PLoS Biol. 2009;7:e72. doi: 10.1371/journal.pbio.1000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Welte MA. Bidirectional transport along microtubules. Curr Biol. 2004;14:R525–537. doi: 10.1016/j.cub.2004.06.045. [DOI] [PubMed] [Google Scholar]
- 47″″.Ally S, Larson AG, Barlan K, Rice SE, Gelfand VI. Opposite-polarity motors activate one another to trigger cargo transport in live cells. J Cell Biol. 2009;187:1071–1082. doi: 10.1083/jcb.200908075. This elegant study uses artificial targeting of motors to membrane organelles to demonstrate that microtubule-based transport in Drosophila cells critically depends on the presence of opposite polarity motors on the same cargo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muller MJ, Klumpp S, Lipowsky R. Tug-of-war as a cooperative mechanism for bidirectional cargo transport by molecular motors. Proc Natl Acad Sci U S A. 2008;105:4609–4614. doi: 10.1073/pnas.0706825105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berezuk MA, Schroer TA. Dynactin enhances the processivity of kinesin-2. Traffic. 2007;8:124–129. doi: 10.1111/j.1600-0854.2006.00517.x. [DOI] [PubMed] [Google Scholar]
- 50.Yamada M, Toba S, Takitoh T, Yoshida Y, Mori D, Nakamura T, Iwane AH, Yanagida T, Imai H, Yu-Lee LY, et al. mNUDC is required for plus-end-directed transport of cytoplasmic dynein and dynactins by kinesin-1. Embo J. 2010;29:517–531. doi: 10.1038/emboj.2009.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Twelvetrees AE, Yuen EY, Arancibia-Carcamo IL, MacAskill AF, Rostaing P, Lumb MJ, Humbert S, Triller A, Saudou F, Yan Z, et al. Delivery of GABAARs to synapses is mediated by HAP1-KIF5 and disrupted by mutant huntingtin. Neuron. 2010;65:53–65. doi: 10.1016/j.neuron.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52″.Zhang X, Lei K, Yuan X, Wu X, Zhuang Y, Xu T, Xu R, Han M. SUN1/2 and Syne/Nesprin-1/2 complexes connect centrosome to the nucleus during neurogenesis and neuronal migration in mice. Neuron. 2009;64:173–187. doi: 10.1016/j.neuron.2009.08.018. Through mouse knockout approaches, this paper convincingly demonstrates that SUN domain proteins SUN1/2 and their partners of the Syne/nesprin family mediate nucleus-centrosome coupling during neuronal migration by interacting with both cytoplasmic dynein and kinesin-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pan X, Ou G, Civelekoglu-Scholey G, Blacque OE, Endres NF, Tao L, Mogilner A, Leroux MR, Vale RD, Scholey JM. Mechanism of transport of IFT particles in C. elegans cilia by the concerted action of kinesin-II and OSM-3 motors. J Cell Biol. 2006;174:1035–1045. doi: 10.1083/jcb.200606003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54″″.Semenova I, Burakov A, Berardone N, Zaliapin I, Slepchenko B, Svitkina T, Kashina A, Rodionov V. Actin dynamics is essential for myosin-based transport of membrane organelles. Curr Biol. 2008;18:1581–1586. doi: 10.1016/j.cub.2008.08.070. This is one of the few examples of detailed analysis of the parameters of actin-based organelle movement in vertebrate cells. The authors show that the ability of actin filaments to remodel is beneficial for actin-based transport. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schonteich E, Wilson GM, Burden J, Hopkins CR, Anderson K, Goldenring JR, Prekeris R. The Rip11/Rab11-FIP5 and kinesin II complex regulates endocytic protein recycling. J Cell Sci. 2008;121:3824–3833. doi: 10.1242/jcs.032441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Horgan CP, Hanscom SR, Jolly RS, Futter CE, McCaffrey MW. Rab11-FIP3 links the Rab11 GTPase and cytoplasmic dynein to mediate transport to the endosomal-recycling compartment. J Cell Sci. 2010;123:181–191. doi: 10.1242/jcs.052670. [DOI] [PubMed] [Google Scholar]
- 57.Wu XS, Tsan GL, Hammer JA., 3rd Melanophilin and myosin Va track the microtubule plus end on EB1. J Cell Biol. 2005;171:201–207. doi: 10.1083/jcb.200503028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sheets L, Ransom DG, Mellgren EM, Johnson SL, Schnapp BJ. Zebrafish melanophilin facilitates melanosome dispersion by regulating dynein. Curr Biol. 2007;17:1721–1734. doi: 10.1016/j.cub.2007.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Caviston JP, Holzbaur EL. Huntingtin as an essential integrator of intracellular vesicular trafficking. Trends Cell Biol. 2009;19:147–155. doi: 10.1016/j.tcb.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hodges AR, Bookwalter CS, Krementsova EB, Trybus KM. A nonprocessive class V myosin drives cargo processively when a kinesin- related protein is a passenger. Curr Biol. 2009;19:2121–2125. doi: 10.1016/j.cub.2009.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Woolner S, Bement WM. Unconventional myosins acting unconventionally. Trends Cell Biol. 2009;19:245–252. doi: 10.1016/j.tcb.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Figueiredo AC, Wasmeier C, Tarafder AK, Ramalho JS, Baron RA, Seabra MC. Rab3GEP is the non-redundant guanine nucleotide exchange factor for Rab27a in melanocytes. J Biol Chem. 2008;283:23209–23216. doi: 10.1074/jbc.M804134200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Itoh T, Fukuda M. Identification of EPI64 as a GTPase-activating protein specific for Rab27A. J Biol Chem. 2006;281:31823–31831. doi: 10.1074/jbc.M603808200. [DOI] [PubMed] [Google Scholar]
- 64.Tang F, Kauffman EJ, Novak JL, Nau JJ, Catlett NL, Weisman LS. Regulated degradation of a class V myosin receptor directs movement of the yeast vacuole. Nature. 2003;422:87–92. doi: 10.1038/nature01453. [DOI] [PubMed] [Google Scholar]
- 65.Kashina AS, Semenova IV, Ivanov PA, Potekhina ES, Zaliapin I, Rodionov VI. Protein kinase A, which regulates intracellular transport, forms complexes with molecular motors on organelles. Curr Biol. 2004;14:1877–1881. doi: 10.1016/j.cub.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 66″″.Guillaud L, Wong R, Hirokawa N. Disruption of KIF17-Mint1 interaction by CaMKII-dependent phosphorylation: a molecular model of kinesin-cargo release. Nat Cell Biol. 2008;10:19–29. doi: 10.1038/ncb1665. This paper provides an important example of locally regulated signal-mediated disassembly of motor: cargo complexes at their destination site. [DOI] [PubMed] [Google Scholar]
- 67.Hoogenraad CC, Feliu-Mojer MI, Spangler SA, Milstein AD, Dunah AW, Hung AY, Sheng M. Liprinalpha1 degradation by calcium/calmodulin-dependent protein kinase II regulates LAR receptor tyrosine phosphatase distribution and dendrite development. Dev Cell. 2007;12:587–602. doi: 10.1016/j.devcel.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 68.Karcher RL, Roland JT, Zappacosta F, Huddleston MJ, Annan RS, Carr SA, Gelfand VI. Cell cycle regulation of myosin-V by calcium/calmodulin-dependent protein kinase II. Science. 2001;293:1317–1320. doi: 10.1126/science.1061086. [DOI] [PubMed] [Google Scholar]
- 69″.Peng Y, Weisman LS. The cyclin-dependent kinase Cdk1 directly regulates vacuole inheritance. Dev Cell. 2008;15:478–485. doi: 10.1016/j.devcel.2008.07.007. The process of cell division in yeast, cell budding, places a high demand on the active inheritance of organelles into the bud, a demand that falls largely on the shoulders of the class V myosin Myo2. Here the authors show that the cyclin-dependent kinase Cdk1 phosphorylates the adaptor Vac17, which links Myo2 to Vac8 in the vacuole membrane, and that this phosphorylation controls the timing of vacuole inheritance in part by increasing the affinity of Vac17 for Myo2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Macaskill AF, Rinholm JE, Twelvetrees AE, Arancibia-Carcamo IL, Muir J, Fransson A, Aspenstrom P, Attwell D, Kittler JT. Miro1 is a calcium sensor for glutamate receptor-dependent localization of mitochondria at synapses. Neuron. 2009;61:541–555. doi: 10.1016/j.neuron.2009.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71″.Wang X, Schwarz TL. The mechanism of Ca2+-dependent regulation of kinesin-mediated mitochondrial motility. Cell. 2009;136:163–174. doi: 10.1016/j.cell.2008.11.046. Together with reference [70″], this paper provides an interesting example of how mitochondrial motility can be directly regulated by calcium levels through conformational changes in the calcium sensor Miro. It should be noted that the molecular details of the proposed regulatory processes, such as the importance of the adaptor protein Milton, are different between theses two studies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Satoh AK, Li BX, Xia H, Ready DF. Calcium-activated Myosin V closes the Drosophila pupil. Curr Biol. 2008;18:951–955. doi: 10.1016/j.cub.2008.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gross SP, Guo Y, Martinez JE, Welte MA. A determinant for directionality of organelle transport in Drosophila embryos. Curr Biol. 2003;13:1660–1668. doi: 10.1016/j.cub.2003.08.032. [DOI] [PubMed] [Google Scholar]
- 74.Wu X, Sakamoto T, Zhang F, Sellers JR, Hammer JA., 3rd In vitro reconstitution of a transport complex containing Rab27a, melanophilin and myosin Va. FEBS Lett. 2006;580:5863–5868. doi: 10.1016/j.febslet.2006.09.047. [DOI] [PubMed] [Google Scholar]
- 75″″.Ohya T, Miaczynska M, Coskun U, Lommer B, Runge A, Drechsel D, Kalaidzidis Y, Zerial M. Reconstitution of Rab- and SNARE-dependent membrane fusion by synthetic endosomes. Nature. 2009;459:1091–1097. doi: 10.1038/nature08107. In this tour de force of in vitro reconstitution, the authors recapitulate membrane fusion by early endosome machinery on synthetic liposomes in a system that includes SNAREs, the small GTPase Rab5, its key regulators and effectors. This study shows that complex Rab GTPase-dependent events, such as specific and efficient membrane tethering and fusion, can in principle be reconstituted in vitro, raising hope that similar approaches can be applied in future to membrane-motor complexes. [DOI] [PubMed] [Google Scholar]
- 76.Pashkova N, Jin Y, Ramaswamy S, Weisman LS. Structural basis for myosin V discrimination between distinct cargoes. Embo J. 2006;25:693–700. doi: 10.1038/sj.emboj.7600965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li XD, Ikebe R, Ikebe M. Activation of myosin Va function by melanophilin, a specific docking partner of myosin Va. J Biol Chem. 2005;280:17815–17822. doi: 10.1074/jbc.M413295200. [DOI] [PubMed] [Google Scholar]
- 78.Li BX, Satoh AK, Ready DF. Myosin V, Rab11, and dRip11 direct apical secretion and cellular morphogenesis in developing Drosophila photoreceptors. J Cell Biol. 2007;177:659–669. doi: 10.1083/jcb.200610157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Osterweil E, Wells DG, Mooseker MS. A role for myosin VI in postsynaptic structure and glutamate receptor endocytosis. J Cell Biol. 2005;168:329–338. doi: 10.1083/jcb.200410091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Niwa S, Tanaka Y, Hirokawa N. KIF1Bbeta- and KIF1A-mediated axonal transport of presynaptic regulator Rab3 occurs in a GTP-dependent manner through DENN/MADD. Nat Cell Biol. 2008;10:1269–1279. doi: 10.1038/ncb1785. [DOI] [PubMed] [Google Scholar]