Abstract
Background
Many patients with chronic obstructive pulmonary disease (COPD) continue to suffer exacerbations, even when treated with maximum recommended therapy (eg, inhaled combinations of long-acting β2-agonist and high dose inhaled corticosteroids, with or without a long-acting anticholinergic [long-acting muscarinic antagonist]). Roflumilast is approved to treat severe COPD in patients with chronic bronchitis – and a history of frequent exacerbations – as an add-on to bronchodilators.
Purpose
The REACT (Roflumilast in the Prevention of COPD Exacerbations While Taking Appropriate Combination Treatment) study (identification number RO-2455-404-RD, clinicaltrials. gov identifier NCT01329029) will investigate whether roflumilast further reduces exacerbations when added to inhaled combination therapy in patients still suffering from frequent exacerbations.
Patients and methods
REACT is a 1-year randomized, double-blind, multicenter, phase III/IV study of roflumilast 500 μg once daily or placebo on top of a fixed long-acting β2-agonist/inhaled corticosteroid combination. A concomitant long-acting muscarinic antagonist will be allowed at stable doses. The primary outcome is the rate of moderate or severe COPD exacerbations. Using a Poisson regression model with a two-sided significance level of 5%, a sample size of 967 patients per treatment group is needed for 90% power. COPD patients with severe to very severe airflow limitation, symptoms of chronic bronchitis, and at least two exacerbations in the previous year will be recruited.
Conclusion
It is hypothesized that because roflumilast (a phosphodiesterase-4 inhibitor) has a different mode of action to bronchodilators and inhaled corticosteroids, it may provide additional benefits when added to these treatments in frequent exacerbators. REACT will be important to determine the role of roflumilast in COPD management. Here, the design and rationale for this important study is described.
Keywords: chronic obstructive pulmonary disease, roflumilast, protocol, LABA, ICS, exacerbation
Introduction
There is a wide variety of pharmacological treatments currently available for chronic obstructive pulmonary disease (COPD). However, it is clear that even dual or triple combination therapy with long-acting bronchodilators and inhaled steroids is insufficient to prevent exacerbations in patients with severe to very severe disease.
Although COPD care has improved over the last decade,1 there is still a huge unmet burden of disease. Exacerbations in particular can be distressing for patients2 and have serious consequences on their long-term health, such as increased disease progression,3 increased risk of cardiovascular events,4 and increased mortality rates.5,6 Management of stable COPD should be based not only on addressing the current disease impact (determined mainly by symptoms and activity limitation) but also by reducing the patient’s future risk of disease progression (determined primarily by exacerbation frequency).7 In clinical practice, patients still suffering from frequent exacerbations may already be treated with a combination of long-acting β2-agonists (LABA) and inhaled corticosteroids (ICS), often in addition to tiotropium (a long-acting muscarinic antagonist [LAMA]). Since frequent exacerbations are associated with a high level of inflammation,8,9 it is logical to add an antiinflammatory therapy to combination treatment in order to further reduce exacerbation risk.
Roflumilast is an antiinflammatory treatment that reduces exacerbation frequency in a specific subgroup of COPD patients with severe airflow limitation, a previous history of exacerbations, and symptoms of chronic cough and sputum.10,11 These features are typical of patients in group D as classified in the latest recommendations from Global Initiative for Obstructive Lung Disease (GOLD).12 In this patient population, the effects of roflumilast are additive when it is added on top of monotherapy with either LABA or LAMA,10,11 and even when used together with ICS.13 However, the effects of roflumilast added on top of dual or triple therapy have not been studied in a clinical trial setting. It is hypothesized that roflumilast will provide additional benefit when used with these other therapeutic combinations.
The REACT (Roflumilast in the Prevention of COPD Exacerbations While Taking Appropriate Combination Treatment) study (trial identification number RO-2455-404-RD, clinicaltrials.gov identifier NCT01329029) is designed to test this hypothesis and also to set in context the side effect profile of roflumilast identified in earlier investigations. This study will involve a double-blind placebo-controlled parallel group. Exposure to roflumilast will be over 12 months, with an extended follow-up period of 3 months, to determine both the subsequent occurrence of exacerbations and the reversibility of any adverse effects. The data from this trial will give insight as to whether drug therapies of different classes can be effectively combined to prevent exacerbations.
Material and methods
REACT study design
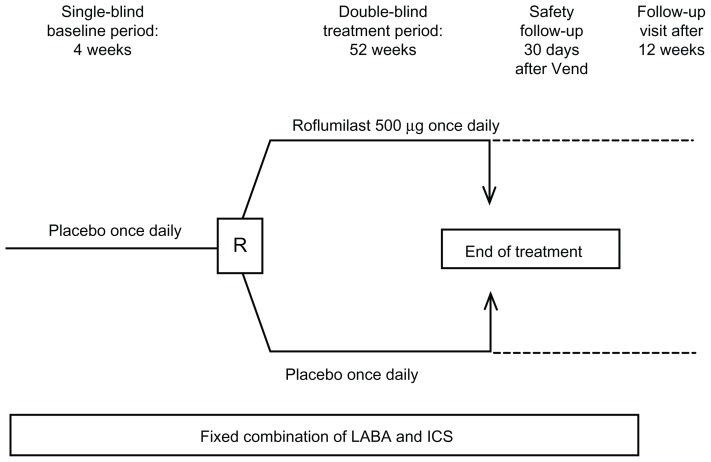
REACT is a 52-week randomized, double-blind, parallel group, multicenter, phase III/IV study (Figure 1). It includes a 4-week run-in period during which all patients will receive placebo in addition to their existing fixed LABA/ICS combination. This is followed by a 52-week treatment period during which patients will be randomized 1:1 to placebo or once-daily roflumilast 500 μg in addition to the fixed LABA/ICS combination. Any commercially available fixed LABA/ICS combination may be used, at the maximum dosage approved in each country. This is the medication previously used by the patient and will not be supplied by the sponsor. Allowed concomitant medications include a LAMA – salbutamol – as rescue medication, and antibiotics and glucocorticoids may be used to treat exacerbations. Drugs to treat concurrent diseases are also allowed. A 30-day follow-up period is included to monitor any ongoing adverse events, with a final follow-up visit 12 weeks after the end of treatment. Compliance will be determined based on the number of tablets taken and the number of days in the treatment period. Compliance must be ≥80% and ≤125% during the 4-week baseline period in order for patients to be randomized (Appendix 1), and must be ≥70% and ≤143% during the 1-year treatment period.
Figure 1.
REACT (Roflumilast in the Prevention of Chronic Obstructive Pulmonary Disease Exacerbations While Taking Appropriate Combination Treatment) study design.
Abbreviations: ICS, inhaled corticosteroid; LABA, long-acting β2-agonist; R, randomization; Vend, last visit.
Patient participation
A total of 1934 patients will be randomized (967 per treatment arm). Patients will be recruited from approximately 200 centers in 21 countries worldwide. All patients must have COPD with severe airflow limitation (confirmed by a post-bronchodilator forced expiratory volume in 1 second [FEV1]/forced vital capacity ratio of 0.70 and a postbronchodilator FEV1 of <50% predicted), symptoms of chronic bronchitis and a history of frequent exacerbations (at least two in the previous year), and must have been on stable treatment with a LABA/ICS fixed combination for ≥12 months prior to study start. A list of inclusion and exclusion criteria is provided in Appendix 2. This study will be conducted in compliance with the Clinical Trial Protocol, the International Conference on Harmonization Consolidated Good Clinical Practices Guideline, the Declaration of Helsinki, and any applicable national regulations. All patients will give informed consent to participate in the study.
Endpoints
The primary endpoint is the rate of moderate or severe COPD exacerbations per patient per year. Moderate exacerbations are defined as requiring oral or parenteral glucocorticoids. Severe exacerbations are defined as requiring hospitalization and/or leading to death. The key secondary endpoint measured in REACT is the change in postbronchodilator FEV1 from randomization to the end of the 52-week treatment period, assessed by spirometry. Other endpoints are described in Appendix 3. Quality of life will be measured using the COPD Assessment Test™ (GlaxoSmithKline, Middlesex, United Kingdom). Safety endpoints include the number of adverse events and changes in body weight/body mass index.
COPD exacerbations
Patients experiencing an exacerbation during the run-in period cannot be entered into the treatment period until after the exacerbation has resolved. COPD symptoms (cough, sputum) and use of rescue medication will be recorded on a daily basis in the patient diary to increase the patient’s awareness of worsening COPD symptoms. To ensure that all exacerbations are captured, patients will be encouraged to contact their study doctor in case of a deterioration of COPD symptoms. All exacerbations will be captured in the exacerbation section of the case report form, including description of severity, whether treatment was required, whether the patient was withdrawn from the study, and start/end date of the exacerbation. The number of moderate or severe exacerbations during the treatment period will be recorded and analyzed as the primary endpoint. A number of other exacerbation endpoints will also be assessed, including the proportion of patients experiencing an exacerbation and time to first, second, and third exacerbation.
Statistical analysis
The primary endpoint rate of moderate or severe COPD exacerbations will be analyzed with a Poisson regression model with the number of exacerbations per patient as the dependent variable. The model will include an offset variable representing the duration of a patient in the study. Treatment will be included as independent variable in the model. A correction for overdispersion will be considered.
Assuming a mean exacerbation rate of 1.25 per patient per year in the placebo group and a reduction in exacerbations of 20% with roflumilast 500 μg, and using a Poisson regression model with a correction for overdispersion of two based on previous data,10 it was estimated that 967 patients per treatment group would provide 90% power to detect a significant difference between treatments with a two-sided α level of 0.05.
To evaluate the effect of study medication discontinuation on the primary endpoint, results including all exacerbations occurring during the 1-year study period (on- and off-treatment) will be provided as a key sensitivity analysis.
The secondary endpoints from spirometry, use of rescue medication, symptom scores, as well as the COPD Assessment Test will be analyzed using a repeated measurements model. The dependent variable will be the change from randomization to each scheduled postrandomization visit. As independent variables, the following factors and covariates (all fixed) will be included: treatment, baseline value, time, and a treatment-by-time interaction.
Time to first, second, and third COPD exacerbation (combined in one analysis) will be analyzed according to the method of Wei–Lin–Weissfeld.14 In addition, time to first, second, and third exacerbation (separate analyses) as well as time to mortality and time to study withdrawal will be analyzed using the Cox proportional hazards regression. A responder analysis of patients with at least one COPD exacerbation (exacerbators) or without any COPD exacerbation (nonexacerbators) will be performed based on both on- and off-treatment data.
Safety analyses will be performed descriptively.
Discussion
The REACT study will address a most important clinically relevant question: whether an oral antiinflammatory phosphodiesterase-4 (PDE4) inhibitor will provide additional benefit in patients already treated and not fully controlled with inhaled LABA/ICS combination therapy and, in a proportion of patients, tiotropium (LAMA). In addition, the study will provide important new information on the safety of roflumilast in these patients.
Over the last decade, large pharmacological intervention studies have not only determined the evidence base for current COPD therapy, but have also allowed a series of problems relevant to the daily life of COPD patients to be addressed. In addition, trials of new treatments have taken place.15 There is now a clear understanding of the interrelations between exacerbations and impaired health status, the patterns of mortality in COPD,16,17 the significance of withdrawing from clinical trials,18 and the most appropriate way of expressing exacerbation rates in interventional and observational studies.19,20 To date, the majority of these large clinical studies have involved inhaled therapies such as ICS, LABA, LAMA, and combinations of these agents in the same and different inhalers. Studies of PDE4 inhibitors have also contributed to the knowledge of COPD, with data from biopsy studies with cilomilast21 and induced sputum data with roflumilast and cilomilast22 showing that these antiinflammatory agents can affect the cellular profile in patients with stable disease, unlike exposure to ICS monotherapy over a similar period of a few weeks.23,24 These studies confirmed the findings of in vitro experiments which showed that PDE4 inhibitors have multiple potential actions on cells involved in persistent inflammation. However, translating these observations into clinical practice is challenging and has provided some further insight into how agents of different classes should be tested.
Although several PDE4 inhibitors have been developed by many pharmaceutical companies, only cilomilast and roflumilast eventually proceeded to large scale trials in humans. The initially encouraging results seen after 6 weeks of treatment with cilomilast in COPD patients of moderate severity were not confirmed in a consistent way in 6-month studies conducted in patients with more severe disease; although positive effects on inflammatory markers in sputum have been reported.25 The changes in lung function in these studies were modest in contrast to the changes in FEV1 seen with roflumilast in the dose-ranging RECORD study.26 The majority of patients in this clinical trial had GOLD Stage II COPD and the number reporting exacerbations requiring treatment with corticosteroids and/or antibiotics was small. In the next phase, using the 500 μg once-daily dose of roflumilast, which is the approved treatment dose, the results were disappointing. A statistically significant improvement in FEV1 was seen in patients with GOLD Stage III/IV COPD but there was no statistically significant difference in the number of exacerbations in those treated with roflumilast compared with placebo.27 The exacerbation rate in this study was lower than anticipated and when subgroups with more severe disease and a history of chronic bronchitis symptoms were studied, where the exacerbation rate was higher, then there was evidence of a positive treatment effect.13 Subsequently, two identical prospective clinical trials were conducted to test the hypothesis that, in a specific subset of patients prone to exacerbations, roflumilast may reduce the exacerbation rate. COPD patients with a history of prior exacerbations requiring medical treatment, confirmed symptoms of chronic bronchitis, and an FEV1 of <50% predicted were recruited and randomized to treatment over 1 year. In this study, patients were asked not to take ICS in case this confounded the action of the antiinflammatory drug, but 50% were allowed to continue with LABA. The results of these studies have been published10 and confirm that there was a positive improvement in both of the primary endpoints in each case. A subsequent prespecified analysis revealed that in patients concomitantly treated with LABA, the addition of roflumilast further reduced the mean exacerbation rate by 21% (relative risk 0.79; P < 0.001).28 A posthoc analysis of the early roflumilast trials, in which concomitant treatment with ICS at a stable dose was allowed, showed that roflumilast provides further reductions in exacerbations also in patients using ICS.13 Finally, in two 6-month trials in patients with GOLD Stage II–IV COPD, an additive effect was seen in terms of lung function improvement when roflumilast was added either to salmeterol (LABA) or tiotropium (LAMA).11
However, as COPD patients often are treated with a combination of drug classes, additional data are clearly needed about whether the addition of roflumilast can further reduce exacerbation frequency in patients who have exacerbations and meet the other criteria already noted despite taking a LABA/ICS combination or triple therapy with LABA/ICS and tiotropium. Based on the pharmacology of roflumilast, the pathways modified are different from those changed by bronchodilators and ICS, but this is no guarantee that this will translate into clinically meaningful improvement. It is reassuring that analyses of previous studies which allowed concomitant therapy suggested incremental efficacy with roflumilast when used either with bronchodilators11,28 or ICS.13 However, these studies could not provide insight into the benefit of roflumilast added to various combinations of different therapeutic drug classes such as LABA/ICS therapy or LABA/ICS/LAMA triple therapy, which is the goal of the current study. The LABA/ICS combinations used in REACT may be any commercially available fixed dose combination, used at the maximum dose approved in each country. While this introduces some variability in the study, this design will reflect that in real life roflumilast will not be added to the same drug regimen in every patient, which means that the results are relevant to patients around the world.
Most oral therapies are associated with side effects and issues of patient tolerance, and roflumilast is associated with a series of pharmacologically predictable side effects. When data were pooled from 14 randomized, double-blind, placebo-controlled studies of roflumilast in COPD patients (5766 patients in total treated with roflumilast and 5491 with placebo), the most frequent adverse events occurring more commonly with roflumilast versus placebo were diarrhea (10.1% versus 2.6%), weight decrease (6.8% versus 1.8%), nausea (5.2% versus 1.4%), and headache (4.6% versus 2.0%). Apart from weight decrease, the majority of these adverse events resolved within 3 weeks (Table 1). Pooling safety data in this way has revealed an established side effect profile that will be monitored in REACT to see if it is modified by concomitant use of COPD treatment combinations.
Table 1.
Adverse events reported with once-daily placebo or roflumilast in the chronic obstructive pulmonary disease safety pool
| Duration of adverse events, weeks | Diarrhea | Weight decrease | Nausea | Headache | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| PBO (n = 5491) | ROF (n = 5766) | PBO (n = 5491) | ROF (n = 5766) | PBO (n = 5491) | ROF (n = 5766) | PBO (n = 5491) | ROF (n = 5766) | |
| <1 | 88 (55.3) | 228 (32.5) | 0 (0) | 0 (0) | 38 (45.2) | 102 (30.5) | 72 (57.1) | 114 (38.8) |
| 1–3 | 41 (25.8) | 258 (36.8) | 3 (3.0) | 24 (6.0) | 28 (33.3) | 144 (43.1) | 24 (19.0) | 93 (31.6) |
| 4–12 | 20 (12.6) | 140 (20.0) | 16 (15.8) | 82 (20.4) | 12 (14.3) | 59 (17.7) | 15 (11.9) | 55 (18.7) |
| 13–25 | 4 (2.5) | 46 (6.6) | 15 (14.9) | 83 (20.7) | 3 (3.6) | 18 (5.4) | 4 (3.2) | 11 (3.7) |
| ≥26 | 2 (1.3) | 14 (2.0) | 16 (15.8) | 88 (21.9) | 1 (1.2) | 4 (1.2) | 3 (2.4) | 8 (2.7) |
| Ongoing or missing recovery date | 4 (2.5) | 15 (2.2) | 51 (50.5) | 124 (30.9) | 2 (2.4) | 7 (2.1) | 8 (6.3) | 13 (4.4) |
| Total, n | 159 | 701 | 101 | 401 | 84 | 334 | 126 | 294 |
Notes: The table includes pooled data from 14 placebo-controlled, double-blind phase II/III studies of roflumilast 500 μg or 250 μg once daily versus placebo in subjects with moderate-to-very severe chronic obstructive pulmonary disease. Eight of the 14 studies have been published previously.10,11,26,27,33 Data are presented as number of patients (percent of events in each category).
Abbreviations: PBO, placebo; ROF, roflumilast.
With regard to weight decrease, the absolute magnitude was greatest in those people who were heaviest, specifically in those who would be classed as obese with a body mass index greater than 30. A low body mass index is widely regarded as a poor prognostic feature in COPD,29,30 and so any drug which reduces body weight needs to be fully understood. There are very limited data in the current safety database about patients followed after the end of the clinical trial. Those data suggest that the patients who have lost weight will regain that weight, but clearly more careful prospectively collected information is needed.31 For this reason, the REACT study includes an extended period of follow-up of the study medication. The occurrence of exacerbations will also be monitored, as withdrawal of ICS has been associated with an increase in exacerbations.32 This will provide further insight, not only into the action of this drug, but also as to whether withdrawal studies could be seen as a more cost-efficient way of evaluating new treatments.
Conclusion
In summary, data from the REACT trial will add considerably to the knowledge of COPD in symptomatic patients with severe airflow limitation who are particularly at risk of exacerbating despite treatment with LABA/ICS combination or LABA/ICS/LAMA triple therapy. It will be determined whether there is a ceiling effect for pharmacological treatment in this condition, or whether – despite current best efforts – more can be offered to these high risk patients who continue to suffer from exacerbations. Moreover, the profile of adverse events should be able to be better understood now that it is clearer which events occur most often and which require objective monitoring. Studies such as this will continue to advance knowledge in COPD and ensure that future treatment guidance is based on firm evidence from carefully conducted clinical trials.
Supplementary data
Appendix 1: randomization criteria
No moderate or severe exacerbation, and/or exacerbation treated with antibiotics, during single-blind baseline period
Tablet compliance ≥80% and ≤125%
Total cough and sputum score ≥14 during the last week directly preceding randomization visit
A postbronchodilator forced expiratory volume in 1 second (FEV1) of ≤50% predicted.
Appendix 2: inclusion and exclusion criteria for the REACT (Roflumilast in the Prevention of Chronic Obstructive Pulmonary Disease Exacerbations While Taking Appropriate Combination Treatment) study
Key inclusion criteria
Male or female aged ≥40 years
Current or former smoker with a smoking history of ≥20 pack years
History of chronic obstructive pulmonary disease (diagnosed ≥12 months prior to study start) associated with chronic productive cough (for 3 months in each of the 2 years prior to study start)
Postbronchodilator FEV1/forced vital capacity ratio of <70% predicted and FEV1 of ≤50% predicted
History of at least two exacerbations in the past year
Treated with fixed long-acting β2-agonist/inhaled corticosteroid combination at a constant dose (the maximum approved strength) for ≥12 months prior to study start.
Key exclusion criteria
Ongoing exacerbation at study start (moderate or severe, or requiring antibiotics)
Lower respiratory tract infection not resolved within 4 weeks prior to study start
Diagnosis of asthma or other relevant lung disease
Current or recent (within 3 months of study start) participation in a pulmonary rehabilitation program
Known α1-antitrypsin deficiency.
Appendix 3: secondary and other endpoints in the REACT (Roflumilast in the Prevention of Chronic Obstructive Pulmonary Disease Exacerbations While Taking Appropriate Combination Treatment) study
Secondary efficacy endpoints
Change in postbronchodilator FEV1 (key secondary endpoint)
Other chronic obstructive pulmonary disease exacerbation (mild, increase in rescue medication of at least three puffs per day on at least two consecutive days during the double-blind treatment period; moderate, requiring oral or parenteral glucocorticoid therapy; and severe, requiring hospitalization and/or leading to death) endpoints
Postbronchodilator lung function measurements
Patient diary endpoints
Quality of life (COPD Assessment Test™; GlaxoSmith-Kline, Middlesex, United Kingdom)
Mortality
Time to withdrawal.
Other endpoints
Pharmacokinetics and pharmacodynamics of roflumilast and roflumilast amine oxide
Safety
Adverse events
Changes in laboratory values, vital signs, and physical examination findings (eg, electrocardiogram)
Changes in body weight and body mass index.
Footnotes
Disclosure
This manuscript was prepared by PMAC on behalf of members of the REACT Steering Committee (PMAC, FJM, LMF, and KFR), all of whom participated in development of the final manuscript. This study is funded by Takeda Pharmaceuticals International GmbH. Medical writing support was provided by Sarah Nelson of Synergy Vision (United Kingdom) and was funded by Takeda.
PMAC has served on the scientific advisory boards of AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKline, Novartis, and Nycomed (a Takeda company) and has received research funding from AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKline, and Nycomed.
FJM has participated in advisory boards in COPD development for Actelion, Almirall, American Institutes for Research, Astra Zeneca, Bayer, BoomComm, Forest, GlaxoSmithKline, Ikaria, MedImmune, Merck, Novartis, Nycomed, Pearl, Pfizer, and Schering. He has been a member of the steering committee for COPD studies sponsored by Actelion, GlaxoSmithKline, Forest, MPex, and Nycomed. He has participated in Food and Drug Administration mock panels for Boehringer Ingelheim and Forest. The University of Michigan received funds from Boehringer Ingelheim for a COPD study. He has served on speaker’s bureaus or in continuing medical education activities sponsored by American College of Chest Physicians, American Lung Association, Almirall, Astra Zeneca, Beaumont, Boehringer Ingelheim, Center for Health Care Education, CME Incite, ePocrates, Forest, France Foundation, GlaxoSmithKline, Lovelace, MedEd, NACE, Nycomed, Potomac, Prescott, Sanofi Aventis, St Luke’s, and UpToDate. He has received royalties from Associates in Medical Marketing and Castle Connolly.
LMF has received payment for consultancy from Boehringer Ingelheim, Chiesi Farmaceutici, GlaxoSmithKline Beech, Merck Sharp and Dohme, Novartis, Nycomed, Pearl Therapeutics, SigmaTau, Sterna, Peer Voice Europe, Pearl Therapeutic, OM Pharma Sa, and TEVA. He has received payment for lectures, advisory boards, or travel expense reimbursements from AstraZeneca, Dey Pharma, Novartis, Schering Plough, SigmaTau, Roche, German Aerospace Center, Mundipharma International, Genetech Inc, Elevation Pharmaceutical, and Ferrer Group. His institution received grants from Boehringer Ingelheim, ScheringPlough, Pfizer, Nycomed, Menarini Industrie Farmaceutiche, Chiesi Farmaceutici, GlaxoSmithKline, Merck Sharp and Dohme, Roche, AstraZeneca, Novartis, SigmaTau, Italian Ministry for University and Research, and Italian Ministry of Health.
UMG is an employee of Takeda Pharmaceuticals International GmbH.
KFR has received payment for consultancy for AstraZeneca, Chiesi Pharmaceutical, Novartis, MSD, GlaxoSmithKline, and Nycomed. He has received research funding from Altana Pharma, Novartis, AstraZeneca, MSD, and Nycomed.
References
- 1.Almagro P, Salvado M, Garcia-Vidal C, et al. Recent improvement in long-term survival after a COPD hospitalisation. Thorax. 2010;65(4):298–302. doi: 10.1136/thx.2009.124818. [DOI] [PubMed] [Google Scholar]
- 2.Kessler R, Stahl E, Vogelmeier C, et al. Patient understanding, detection, and experience of COPD exacerbations: an observational, interview-based study. Chest. 2006;130(1):133–142. doi: 10.1378/chest.130.1.133. [DOI] [PubMed] [Google Scholar]
- 3.Decramer M, Celli B, Kesten S, Liu D, Tashkin DP. Frequency of exacerbations adversely impacts the course of COPD. Am J Respir Crit Care Med. 2010;181:A1526. [Google Scholar]
- 4.Donaldson GC, Hurst JR, Smith CJ, Hubbard RB, Wedzicha JA. Increased risk of myocardial infarction and stroke following exacerbation of COPD. Chest. 2010;137(5):1091–1097. doi: 10.1378/chest.09-2029. [DOI] [PubMed] [Google Scholar]
- 5.Wedzicha JA, Seemungal TA. COPD exacerbations: defining their cause and prevention. Lancet. 2007;370(9589):786–796. doi: 10.1016/S0140-6736(07)61382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soler-Cataluna JJ, Martinez-Garcia MA, Roman Sanchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925–931. doi: 10.1136/thx.2005.040527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Postma D, Anzueto A, Calverley P, et al. A new perspective on optimal care for patients with COPD. Prim Care Respir J. 2011;20(2):205–209. doi: 10.4104/pcrj.2011.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perera WR, Hurst JR, Wilkinson TM, et al. Inflammatory changes, recovery and recurrence at COPD exacerbation. Eur Respir J. 2007;29(3):527–534. doi: 10.1183/09031936.00092506. [DOI] [PubMed] [Google Scholar]
- 9.Bhowmik A, Seemungal TA, Sapsford RJ, Wedzicha JA. Relation of sputum inflammatory markers to symptoms and lung function changes in COPD exacerbations. Thorax. 2000;55(2):114–120. doi: 10.1136/thorax.55.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calverley PM, Rabe KF, Goehring UM, Kristiansen S, Fabbri LM, Martinez FJ. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet. 2009;374(9691):685–694. doi: 10.1016/S0140-6736(09)61255-1. [DOI] [PubMed] [Google Scholar]
- 11.Fabbri LM, Calverley PM, Izquierdo-Alonso JL, et al. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomised clinical trials. Lancet. 2009;374(9691):695–703. doi: 10.1016/S0140-6736(09)61252-6. [DOI] [PubMed] [Google Scholar]
- 12.Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of COPD. Dec, 2011. [Accessed April 19, 2012]. Available from: http://www.goldcopd.org.
- 13.Rennard SI, Calverley PM, Goehring UM, Bredenbroker D, Martinez FJ. Reduction of exacerbations by the PDE4 inhibitor roflumilast – the importance of defining different subsets of patients with COPD. Respir Res. 2011;12:18. doi: 10.1186/1465-9921-12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei LJ, Lin DY, Weissfeld L. Regression analysis of multivariate incomplete failure time data by modeling marginal distributions. J Am Stat Assoc. 1989;84(408):1065–1073. [Google Scholar]
- 15.Calverley PM, Rennard SI. What have we learned from large drug treatment trials in COPD? Lancet. 2007;370(9589):774–785. doi: 10.1016/S0140-6736(07)61381-6. [DOI] [PubMed] [Google Scholar]
- 16.Anthonisen NR, Skeans MA, Wise RA, Manfreda J, Kanner RE, Connett JE. The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann Intern Med. 2005;142(4):233–239. doi: 10.7326/0003-4819-142-4-200502150-00005. [DOI] [PubMed] [Google Scholar]
- 17.McGarvey LP, John M, Anderson JA, Zvarich MT, Wise RA. Ascertainment of cause-specific mortality in COPD: operations of the TORCH Clinical Endpoint Committee. Thorax. 2007;62(5):411–415. doi: 10.1136/thx.2006.072348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calverley PM, Spencer S, Willits L, Burge PS, Jones PW. Withdrawal from treatment as an outcome in the ISOLDE study of COPD. Chest. 2003;124(4):1350–1356. doi: 10.1378/chest.124.4.1350. [DOI] [PubMed] [Google Scholar]
- 19.Keene ON, Calverley PM, Jones PW, Vestbo J, Anderson JA. Statistical analysis of exacerbation rates in COPD: TRISTAN and ISOLDE revisited. Eur Respir J. 2008;32(1):17–24. doi: 10.1183/09031936.00161507. [DOI] [PubMed] [Google Scholar]
- 20.Suissa S. Statistical treatment of exacerbations in therapeutic trials of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173(8):842–846. doi: 10.1164/rccm.200508-1338PP. [DOI] [PubMed] [Google Scholar]
- 21.Gamble E, Grootendorst DC, Brightling CE, et al. Antiinflammatory effects of the phosphodiesterase-4 inhibitor cilomilast (Ariflo) in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;168(8):976–982. doi: 10.1164/rccm.200212-1490OC. [DOI] [PubMed] [Google Scholar]
- 22.Grootendorst DC, Gauw SA, Verhoosel RM, et al. Reduction in sputum neutrophil and eosinophil numbers by the PDE4 inhibitor roflumilast in patients with COPD. Thorax. 2007;62(12):1081–1087. doi: 10.1136/thx.2006.075937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bourbeau J, Christodoulopoulos P, Maltais F, Yamauchi Y, Olivenstein R, Hamid Q. Effect of salmeterol/fluticasone propionate on airway inflammation in COPD: a randomised controlled trial. Thorax. 2007;62(11):938–943. doi: 10.1136/thx.2006.071068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hattotuwa K, Gizycki MJ, Ansari TW, Jeffrey PK, Barnes NC. The effects of inhaled fluticasone on airway inflammation in chronic obstructive pulmonary disease: a double-blind, placebo-controlled biopsy study. Am J Respir Crit Care Med. 2002;165(12):1592–1596. doi: 10.1164/rccm.2105025. [DOI] [PubMed] [Google Scholar]
- 25.Compton CH, Gubb J, Nieman R, et al. Cilomilast, a selective phosphodiesterase-4 inhibitor for treatment of patients with chronic obstructive pulmonary disease: a randomised, dose-ranging study. Lancet. 2001;358(9278):265–270. doi: 10.1016/S0140-6736(01)05481-2. [DOI] [PubMed] [Google Scholar]
- 26.Rabe KF, Bateman ED, O’Donnell D, Witte S, Bredenbroker D, Bethke TD. Roflumilast – an oral anti-inflammatory treatment for chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2005;366(9485):563–571. doi: 10.1016/S0140-6736(05)67100-0. [DOI] [PubMed] [Google Scholar]
- 27.Calverley PM, Sanchez-Toril F, McIvor A, Teichmann P, Bredenbroeker D, Fabbri LM. Effect of 1-year treatment with roflumilast in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;176(2):154–161. doi: 10.1164/rccm.200610-1563OC. [DOI] [PubMed] [Google Scholar]
- 28.Bateman ED, Rabe KF, Calverley PM, et al. Roflumilast with long-acting β2-agonists for COPD: influence of exacerbation history. Eur Respir J. 2011;38(3):553–560. doi: 10.1183/09031936.00178710. [DOI] [PubMed] [Google Scholar]
- 29.Iversen KK, Kjaergaard J, Akkan D, et al. Chronic obstructive pulmonary disease in patients admitted with heart failure. J Intern Med. 2008;264(4):361–369. doi: 10.1111/j.1365-2796.2008.01975.x. [DOI] [PubMed] [Google Scholar]
- 30.Landbo C, Prescott E, Lange P, Vestbo J, Almdal TP. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160(6):1856–1861. doi: 10.1164/ajrccm.160.6.9902115. [DOI] [PubMed] [Google Scholar]
- 31.Martinez FJ, Rabe KF, Wouters EFM, et al. Time course and reversibility of weight decrease with roflumilast, a phosphodiesterase 4 inhibitor. Am J Respir Crit Care Med. 2010;181:A4441. [Google Scholar]
- 32.Choudhury AB, Dawson CM, Kilvington HE, et al. Withdrawal of inhaled corticosteroids in people with COPD in primary care: a randomised controlled trial. Respir Res. 2007;8:93. doi: 10.1186/1465-9921-8-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bredenbroker D, Syed J, Leichtl S, Rathgeb F, Wurst W. Safety of once-daily roflumilast, a new, orally active selective phosphodiesterase 4 inhibitor, in patients with COPD. Am J Respir Crit Care Med. 2002;165:A595. [Google Scholar]