Abstract
The filoviruses, Marburg virus (MARV) and Ebola virus (EBOV), are causative agents of severe hemorrhagic fever with high mortality rates in humans and non-human primates. Sporadic outbreaks of filovirus infection have occurred in Central Africa and parts of Asia. Identification of the natural reservoir animals that are unknown yet and epidemiological investigations are current challenges to forestall outbreaks of filovirus diseases. The filovirus species identified currently include one in the MARV group and five in the EBOV group, with large genetic variations found among the species. Therefore, it has been difficult to develop a single sensitive assay to detect all filovirus species, which would advance laboratory diagnosis greatly in endemic areas. In this study, a highly sensitive universal RT-PCR assay targeting the nucleo-protein (NP) gene of filoviruses was developed. The genomic RNAs of all known MARV and EBOV species were detected by using an NP-specific primer set. In addition, this RT-PCR procedure was verified further for its application to detect viral RNAs in tissue samples of animals infected experimentally and blood specimens of infected patients. This assay will be a useful method for diagnostics and epidemiological studies of filovirus infections.
Keywords: Filovirus, Marburg virus, Ebola virus, Diagnosis, RT-PCR
Marburg virus (MARV) and Ebola virus (EBOV) are enveloped, single-stranded, negative-sense RNA viruses classified into two genera, Marburgvirus and Ebolavirus, in the family Filoviridae, order Mononegavirales (Feldmann et al., 2004). These viruses are causative agents of severe hemorrhagic fever with high mortality rates in humans and non-human primates (Feldmann et al., 2003). There is a single Marburgvirus species, Lake Victoria marburgvirus (LVMARV), whereas there are four known Ebolavirus species, Zaire ebolavirus (ZEBOV), Sudan ebolavirus (SEBOV), Reston ebolavirus (REBOV) and Cote d'Ivoire ebolavirus (CIEBOV) (Feldmann et al., 2004). The genomic structures of filoviruses are very similar and approximately 19 kilobases in length, containing seven genes arranged sequentially in the order nucleoprotein (NP)-viral protein (VP) 35-VP40-glycoprotein-VP30-VP24-RNA polymerase (L) (Sanchez et al., 2006).
Since the discovery of Marburg hemorrhagic fever in Germany in 1967, sporadic outbreaks of Marburg and Ebola hemorrhagic fever have been reported from different countries in Central Africa (Feldmann et al., 2003). Incidences have increased in Central Africa since the beginning of the new millennium (Centers for Disease Control and Prevention, 2010a, b), and Bundibugyo ebolavirus (BEBOV) which has been proposed as a fifth species of EBOV was discovered recently in Uganda (Towner et al., 2008). Furthermore, two imported cases of Marburg hemorrhagic fever (in the Netherlands and the United States) (Centers for Disease Control and Prevention, 2009; Timen et al., 2009) and one of Ebola hemorrhagic fever (in South Africa) (World Health Organization, 1997) in travelers, have been reported, emphasizing the risk of filovirus infection in non-endemic countries.
Real-time RT-PCR (Drosten et al., 2002; Gibb et al., 2001; Weidmann et al., 2004) and reverse transcription-loop-mediated isothermal amplification (RT-LAMP) methods (Kurosaki et al., 2007, 2010) have been published recently for the diagnosis of filovirus infections. However, the real-time RT-PCR requires expensive, sophisticated equipment and thus does not seem to be practical for routine use in endemic areas such as Africa, and false-positive reactions in RT-LAMP cannot be discriminated since it does not provide valuable nucleotide sequence information on its products. More importantly, these methods established previously are relatively species-specific and cannot be applied reliably in diagnostics and field studies for the broad detection of filoviruses, including potential new species. For example, this was the case for BEBOV infections, which were not detected initially by using real-time RTPCR specific for the filoviruses species known previously, ZEBOV, SEBOV, and MARV (Towner et al., 2008). In fact, only one sample out of 20 human blood specimens suspected was positive in conventional RT-PCR using the currently available universal filovirus primers, FILO-A and FILO-B (Sanchez et al., 1999; Towner et al., 2008). Therefore, it is important to design new primers for RT-PCR allowing the broad detection of filovirus genomic RNA. In this study, nucleotide sequences of all known filovirus species were compared and a new assay for the detection of all known filoviruses using a primer set targeting a highly conserved region in the NP gene was evaluated.
One-step RT-PCR was carried out using a QIAGEN OneStep RT-PCR Kit (QIAGEN GmbH, Hilden, Germany) according to the manufacturer's instructions. A total volume of 25 μl of reaction mixture containing 0.2 μM of each primer and 1 μl of template RNA was used. Four primers targeting MARV and EBOV NP genes (FiloNP primers) were designed (Table 1). The one-step RT-PCR program consisted of reverse transcription at 50 °C for 30 min, initial PCR activation at 95 °C for 15 min, followed by 50 cycles of denaturation at 94 °C for 15 s, annealing at 53 °C for 30 s, extension at 72 °C for 30 s, and final extension at 72 °C for 7 min (Veriti 200 thermal cycler; Life Technologies Co., Carlsbad, CA). For comparison, the primers published previously, FILO-A and FILO-B, were used under the same conditions.
Table 1.
Primers used in this study.
| Primer | Sequence (5′–3′) | Target gene (position) | Product size | Reference strain (accession no.a) | Reference |
|---|---|---|---|---|---|
| FiloNP-Fm | TGGCTTACYACAGGYCACATGAAAGT | MARV NP (620–645) | 594 bp | MARV Musoke (NC_001608) | This study |
| FiloNP-Rm | GTGTGTGATTTCAGTTTTYTGGAGGTGGAA | MARVNP(l213–1184) | |||
| FiloNP-Fe | TGGCAATCAGTDGGACACATGATGGT | EBOV NP (1040–1065) | 594 bp | EBOV Mayinga (NC_002549) | This study |
| FiloNP-Re | GAAGCTGATTTCRTTCTTYTTCTGATGGAA | EBOV NP(l633-1604) | |||
| FILO-A | ATCGGAATTTTTCTTTCTCATT | Filovirus L (13123–13144) | 419 bp | MARV Musoke (NC_001608) | Sanchez et al. (1999) |
| FILO-B | ATGTGGTGGGTTATAATAATCACTGACATG | Filovirus L (13541–13512) |
Accession no. indicates Genbank accession number of reference sequence.
LVMARV (Popp, Ozolin, Musoke, Ravn, and Angola #368), ZEBOV (Mayinga and Kikwit), SEBOV (Boniface), CIEBOV (Cote d'Ivoire), BEBOV, and REBOV (Pennsylvania) strains were used in this study. These viruses were propagated in Vero E6 cells, and viral RNAs were extracted from 250 μl aliquots of culture supernatants using TRIzol-LS reagent (Invitrogen Co., Carlsbad, CA) according to the manufacturer's instructions. The extracted RNA was dissolved in 20 μl of nuclease-free distilled water. All infectious materials were handled in the biosafety level 4 facility of the National Microbiology Laboratory, Public Health Agency of Canada. Lassa virus, hantavirus, dengue 2 virus, and leptospira interrogans RNAs were used as specificity controls because of the similarity of disease symptoms and/or potential endemic area shared with filoviruses.
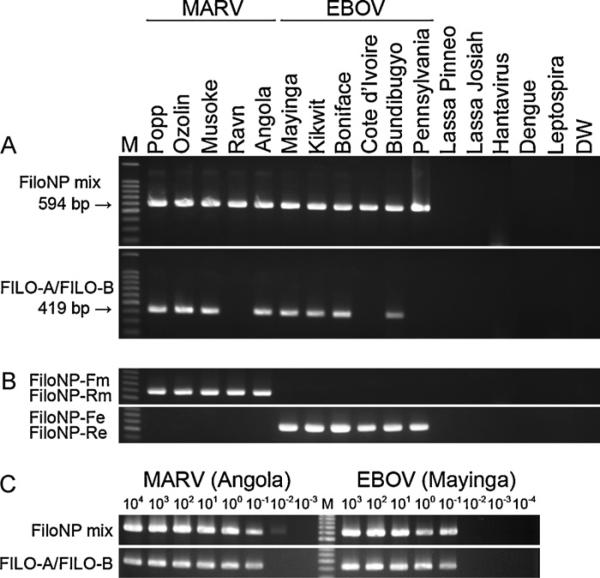
As shown in Fig. 1A, NP gene fragments of 5 MARV strains and 6 EBOV strains were amplified similarly as 594 bp products by the NP primer combination designed in this study. Nucleotide sequences of all the products were determined and identified as those derived from the respective template strains (data not shown). There was no nonspecific amplification of the Lassa virus and hantavirus RNAs tested. Furthermore, assays using FiloNP-Fm and FiloNP-Rm or FiloNP-Fe and FiloNP-Re primer combinations amplified separately MARV and EBOV RNAs, respectively (Fig. 1B), demonstrating the usefulness of these primer sets in differentiating between MARV and EBOV strains. In contrast, the primers published previously, FILO-A and FILO-B, failed to detect LVMARV Ravn, CIEBOV Cote d'Ivoire, and REBOV Pennsylvania strains (Fig. 1A). The lower stability of the primer match of FILO-A and FILO-B than NP primers, as seen in the alignments between primers and virus genomes, likely influenced the efficiency of amplification (Fig. 2).
Fig. 1.
Specificity and sensitivity of RT-PCR for the detection of MARV and EBOV. (A) Filovirus NP gene fragments were amplified by RT-PCR using a mixture of 4 primers, FiloNP-Fm, FiloNP-Rm, FiloNP-Fe, and FiloNP-Re (upper panel), or FILO-A and FILO-B (lower panel). Lassa virus (strains Pinneo and Josiah), hantavirus (species Dobraba, strain Slovenia), dengue 2 virus (strain VNHCM18-C/02), and leptospira interrogans (serovar Manilae, strain UP-MMC-NIID) RNAs were used to verify the specificity of the RT-PCR. Amplification of these control RNAs by RT-PCR was confirmed by using specific primers for the respective pathogens (data not shown). Nuclease-free distilled water (DW) was used as a negative control. Data are representative of two independent experiments. (B) MARV and EBOV NP genes were detected by FiloNPFm and FiloNP-Rm (upper panel) and FiloNP-Fe and FiloNP-Re (lower panel) primer sets, respectively. Lassa virus and hantavirus RNAs were used to verify the specificity of the RT-PCR. Data are representative of two independent experiments. (C) Tenfold serial dilutions of RNA derived from LVMARV, strain Angola (left) and ZEBOV, strain Mayinga (right) were analyzed; approximate virus titers (PFU for strain Angola and FFU for strain Mayinga) are shown at the top of the panel. Primers FiloNP-Fm, FiloNP-Rm, FiloNP-Fe, and FiloNP-Re (upper panel) and FILO-A and FILO-B (lower panel) were used for amplification. Data are representative of three independent experiments. Lane M: 100-bp DNA ladder.
Fig. 2.
Alignment of filovirus NP and L gene sequences and primer sequences used in this study. Reverse primers are shown as complementary sequences. Dots indicate the positions identical to LVMARV strain Popp, or ZEBOV strain Mayinga sequences. The numbers on the left and right indicate the respective nucleotide positions in the Popp and Mayinga strain genome sequences. Asterisks indicate the positions matching the primer sequences. Genbank accession numbers of the nucleotide sequences used in this study are Z29337 (Popp), AY358025 (Ozolin), NC 001608 (Musoke), EF446131 (Ravn), DQ447656 (Angola), NC 002549 (Mayinga), AY354458 (Kikwit), FJ968794 (Boniface), FJ217162 (Cote d'Ivoire), FJ217161 (Bundibugyo), and AY769362 (Pennsylvania).
To determine the sensitivity of the RT-PCR assay using NP primers, viral RNAs derived from supernatants of samples infected LVMARV strain Angola #368 (approximately 107 plaque-forming units (PFU)/ml) (Geisbert et al., 2007), and ZEBOV strain Mayinga (approximately 107 focus forming units (FFU)/ml), were diluted serially 10-fold in nuclease-free distilled water, and used as templates for amplification. The detection limits for LVMARV strain Angola #368, and ZEBOV strain Mayinga were approximately 10–2 to 10–1 PFU or FFU/reaction (Fig. 1C) and thus equivalent to the reported sensitivity for the universal primers designed previously (i.e. FILO-A and FILO-B). Although the RT-PCR may not be more sensitive than the TaqMan RT-PCR (Weidmann et al., 2004) and RT-LAMP (Kurosaki et al., 2007, 2010) established previously, the simplicity and cross-reactivity among all known filovirus strains provides an advantage for rapid diagnostics in reference centers and field settings.
Finally, the applicability of RT-PCR using NP primers to in vivo diagnostics was studied. Total RNA was extracted from 100 μl of a 10% (w/v) spleen homogenate derived from mice infected with mouse-adapted ZEBOV (titer approximately 107 FFU/g) (Ebihara et al., 2006) by using TRIzol-LS reagent (Invitrogen Co.) according to the manufacturer's instructions. The extracted RNA, dissolved in 30 μl of nuclease-free distilled water, was diluted serially 10-fold in nuclease-free distilled water and used as a template. Viral gene fragments were amplified successfully with the detection limit of approximately 10–3 FFU/reaction (Fig. 3A), demonstrating that the sensitivity of the RT-PCR assay using NP primers was equivalent to that of the RT-PCR using FILO-A and FILO-B reported previously (Sanchez et al., 1999). Subsequently, whole-blood samples from Marburg hemorrhagic fever cases in Angola in 2004/05 (World Health Organization, 2005) were analyzed. Total RNA was extracted from 100 μl of patient blood samples and dissolved in 10 μl of nuclease-free distilled water. Specific amplification of MARV NP gene sequences by the NP primer set was confirmed in all the samples tested, as with the primer set of FILO-A and FILO-B reported previously (Fig. 3B), demonstrating the applicability of this assay to human diagnostics.
Fig. 3.
Detection of EBOV and MARV in experimental animal and human specimens. The NP gene fragments were amplified by RT-PCR using a combination of 4 primers, FiloNP-Fm, FiloNP-Rm, FiloNP-Fe, and FiloNP-Re (upper panels), or FILO-A and FILO-B (lower panels). (A) Tenfold serial dilutions of RNA extracted from ZEBOV-infected mouse spleen were used as templates; approximate virus titers (FFU) used in each reaction are shown at the top of the panel. Data are representative of three independent experiments. (B) MARV-infected human blood specimens obtained during the 2004/05 Marburg hemorrhagic fever outbreak in Angola were analyzed for the presence of MARV RNA. Blood collection from humans during the outbreak in Angola was approved under a special response protocol established between the World Health Organization and national authorities. Lane M: 100-bp DNA ladder.
In this study, NP-gene-specific primers were designed. NP transcripts were detected in vitro as early as 7 h after infection and the number of NP transcript copies was abundant, in contrast to the much lower copy numbers of RNA transcripts derived from the L gene (Sanchez and Kiley, 1987). Thus, this RT-PCR assay using primer sets targeting the NP gene is expected to be more sensitive than those based on L-gene-specific primers to detect cell-associated filovirus RNA transcripts in infected primary target cells such as peripheral monocytes in the early stage of infection. The broad cross-reactivity of RT-PCR with the NP primers designed in this study compared to the ones reported previously will enhance filovirus PCR diagnostics and thus provide a novel tool for public health and biodefense. This combined with its simplicity will also improve ecological and epidemiological field studies in regions with poor infrastructure in Central Africa.
Acknowledgments
We thank Aiko Ohnuma for technical assistance and Kim Barrymore for editing the manuscript. We also thank the Special Pathogens Branch, Centers for Disease Control and Prevention, and Dr. Jiro Arikawa and Dr. Kumiko Yoshimatsu (Hokkaido University), Dr. Kouichi Morita (Nagasaki University), and Dr. Nobuo Koizumi (National Institute of Infectious Diseases) for providing the Bundibugyo ebolavirus isolate, hantavirus, dengue 2 virus, and leptospira RNAs, respectively. This work was supported by a grant-in-aid from the Ministry of Health, Labor and Welfare of Japan, and in part by the Program of Founding Research Centers for Emerging and Reemerging Infectious Diseases and the Global COE Program “Establishment of International Collaboration Centers for Zoonosis Control” from the Ministry of Education, Culture, Sports, Science, and Technology, Japan. In addition, the study was supported by the National Microbiology Laboratory of the Public Health Agency of Canada, and the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institute of Health.
References
- Centers for Disease Control Prevention Imported case of Marburg hemorrhagic fever – Colorado, 2008. MMWR Morb. Mortal. Wkly. Rep. 2009;58:1377–1381. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Known cases and outbreaks of Ebola hemorrhagic fever, in chronological order. 2010a Available at: http://www.cdc.gov/ncidod/dvrd/spb/mnpages/dispages/ebola/ebolatable.htm.
- Centers for Disease Control and Prevention Known cases and outbreaks of Marburg hemorrhagic fever, in chronological order. 2010b Available at: http://www.cdc.gov/ncidod/dvrd/spb/mnpages/dispages/marburg/marburgtable.htm.
- Drosten C, Gottig S, Schilling S, Asper M, Panning M, Schmitz H, Gunther S. Rapid detection and quantification of RNA of Ebola and Marburg viruses, Lassa virus, Crimean-Congo hemorrhagic fever virus, Rift Valley fever virus, dengue virus, and yellow fever virus by real-time reverse transcription-PCR. J. Clin. Microbiol. 2002;40:2323–2330. doi: 10.1128/JCM.40.7.2323-2330.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebihara H, Takada A, Kobasa D, Jones S, Neumann G, Theriault S, Bray M, Feldmann H, Kawaoka Y. Molecular determinants of Ebola virus virulence in mice. PLoS Pathog. 2006;2:e73. doi: 10.1371/journal.ppat.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann H, Geisbert TW, Jahrling PB. Filoviridae. In: Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA, editors. Virus Taxonomy: Eighth Report of the International Committee on Taxonomy of Viruses. Elsevier Academic Press; London: 2004. pp. 645–653. [Google Scholar]
- Feldmann H, Jones S, Klenk HD, Schnittler HJ. Ebola virus: from discovery to vaccine. Nat. Rev. Immunol. 2003;3:677–685. doi: 10.1038/nri1154. [DOI] [PubMed] [Google Scholar]
- Geisbert TW, Daddario-DiCaprio KM, Geisbert JB, Young HA, Formenty P, Fritz EA, Larsen T, Hensley LE. Marburg virus Angola infection of rhesus macaques: pathogenesis and treatment with recombinant nematode anticoagulant protein c2. J. Infect. Dis. 2007;196(Suppl. 2):S372–381. doi: 10.1086/520608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb TR, Norwood DA, Jr., Woollen N, Henchal EA. Development and evaluation of a fluorogenic 5′-nuclease assay to identify Marburg virus. Mol. Cell. Probes. 2001;15:259–266. doi: 10.1006/mcpr.2001.0369. [DOI] [PubMed] [Google Scholar]
- Kurosaki Y, Grolla A, Fukuma A, Feldmann H, Yasuda J. Development and evaluation of the simple diagnostic assay for Marburg virus using reverse transcription-loop-mediated isothermal amplification method. J. Clin. Microbiol. 2010;48:2330–2336. doi: 10.1128/JCM.01224-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosaki Y, Takada A, Ebihara H, Grolla A, Kamo N, Feldmann H, Kawaoka Y, Yasuda J. Rapid and simple detection of Ebola virus by reverse transcription-loop-mediated isothermal amplification. J. Virol. Methods. 2007;141:78–83. doi: 10.1016/j.jviromet.2006.11.031. [DOI] [PubMed] [Google Scholar]
- Sanchez A, Geisbert TW, Feldmann H. Filoviridae: Marburg and Ebola viruses. In: David MK, Peter MH, editors. Fields Virology. Lippincott Williams and Wilkins, a Wolters Kluwer Business; Philadelphia: 2006. pp. 1409–1448. [Google Scholar]
- Sanchez A, Kiley MP. Identification and analysis of Ebola virus messenger RNA. Virology. 1987;157:414–420. doi: 10.1016/0042-6822(87)90283-2. [DOI] [PubMed] [Google Scholar]
- Sanchez A, Ksiazek TG, Rollin PE, Miranda ME, Trappier SG, Khan AS, Peters CJ, Nichol ST. Detection and molecular characterization of Ebola viruses causing disease in human and nonhuman primates. J. Infect. Dis. 1999;179(Suppl. 1):S164–S169. doi: 10.1086/514282. [DOI] [PubMed] [Google Scholar]
- Timen A, Koopmans MP, Vossen AC, van Doornum GJ, Gunther S, van den Berkmortel F, Verduin KM, Dittrich S, Emmerich P, Osterhaus AD, Dissel JT, Coutinho RA. Response to imported case of Marburg hemorrhagic fever, the Netherland. Emerg. Infect. Dis. 2009;15:1171–1175. doi: 10.3201/eid1508.090051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towner JS, Sealy TK, Khristova ML, Albarino CG, Conlan S, Reeder SA, Quan PL, Lipkin WI, Downing R, Tappero JW, Okware S, Lutwama J, Bakamutumaho B, Kayiwa J, Comer JA, Rollin PE, Ksiazek TG, Nichol ST. Newly discovered Ebola virus associated with hemorrhagic fever outbreak in Uganda. PLoS Pathog. 2008;4:e1000212. doi: 10.1371/journal.ppat.1000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidmann M, Muhlberger E, Hufert FT. Rapid detection protocol for filoviruses. J. Clin. Virol. 2004;30:94–99. doi: 10.1016/j.jcv.2003.09.004. [DOI] [PubMed] [Google Scholar]
- World Health Organization Ebola haemorrhagic fever. A summary of the outbreak in Gabon. Wkly. Epidemiol. Rec. 1997;72:7–8. [PubMed] [Google Scholar]
- World Health Organization Marburg haemorrhagic fever, Angola. Wkly. Epidemiol. Rec. 2005;80:158–159. [PubMed] [Google Scholar]