Abstract
In the title molecule, C24H23Cl2N3O7, the central benzene ring forms dihedral angles of 65.71 (1) and 44.42 (1)° with the pyrimidine ring and the terminal benzene ring, respectively. In the crystal, molecules are linked via C—H⋯O hydrogen bonds.
Related literature
For reference bond-length data, see: Allen et al. (1987 ▶). For the synthesis of 4-(4,6-dimethoxypyrimidin-2-yloxy)benzenamine, see: Jin et al. (2011 ▶). For biological properties of fungicides, see: Gozzo & Garlaschelli (1985 ▶).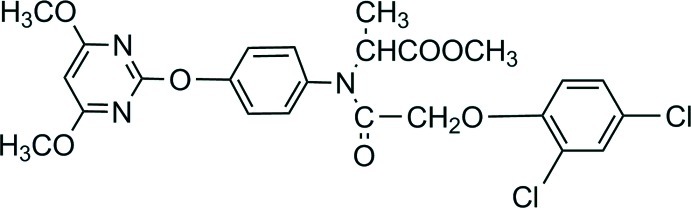
Experimental
Crystal data
C24H23Cl2N3O7
M r = 536.35
Triclinic,

a = 8.2438 (9) Å
b = 11.2405 (12) Å
c = 14.2502 (15) Å
α = 85.178 (2)°
β = 78.702 (2)°
γ = 80.032 (2)°
V = 1273.6 (2) Å3
Z = 2
Mo Kα radiation
μ = 0.30 mm−1
T = 298 K
0.16 × 0.12 × 0.10 mm
Data collection
Bruker SMART APEX CCD area-detector diffractometer
15581 measured reflections
6204 independent reflections
4390 reflections with I > 2σ(I)
R int = 0.050
Refinement
R[F 2 > 2σ(F 2)] = 0.056
wR(F 2) = 0.174
S = 1.06
6204 reflections
329 parameters
H-atom parameters constrained
Δρmax = 0.77 e Å−3
Δρmin = −0.35 e Å−3
Data collection: SMART (Bruker, 2001 ▶); cell refinement: SAINT-Plus (Bruker, 2001 ▶); data reduction: SAINT-Plus; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: PLATON (Spek, 2009 ▶); software used to prepare material for publication: PLATON.
Supplementary Material
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536812025494/wn2477sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536812025494/wn2477Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536812025494/wn2477Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C9—H9⋯O4i | 0.93 | 2.57 | 3.402 (3) | 150 |
Symmetry code: (i) 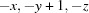 .
.
Acknowledgments
We gratefully acknowledge financial support of this work by the National Basic Research Program of China (2010CB126100) and the National Natural Science Foundation of China (Nos. 21172090 and 21002037). The research was supported in part by the PCSIRT (No. IRT0953) and the self-determined research funds of CCNU from the college’s basic research and operation of MOE (No. CCNU10A01007).
supplementary crystallographic information
Comment
N-acylalanine fungicides are mainly used in crop protection because of their systemic properties, with both curative and protective activity against fungal pathogens of the Peronosporales (Gozzo & Garlaschelli, 1985). The pyrimidinyl group is widely used in fungicides and drug molecular design (Jin et al., 2011), so we have introduced the pyrimidinyl group into acylalanine derivatives in order to decrease resistance and increase activity.
Here we report the crystal structure of the title compound (Fig.1). The bond lengths (Allen et al., 1987) and angles show normal values. The central benzene ring forms dihedral angles of 65.71 (1)° and 44.42 (1)° with the pyrimidinyl ring and the terminal benzene ring, respectively. The C9—H9···O4 intermolecular hydrogen bond plays an important role in determining the crystal structure.
Experimental
4-(4,6-Dimethoxypyrimidin-2-yloxy)benzenamine (Jin et al., 2011) (1 mmol) and methyl 2-chloropropanoate (1.2 mmol) were dissolved in 15 ml dimethylformamide, then 1 mmol K2CO3 was added with constant stirring. The temperature was maintained at 100 °C for 10 h. The reaction mixture was poured into water and extracted with ethyl acetate, dried with Na2SO4, then purified by column chromatography on silica gel with petroleum ether/ethyl acetate (4:1) to give the compound methyl 2-(N-(4-(4,6-dimethoxypyrimidin-2-yloxy)phenyl)amino)propanoate.
To a mixture of methyl 2-(N-(4-(4,6-dimethoxypyrimidin-2-yloxy) phenyl)amino)propanoate (2 mmol) and triethylamine (2 mmol) in CH2Cl2 (20 ml), 2,4-dichlorophenoxyacetyl chloride (2 mmol) was added at 2–5 °C and the mixture was stirred for another 3 h, then washed with saturated sodium hydrogen carbonate solution and dried with Na2SO4. The residue was purified by column chromatography on silica gel with petroleum ether/ethyl acetate (3:1) to give the pure title compound as a white solid. Recrystallization from ethanol over a period of one week gave colourless crystals of the title compound.
Refinement
H atoms were geometrically positioned (Csp2—H = 0.93 Å, Cmethine—H = 0.98 Å, Cmethylene—H = 0.97 Å, Cmethyl—H = 0.96 Å) and refined as riding, with Uiso(H) = xUeq(C), where x = 1.5 for methyl H and 1.2 for all other H atoms.
Figures
Fig. 1.
Molecular structure of the title compound, with 50% probability displacement ellipsoids. H atoms are shown as spheres of arbitrary radius.
Fig. 2.
Part of the crystal packing, showing the intermolecular hydrogen bonds as dashed lines.
Crystal data
| C24H23Cl2N3O7 | Z = 2 |
| Mr = 536.35 | F(000) = 556 |
| Triclinic, P1 | Dx = 1.399 Mg m−3 |
| a = 8.2438 (9) Å | Mo Kα radiation, λ = 0.71073 Å |
| b = 11.2405 (12) Å | Cell parameters from 5848 reflections |
| c = 14.2502 (15) Å | θ = 2.4–27.7° |
| α = 85.178 (2)° | µ = 0.30 mm−1 |
| β = 78.702 (2)° | T = 298 K |
| γ = 80.032 (2)° | Block, colourless |
| V = 1273.6 (2) Å3 | 0.16 × 0.12 × 0.10 mm |
Data collection
| Bruker SMART APEX CCD area-detector diffractometer | 4390 reflections with I > 2σ(I) |
| Radiation source: fine-focus sealed tube | Rint = 0.050 |
| Graphite monochromator | θmax = 28.3°, θmin = 1.8° |
| phi and ω scans | h = −10→10 |
| 15581 measured reflections | k = −14→14 |
| 6204 independent reflections | l = −18→18 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.056 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.174 | H-atom parameters constrained |
| S = 1.06 | w = 1/[σ2(Fo2) + (0.0812P)2 + 0.2621P] where P = (Fo2 + 2Fc2)/3 |
| 6204 reflections | (Δ/σ)max < 0.001 |
| 329 parameters | Δρmax = 0.77 e Å−3 |
| 0 restraints | Δρmin = −0.35 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1 | −0.1814 (4) | 0.1820 (3) | 0.55913 (16) | 0.0750 (7) | |
| C2 | −0.1019 (5) | 0.0866 (3) | 0.60971 (18) | 0.0888 (9) | |
| H2 | −0.1613 | 0.0343 | 0.6514 | 0.107* | |
| C3 | 0.0693 (5) | 0.0735 (2) | 0.59483 (17) | 0.0809 (8) | |
| C4 | 0.0665 (3) | 0.2312 (2) | 0.49075 (15) | 0.0619 (5) | |
| C5 | −0.4294 (4) | 0.3007 (4) | 0.5202 (3) | 0.1131 (12) | |
| H5A | −0.4154 | 0.3749 | 0.5442 | 0.170* | |
| H5B | −0.5467 | 0.2964 | 0.5285 | 0.170* | |
| H5C | −0.3805 | 0.2983 | 0.4533 | 0.170* | |
| C6 | 0.3282 (7) | −0.0322 (4) | 0.6277 (3) | 0.1322 (16) | |
| H6A | 0.3745 | −0.0543 | 0.5632 | 0.198* | |
| H6B | 0.3690 | −0.0945 | 0.6716 | 0.198* | |
| H6C | 0.3610 | 0.0426 | 0.6385 | 0.198* | |
| C7 | 0.0948 (3) | 0.3856 (2) | 0.36520 (15) | 0.0582 (5) | |
| C8 | 0.0267 (3) | 0.34724 (19) | 0.29442 (15) | 0.0576 (5) | |
| H8 | 0.0106 | 0.2673 | 0.2953 | 0.069* | |
| C9 | −0.0170 (3) | 0.42863 (18) | 0.22240 (14) | 0.0529 (5) | |
| H9 | −0.0651 | 0.4042 | 0.1749 | 0.063* | |
| C10 | 0.0107 (2) | 0.54753 (17) | 0.22072 (14) | 0.0508 (4) | |
| C11 | 0.0755 (3) | 0.5849 (2) | 0.29330 (16) | 0.0621 (5) | |
| H11 | 0.0911 | 0.6649 | 0.2932 | 0.075* | |
| C12 | 0.1170 (3) | 0.5032 (2) | 0.36626 (16) | 0.0646 (6) | |
| H12 | 0.1599 | 0.5281 | 0.4157 | 0.077* | |
| C13 | 0.0662 (2) | 0.62200 (17) | 0.05341 (15) | 0.0512 (4) | |
| C14 | 0.2208 (3) | 0.52456 (19) | 0.04013 (15) | 0.0557 (5) | |
| H14A | 0.2863 | 0.5293 | 0.0889 | 0.067* | |
| H14B | 0.1868 | 0.4454 | 0.0472 | 0.067* | |
| C15 | 0.3997 (2) | 0.63889 (18) | −0.06993 (14) | 0.0522 (5) | |
| C16 | 0.4732 (2) | 0.6621 (2) | −0.16498 (15) | 0.0564 (5) | |
| C17 | 0.5604 (3) | 0.7573 (2) | −0.19099 (17) | 0.0650 (6) | |
| H17 | 0.6083 | 0.7721 | −0.2547 | 0.078* | |
| C18 | 0.5757 (3) | 0.8305 (2) | −0.1213 (2) | 0.0724 (7) | |
| C19 | 0.5045 (3) | 0.8096 (2) | −0.0276 (2) | 0.0744 (6) | |
| H19 | 0.5156 | 0.8594 | 0.0189 | 0.089* | |
| C20 | 0.4161 (3) | 0.7144 (2) | −0.00201 (17) | 0.0644 (6) | |
| H20 | 0.3672 | 0.7011 | 0.0617 | 0.077* | |
| C21 | −0.1595 (3) | 0.7360 (2) | 0.1557 (2) | 0.0687 (6) | |
| H21 | −0.2150 | 0.7427 | 0.1001 | 0.082* | |
| C22 | −0.2918 (4) | 0.7281 (3) | 0.2402 (3) | 0.1095 (12) | |
| H22A | −0.3292 | 0.6513 | 0.2438 | 0.164* | |
| H22B | −0.3842 | 0.7919 | 0.2355 | 0.164* | |
| H22C | −0.2486 | 0.7360 | 0.2969 | 0.164* | |
| C23 | −0.0768 (3) | 0.84909 (18) | 0.14879 (15) | 0.0550 (5) | |
| C24 | −0.1183 (4) | 1.0605 (2) | 0.1175 (2) | 0.0823 (8) | |
| H24A | −0.0790 | 1.0730 | 0.1744 | 0.124* | |
| H24B | −0.2063 | 1.1253 | 0.1070 | 0.124* | |
| H24C | −0.0275 | 1.0584 | 0.0636 | 0.124* | |
| Cl1 | 0.45524 (9) | 0.57030 (7) | −0.25189 (5) | 0.0857 (2) | |
| Cl2 | 0.68469 (11) | 0.95120 (8) | −0.15360 (8) | 0.1130 (3) | |
| N1 | −0.0971 (3) | 0.25673 (18) | 0.49818 (12) | 0.0639 (5) | |
| N2 | 0.1583 (3) | 0.14535 (19) | 0.53514 (13) | 0.0715 (5) | |
| N3 | −0.0284 (2) | 0.62954 (15) | 0.14216 (13) | 0.0542 (4) | |
| O1 | −0.3482 (3) | 0.2002 (2) | 0.57170 (15) | 0.1022 (7) | |
| O2 | 0.1513 (4) | −0.0183 (2) | 0.64236 (15) | 0.1119 (8) | |
| O3 | 0.1632 (2) | 0.30374 (17) | 0.43166 (13) | 0.0761 (5) | |
| O4 | 0.03226 (19) | 0.69051 (14) | −0.01316 (11) | 0.0641 (4) | |
| O5 | 0.32022 (19) | 0.54002 (13) | −0.05232 (10) | 0.0600 (4) | |
| O6 | −0.1820 (2) | 0.94630 (14) | 0.12870 (13) | 0.0702 (4) | |
| O7 | 0.0634 (2) | 0.84994 (14) | 0.16004 (13) | 0.0695 (4) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.0984 (19) | 0.0862 (17) | 0.0475 (12) | −0.0416 (15) | −0.0091 (12) | 0.0017 (11) |
| C2 | 0.137 (3) | 0.0818 (19) | 0.0513 (13) | −0.0444 (18) | −0.0113 (15) | 0.0159 (12) |
| C3 | 0.131 (3) | 0.0653 (15) | 0.0478 (12) | −0.0171 (16) | −0.0222 (14) | 0.0056 (11) |
| C4 | 0.0814 (16) | 0.0601 (13) | 0.0479 (11) | −0.0198 (11) | −0.0157 (10) | 0.0018 (9) |
| C5 | 0.084 (2) | 0.147 (3) | 0.109 (3) | −0.036 (2) | −0.0128 (18) | 0.016 (2) |
| C6 | 0.172 (4) | 0.110 (3) | 0.093 (2) | 0.044 (3) | −0.037 (3) | 0.011 (2) |
| C7 | 0.0544 (11) | 0.0615 (13) | 0.0562 (11) | −0.0144 (9) | −0.0044 (9) | 0.0091 (9) |
| C8 | 0.0619 (12) | 0.0471 (11) | 0.0625 (12) | −0.0166 (9) | −0.0049 (9) | 0.0055 (9) |
| C9 | 0.0548 (11) | 0.0507 (11) | 0.0524 (10) | −0.0144 (9) | −0.0041 (8) | −0.0001 (8) |
| C10 | 0.0508 (10) | 0.0454 (10) | 0.0512 (10) | −0.0091 (8) | 0.0024 (8) | 0.0015 (8) |
| C11 | 0.0707 (14) | 0.0491 (11) | 0.0658 (13) | −0.0169 (10) | −0.0047 (10) | −0.0026 (10) |
| C12 | 0.0714 (14) | 0.0676 (14) | 0.0577 (12) | −0.0214 (11) | −0.0097 (10) | −0.0045 (10) |
| C13 | 0.0521 (10) | 0.0417 (10) | 0.0588 (11) | −0.0099 (8) | −0.0093 (8) | 0.0053 (8) |
| C14 | 0.0563 (11) | 0.0481 (11) | 0.0544 (11) | −0.0052 (8) | 0.0018 (9) | 0.0098 (8) |
| C15 | 0.0463 (10) | 0.0480 (10) | 0.0555 (11) | −0.0004 (8) | −0.0037 (8) | 0.0094 (8) |
| C16 | 0.0437 (10) | 0.0631 (13) | 0.0553 (11) | −0.0009 (9) | −0.0026 (8) | 0.0052 (9) |
| C17 | 0.0448 (11) | 0.0729 (15) | 0.0679 (13) | −0.0074 (10) | 0.0026 (9) | 0.0153 (11) |
| C18 | 0.0541 (13) | 0.0674 (15) | 0.0905 (18) | −0.0168 (11) | 0.0003 (11) | 0.0081 (13) |
| C19 | 0.0710 (15) | 0.0701 (15) | 0.0822 (16) | −0.0164 (12) | −0.0097 (12) | −0.0049 (13) |
| C20 | 0.0667 (13) | 0.0620 (13) | 0.0588 (12) | −0.0073 (10) | −0.0039 (10) | 0.0059 (10) |
| C21 | 0.0489 (11) | 0.0513 (12) | 0.0969 (17) | −0.0032 (9) | 0.0011 (11) | 0.0020 (11) |
| C22 | 0.0783 (18) | 0.0708 (18) | 0.159 (3) | −0.0151 (14) | 0.0354 (19) | −0.0167 (19) |
| C23 | 0.0559 (12) | 0.0469 (11) | 0.0537 (11) | −0.0005 (9) | −0.0002 (9) | 0.0059 (8) |
| C24 | 0.0913 (18) | 0.0461 (12) | 0.0980 (19) | −0.0044 (12) | −0.0036 (14) | 0.0163 (12) |
| Cl1 | 0.0820 (4) | 0.1117 (6) | 0.0616 (4) | −0.0311 (4) | 0.0092 (3) | −0.0140 (3) |
| Cl2 | 0.1001 (6) | 0.0934 (6) | 0.1411 (8) | −0.0505 (5) | 0.0159 (5) | 0.0014 (5) |
| N1 | 0.0801 (13) | 0.0671 (12) | 0.0487 (9) | −0.0268 (10) | −0.0111 (8) | 0.0028 (8) |
| N2 | 0.1018 (16) | 0.0628 (12) | 0.0512 (10) | −0.0095 (11) | −0.0233 (10) | 0.0038 (9) |
| N3 | 0.0520 (9) | 0.0426 (9) | 0.0618 (10) | −0.0045 (7) | −0.0012 (7) | 0.0035 (7) |
| O1 | 0.0946 (15) | 0.137 (2) | 0.0777 (12) | −0.0521 (14) | −0.0042 (11) | 0.0191 (12) |
| O2 | 0.174 (3) | 0.0830 (14) | 0.0698 (12) | −0.0018 (15) | −0.0302 (14) | 0.0235 (10) |
| O3 | 0.0694 (10) | 0.0823 (12) | 0.0788 (11) | −0.0230 (9) | −0.0233 (8) | 0.0288 (9) |
| O4 | 0.0676 (9) | 0.0551 (8) | 0.0673 (9) | −0.0059 (7) | −0.0180 (7) | 0.0156 (7) |
| O5 | 0.0649 (9) | 0.0527 (8) | 0.0548 (8) | −0.0097 (7) | 0.0049 (6) | 0.0042 (6) |
| O6 | 0.0625 (9) | 0.0498 (9) | 0.0895 (11) | 0.0002 (7) | −0.0083 (8) | 0.0141 (8) |
| O7 | 0.0671 (10) | 0.0522 (9) | 0.0904 (12) | −0.0080 (7) | −0.0223 (8) | 0.0041 (8) |
Geometric parameters (Å, º)
| C1—O1 | 1.334 (3) | C13—N3 | 1.350 (3) |
| C1—N1 | 1.336 (3) | C13—C14 | 1.522 (3) |
| C1—C2 | 1.382 (4) | C14—O5 | 1.423 (2) |
| C2—C3 | 1.369 (4) | C14—H14A | 0.9700 |
| C2—H2 | 0.9300 | C14—H14B | 0.9700 |
| C3—N2 | 1.326 (4) | C15—O5 | 1.367 (2) |
| C3—O2 | 1.342 (3) | C15—C20 | 1.380 (3) |
| C4—N1 | 1.315 (3) | C15—C16 | 1.394 (3) |
| C4—N2 | 1.317 (3) | C16—C17 | 1.376 (3) |
| C4—O3 | 1.363 (3) | C16—Cl1 | 1.721 (2) |
| C5—O1 | 1.433 (4) | C17—C18 | 1.379 (4) |
| C5—H5A | 0.9600 | C17—H17 | 0.9300 |
| C5—H5B | 0.9600 | C18—C19 | 1.368 (4) |
| C5—H5C | 0.9600 | C18—Cl2 | 1.737 (2) |
| C6—O2 | 1.415 (5) | C19—C20 | 1.381 (3) |
| C6—H6A | 0.9600 | C19—H19 | 0.9300 |
| C6—H6B | 0.9600 | C20—H20 | 0.9300 |
| C6—H6C | 0.9600 | C21—N3 | 1.465 (3) |
| C7—C12 | 1.368 (3) | C21—C22 | 1.466 (4) |
| C7—C8 | 1.377 (3) | C21—C23 | 1.531 (3) |
| C7—O3 | 1.395 (3) | C21—H21 | 0.9800 |
| C8—C9 | 1.376 (3) | C22—H22A | 0.9600 |
| C8—H8 | 0.9300 | C22—H22B | 0.9600 |
| C9—C10 | 1.392 (3) | C22—H22C | 0.9600 |
| C9—H9 | 0.9300 | C23—O7 | 1.199 (3) |
| C10—C11 | 1.378 (3) | C23—O6 | 1.321 (2) |
| C10—N3 | 1.439 (3) | C24—O6 | 1.454 (3) |
| C11—C12 | 1.383 (3) | C24—H24A | 0.9600 |
| C11—H11 | 0.9300 | C24—H24B | 0.9600 |
| C12—H12 | 0.9300 | C24—H24C | 0.9600 |
| C13—O4 | 1.216 (2) | ||
| O1—C1—N1 | 118.8 (2) | H14A—C14—H14B | 108.1 |
| O1—C1—C2 | 118.8 (2) | O5—C15—C20 | 125.74 (18) |
| N1—C1—C2 | 122.4 (3) | O5—C15—C16 | 116.07 (19) |
| C3—C2—C1 | 116.1 (2) | C20—C15—C16 | 118.2 (2) |
| C3—C2—H2 | 121.9 | C17—C16—C15 | 121.3 (2) |
| C1—C2—H2 | 121.9 | C17—C16—Cl1 | 119.07 (17) |
| N2—C3—O2 | 118.4 (3) | C15—C16—Cl1 | 119.64 (17) |
| N2—C3—C2 | 123.6 (2) | C16—C17—C18 | 119.1 (2) |
| O2—C3—C2 | 118.0 (3) | C16—C17—H17 | 120.5 |
| N1—C4—N2 | 130.0 (2) | C18—C17—H17 | 120.5 |
| N1—C4—O3 | 118.5 (2) | C19—C18—C17 | 120.7 (2) |
| N2—C4—O3 | 111.5 (2) | C19—C18—Cl2 | 119.9 (2) |
| O1—C5—H5A | 109.5 | C17—C18—Cl2 | 119.39 (19) |
| O1—C5—H5B | 109.5 | C18—C19—C20 | 120.0 (3) |
| H5A—C5—H5B | 109.5 | C18—C19—H19 | 120.0 |
| O1—C5—H5C | 109.5 | C20—C19—H19 | 120.0 |
| H5A—C5—H5C | 109.5 | C15—C20—C19 | 120.8 (2) |
| H5B—C5—H5C | 109.5 | C15—C20—H20 | 119.6 |
| O2—C6—H6A | 109.5 | C19—C20—H20 | 119.6 |
| O2—C6—H6B | 109.5 | N3—C21—C22 | 115.3 (2) |
| H6A—C6—H6B | 109.5 | N3—C21—C23 | 108.87 (17) |
| O2—C6—H6C | 109.5 | C22—C21—C23 | 114.0 (2) |
| H6A—C6—H6C | 109.5 | N3—C21—H21 | 105.9 |
| H6B—C6—H6C | 109.5 | C22—C21—H21 | 105.9 |
| C12—C7—C8 | 121.4 (2) | C23—C21—H21 | 105.9 |
| C12—C7—O3 | 116.8 (2) | C21—C22—H22A | 109.5 |
| C8—C7—O3 | 121.4 (2) | C21—C22—H22B | 109.5 |
| C9—C8—C7 | 119.2 (2) | H22A—C22—H22B | 109.5 |
| C9—C8—H8 | 120.4 | C21—C22—H22C | 109.5 |
| C7—C8—H8 | 120.4 | H22A—C22—H22C | 109.5 |
| C8—C9—C10 | 119.9 (2) | H22B—C22—H22C | 109.5 |
| C8—C9—H9 | 120.0 | O7—C23—O6 | 124.5 (2) |
| C10—C9—H9 | 120.0 | O7—C23—C21 | 125.12 (18) |
| C11—C10—C9 | 119.99 (19) | O6—C23—C21 | 110.36 (19) |
| C11—C10—N3 | 121.04 (18) | O6—C24—H24A | 109.5 |
| C9—C10—N3 | 118.97 (18) | O6—C24—H24B | 109.5 |
| C10—C11—C12 | 119.8 (2) | H24A—C24—H24B | 109.5 |
| C10—C11—H11 | 120.1 | O6—C24—H24C | 109.5 |
| C12—C11—H11 | 120.1 | H24A—C24—H24C | 109.5 |
| C7—C12—C11 | 119.6 (2) | H24B—C24—H24C | 109.5 |
| C7—C12—H12 | 120.2 | C4—N1—C1 | 114.2 (2) |
| C11—C12—H12 | 120.2 | C4—N2—C3 | 113.7 (2) |
| O4—C13—N3 | 122.16 (19) | C13—N3—C10 | 122.15 (16) |
| O4—C13—C14 | 120.86 (18) | C13—N3—C21 | 115.18 (17) |
| N3—C13—C14 | 116.98 (17) | C10—N3—C21 | 122.20 (18) |
| O5—C14—C13 | 110.13 (16) | C1—O1—C5 | 118.3 (2) |
| O5—C14—H14A | 109.6 | C3—O2—C6 | 118.4 (3) |
| C13—C14—H14A | 109.6 | C4—O3—C7 | 120.35 (18) |
| O5—C14—H14B | 109.6 | C15—O5—C14 | 117.63 (16) |
| C13—C14—H14B | 109.6 | C23—O6—C24 | 116.33 (18) |
| O1—C1—C2—C3 | −179.1 (2) | N2—C4—N1—C1 | −1.4 (4) |
| N1—C1—C2—C3 | 0.8 (4) | O3—C4—N1—C1 | −178.7 (2) |
| C1—C2—C3—N2 | −0.8 (4) | O1—C1—N1—C4 | −179.9 (2) |
| C1—C2—C3—O2 | −179.8 (2) | C2—C1—N1—C4 | 0.1 (3) |
| C12—C7—C8—C9 | 1.1 (3) | N1—C4—N2—C3 | 1.4 (4) |
| O3—C7—C8—C9 | −170.65 (19) | O3—C4—N2—C3 | 178.9 (2) |
| C7—C8—C9—C10 | 1.3 (3) | O2—C3—N2—C4 | 178.8 (2) |
| C8—C9—C10—C11 | −2.7 (3) | C2—C3—N2—C4 | −0.2 (4) |
| C8—C9—C10—N3 | 176.97 (18) | O4—C13—N3—C10 | 179.28 (19) |
| C9—C10—C11—C12 | 1.8 (3) | C14—C13—N3—C10 | −1.8 (3) |
| N3—C10—C11—C12 | −177.89 (19) | O4—C13—N3—C21 | −8.4 (3) |
| C8—C7—C12—C11 | −2.0 (3) | C14—C13—N3—C21 | 170.50 (19) |
| O3—C7—C12—C11 | 170.1 (2) | C11—C10—N3—C13 | 107.0 (2) |
| C10—C11—C12—C7 | 0.6 (3) | C9—C10—N3—C13 | −72.7 (3) |
| O4—C13—C14—O5 | 7.9 (3) | C11—C10—N3—C21 | −64.8 (3) |
| N3—C13—C14—O5 | −171.06 (18) | C9—C10—N3—C21 | 115.5 (2) |
| O5—C15—C16—C17 | −178.33 (17) | C22—C21—N3—C13 | 162.8 (3) |
| C20—C15—C16—C17 | 0.1 (3) | C23—C21—N3—C13 | −67.6 (3) |
| O5—C15—C16—Cl1 | 1.7 (2) | C22—C21—N3—C10 | −24.9 (3) |
| C20—C15—C16—Cl1 | −179.85 (16) | C23—C21—N3—C10 | 104.8 (2) |
| C15—C16—C17—C18 | 0.4 (3) | N1—C1—O1—C5 | −1.0 (4) |
| Cl1—C16—C17—C18 | −179.63 (18) | C2—C1—O1—C5 | 178.9 (3) |
| C16—C17—C18—C19 | −0.4 (4) | N2—C3—O2—C6 | 1.2 (4) |
| C16—C17—C18—Cl2 | −179.73 (16) | C2—C3—O2—C6 | −179.7 (3) |
| C17—C18—C19—C20 | 0.0 (4) | N1—C4—O3—C7 | −14.1 (3) |
| Cl2—C18—C19—C20 | 179.26 (19) | N2—C4—O3—C7 | 168.1 (2) |
| O5—C15—C20—C19 | 177.7 (2) | C12—C7—O3—C4 | 127.6 (2) |
| C16—C15—C20—C19 | −0.6 (3) | C8—C7—O3—C4 | −60.3 (3) |
| C18—C19—C20—C15 | 0.6 (4) | C20—C15—O5—C14 | 11.7 (3) |
| N3—C21—C23—O7 | −20.9 (3) | C16—C15—O5—C14 | −169.97 (17) |
| C22—C21—C23—O7 | 109.4 (3) | C13—C14—O5—C15 | 68.7 (2) |
| N3—C21—C23—O6 | 159.17 (19) | O7—C23—O6—C24 | 1.6 (3) |
| C22—C21—C23—O6 | −70.5 (3) | C21—C23—O6—C24 | −178.4 (2) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C9—H9···O4i | 0.93 | 2.57 | 3.402 (3) | 150 |
Symmetry code: (i) −x, −y+1, −z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: WN2477).
References
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans. 2, pp. S1–19.
- Bruker (2001). SMART and SAINT-Plus Bruker AXS Inc., Madison, Wisconsin, USA.
- Gozzo, F. & Garlaschelli, L. (1985). Pestic. Sci. 16, 227–286.
- Jin, C. F., Liang, Y. J., He, H. W. & Fu, L. W. (2011). Eur. J. Med. Chem. 46, 429–432. [DOI] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536812025494/wn2477sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536812025494/wn2477Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536812025494/wn2477Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report




