Abstract
The aim of the present study was to assess recovery from hematopoietic and gastrointestinal damage by Ex-RAD®, also known as ON01210.Na (4-carboxystyryl-4-chlorobenzylsulfone, sodium salt), after total body radiation. In our previous study, we reported that Ex-RAD, a small-molecule radioprotectant, enhances survival of mice exposed to gamma radiation, and prevents radiation-induced apoptosis as measured by the inhibition of radiation-induced protein 53 (p53) expression in cultured cells. We have expanded this study to determine best effective dose, dose-reduction factor (DRF), hematological and gastrointestinal protection, and in vivo inhibition of p53 signaling. A total of 500 mg/kg of Ex-RAD administered at 24 h and 15 min before radiation resulted in a DRF of 1.16. Ex-RAD ameliorated radiation-induced hematopoietic damage as monitored by the accelerated recovery of peripheral blood cells, and protection of granulocyte macrophage colony-forming units (GM-CFU) in bone marrow. Western blot analysis on spleen indicated that Ex-RAD treatment inhibited p53 phosphorylation. Ex-RAD treatment reduces terminal deoxynucleotidyl transferase mediated dUTP nick end labeling assay (TUNEL)-positive cells in jejunum compared with vehicle-treated mice after radiation injury. Finally, Ex-RAD preserved intestinal crypt cells compared with the vehicle control at 13 and 14 Gy. The results demonstrated that Ex-RAD ameliorates radiation-induced peripheral blood cell depletion, promotes bone marrow recovery, reduces p53 signaling in spleen and protects intestine from radiation injury.
Keywords: Ex-RAD, GM-CFU, gastrointestinal, radioprotection
INTRODUCTION
Protection from normal tissue injury is a matter of concern in therapeutic radiology [1]. Other instances of radiation exposure include accidents that can occur in nuclear power plants, potential terrorist attack involving improvised explosive devices (IEDs) containing radioactive materials, and extended space explorations by the National Aeronautics and Space Administration (NASA) [2]. Also, the development of a radiation countermeasure drug that can be used before radiation exposure may have dual uses: protecting cancer patients from normal tissue injury during radiation therapy, and for first responders who may need to enter a radiation-exposure field for rescue operations.
Exposure to ionizing radiation results in multi-organ dysfunction syndrome (MODS), which can lead to acute radiation syndrome (ARS) or long-term health effects like cancer or pulmonary fibrosis, depending on the radiation dose rate and total dose [2, 3]. ARS includes hematopoietic and gastrointestinal (GI) subsyndromes [2, 4] manifested as peripheral blood pancytopenia, depletion of bone marrow progenitor cells and impairment of intestinal crypt cell regeneration. Therefore, strategies for radiation countermeasure development are based on amelioration of peripheral blood cell depletion, restoration of bone marrow progenitor cells and regeneration of intestinal crypt cells.
Cytokines and hematopoietic growth factors are used routinely to protect from the hematopoietic subsyndrome [5, 6]. Other radiation-countermeasure drugs that showed promise include 5-androstenediol (5-AED) [7] and angiotensin peptides [8]. We reported that gamma-tocotrienol, a naturally occurring vitamin E analog, protected mice from radiation-induced pancytopenia [9], restored bone marrow progenitor cells [10] and accelerated the regeneration of intestinal crypt cells [11]. Keratinocyte growth factor (KGF) [12] was also shown to protect both bone marrow and bowel cells. Since accumulated oxidative damage also causes radiation-induced lethality, various other families of antioxidants and thiols were found to moderate the harmful effects of radiation on hematopoietic tissue. Such compounds include tocopherols, tocotrienols, amifostine and genistein [9–11, 13–15].
Agents that prevent GI subsyndrome include fibroblast growth factor (FGF2) [16], cytokines including interleukins, stem cell factor (SCF ), transforming growth factor (TGFβ3) and KGF [17]. These agents specifically provided protection against crypt cell depletion and GI damage-induced mortality [18, 19]. Although cytokine therapy is available for treating radiation injury [19], their relatively short shelf-life, high cost and pleiotropic effects have somewhat limited their use. Currently, there is no Food and Drug Administration (FDA) approved drug available for the protection against lethal effects of radiation. We reported that Ex-RAD® [4-carboxystyryl-4-chlorobenzylsulfone, sodium salt; or ON 01210.Na] is a synthetic small-molecule radioprotective compound (from Onconova Therapeutics, Inc. [OTI]) that is effective in male C3H mice [20] when administered 24 h and 15 min (two injections) before total body irradiation (TBI). Although Ex-RAD had been shown to be an inhibitor of apoptosis in vitro, it is not known whether a similar mechanism is occuring in vivo [20]. In this paper, we report that Ex-RAD accelerates peripheral blood recovery, protects bone marrow colony forming units (CFUs) and intestinal crypts, and also protects the spleen by inhibiting phosphorylation of protein 53 (p53).
MATERIALS AND METHODS
Chemicals and reagents
Ex-RAD was obtained from OTI (Newtown, PA, USA). Chemicals for electrophoresis and immunoblots were purchased from Invitrogen (Frederick, MD, USA). All other chemicals were purchased from Sigma-Aldrich Chemical Company (St. Louis, MO, USA). Antibodies for p53 (DO-1) and phosphorylated p53, goat anti-mouse Immunoglobulin G-horseradish peroxidase (IgG-HRP) and goat anti-rabbit IgG-HRP were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Animals
Six- to eight-week-old male C3H/HeN mice were purchased from the National Cancer Institute (Frederick, MD, USA), and were housed (eight per cage) in an air-conditioned facility at the Armed Forces Radiobiology Research Institute [20]. Temperature, humidity and light/dark cycle were standardized to prevent any variation in natural circadian rhythm. All animals were maintained on acidified water (pH 2.5–3.0) to prevent growth of pseudomonas. All animal procedures were performed in accordance with a protocol approved by the Armed Forces Radiobiology Research Institute's Animal Care and Use Committee (IACUC) using the principles and procedures outlined in the National Research Council's Guide for the Care and Use of Laboratory Animals.
Drug administration
White crystalline Ex-RAD was suspended in a vehicle containing 1% Tween-80, 0.1 M potassium phosphate buffer (KP) of pH 8.2 and 15 mM sodium chloride (NaCl). Each mouse received 0.25 ml of either the drug (25–500 mg/kg) or the vehicle subcutaneously. The drug was administered 24 h and 15 min (two doses) before irradiation, unless otherwise mentioned. All subcutaneous (sc) injections of the drug and vehicle in animals were done aseptically at the nape of the neck with a 23-G needle before irradiation. No infection or local reaction was observed at the site of injection. At least 16 animals were used per group in all experiments.
Irradiation
All irradiations were done at the cobalt-60 gamma-radiation facility of the Armed Forces Radiobiology Research Institute (Bethesda, MD, USA). Mice were bilaterally irradiated in well ventilated Plexiglas boxes (eight mice in each box) at a dose rate of 0.6 Gy/min. The alanine/ electron spin resonance (ESR ) dosimetry system (American Society for Testing and Material Standard E 1607) was used to measure dose rates (to water) in the cores of acrylic (Plexiglas) mouse phantoms [20]. After irradiation, mice were returned to their original cages with access to food and water ad libitum.
Determination of effective dose of Ex-RAD
Each group of mice (n = 16 per group) was given sc one of five doses of Ex-RAD (25, 50, 100, 250 and 500 mg/kg of body weight) 24 h and 15 min before TBI at 7.5 Gy. This radiation dose was selected to induce hematopoietic syndrome after TBI. Mice were returned to cages after radiation with free access to food and water. Weight loss, apparent behavioral deficit and survival of these mice were monitored for a period of 30 days.
Determination of dose reduction factor (DRF)
The method used for DRF determination essentially was the same as described in our previous study [9]. Five groups of mice (n = 16 per group) were injected with vehicle and another five groups were injected sc with 500 mg/kg of Ex-RAD. The vehicle-injected groups were irradiated at 6.25, 7.0, 7.5, 8.0 and 8.5 Gy. The Ex-RAD-injected groups were irradiated at 7, 7.5, 8.0, 8.5 and 9.0 Gy. The range of radiation doses for vehicle- or Ex-RAD-treated groups were selected based on our previous observations [20] so that the lowest radiation dose would result in 100% survival and the highest dose would result in 100% lethality. Data obtained from these studies were used to calculate LD50/30 (lethal radiation dose that results in 50% mortality in 30 days) for vehicle- and Ex-RAD-treated mice. DRF was calculated as the ratio of the LD50/30 radiation dose of Ex-RAD-treated mice to the LD50/30 radiation dose of vehicle-treated mice [9, 21].
Hematological studies of peripheral blood
These studies were done as reported earlier [9]. C3H mice (n = 10 per group) were pretreated with 500 mg/kg of Ex-RAD (or vehicle) sc 24 h and 15 min prior to sub-lethal TBI of 6 Gy and anesthetized humanely with an overdose of isoflurane (Hospira Inc., Lake Forest, IL, USA). Blood (0.6–1.0 ml) was collected from the posterior vena cava using a 23-G needle on Days 0, 1, 4, 7, 17 and 30, with Day 0 being the day of irradiation. Blood was transferred immediately into ethylenediamine tetraacetic acid (EDTA, Sigma, St. Louis, MO, USA) treated blood collection tubes, and mixed gently on a rotary shaker until analysis was performed. This sub-lethal dose was chosen based on prior experience, since it is sufficient to cause rapid depletion of peripheral blood counts, yet all animals in both vehicle- and Ex-RAD-treated groups would survived for the entire experimental period of 30 days. Total white blood cells (WBC), red blood cells (RBC), absolute neutrophil counts (ANC), monocytes (MONO) and platelets (PLT) were counted using an Advia 120 cell counter (Bayer Corporation, Terrytown, NY, USA) and data were generated using the MS software, version 5.9.
Granulocyte-macrophage colony-forming unit (GM-CFU) assay
Hematopoietic progenitor cells committed to granulocyte-macrophage differentiation (GM-colony forming units or GM-CFU) in mouse bone marrow were assayed using a standard protocol [22]. Four groups (n = 3 per group) of mice (unirradiated vehicle, unirradiated drug, irradiated vehicle and irradiated drug) were injected with 500 mg/kg of Ex-RAD or vehicle twice, 24 h and 15 min before 7 Gy TBI. Femoral bone marrow was extracted 4, 8 and 14 days post-TBI and cell suspensions were prepared by flushing bones with 2 ml sterile phosphate buffered saline (PBS) containing 3% fetal bovine serum (FBS, Invitrogen, Frederick, MD, USA). Each cell suspension represented a pool of marrow from four femurs of two mice. The total number of nucleated cells in each suspension was determined with a Coulter counter. Cells were washed twice with Iscove's Modified Dulbecco's Medium (IMDM, Stem Cell Technologies, Vancouver, Canada) before assays and 1–5 × 105 cells/dish were seeded in 35-cm cell culture dishes with methocult® media (M3534, Stem Cell Technologies, Vancouver, Canada) supplemented with murine granulocyte macrophage-colony stimulating factor (10 ng/ml) as per the instruction in the kit. Plates were scored for colonies after culturing for 14 days at 37°C, 5% CO2.
Intestinal crypt colony assay
A microcolony crypt survival assay was performed as described by Withers and Elkind [23] and modified by Potten and Hendry [24]. Mice (n = 8 per group) were sacrificed 12 h and 3.5 days after TBI (13 and 14 Gy). Two 5-cm segments of jejunum were dissected and cleaned by flushing with PBS and fixed in Carnoy's fixative for 20–30 minutes. The intestine segments were transferred into 70% ethanol, embedded with paraffin and at least 10 sections of 3–5 µM thickness were cut from each segment, and stained with hematoxyllin and eosin (H&E) [25]. Stained sections were screened blindly by two individuals microscopically for crypts with at least 10 cells. An automated screening program by Bioquant was used to enumerate the crypts. The microcolony survival was expressed as the average number of surviving crypts from five sections. The average from each mouse was considered as a single value for statistical purposes.
TUNEL apoptosis detection assay
Jejunum was collected 4 and 24 h post-TBI (6 Gy) and Terminal deoxynucleotidyl transferase mediated dUTP nick end labeling assay (TUNEL) staining was performed using a TUNEL Apoptosis Detection Kit (GenScript, Piscataway, NJ, USA) by following the manufacturer's protocol. Briefly, cross-sections of paraffin-embedded jejunum were deparaffinized, rehydrated in gradient ethanol then digested with Proteinase K. Slides were incubated with fluorescein-labeled dUTP, and mounted with 4′,6-diamidino-2-phenylindole (DAPI). Jejunal sections were scored blindly for all TUNEL positive nuclei of the villi using 50 villi per mouse and three mice per group.
Preparation of protein extracts from mouse spleen
Spleens (n = 3 per group) were collected at 4 and 24 h post-TBI. For preparation of total protein extracts, 1 g of spleen was disrupted by tissue homogenizer (Fast Prep®-24, MP Biomedicals, Solon, OH) in 1X radioimmunoprecipitation assay (RIPA) buffer (Santa Cruz Biotechnology, Santa Cruz, CA, USA) supplemented with complete protease inhibitor (Roche Diagnostics Corporation, Indianapolis, IN, USA). After centrifugation (10 min, 10 000 × g, 4°C), the pellet was discarded and the supernatant was used for protein determination using the (bicinchoninic acid) BCA protein assay kit (Thermo Scientific, Asheville, NC, USA).
SDS-PAGE and western blot analysis
Protein extracts of spleen were separated by SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis). Samples were prepared using 4X NuPAGE loading sample (LDS) buffer (Invitrogen, Frederick, MD, USA), containing 3% β-mercaptoethanol and heated at 70°C for 10 min. Fifty micrograms of each sample were loaded into a NuPAGE 4–12% Bis-Tris gel (Invitrogen, Frederick, MD, USA). Subsequently, proteins were transferred to a polyvinylidene fluoride (PVDF) membrane using the 7 min iBlot transfer method (Invitrogen, Frederick, MD, USA). After completion of the transfer, membranes were blocked in 5% nonfat dry milk in tris buffered saline (TBS)-Tween (0.1%) for 1 h. Membranes were incubated with primary antibodies, p53 (DO-1; 1:200; Santa Cruz Biotechnology, Santa Cruz, CA, USA), phospho p53 (Ser 15; 1:1000; cell signaling, Danvers, MA, USA) and β-actin (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight at 4°C. Incubation with peroxidase-conjugated anti-mouse or anti-rabbit secondary antibody (1:2000) was performed for 2 h at room temperature. Bound antibodies were then detected using Super Signal West Pico Chemiluminescent Substrate electrochemiluminescence (ECL) kit (Thermo Scientific, Asheville, NC, USA) and an LAS3000 Fuji imager. Experiments were repeated three times and band densities were analyzed using Multi gauge V3.1 analysis software from Fuji Film. The p53 and p-p53 levels were normalized to actin levels in each sample.
Statistical analysis
A Kaplan–Meier survival plot was drawn using PASW Statistics 18 program software. For survival data, Fisher's exact test was used to compare survival at 30 days and a log-rank test was used to compare survival curves. Means and standard errors were reported for cytopenia and marrow response data. Analysis of variance (ANOVA) was used to determine if there was a significant difference among different groups. For a given day, if there was a significant difference among the groups, a pair-wise comparison was done using the Tukey–Kramer method. A significance level was set at 5% for each test (P < 0.05). All statistical tests were two-sided. Statistical software, PC SAS, was used for statistical analyses.
RESULTS
Radioprotective efficacy and DRF of Ex-RAD
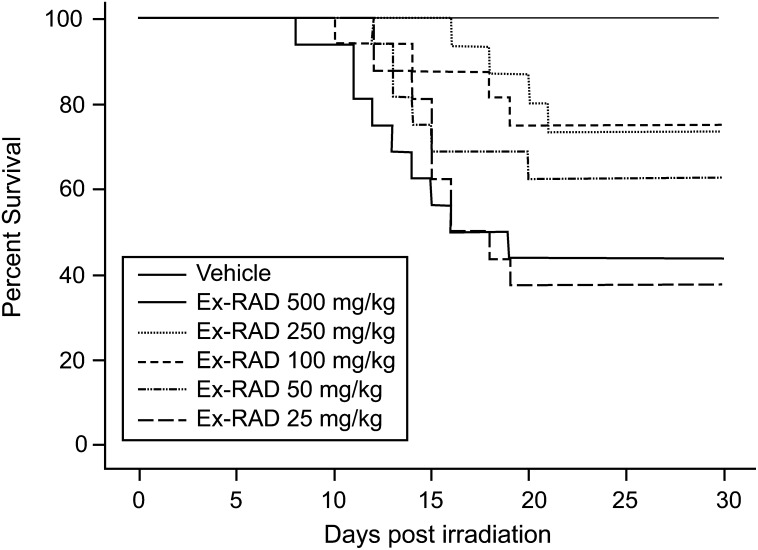
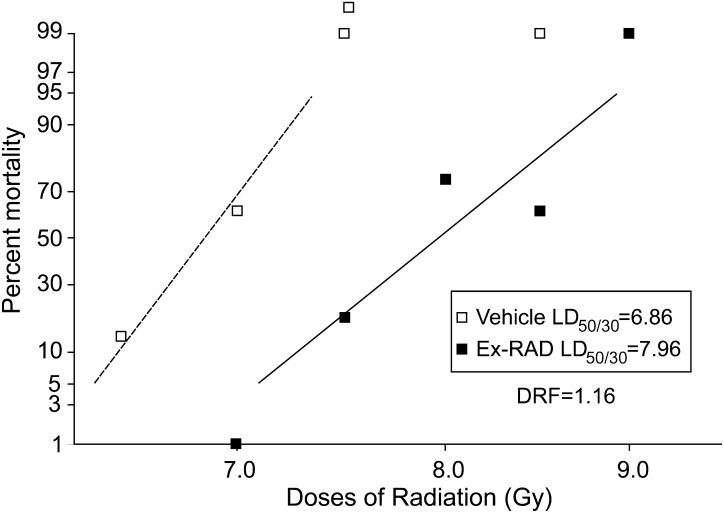
As shown in Fig. 1, Kaplan–Meier survival plots for five doses of Ex-RAD (25–500 mg/kg), 500 mg/kg of Ex-RAD, administered 24 h and 15 min before radiation, was found to provide the highest survival-protection at 7.5 Gy (0.6 Gy/min) of cobalt-60 gamma radiation (100% survival compared with 40% for the radiation control group at 30 days). Both 250 and 100 mg/kg of Ex-RAD showed about 75% survival, and 50 mg/kg provided 60% survival compared with the irradiated control group. The lowest dose, 25 mg/kg did not result in any survival benefit. Mice that received Ex-RAD doses of 500 mg/kg exhibited a significant increase in survival as compared with either vehicle control group or the lowest dose of Ex-RAD, 25 mg/kg treated group (P < 0.0033, Fisher's exact test). No apparent toxicity was observed with the highest dose (500 mg/kg) used in this study. Surviving animals were healthy at the end of the study (30 day after radiation). The optimal 500 mg/kg dose was used to obtain the DRF for Ex-RAD. The drug was administered to mice 24 h and 15 min (two doses) before TBI at various radiation doses, and survival data were used to evaluate the DRF. The LD50/30 radiation doses of 6.86 Gy for vehicle and 7.96 Gy for Ex-RAD were calculated from percent mortality observed in 30 days for groups treated with vehicle and Ex-RAD (Fig. 2). The DRF calculated from these LD50/30 values was 1.16 (the ratio of LD50/30 of Ex-RAD-treated to vehicle-treated mice) with 95% confidence interval (CI) 1.13–1.20. The 95% CI is 7.80, 8.22 for Ex-RAD LD50/30, and 6.66, 7.00 for vehicle LD50/30, respectively.
Fig. 1.
Dose response of Ex-RAD for radioprotection. Kaplan–Meier 30-day survival rates were observed in male C3H/HeN mice (n = 16 per group) treated twice sc, 24 h and 15 min before radiation at 7.5 Gy of cobalt-60 gamma radiation with vehicle (1% Tween-80, 0.1 M KP buffer, 15 mM NaCl, pH 8.2) or Ex-RAD (25–500 mg/kg body weight). Mice that received Ex-RAD dose of 500 mg/kg exhibited a significant increase in survival as compared with vehicle control group and lowest dose of Ex-RAD 25 mg/kg (P < 0.0033, Fisher's exact test). Note: There was a significant difference among the survival curves (P = 0.0014, df = 5, Chi Squared value = 19.67, logrank test).
Fig. 2.
Dose reduction factor (DRF) determination. Mice (n = 16 per group) were treated sc twice, 24 h and 15 min before radiation with vehicle (1% Tween-80, 0.1 M K-P buffer, 15 mM NaCl, pH 8.2) or Ex-RAD, 500 mg/kg body weight. Radiation doses for the vehicle group were 6.25, 7.0, 7.5, 8.0 and 8.5 Gy; doses for Ex-RAD-treated group were 7, 7.5, 8.0, 8.5 and 9.0 Gy. Probit mortality curves were generated and a DRF of 1.16 with 95% confidence interval (CI, 1.13–1.20) was calculated from the ratio of LD50/30 of Ex-RAD-treated to vehicle-treated mice. Note: The 95% CI is 7.80, 8.22 for Ex-RAD LD50/30 and 6.66, 7.00 for vehicle LD50/30, respectively.
Ex-RAD accelerates recovery from radiation-induced neutropenia
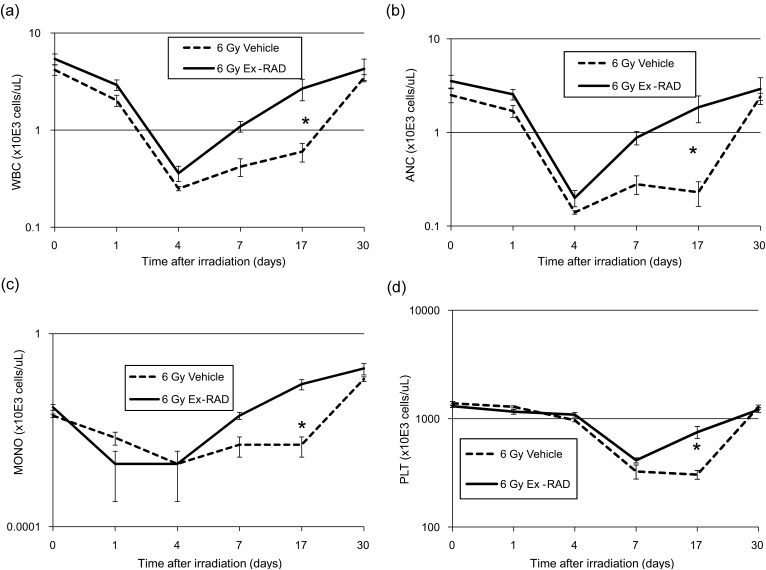
Figure 3 shows the protective effects of Ex-RAD prophylaxis on acute radiation-induced cytopenia. We used a sublethal dose of 6 Gy in this experiment (LD50/30 of vehicle-treated C3H mice = 6.86 Gy). We were able to see full recovery from radiation-induced depletion of peripheral blood in 30 days with sufficient mice allowing for robust statistical evaluation.
Fig. 3.
Ex-RAD pretreatment enhances recovery of peripheral blood cells in irradiated C3H mice. Effect of Ex-RAD on (a) total white blood cells (WBC), (b) absolute neutrophil count (ANC), (c) monocytes (MONO) and (d) platelets (PLT) in mice (n = 10 per group) subjected to total-body gamma radiation with a sublethal dose of 6 Gy (0.6 Gy/min) was measured. Mice were administered sc injection of vehicle (1% Tween-80, 0.1 M KP buffer, 15 mM NaCl, pH 8.2) or Ex-RAD 500 mg/kg body weight, 24 h and 15 min prior to gamma radiation. Day 0 represents the day of irradiation. Data represented are means ± standard error of the mean (SEM) for n = 10 mice for each time point. The marked group (*) indicates significant difference (P < 0.05) between irradiated Ex-RAD compared with irradiated vehicle group by Tukey–Kramer method. Note: Some error bars are very small and hence not visible in the figure.
Radiation-induced severe depletion of blood cell counts were obtained in mice shortly following 6 Gy TBI. Total WBC, ANC and MONO counts began to decline shortly after radiation, reaching their nadirs on Day 4 in both Ex-RAD-treated and vehicle control groups (Fig. 3a–c). ANC counts were less than 500 cells/µl, which led to severe neutropenia on Day 4 post-TBI. For platelets, the nadir was observed between Days 7 to 17 post-TBI in the vehicle-treated group (Fig. 3d). Peripheral blood cell counts recovered to normal levels by Day 30 post-TBI. However, recovery was accelerated in Ex-RAD-treated animals after Day 4 post-TBI for WBC, MONO and ANC counts. WBC (Fig. 3a), ANC (Fig. 3b) and MONO (Fig. 3c) counts on Day 17 after radiation were significantly higher (P < 0.05) in the drug-treated animals compared with the vehicle-treated controls, indicating faster regeneration of blood elements. Although PLT counts after irradiation were found to be the lowest between Days 7 and 17 in vehicle-treated animals, there was an accelerated recovery in the drug-treated group after the 7th day of radiation, and rate of recovery was significantly higher on Day 17 post-TBI (P < 0.05, Fig. 3d) compared with the vehicle-treated group. The nadir for RBC counts was also at Day 4 post-TBI, however recovery with Ex-RAD treatment by Day 17 was not statistically significantly different from those of vehicle controls (data not shown).
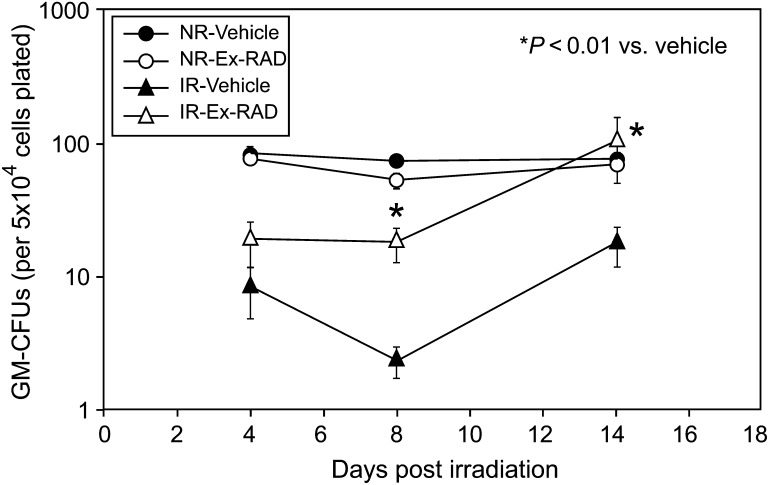
Ex-RAD protects clonal subpopulation of bone marrow progenitor cells after ionizing radiation
To test Ex-RAD's ability to alleviate radiation-induced defects in hematopoiesis, mice were treated with 500 mg/kg of Ex-RAD before exposure to 7 Gy TBI. Femoral marrow GM-CFUs were counted at Days 4, 8 and 14 post-TBI. GM-CFU reduced significantly, achieving a nadir at ∼8 days post-exposure, in the marrow of vehicle-treated animals (Fig. 4). Ex-RAD prophylaxis promoted recovery of the progenitor subpopulations in irradiated mice 8–14 days following the exposure. No effect of Ex-RAD was observed in sham-irradiated animals (Fig. 4).
Fig. 4.
Ex-RAD protects bone marrow granulocyte-macrophage colony-forming units (GM-CFUs). Ex-RAD (500 mg/kg) or vehicle (1% Tween-80, 0.1 M KP buffer, 15 mM NaCl, pH 8.2) was injected sc twice, 24 h and 15 min before exposure to 7 Gy TBI or sham irradiation. A significant (*P < 0.01) increase in colony size was observed in bone marrow cells harvested from Ex-RAD-treated animals on Days 8 and 14 compared with vehicle-treated animals. No differences in GM-CFUs were observed in sham-irradiated, vehicle-treated and drug-treated animals. Each point represents the mean ± SEM of three pools of marrow. Each pool comprised bone marrow from four femurs.
Ex-RAD treatment enhances intestinal crypt survival in mice
In addition to the hematopoietic system, supralethal doses of radiation also affect the GI system. Radiation-induced gastrointestinal syndrome is primarily due to the death of epithelial stem cells of the crypts, which proliferate rapidly and hence are prone to damage by radiation. The early mitotic arrest occurring in these cells, coupled with a continued maturation and migration of surviving cells toward the extrusion zone at the villus apex, results not only in cellular depletion of the crypt but also, with time, in a reduction in villus supporting the epithelium [23]. Shrinkage of the villus commences after crypt depletion, leading to involution of the mucosa with consequent metabolite and electrolyte imbalance [26].
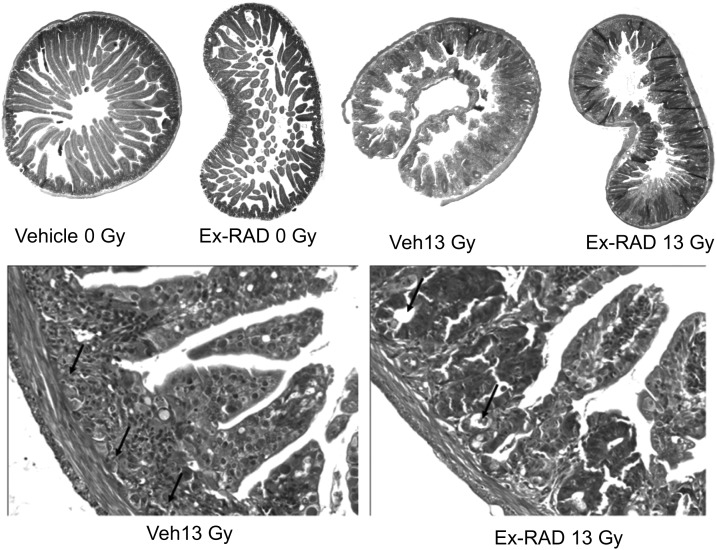
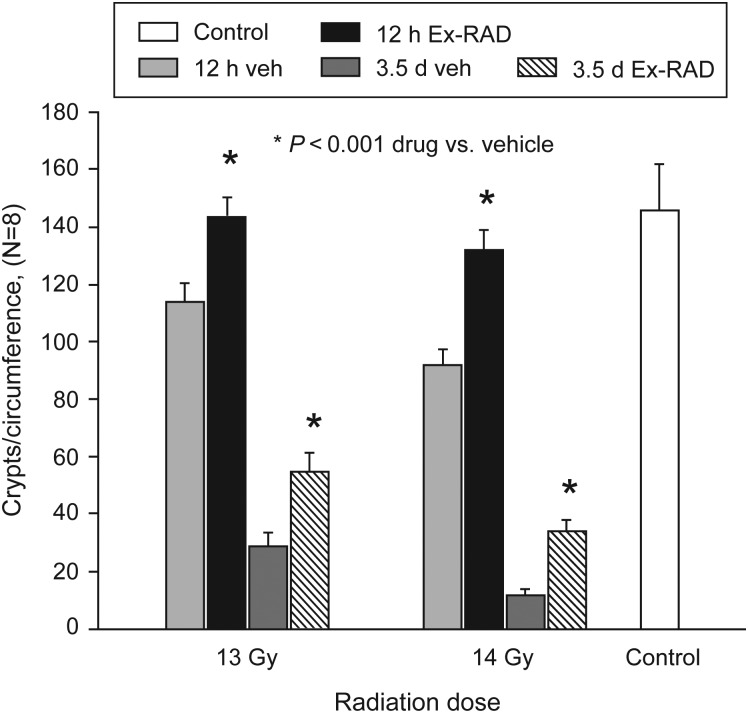
Apoptosis in crypt cells results in disappearance of crypts (villi) within 2 days post-TBI. Surviving regenerative cells continue to grow and form crypt-like structures over several days. We used two radiation doses, 13 and 14 Gy (TBI), and examined crypt survival 12 h and 3.5 days post-TBI. Ex-RAD treatment alone did not alter the histology of jejunum when compared with the sham-irradiated control groups (Fig. 5). The morphology of villi in vehicle-treated groups was distorted significantly after radiation, as evidenced by increasing frequency of crypt degeneration and significant reduction in villus height (Fig. 5). There was also an increase in the number of dead and goblet cells. In contrast, the jejunum from mice pretreated with Ex-RAD appeared to have normal villus height, considerable improvement in overall conservation of crypt morphology (Fig. 5) and reduction in dead and goblet cells when compared with the vehicle-treated irradiated group. Quantification of the crypts indicated that at both time points (12 h and 3.5 days post-TBI) and at both the radiation doses (13 and 14 Gy), the number of crypt cells in the Ex-RAD-treated group was significantly higher (P < 0.001) than in the vehicle-treated group (Fig. 6).
Fig. 5.
Ex-RAD protects mouse jejunum crypt cells from radiation damage post-TBI. Eight-week-old mice were injected sc with vehicle or Ex-RAD before TBI with 13 Gy. Photomicrograph of jejunum sections of mice harvested 3.5 days after irradiation is shown in the top panel. Higher magnification (40×) images of vehicle-treated mice showed shortened, irregular and thickened villi (arrows) with increased number of goblet cells; whereas Ex-RAD-treated mice showed normal villi, restoration of crypt cells (arrow) and normal distribution of goblet cells.
Fig. 6.
Crypts per circumference of jejunum sections from Ex-RAD-treated and vehicle-treated groups 12 h and 3.5 days post radiation (13 and 14 Gy). The number of crypt cells in the Ex-RAD-treated group was significantly higher at both times (*P < 0.001, n = 8) than the vehicle-treated group. Values are means ± SE of the mean of surviving crypts per cross-section.
Ex-RAD treatment reduces apoptosis in mouse jejunum 24 h after radiation
Jejunum collected 24 h post irradiation (6 Gy) were analyzed for apoptotic nuclei using TUNEL staining.
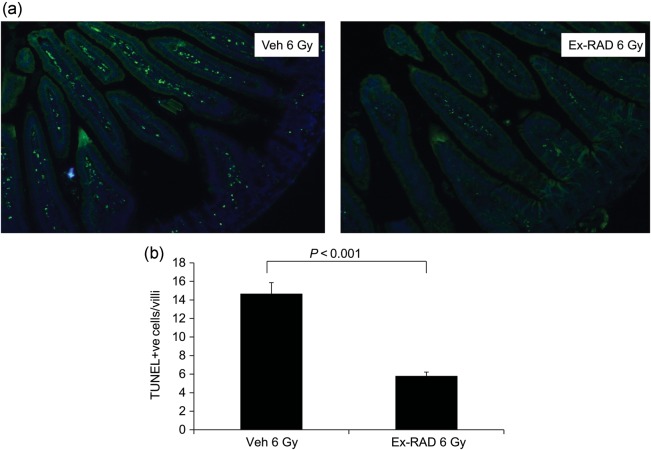
Figure 7 shows representative images of jejunum samples stained with FITC for nicked DNA. Irradiation resulted in a dramatic increase in TUNEL staining compared with naïve (data not shown). However, Ex-RAD-treated irradiated group showed significantly lower numbers of TUNEL-positive apoptotic cells in the villi compared with the vehicle-treated irradiated group (Fig. 7). We did not observe any significant difference in TUNEL-positive cells between vehicle- and drug-treated groups in samples collected 4 h post-radiation (data not shown).
Fig. 7.
TUNEL staining in the jejunum sections from Ex-RAD-treated and vehicle-treated groups 24 h post-TBI (6 Gy). Mice were sacrificed at the indicated time post-TBI. (a) Cell death in the jejunum sections after IR was assessed by TUNEL staining (magnification 10×), (b) Quantification of TUNEL positive cells in 50 villi/mouse. Values are means ± SEM; n = 3 in each group. * indicates P < 0.0001 compared with vehicle control.
Ex-RAD modulates the expression of Phospho-p53 (ser15) in spleen
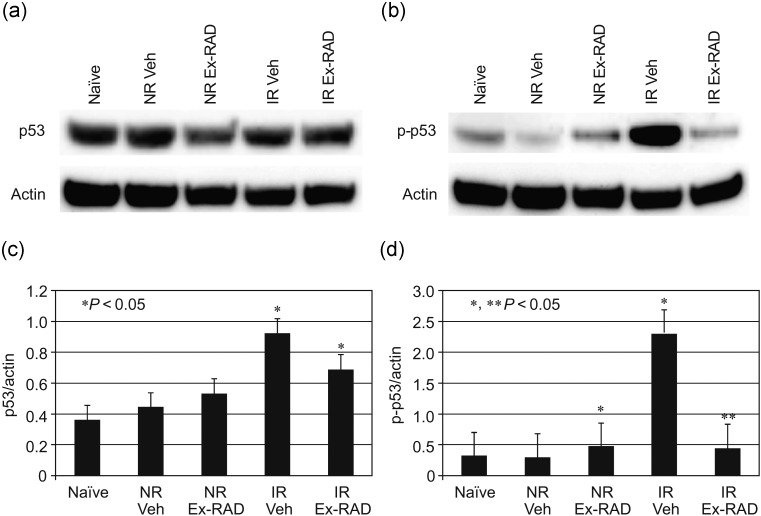
We investigated expression levels of p53 and its phosphorylation status (Ser 15, pro-apoptotic form) in spleens of sham-irradiated and irradiated mice from vehicle-treated and Ex-RAD-treated groups. Western blot analysis of spleens collected at 4 and 24 h after exposure to 6 Gy TBI showed increased levels of p53 and phospho-p53 (p-p53). Total p53 expression was slightly lower in the Ex-RAD-treated group compared with the vehicle-treated group (Fig. 8a & c) at 24 h post-TBI. However, the expression level of p-p53 was significantly (P < 0.05) lower in the Ex-RAD group compared with the vehicle-treated irradiated mice (Fig. 8b & d). Samples collected 4 h post-TBI did not show any significant difference between the irradiated treated groups (vehicle vs. Ex-RAD).
Fig. 8.
Ex-RAD inhibits gamma radiation-induced phospho-p53 (p-p53) activity in spleens of mice. Mice were treated with Ex-RAD (500 mg/kg) before radiation at 6 Gy and spleens were harvested 24 h post-radiation. Spleen homogenates were prepared for total protein for five groups (Naïve, Non-radiated vehicle, Non-radiated Ex-RAD, Irradiated vehicle and Irradiated Ex-RAD; shown as Naïve, NR Veh, NR Ex-RAD, IR Veh and IR Ex-RAD). Endogenous actin expression was shown as control. (a) p53 expression, inhibited by Ex-RAD; (b) p-p53 expression, significantly inhibited by the drug; (c and d) specific p53 and p-p53 bands were quantitated densitometrically, p53 and p-53 levels were normalized to actin levels in each group. *P < 0.05 compared with NR Veh, ** P < 0.05 compared with IR Veh. Error bar indicates SEM for n = 3 independent experiments.
DISCUSSION
Ionizing radiation can cause injury to both hematopoietic and GI systems depending on the radiation dose, dose rate and radiation quality [27, 28]. Sepsis is the primary cause of mortality during the early phase of the radiation-induced hematopoietic syndrome. Low levels of circulating immune blood cells and increased translocation of bacteria from the gastrointestinal tract into circulation and other tissues can lead to opportunistic infections and sepsis [3, 4, 29].
Occurrence of thrombocytopenia, hemorrhage and defects in the adaptive immune system resulting from rapid depletion of lymphocytes can lead to further complications [3]. We have previously demonstrated that Ex-RAD protects mice from radiation-induced mortality [20]. Extensive toxicity studies have been conducted in rodent and non-rodent species to establish the safety of the compound (unpublished data from OTI). No toxicity was observed with the highest dose used during this study. We demonstrated that 500 mg/kg was the most effective dose of Ex-RAD in mice when given 24 h and 15 min (two doses) before irradiation, this regimen was then used to evaluate whether Ex-RAD protected mice over a range of radiation doses and determined dose reduction or modification factor (DRF). An evaluation of DRF for 30-day survival (LD50/30) allows for a comparison of potential agents that protect against radiation-induced hematopoietic mortality [30] in mouse model. Naturally occurring vitamin E, alpha tocopherol, demonstrated a DRF of 1.11 (95% CI) when given within 15 min after TBI in CD2F1 mice [31]. In this work, we demonstrated that Ex-RAD afforded a better DRF (1.16) when given 24 h and 15 min (two administrations) before TBI. Thirty-day survival in mice is the standard endpoint for studying hematopoietic protection after radiation injury [32]. We have chosen sublethal dose 6 Gy for hematological study of peripheral blood based on the DRF study data (LD50/30 = 6.86) with the same strain of mice to ensure the availability of the surviving animals, which can be used as irradiated controls for 30 days. Ex-RAD efficiently accelerated recovery from neutropenia and restored blood platelets after near-lethal doses of ionizing irradiation. Our data also indicate that pretreatment with Ex-RAD significantly protected and restored bone marrow progenitor cells committed to granulocyte and monocyte production in irradiated mice. The precise mechanism by which Ex-RAD protects from potentially lethal effects of acute radiation remains uncertain. However, a principal mechanism of radioprotection by Ex-RAD was an enhanced restoration of the innate immune system. Our current study demonstrates the role of Ex-RAD in hematopoietic recovery. Radioprotection by Ex-RAD was associated with significant protection of leukocytes and platelets in peripheral blood and GM-CFU in bone marrow. Therefore, Ex-RAD may improve the survival of irradiated animals by attenuating the deleterious effects of radiation on the host immune system.
Dysfunction of intestinal crypt epithelial cells is a critical element in the pathogenesis of the GI syndrome [33]. Unlike the conventional belief that GI syndrome is manifested from 10 Gy to 14 Gy in mice, recent studies indicate that intestinal damage is dose-dependent and can be observed at much lower radiation doses [33, 11]. Apoptotic death of crypt stem cells can be detected 4.5 h after exposure to 1 Gy, progressively increasing to 10 Gy [33]. When the radiation dose increases to 8 Gy and higher, functional changes accompany damage to the crypts [11]; these changes may include malabsorption expressed as acute bowel reactions, including radiation proctitis [34]. These symptoms result from damage to the epithelial cells, which ultimately result in fluid and electrolyte imbalance, bacteremia, endotoxemia and subsequent lethality [35]. Protection of intestinal crypt stem cells and epithelial cells prevents GI syndrome. Interleukin-11 (IL-11) has been shown to protect mouse intestinal crypt cells from radiation-induced lethality [36]. Although IL-11 has several activities outside the hematopoietic system, its most substantial non-hematopoietic function may be its ability to stimulate the recovery of GI epithelial cells after radiation exposure [36]. A number of agents other than cytokines also have been shown to protect against radiation-induced GI injury [11, 37]. Among these, aminothiols (phosphorothioates) are very effective in animal models with DRFs ranging from 1.23 to 1.8, depending upon the aminothiol used. Other agents include prostanoids (prostaglandins and prostacyclins), nutraceuticals (vitamin E and vitamin A) and other nutritional support [38], and methyl xanthenes [39].
Damage to the intestine from irradiation appears as early as 30 min after acute supra-lethal radiation exposure (12–14 Gy). But, repair and regeneration of crypt cells is complete within 3 to 4 days [40]. Therefore, early and accurate assessment of intestinal injury can be conducted 3–4 days after 12 Gy of TBI. In recent studies, a conventional crypt cell assay was used as the indicator of radioprotective efficacy. We observed that 13 Gy of radiation caused severe mucosal structural injury, including significant loss of crypt cells and severe disruption of mucosal integrity. Both of these pathologic processes were prevented significantly by Ex-RAD, indicating that the drug had effectively protected against evolving post-TBI GI injury. This may result from protection of the intestinal epithelium, which would have allowed for the proper absorption of essential nutrients as well as enhanced regeneration of crypt cells. Similar to the Ex-RAD-evoked radioprotective responses, the tocol antioxidant, gamma-tocotrienol, has been shown to alleviate intestinal radiation injury and reduce vascular oxidative stress after TBI (8 Gy) [11]. We demonstrate that even at 6 Gy, epithelial cells undergo massive apoptosis within 24 h post-irradiation, thereby inflicting severe damage to the functioning villi. Ex-RAD administration significantly reduces radiation-induced apoptosis in the villi.
The radioprotective mechanism of Ex-RAD may involve prevention of p53-dependent apoptosis [20]. We had previously shown that Ex-RAD down-regulated the p53-dependent apoptotic pathway by inhibiting p53, p21 (cyclin-dependent kinase inhibitor 1) and Bax (Bcl2 associated X protein) in cultured human umbilical vein endothelial cells (HUVEC) and AG1522 (human skin fibroblast) cells [20]. Analysis of the tumor suppressor protein p53 in the hematopoietic tissue (spleen) from irradiated animals demonstrated that Ex-RAD may inhibit p53-signaling. Our data indicate that the inhibition of p53 signaling in spleen and apoptosis (as measured by TUNEL assay) in jejunum may partially contribute to protection of hematopoietic and GI systems from radiation damage by Ex-RAD. These results and other toxicology/pharmacokinetic characteristics have contributed to the approval of Ex-RAD by the FDA as an investigational new drug in December 2008. The drug is currently in Phase I clinical trials in humans. In conclusion, Ex-RAD treatment mitigates potentially life-threatening neutropenia and bone marrow suppression and, in turn, promotes marrow recovery, reduces radiation-induced phosphorylation of p53 signaling, and enhances survival of acutely irradiated mice. In addition to attenuating of hematopoietic injury, Ex-RAD also reduces intestinal injury. However, the molecular mechanisms involved in Ex-RAD's promotion of recovery of hematopoietic and GI tissues warrant further study.
CONFLICT OF INTEREST
M.M. is an employee of OTI. No other authors have financial obligations to OTI.
ACKNOWLEDGEMENTS
The authors wish to thank Ethery Amary for technical help and Amanda Gillum (OTI) for proofreading the manuscript. This work was supported by a grant (DAMD17-03-2-0027 to K.S.K.) from the US Army Medical Research Acquisition Activity, 82D Chandler St., Fort Detrick, MD 21702-5014, United States Army Medical Research and Material Command, administered by the The Henry M. Jackson Foundation for the Advancement of Military Medicine, Rockville, MD. The content of this manuscript does not necessarily reflect the position or policy of the US Government, and no official endorsement should be inferred.
REFERENCES
- 1.Reeves GI. Radiation injuries. Crit Care Clin. 1999;15:457–473. doi: 10.1016/s0749-0704(05)70063-4. [DOI] [PubMed] [Google Scholar]
- 2.Fliedner TM, Nothdurft W, Heit H. Biological factors affecting the occurrence of radiation syndromes. In: Broerse JJ, MacVittie TJ, editors. Response of Different Species to Total Body Irradiation. Leiden: Martinas Nijhoff Publishers; 1984. pp. 209–219. [Google Scholar]
- 3.Hendry JH, Feng-Tong Y. Response of bone marrow to low LET irradiation. In: Hendry JH, Lord BI, editors. Radiation Toxicology: Bone Marrow and Leukemia. London: Taylor and Francis; 1995. pp. 91–116. [Google Scholar]
- 4.Mauch P, Constine L, Greenberger J, et al. Hematopoietic stem cell compartment: acute and late effects of radiation therapy and chemotherapy. Int J Radiat Oncol Biol Physet. 1995;31:1319–39. doi: 10.1016/0360-3016(94)00430-S. [DOI] [PubMed] [Google Scholar]
- 5.Neta R, Oppenheim JJ, Douches SD. Interdependence of the radioprotective effects of human recombinant interleukin 1 alpha, tumor necrosis factor alpha, granulocyte colony-stimulating factor, and murine recombinant granulocyte-macrophage colony-stimulating factor. J Immunol. 1988;140:108–11. [PubMed] [Google Scholar]
- 6.Waddick KG, Song CW, Souza L, et al. Comparative analysis of the in vivo radioprotective effects of recombinant granulocyte colony-stimulating factor (G-CSF), recombinant granulocyte-macrophage CSF, and their combination. Blood. 1991;77:2364–71. [PubMed] [Google Scholar]
- 7.Whitnall MH, Wilhelmsen CL, McKinney L, et al. Radioprotective efficacy and acute toxicity of 5-androstenediol after subcutaneous or oral administration in mice. Immunopharmacol Immunotoxicol. 2002;24:595–626. doi: 10.1081/iph-120016038. [DOI] [PubMed] [Google Scholar]
- 8.Rodgers KE, Xiong S, diZerega GS. Accelerated recovery from irradiation injury by angiotensin peptides. Cancer Chemother Pharmacol. 2002;49:403–11. doi: 10.1007/s00280-002-0434-6. [DOI] [PubMed] [Google Scholar]
- 9.Ghosh SP, Kulkarni S, Hieber K, et al. Gamma-tocotrienol, a tocol antioxidant as a potent radioprotector. Int J Radiat Biol. 2009;85:598–606. doi: 10.1080/09553000902985128. [DOI] [PubMed] [Google Scholar]
- 10.Kulkarni S, Ghosh SP, Satyamitra M, et al. Gamma-tocotrienol protects hematopoietic stem and progenitor cells in mice after total-body irradiation. Radiation Research. 2010;173:738–47. doi: 10.1667/RR1824.1. [DOI] [PubMed] [Google Scholar]
- 11.Berbee M, Fu Q, Boerma M, et al. Gamma-Tocotrienol ameliorates intestinal radiation injury and reduces vascular oxidative stress after total-body irradiation by an HMG-CoA reductase-dependent mechanism. Radiation Research. 2009;171:596–605. doi: 10.1667/RR1632.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okunieff P, Li M, Liu W, et al. Keratinocyte growth factors radioprotect bowel and bone marrow but not KHT sarcoma. Am J Clin Oncol. 2001;24:491–5. doi: 10.1097/00000421-200110000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Kumar KS, Srinivasan V, Toles R, et al. Nutritional approaches to radioprotection :vitamin E. Military Medicine. 2002;167(Suppl 1):57–9. [PubMed] [Google Scholar]
- 14.Landauer MR, Srinivasan V, Seed TM. Genistein treatment protects mice from ionizing radiation injury. J Appl Toxicol. 2003;23:379–385. doi: 10.1002/jat.904. [DOI] [PubMed] [Google Scholar]
- 15.Srinivasan V, Pendergrass JA, Jr, Kumar KS, et al. Radioprotection, pharmacokinetic and behavioural studies in mouse implanted with biodegradable drug (amifostine) pellets. Int J Radiat Biol. 2002;78:535–43. doi: 10.1080/095530002317577358. [DOI] [PubMed] [Google Scholar]
- 16.Paris F, Fuks Z, Kang A, et al. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001;293:293–297. doi: 10.1126/science.1060191. [DOI] [PubMed] [Google Scholar]
- 17.Dorr W, Spekl K, Farrell CL. Amelioration of acute oral mucositis by keratinocyte growth factor: fractionated irradiation. Int J Radiat Oncol Biol Phys. 2002;54:245–251. doi: 10.1016/s0360-3016(02)02918-8. [DOI] [PubMed] [Google Scholar]
- 18.Maj JG, Paris F, Haimovitz-Friedman A, et al. Microvascular function regulates intestinal crypt response to radiation. Cancer Res. 2003;63:4338–4341. [PubMed] [Google Scholar]
- 19.Potten CS. Radiation injury of the gastrointestinal epithelium: current research on treatment, management, and prevention. In: Ricks RC, Berger ME, O'Hara FM, editors. The Medical Basis for Radiation Accident Preparedness: The Clinical Care of Victims. Boca Raton, FL: Parthenon Publishing Group; 2002. pp. 139–48. [Google Scholar]
- 20.Ghosh SP, Perkins MW, Hieber K, et al. Radiation protection by a new chemical entity, Ex-Rad: efficacy and mechanisms. Radiat Res. 2009;171:173–9. doi: 10.1667/RR1367.1. [DOI] [PubMed] [Google Scholar]
- 21.Whitnall MH, Elliott TB, Harding RA, et al. Androstenediol stimulates myelopoiesis and enhances resistance to infection in gamma-irradiated mice. Int J Immunopharmacol. 2000;22:1–14. doi: 10.1016/s0192-0561(99)00059-4. [DOI] [PubMed] [Google Scholar]
- 22.De Kruijf EJ, van Pel M, Hagoort H, et al. Repeated hematopoietic stem and progenitor cell mobilization without depletion of the bone marrow stem and progenitor cell pool in mice after repeated administration of recombinant murine G-CSF. Hum Immunol. 2007;68:368–74. doi: 10.1016/j.humimm.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 23.Withers HR, Elkind MM. Microcolony survival assay for cells of mouse intestinal mucosa exposed to radiation. Int J Radiat Biol Relat Stud Phys Chem Med. 1970;17:261–7. doi: 10.1080/09553007014550291. [DOI] [PubMed] [Google Scholar]
- 24.Potten CS, Hendry JH. The microcolony assay in mouse small intestine. In: Hendry CSPaJH., editor. Cell Clones: Manual of Mammalian Cell Techniques. Edinburgh: Churchill Livingstone; 1985. pp. 50–60. [Google Scholar]
- 25.Wang J, Zheng H, Sung CC, Hauer-Jensen M. The synthetic somatostatin analogue, octreotide, ameliorates acute and delayed intestinal radiation injury. Int J Radiat Oncol Biol Phys. 1999;45:1289–1296. doi: 10.1016/s0360-3016(99)00293-x. [DOI] [PubMed] [Google Scholar]
- 26.Potten CS. A comprehensive study of the radiobiological response of the murine (BDF1) small intestine. Int J Radiat Biol. 1990;58:925–73. doi: 10.1080/09553009014552281. [DOI] [PubMed] [Google Scholar]
- 27.Seed TM, Fritz TE, Tolle DV, et al. Survival patterns and hemopathological responses of dogs under continuous gamma radiation. In: Broerse JJ, MacVittie TJ, editors. Response of Different Species to Total Body Irradiation. Boston, MA: Martinus Nijhoff Publishers; 1984. pp. 137–59. [Google Scholar]
- 28.Seed TM, Kaspar LV. Probing altered hematopoietic progenitor cells of preleukemic dogs with JANUS fission neutrons. Radiat Res. 1991;128:S81–6. [PubMed] [Google Scholar]
- 29.Madonna GS, Ledney GD, Elliott TB, et al. Trehalose dimycolate enhances resistance to infection in neutropenic animals. Infect Immun. 1989;57:2495–501. doi: 10.1128/iai.57.8.2495-2501.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weiss JF, Landauer MR. History and development of radiation-protective agents. Int J Radiat Biol. 2009;85:539–73. doi: 10.1080/09553000902985144. [DOI] [PubMed] [Google Scholar]
- 31.Srinivasan V, Weiss JF. Radioprotection by vitamin E: injectable vitamin E administered alone or with WR-3689 enhances survival of irradiated mice. Int J Radiat Oncol Biol Phys. 1992;23:841–5. doi: 10.1016/0360-3016(92)90657-4. [DOI] [PubMed] [Google Scholar]
- 32.Brown DQ, Graham WJ, III, MacKenzie LJ, et al. Can WR-2721 be improved upon? Pharmacol Ther. 1988;39:157–68. doi: 10.1016/0163-7258(88)90057-5. [DOI] [PubMed] [Google Scholar]
- 33.Potten CS, Grant HK. The relationship between ionizing radiation-induced apoptosis and stem cells in the small and large intestine. Br J Cancer. 1998;78:993–1003. doi: 10.1038/bjc.1998.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hovdenak N., Fajardo LF, Hauer-Jensen M. Acute radiation proctitis: a sequential clinicopathologic study during pelvic radiotherapy. Int J Radiat Oncol Biol Phys. 2000;48:1111–17. doi: 10.1016/s0360-3016(00)00744-6. [DOI] [PubMed] [Google Scholar]
- 35.Buell MG, Harding RK. Proinflammatory effects of local abdominal irradiation on rat gastrointestinal tract. Dig Dis Sci. 1989;34:390–9. doi: 10.1007/BF01536261. [DOI] [PubMed] [Google Scholar]
- 36.Potten CS. Interleukin-11 protects the clonogenic stem cells in murine small-intestinal crypts from impairment of their reproductive capacity by radiation. Int J Cancer. 1995;62:356–61. doi: 10.1002/ijc.2910620321. [DOI] [PubMed] [Google Scholar]
- 37.Weiss JF, et al. Effect of radioprotective agents on survival after acute intestinal radiation injury, In. In: Dubois A, King GL, Livengood DR, editors. Radiation and the Gastrointestinal Tract. London: Academic Press; 1993. pp. 183–99. [Google Scholar]
- 38.Srinivasan V, Dubois A. Nutritional support of irradiated medicine, In. In: Dubois A, King GL, Livengood DR, editors. Radiation and the Gastrointestinal Tract. London: Academic Press; 1993. pp. 201–13. [Google Scholar]
- 39.Lehnert S. Radioprotection of mouse intestine by inhibitors of cyclic AMP phosphodiesterase. Int J Radiat Oncol Biol Phys. 1979;5:825–33. doi: 10.1016/0360-3016(79)90067-1. [DOI] [PubMed] [Google Scholar]
- 40.Cai WB. The number of clonogenic cells in crypts in three regions of murine large intestine. Int J Radiat Biol. 1997;71:573–9. doi: 10.1080/095530097143905. [DOI] [PubMed] [Google Scholar]