Abstract
In the title compound, C14H20N2O2S, the sulfonamide O atoms lie to one side of the benzene ring and the aminobicycloheptanyl to the other side [Car—S—N—C torsion angle = −57.93 (11)°; ar = aromatic]. An intramolecular N—H⋯N hydrogen bond is formed. In the crystal, a supramolecular chain is formed along the b axis via N—H⋯O and N—H⋯N hydrogen bonds.
Related literature
For chiral ligands in asymmetric catalytic reactions, see: Seo et al. (2001 ▶); Abdel-Aziz et al. (2004 ▶); Matsunaga et al. (2005 ▶); Yamakuchi et al. (2005 ▶).
Experimental
Crystal data
C14H20N2O2S
M r = 280.38
Monoclinic,

a = 10.1715 (2) Å
b = 6.1169 (1) Å
c = 11.5150 (3) Å
β = 110.332 (2)°
V = 671.80 (2) Å3
Z = 2
Cu Kα radiation
μ = 2.14 mm−1
T = 100 K
0.30 × 0.20 × 0.10 mm
Data collection
Agilent SuperNova Dual diffractometer with Atlas detector
Absorption correction: multi-scan (CrysAlis PRO; Agilent, 2012 ▶) T min = 0.566, T max = 0.814
4721 measured reflections
2750 independent reflections
2731 reflections with I > 2σ(I)
R int = 0.014
Refinement
R[F 2 > 2σ(F 2)] = 0.024
wR(F 2) = 0.066
S = 1.03
2750 reflections
185 parameters
4 restraints
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.22 e Å−3
Δρmin = −0.26 e Å−3
Absolute structure: Flack (1983 ▶), 1217 Friedel pairs
Flack parameter: −0.001 (10)
Data collection: CrysAlis PRO (Agilent, 2012 ▶); cell refinement: CrysAlis PRO; data reduction: CrysAlis PRO; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 (Farrugia, 1997 ▶) and DIAMOND (Brandenburg, 2006 ▶); software used to prepare material for publication: publCIF (Westrip, 2010 ▶).
Supplementary Material
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536812025421/lh5485sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536812025421/lh5485Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536812025421/lh5485Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N1—H1n⋯O1i | 0.88 (1) | 2.20 (1) | 2.976 (2) | 148 (2) |
| N1—H2n⋯N2 | 0.87 (1) | 2.39 (2) | 2.752 (2) | 105 (2) |
| N2—H3n⋯N1i | 0.89 (1) | 2.04 (1) | 2.907 (2) | 166 (2) |
Symmetry code: (i)  .
.
Acknowledgments
The authors extend their appreciation to the Research Center of Pharmacy, King Saud University, for funding this work. They also thank the Ministry of Higher Education (Malaysia) for funding structural studies through the High-Impact Research scheme (UM·C/HIR/MOHE/SC/12).
supplementary crystallographic information
Comment
The title compound (I) was synthesized in the context of the development of chiral ligands for asymmetric catalytic reactions (Seo et al., 2001; Abdel-Aziz et al., 2004; Matsunaga et al., 2005; Yamakuchi et al. 2005).
In (I), Fig. 1, the two S-bound O atoms lie to one side of the adjacent benzene ring with the O1 and O2 atoms lying -0.466 (1) and -0.771 (1) Å out of the plane, respectively, and the aminobicycloheptanyl residue lying to the other side, the C8—S1—N2—C2 torsion being -57.93 (11)°. An intramolecular N—H···N hydrogen bond is noted, Table 1.
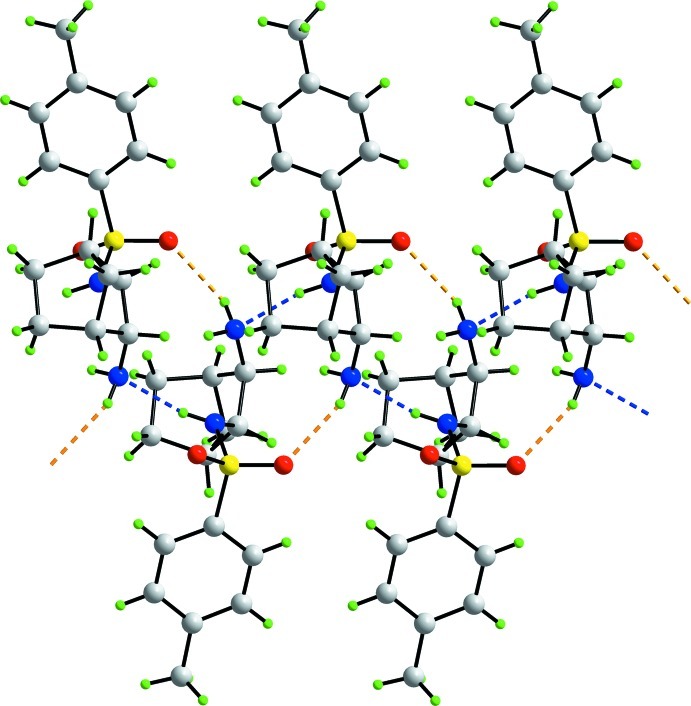
Molecules are connected into a supramolecular chain along the b axis via N—H···O and N—H···N hydrogen bonds that generate 12-membered {···HNC2NH···OSNC2N} synthons, Fig. 2 and Table 1. The chains pack into a three-dimensional architecture without specific interactions between them, Fig. 3.
Experimental
To the mixture of 2-imidazolidinone (2.0 ml), water (2 ml), ethanol (6 ml) and Ba(OH)2.8H2O (20 ml) were added. This was heated at 413 K in a glass sealed tube for 24 h. The solvents were evaporated and the precipitate extracted three times with chloroform (10 ml × 3). The organic extract was dried and crystallized from ethanol to afford the title compound.
Refinement
Carbon-bound H-atoms were placed in calculated positions [C—H = 0.95 to 1.00 Å, Uiso(H) = 1.2–1.5Ueq(C)] and were included in the refinement in the riding model approximation. The amino H-atoms were refined with N—H = 0.88±0.01 Å.
Figures
Fig. 1.
The molecular structure of (I) showing the atom-labelling scheme and displacement ellipsoids at the 50% probability level.
Fig. 2.
A view of the supramolecular helical chain along the b axis in (I). The N—H···O and N—H···N hydrogen bonds are shown as orange and blue dashed lines, respectively.
Fig. 3.
A view in projection down the b axis of the unit-cell contents for (I). The N—H···O and N—H···N (obscured) hydrogen bonds are shown as orange and blue dashed lines, respectively.
Crystal data
| C14H20N2O2S | F(000) = 300 |
| Mr = 280.38 | Dx = 1.386 Mg m−3 |
| Monoclinic, P21 | Cu Kα radiation, λ = 1.54184 Å |
| Hall symbol: P 2yb | Cell parameters from 3574 reflections |
| a = 10.1715 (2) Å | θ = 4.1–76.4° |
| b = 6.1169 (1) Å | µ = 2.14 mm−1 |
| c = 11.5150 (3) Å | T = 100 K |
| β = 110.332 (2)° | Prism, colourless |
| V = 671.80 (2) Å3 | 0.30 × 0.20 × 0.10 mm |
| Z = 2 |
Data collection
| Agilent SuperNova Dual diffractometer with Atlas detector | 2750 independent reflections |
| Radiation source: SuperNova (Cu) X-ray Source | 2731 reflections with I > 2σ(I) |
| Mirror monochromator | Rint = 0.014 |
| Detector resolution: 10.4041 pixels mm-1 | θmax = 76.6°, θmin = 4.1° |
| ω scan | h = −11→12 |
| Absorption correction: multi-scan (CrysAlis PRO; Agilent, 2012) | k = −7→7 |
| Tmin = 0.566, Tmax = 0.814 | l = −14→12 |
| 4721 measured reflections |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.024 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.066 | w = 1/[σ2(Fo2) + (0.049P)2 + 0.0858P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.03 | (Δ/σ)max = 0.001 |
| 2750 reflections | Δρmax = 0.22 e Å−3 |
| 185 parameters | Δρmin = −0.26 e Å−3 |
| 4 restraints | Absolute structure: Flack (1983), 1217 Friedel pairs |
| Primary atom site location: structure-invariant direct methods | Flack parameter: −0.001 (10) |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| S1 | 0.26041 (3) | 0.50378 (5) | 0.72818 (2) | 0.01602 (9) | |
| O1 | 0.26695 (10) | 0.73734 (17) | 0.71708 (9) | 0.0218 (2) | |
| N1 | 0.52705 (11) | 0.5254 (2) | 1.10027 (10) | 0.0184 (2) | |
| N2 | 0.32587 (11) | 0.44016 (18) | 0.87334 (10) | 0.0160 (2) | |
| O2 | 0.32988 (10) | 0.36603 (18) | 0.66642 (9) | 0.0215 (2) | |
| C1 | 0.38324 (13) | 0.5685 (2) | 1.09495 (11) | 0.0168 (3) | |
| H1 | 0.3790 | 0.7246 | 1.1192 | 0.020* | |
| C2 | 0.26899 (13) | 0.5369 (2) | 0.96230 (11) | 0.0153 (2) | |
| H2 | 0.2286 | 0.6832 | 0.9304 | 0.018* | |
| C3 | 0.15457 (14) | 0.4000 (2) | 0.98927 (13) | 0.0190 (3) | |
| H3 | 0.0587 | 0.4149 | 0.9256 | 0.023* | |
| C4 | 0.16743 (13) | 0.4842 (3) | 1.11857 (12) | 0.0224 (3) | |
| H4A | 0.1085 | 0.4015 | 1.1559 | 0.027* | |
| H4B | 0.1486 | 0.6429 | 1.1196 | 0.027* | |
| C5 | 0.32467 (14) | 0.4279 (2) | 1.17705 (12) | 0.0198 (3) | |
| H5 | 0.3666 | 0.4604 | 1.2676 | 0.024* | |
| C6 | 0.32835 (14) | 0.1852 (2) | 1.14384 (13) | 0.0213 (3) | |
| H6A | 0.3138 | 0.0901 | 1.2079 | 0.026* | |
| H6B | 0.4187 | 0.1470 | 1.1345 | 0.026* | |
| C7 | 0.20485 (14) | 0.1631 (2) | 1.01838 (13) | 0.0220 (3) | |
| H7A | 0.2373 | 0.1024 | 0.9533 | 0.026* | |
| H7B | 0.1296 | 0.0687 | 1.0268 | 0.026* | |
| C8 | 0.07959 (14) | 0.4382 (2) | 0.67418 (11) | 0.0164 (2) | |
| C9 | −0.01687 (14) | 0.6042 (2) | 0.66650 (12) | 0.0200 (3) | |
| H9 | 0.0144 | 0.7488 | 0.6912 | 0.024* | |
| C10 | −0.15935 (14) | 0.5562 (2) | 0.62230 (12) | 0.0205 (3) | |
| H10 | −0.2253 | 0.6691 | 0.6170 | 0.025* | |
| C11 | −0.20671 (13) | 0.3446 (2) | 0.58568 (12) | 0.0185 (3) | |
| C12 | −0.10771 (14) | 0.1807 (2) | 0.59531 (12) | 0.0190 (3) | |
| H12 | −0.1387 | 0.0356 | 0.5715 | 0.023* | |
| C13 | 0.03540 (14) | 0.2258 (2) | 0.63911 (12) | 0.0180 (3) | |
| H13 | 0.1017 | 0.1131 | 0.6449 | 0.022* | |
| C14 | −0.36173 (14) | 0.2973 (3) | 0.53354 (14) | 0.0244 (3) | |
| H14A | −0.4059 | 0.3911 | 0.4615 | 0.037* | |
| H14B | −0.4036 | 0.3264 | 0.5969 | 0.037* | |
| H14C | −0.3765 | 0.1436 | 0.5083 | 0.037* | |
| H1n | 0.5811 (16) | 0.480 (3) | 1.1733 (11) | 0.025 (4)* | |
| H2n | 0.530 (2) | 0.412 (2) | 1.0552 (16) | 0.033 (5)* | |
| H3n | 0.357 (2) | 0.3032 (19) | 0.882 (2) | 0.035 (5)* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| S1 | 0.01351 (14) | 0.01864 (15) | 0.01503 (14) | −0.00197 (11) | 0.00386 (10) | −0.00003 (11) |
| O1 | 0.0213 (5) | 0.0204 (5) | 0.0199 (5) | −0.0048 (4) | 0.0026 (4) | 0.0027 (4) |
| N1 | 0.0138 (5) | 0.0199 (5) | 0.0187 (5) | −0.0012 (5) | 0.0021 (4) | 0.0008 (5) |
| N2 | 0.0140 (5) | 0.0182 (5) | 0.0151 (5) | 0.0023 (4) | 0.0040 (4) | 0.0002 (4) |
| O2 | 0.0177 (5) | 0.0296 (5) | 0.0185 (4) | 0.0000 (4) | 0.0081 (4) | −0.0028 (4) |
| C1 | 0.0169 (6) | 0.0155 (6) | 0.0161 (6) | 0.0013 (4) | 0.0032 (4) | −0.0005 (4) |
| C2 | 0.0141 (5) | 0.0153 (6) | 0.0160 (5) | 0.0026 (4) | 0.0046 (4) | 0.0002 (5) |
| C3 | 0.0133 (6) | 0.0223 (6) | 0.0217 (6) | 0.0008 (5) | 0.0064 (5) | 0.0013 (5) |
| C4 | 0.0201 (6) | 0.0266 (7) | 0.0240 (6) | 0.0055 (6) | 0.0122 (5) | 0.0031 (6) |
| C5 | 0.0196 (6) | 0.0226 (6) | 0.0176 (6) | 0.0041 (5) | 0.0069 (5) | 0.0024 (5) |
| C6 | 0.0198 (6) | 0.0204 (7) | 0.0259 (7) | 0.0021 (5) | 0.0106 (5) | 0.0057 (5) |
| C7 | 0.0180 (6) | 0.0197 (6) | 0.0306 (7) | −0.0037 (5) | 0.0113 (5) | 0.0006 (6) |
| C8 | 0.0151 (6) | 0.0188 (6) | 0.0141 (6) | −0.0009 (5) | 0.0036 (4) | 0.0007 (4) |
| C9 | 0.0195 (6) | 0.0174 (6) | 0.0216 (6) | −0.0002 (5) | 0.0053 (5) | −0.0012 (5) |
| C10 | 0.0178 (6) | 0.0217 (7) | 0.0216 (6) | 0.0033 (5) | 0.0063 (5) | 0.0020 (5) |
| C11 | 0.0155 (6) | 0.0247 (7) | 0.0146 (6) | −0.0006 (5) | 0.0041 (5) | 0.0001 (5) |
| C12 | 0.0189 (6) | 0.0183 (6) | 0.0177 (6) | −0.0022 (5) | 0.0037 (5) | −0.0010 (5) |
| C13 | 0.0171 (6) | 0.0182 (6) | 0.0175 (6) | 0.0023 (5) | 0.0046 (5) | −0.0003 (5) |
| C14 | 0.0154 (6) | 0.0326 (8) | 0.0238 (7) | −0.0015 (5) | 0.0049 (5) | −0.0024 (6) |
Geometric parameters (Å, º)
| S1—O2 | 1.4367 (10) | C5—H5 | 1.0000 |
| S1—O1 | 1.4380 (11) | C6—C7 | 1.556 (2) |
| S1—N2 | 1.6172 (11) | C6—H6A | 0.9900 |
| S1—C8 | 1.7707 (13) | C6—H6B | 0.9900 |
| N1—C1 | 1.4665 (16) | C7—H7A | 0.9900 |
| N1—H1n | 0.875 (9) | C7—H7B | 0.9900 |
| N1—H2n | 0.873 (9) | C8—C13 | 1.3884 (18) |
| N2—C2 | 1.4649 (16) | C8—C9 | 1.3930 (19) |
| N2—H3n | 0.888 (9) | C9—C10 | 1.3902 (18) |
| C1—C5 | 1.5425 (18) | C9—H9 | 0.9500 |
| C1—C2 | 1.5771 (16) | C10—C11 | 1.394 (2) |
| C1—H1 | 1.0000 | C10—H10 | 0.9500 |
| C2—C3 | 1.5502 (18) | C11—C12 | 1.3977 (19) |
| C2—H2 | 1.0000 | C11—C14 | 1.5076 (18) |
| C3—C7 | 1.5347 (19) | C12—C13 | 1.3925 (19) |
| C3—C4 | 1.5376 (19) | C12—H12 | 0.9500 |
| C3—H3 | 1.0000 | C13—H13 | 0.9500 |
| C4—C5 | 1.5432 (18) | C14—H14A | 0.9800 |
| C4—H4A | 0.9900 | C14—H14B | 0.9800 |
| C4—H4B | 0.9900 | C14—H14C | 0.9800 |
| C5—C6 | 1.537 (2) | ||
| O2—S1—O1 | 119.52 (6) | C6—C5—H5 | 114.4 |
| O2—S1—N2 | 105.89 (6) | C1—C5—H5 | 114.4 |
| O1—S1—N2 | 108.41 (6) | C4—C5—H5 | 114.4 |
| O2—S1—C8 | 108.84 (6) | C5—C6—C7 | 103.51 (11) |
| O1—S1—C8 | 105.56 (6) | C5—C6—H6A | 111.1 |
| N2—S1—C8 | 108.23 (6) | C7—C6—H6A | 111.1 |
| C1—N1—H1n | 112.5 (12) | C5—C6—H6B | 111.1 |
| C1—N1—H2n | 111.1 (13) | C7—C6—H6B | 111.1 |
| H1n—N1—H2n | 100.2 (18) | H6A—C6—H6B | 109.0 |
| C2—N2—S1 | 120.32 (9) | C3—C7—C6 | 102.75 (11) |
| C2—N2—H3n | 120.9 (14) | C3—C7—H7A | 111.2 |
| S1—N2—H3n | 110.2 (14) | C6—C7—H7A | 111.2 |
| N1—C1—C5 | 117.79 (10) | C3—C7—H7B | 111.2 |
| N1—C1—C2 | 114.02 (10) | C6—C7—H7B | 111.2 |
| C5—C1—C2 | 102.34 (10) | H7A—C7—H7B | 109.1 |
| N1—C1—H1 | 107.4 | C13—C8—C9 | 120.92 (13) |
| C5—C1—H1 | 107.4 | C13—C8—S1 | 120.39 (10) |
| C2—C1—H1 | 107.4 | C9—C8—S1 | 118.69 (11) |
| N2—C2—C3 | 115.36 (10) | C10—C9—C8 | 119.41 (13) |
| N2—C2—C1 | 112.93 (10) | C10—C9—H9 | 120.3 |
| C3—C2—C1 | 102.95 (10) | C8—C9—H9 | 120.3 |
| N2—C2—H2 | 108.4 | C9—C10—C11 | 120.88 (13) |
| C3—C2—H2 | 108.4 | C9—C10—H10 | 119.6 |
| C1—C2—H2 | 108.4 | C11—C10—H10 | 119.6 |
| C7—C3—C4 | 101.26 (11) | C10—C11—C12 | 118.56 (12) |
| C7—C3—C2 | 109.72 (11) | C10—C11—C14 | 120.16 (13) |
| C4—C3—C2 | 101.29 (10) | C12—C11—C14 | 121.25 (13) |
| C7—C3—H3 | 114.4 | C13—C12—C11 | 121.36 (13) |
| C4—C3—H3 | 114.4 | C13—C12—H12 | 119.3 |
| C2—C3—H3 | 114.4 | C11—C12—H12 | 119.3 |
| C3—C4—C5 | 94.26 (10) | C8—C13—C12 | 118.85 (12) |
| C3—C4—H4A | 112.9 | C8—C13—H13 | 120.6 |
| C5—C4—H4A | 112.9 | C12—C13—H13 | 120.6 |
| C3—C4—H4B | 112.9 | C11—C14—H14A | 109.5 |
| C5—C4—H4B | 112.9 | C11—C14—H14B | 109.5 |
| H4A—C4—H4B | 110.3 | H14A—C14—H14B | 109.5 |
| C6—C5—C1 | 109.75 (11) | C11—C14—H14C | 109.5 |
| C6—C5—C4 | 102.60 (11) | H14A—C14—H14C | 109.5 |
| C1—C5—C4 | 99.82 (10) | H14B—C14—H14C | 109.5 |
| O2—S1—N2—C2 | −174.49 (10) | C1—C5—C6—C7 | −74.78 (12) |
| O1—S1—N2—C2 | 56.10 (11) | C4—C5—C6—C7 | 30.64 (13) |
| C8—S1—N2—C2 | −57.93 (11) | C4—C3—C7—C6 | −39.25 (12) |
| S1—N2—C2—C3 | 93.87 (12) | C2—C3—C7—C6 | 67.22 (13) |
| S1—N2—C2—C1 | −148.11 (9) | C5—C6—C7—C3 | 5.12 (13) |
| N1—C1—C2—N2 | 8.26 (15) | O2—S1—C8—C13 | 32.18 (13) |
| C5—C1—C2—N2 | −120.07 (12) | O1—S1—C8—C13 | 161.63 (11) |
| N1—C1—C2—C3 | 133.32 (11) | N2—S1—C8—C13 | −82.46 (12) |
| C5—C1—C2—C3 | 5.00 (12) | O2—S1—C8—C9 | −147.16 (10) |
| N2—C2—C3—C7 | 49.21 (14) | O1—S1—C8—C9 | −17.71 (12) |
| C1—C2—C3—C7 | −74.26 (12) | N2—S1—C8—C9 | 98.19 (11) |
| N2—C2—C3—C4 | 155.65 (10) | C13—C8—C9—C10 | −0.4 (2) |
| C1—C2—C3—C4 | 32.18 (12) | S1—C8—C9—C10 | 178.91 (10) |
| C7—C3—C4—C5 | 56.71 (12) | C8—C9—C10—C11 | 0.0 (2) |
| C2—C3—C4—C5 | −56.28 (12) | C9—C10—C11—C12 | 0.5 (2) |
| N1—C1—C5—C6 | −58.83 (14) | C9—C10—C11—C14 | −177.73 (12) |
| C2—C1—C5—C6 | 67.07 (12) | C10—C11—C12—C13 | −0.68 (19) |
| N1—C1—C5—C4 | −166.13 (11) | C14—C11—C12—C13 | 177.57 (12) |
| C2—C1—C5—C4 | −40.23 (12) | C9—C8—C13—C12 | 0.3 (2) |
| C3—C4—C5—C6 | −53.44 (12) | S1—C8—C13—C12 | −179.03 (10) |
| C3—C4—C5—C1 | 59.52 (12) | C11—C12—C13—C8 | 0.3 (2) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H1n···O1i | 0.88 (1) | 2.20 (1) | 2.976 (2) | 148 (2) |
| N1—H2n···N2 | 0.87 (1) | 2.39 (2) | 2.752 (2) | 105 (2) |
| N2—H3n···N1i | 0.89 (1) | 2.04 (1) | 2.907 (2) | 166 (2) |
Symmetry code: (i) −x+1, y−1/2, −z+2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: LH5485).
References
- Abdel-Aziz, A. A.-M., El Bialy, S. A. A., Goda, F. E. & Kunieda, T. (2004). Tetrahedron Lett. 45, 8073–8077.
- Agilent (2012). CrysAlis PRO Agilent Technologies, Yarnton, England.
- Brandenburg, K. (2006). DIAMOND Crystal Impact GbR, Bonn, Germany.
- Farrugia, L. J. (1997). J. Appl. Cryst. 30, 565.
- Flack, H. D. (1983). Acta Cryst. A39, 876–881.
- Matsunaga, H., Ishizuka, T. & Kunieda, T. (2005). Tetrahedron Lett. 46, 3645–3648.
- Seo, R., Ishizuka, T., Abdel-Aziz, A. A.-M. & Kunieda, T. (2001). Tetrahedron Lett. 42, 6353–6355.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
- Yamakuchi, M., Matsunaga, H., Tokuda, R., Ishizuka, T., Nakajima, M. & Kuniedab, T. (2005). Tetrahedron Lett. 46, 4019–4022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536812025421/lh5485sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536812025421/lh5485Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536812025421/lh5485Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report