Abstract
The purpose of this study is to develop a procedure for eradicative brachytherapy that can deliver a curative boost dose to bulky (>4 cm) vaginal stump recurrence of uterine cancer without risk of damaging surrounding organs. We separated risk organs (the rectum and sigmoid) from the target during brachytherapy, with a hyaluronate gel injection into the pararectal space via the percutaneous paraperineal approach under local anesthesia. The rectum anchored to the sacrum by native ligament was expected to shift posteriorly. We encountered a patient with bulky stump recurrence of uterine cancer, approximately 8 cm in maximum diameter. She was complaining of abdominal pain and constipation due to bowel encasement. Following 50 Gy of external beam radiotherapy, we applied a single fraction of brachytherapy under gel separation and delivered 14.5 Gy (50.8 GyE: equivalent dose in 2-Gy fraction calculated with linear quadratic model at α/β = 3) to the target. The gel injection procedure was completed in 30 min without complications. A total irradiation dose of 100.8 GyE was delivered to the target and the cumulative minimum dose to the most irradiated rectosigmoidal volume of 2 cc (cumulative D2cc) was calculated as 58.5 GyE with gel injection, and was estimated to be 96 GyE without. Over three years, the local stump tumor has completely disappeared, with no complications. Brachytherapy with a pararectal gel injection can be a safe and effective eradicative option for bulky vaginal stump recurrence.
Keywords: high dose rate brachytherapy, recurrence, vaginal stump, uterine cancer, hyaluronate
INTRODUCTION
Vaginal stump recurrence (VSR) after surgery is common in uterine body cancer and uterine cancer. The overall incidence of VSR in uterine body cancer after definitive surgery is reported as being 2.4–15% [1, 2]. Prophylaxis such as postoperative vaginal vault brachytherapy [3], additional vaginal cuff excision [4], or external beam radiotherapy with or without chemotherapy is recommended in high-risk patients [5–7]; however, because the stump tumor after hysterectomy is usually closely surrounded by radiosensitive organs (e.g. the rectum, sigmoid colon and/or small intestines [6, 8]), the safe radiation dose is limited and only small tumors are expected to be curable with brachytherapy [9, 10]. For a bulky (>4 cm) VSR, only surgery is considered as definitive treatment. To date, no safe and effective treatment methods have been demonstrated as alternatives to surgery.
Recently developed technologies such as intensity modified radiotherapy—image-guided radiotherapy, which offers accurate and selective dose delivery, still have limited usefulness when the target is closely surrounded by at-risk organs, especially when these are motile. Brachytherapy is essentially free from the set-up errors that occur in daily treatment with external beam radiotherapy (EBRT), and therefore may offer more precise dose distribution and better dose delivery to the target [11]. However, there has been little progress regarding the problem of dose limitation to reduce the involvement of adjacent critical organs.
We previously described a solution to this problem in which the target and risk organs are separated using injection techniques [12–16]. Temporary separation by percutaneous hyaluronate gel injection provided a safe distance for the critical organs during high-dose-rate brachytherapy (HDRBT). We adapted this technique to the reirradiation treatment of prostate cancer [16] and paraaortic lymphnode metastasis [17].
In this paper, we describe a pararectal gel injection technique for eradicative brachytherapy, and present a case of vaginal stump recurrence of uterine cancer causing bowel obstruction that was treated using this method. The feasibility, safety and effectiveness of the technique are discussed.
METHODS
Preparation of needle deployment for HDRBT and gel injection
To prepare the gel, 180 mg of sodium hyaluronate of median molecular weight 3.4 millio Daltons (Suvenyl, Chugai/Roche, Tokyo, Japan) was mixed with saline and 5 ml of contrast medium (Iopamiron 300 mg iodine/ml, Bayer, Germany) to make a volume of 100 ml.
Electrocardiography, arterial oxygen pressure, respiration and blood pressure were monitored throughout the procedure, and the patient received awake sedation with 25 mg of hydroxyzine pamoate to enable her to report any abnormal sensation during needle deployment and gel injection.
Needle deployment via paraperineal approach and gel injection
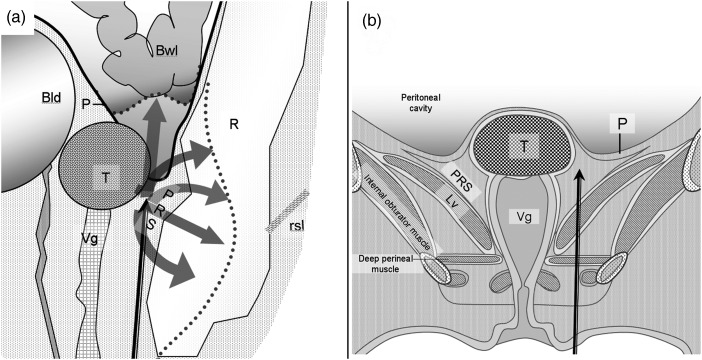
Figure 1 shows the deployment of brachytherapy applicator needles (1.1-mm external diameter; 20 cm long) and gel injection needles (21-gauge; shaped with steepled tops and side holes for improved straight-line insertion), which were advanced by paraperineal percutaneous approach under local anesthesia and X-ray computed tomography (CT) (SCT-7000, Shimazu, Kyoto, Japan) guidance. Hyaluronate gel was injected into the pararectal space to separate surrounding rectum and bowel from the target. The puncture point was the lateral side of the labium major or anal ring. The needles were inserted through subcutaneous adipose tissue, perineal fascia and muscle (superficial transverse perineal muscle and transverse vaginal muscle), and through the levator ani muscle to reach the desired point of the pararectal space. The gel was injected into the pararectal space between the target, the rectum and the peritoneum that includes the bowel. The injected gel pushes the rectum, the peritoneum and the bowel away from the target (thick gray arrows in Fig. 3) while the rectosacral ligament anchors the rectum to the sacrum.
Fig. 1.
Schematic illustrations of gel injection into the pararectal space: In sagittal view (a), the injected gel pushes the rectum, the peritoneum and the bowel away from the target (thick gray arrows) while the rectosacral ligament (rsl) anchors the rectum to the sacrum. In coronal view (b), the gel injection needle inserted from the skin to the pararectal space passes through a few muscle layers. Other abbreviations: T, target; R, rectum; P, Peritoneum; Bwl, bowel; Bld, bladder; Vg, vagina; Lv, levator ani muscle.
Fig. 3.
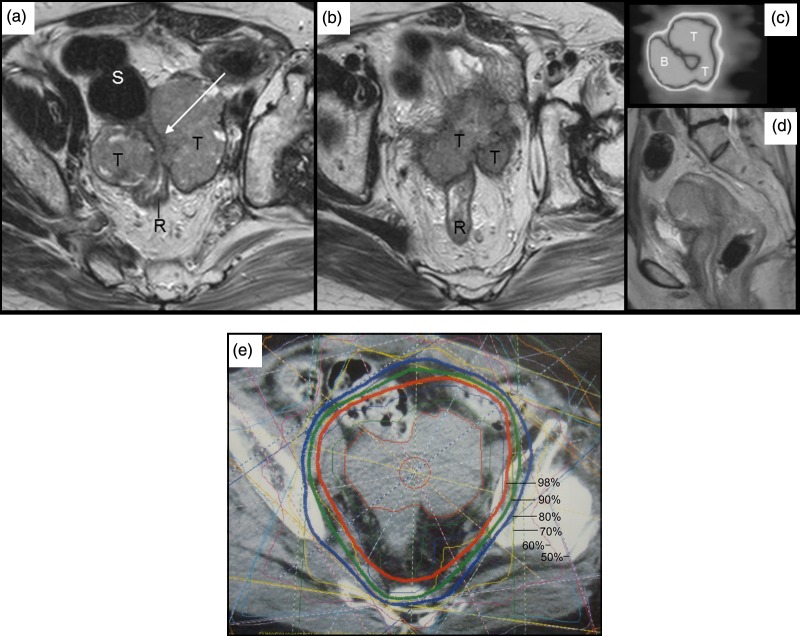
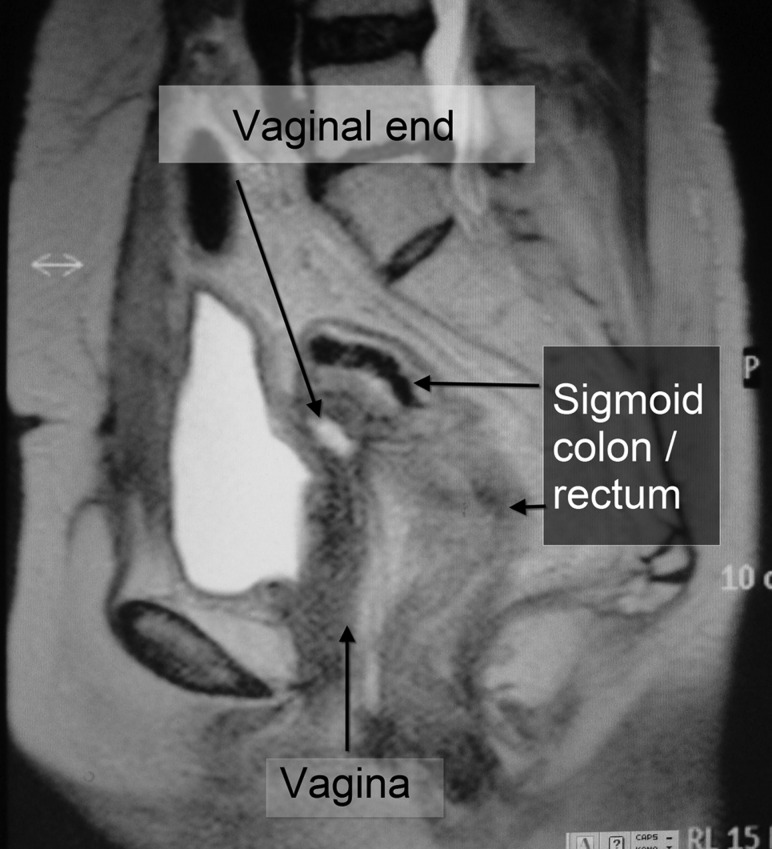
MR images before external beam treatment show recurrent tumor (T) tightly encasing a part of the rectosigmoidal colon (long white arrow) (a, b). Sagittal image of FDG-PET study showing increased accumulation at maximum standardized uptake value of 4.56 (confirming) (c) and a corresponding MR image (d). Dose distributions on X-ray CT image in conformal radiotherapy.
The distance between the target and the closest part of the bowel was determined to keep the accumulated D2cc <75 Gy for colon and rectum [18], and, tentatively < 65 Gy for small intestine. Following injection of 100 ml of gel, the borders of both the tumor and the rectum were clearly contoured on X-ray CT.
Brachytherapy planning and irradiation
Fine-pitch (3-mm) CT images were acquired and transferred to the treatment planning computer, and we created a 3D treatment plan (PLATO version 14.2, Nucletron, Veenendaal, Netherlands) for a safe and eradicative dose delivery.
The planning data were transported to an I-192 remote after-loader system (Microselectron HDR Ir-192, Nucletron) and irradiation was started immediately. Irradiation time was 500 s.
After irradiation, the needles were promptly removed. The patient was then allowed to rest, and she left the clinic on foot when ready. There were no procedure-related complications, no additional medication was required, and the patient was subsequently followed-up at our clinics.
Calculation of equivalent dose and comparison of rectal doses
Equivalent dose in 2-Gy fractions was calculated with the commonly used linear quadratic model at α/β = 3 and expressed with a form: GyELQ2,α/β=3. Cumulative dose was calculated with the equivalent dose when necessary according to the American Brachytherapy Society calculation sheet. We compared the rectal dose with gel injection and without. The latter dose was calculated using the original rectal position. The equivalent dose calculated with the linear quadratic-linear model at α/β = 3, D(T) = 6 was notated with GyELQ2,α/β=3, DT=6 [19]
PATIENT
A 60-year-old female was referred to our radiotherapy department with vaginal stump recurrence of endometrial cancer.
The patient was diagnosed at the age of 55 years with stage IC uterine body cancer of histological grade 1 endometrioid adenocarcinoma. She underwent total abdominal hysterectomy and bilateral salpingo-oophorectomy, and received no adjuvant therapy. She visited hospital monthly for 1.5 years, at which time she suddenly stopped attending.
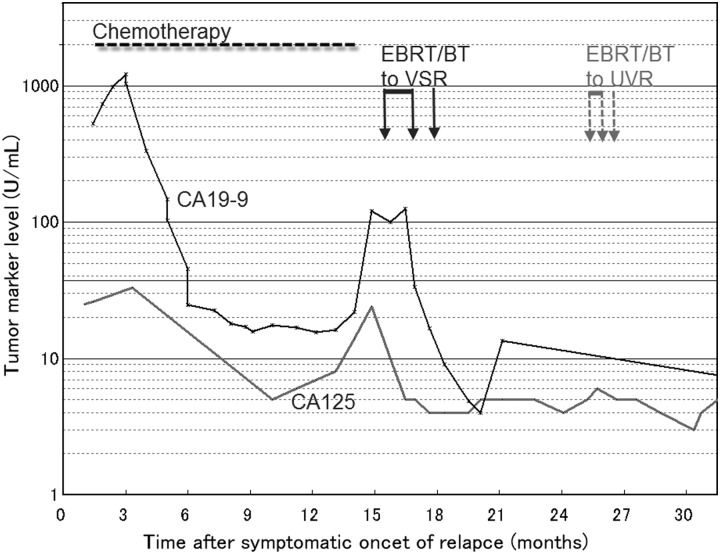
The patient re-presented at hospital at the age of 59 years, following a relapse of genital bleeding. Recurrence was histologically confirmed; her serum CA19-9 level was 1216.4 U/ml. Chemotherapy was started and a total of 17 cycles administered intravenously was carried out for 12 months consisting of four cycles with paclitaxel 90 mg/m2 and carboplatin (area under the plasma concentration time curve [AUC] = 2), one with cisplatin 50 mg/m2 and docetaxel 55 mg/m2, one with cisplatin 70 mg/m2 and docetaxel 70 mg/m2, followed by 11 cycles with docetaxel 55 mg/m2 and carboplatin [AUC] = 5). During the chemotherapy, tumor markers decreased for about 1 year (Fig. 2), while tumor size was stable; the patient then began to complain of abdominal pain and constipation, at which time the tumor marker level showed a steep re-increase up to approximately 8 cm in maximal diameter and 149.8 cc in gross tumor volume, magnetic resonance imaging revealed tumor encasement of the bowel, and positron emission tomography (PET) showed strong fluorodeoxyglucose (FDG) accumulation at the tumor site (Fig. 3).
Fig. 2.
Clinical course. Abbreviations: EBRT, external beam radiotherapy; BT, brachytherapy; VSR, vaginal stump recurrence; UVR, uretovaginal recurrence. Normal ranges: CA125 <35 U/ml, CA19-9 <37 U/m.
We planned two-step radiotherapy: (i) 50 Gy of conformal EBRT in 2 Gy per fraction for 5 weeks aiming to reduce the initial tumor volume (150 ml) and to release the bowel from encasement; and (ii) a boost brachytherapy to the supposedly residual target, with pararectal spacing with gel injection to preserve the bowel. Both treatments were scheduled on an outpatient basis. Informed consent was obtained from the patient prior to treatment, which was performed with standard institutional approval. The interventional and radiotherapeutic procedures were performed by qualified specialists.
RESULTS
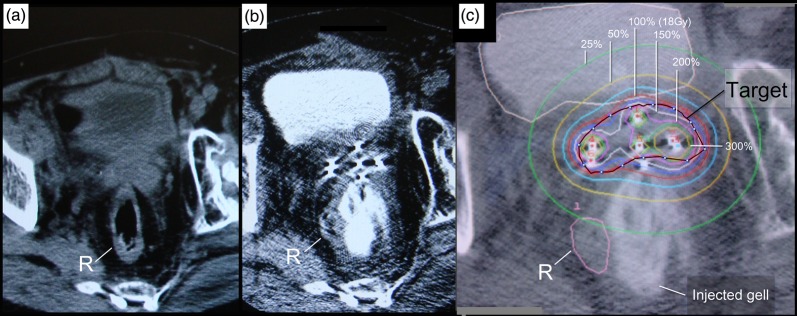
The gel injection procedure took approximately 45 min. Rectum and sigmoid colon located close to the shrunken tumor (20 cc and 4.5 × 3 × 3 cm in diameter) was shifted away from the target by more than 1.5 cm by the injected gel (Fig. 4a, b).
Fig. 4.
Spacing effect of HGI and brachytherapy dose distribution. CT image prior to HGI shows part of the rectum (R) still attached to the tumor surface (a). CT image after needle insertion and gel injection (b) and the treatment plan (c) show the rectum shifted safely away from the target.
A single fraction of 14.5 Gy (50.8 GyELQ2,α/β=3, 36.83 GyELQ2,α/β=3, DT=6) was delivered to the 100% isodose line of the 20-cc planning target volume (PTV) (Fig. 4a).
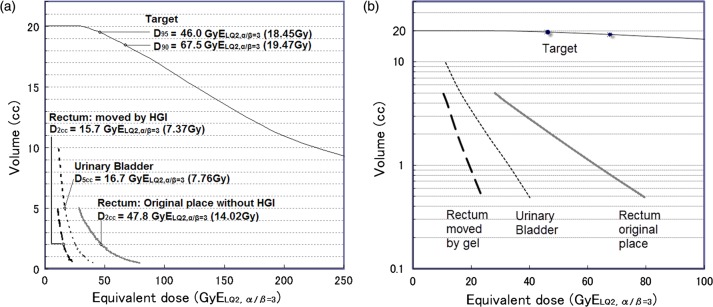
Estimated D2cc (the minimum dose to the most irradiated volume of 2 cc) to the rectum was 15.7 GyELQ2,α/β=3 with gel injection, compared with 47.28 Gy GyELQ2,α/β=3 without, yielding a safety enhancement ratio of 3.12 times (Fig. 5). The dose reduction effect in rectum was larger with a higher dose (Fig. 4b). Cumulative D2cc of EBRT and brachytherapy was 65.7 GyELQ2,α/β=3 with gel injection, compared with 97.28 Gy GyELQ2,α/β=3 without. Bladder D5cc and D2cc were 7.72 Gy (32 GyELQ2,α/β=3) and 9.89 Gy (25.5 Gy ELQ2,α/β=3), respectively.
Fig. 5.
Equivalent dose–volume histograms for the target, rectum, urinary bladder, and rectum without HGI. The rectal dose was reduced by HGI (a) with increasing effect for higher doses (b: semilogarithmic plot). Abbreviations: GyELQ2,α/β = 3, biologically equivalent dose calculated with the linear quadratic model at α/β = 3; D90 and D95, doses that cover 90% and 95% volumes of the clinical target volume, respectively. Physical dose in single fraction (Gy) is in the parentheses.
Irradiation time was 500.6 s, and the whole process took 3.5 h. There were no procedure-related complications.
Clinical outcome
At 3 years after the brachytherapy, the patient currently has good performance status with a small lung metastasis scheduled to be treated. No complications were observed over the 3-year follow-up period and the genital bleeding has not recurred. The size of the stump recurrence is remarkably diminished (Fig. 6), and no FDG accumulation was seen on PET imaging at 6, 12, 18, 24 and 30 months after the brachytherapy. Lower vaginal recurrence occurred 1 year after the brachytherapy, which was successfully treated with EBRT followed by HDRBT.
Fig. 6.
An MR image at 1 year after brachytherapy. A small fluid collection is observed on the MR image but no tumor is seen.
DISCUSSION
High-dose radiotherapy is associated with significant risk of bowel complications in patients with bulky recurrent tumor at the vaginal stump because the topography of the vaginal stump after hysterectomy is dominated by its close proximity to the surrounding bowel organs [6]. In the present patient, the described gel injection technique provided a reasonable solution as a safe and eradicative irradiation for vaginal stump recurrence.
Dosimetric benefit by gel injection technique
The dosimetric merit of this procedure was to decrease the bowel toxicity. The recommended values for D2cc to the sigmoid and D2cc to the rectum are <75 Gy according to the American Brachytherapy Society guidelines [18]. In a case series reported by Potter, 156 patients received a total prescribed mean dose of 93 +/– 13 Gy for 90% of the target (uterine cancer), 65 +/– 9 Gy for rectum and 64 +/– 9 Gy for sigmoid, where grade 3–4 rectal toxicity was observed in 5 of 156 patients [20]. They described the gradual reduction of these dose volume constraints to 70 Gy during the course of the study [20]. In the present case, we provided 100.8 Gy LQ2,α/β=3 (86.8 GyELQL2,α/β=3, DT=6) to the target D100% and 65.7 Gy LQ2,α/β=3, for the rectosigmoidal D2cc. The present technique applied to the present case of stump recurrence may be useful in safely increasing the target dose in similar situations, though further case experiences will be necessary to establish a protocol.
Dose volume constraint for small intestine in single fraction treatment is not yet clearly defined, however, there are related recommendations: < 12.5 Gy (38.75 Gy LQ2,α/β=3) for < 30 ml of small intestinal volume in single fraction EBRT [8], and <15.5 Gy (57.35 Gy LQ2,α/β=3) [21] for D1cc of gastric mucosa. No definitively recommended value is currently available for the bladder, although D2cc <90 Gy or 95 Gy has been previously reported [18, 20, 22].
Pararectal space injection for vaginal stump recurrence
The paraperineal approach to the pararectal space has the merits of easy handling, a wider range of needle angles, and positional stability compared with the vaginal approach. Anatomically, the vaginal stump tumor is close and often attached to the peritoneum, either adhesively or invasively. Detailed observation is necessary to analyze tumor extension. A preliminary course of EBRT may be recommended prior to brachytherapy with gel injection in some cases. The presence of adhesions to varying extents in the postsurgical perivesical and pararectal spaces may cause certain technical difficulties in separating the organs.
Advantages of gel injection
Unlike a forced blunt dissection, one of the expected effects of the injected gel is first to separate the easily detachable layers or zones without invasion. The spacing effect of the gel generally lasts for 1–4 h depending on its concentration and the molecular weight of the hyaluronate. Long-life, artificially stabilized, and harder gel variants of hyaluronate are available [23, 24], but may not necessarily be suitable for this short time application [23–26]. Despite these successful reports, we should be aware of uncertainty of durability of the hyaluronate gel in different areas and different conditions including concentration and tissue structure.
Time and cost-effectiveness of this gel injection procedure
Native-type hyaluronate is much less expensive than the cross-linked type (the cost is approximately 1/60th that of the artificial type) and the time required for gel injection was 45 min. We consider the hyaluronate gel injection procedure is cost- and time-effective.
CONCLUSION
We have described a minimally invasive gel injection procedure performed in the outpatient clinic, in which the patient was safely treated with an eradicative dose by HDRBT, with bowel preservation. We consider that the gel injection procedure enables improvement of the HDRBT therapeutic ratio in eradicative dose treatment of stump relapse of gynecologic cancers.
ACKNOWLEDGEMENTS
This research was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan (MEXT Grant), grant number 23659595. The patient consented to publication prior to submission of the manuscript.
REFERENCES
- 1.Larson DM, Broste SK, Krawisz BR. Surgery without radiotherapy for primary treatment of endometrial cancer. Obstet Gynecol. 1998;91(3):355–9. doi: 10.1016/s0029-7844(97)00698-4. [DOI] [PubMed] [Google Scholar]
- 2.Yoney A, Yildirim C, Bati Y, et al. Low risk stage I endometrial carcinoma: rognostic factors and outcomes. Indian J Cancer. 2011;48(2):204–10. doi: 10.4103/0019-509X.82895. [DOI] [PubMed] [Google Scholar]
- 3.Chadha M, Nanavati PJ, Liu P, et al. Patterns of failure in endometrial carcinoma stage IB grade 3 and IC patients treated with postoperative vaginal vault brachytherapy. Gynecol Oncol. 1999;75(1):103–7. doi: 10.1006/gyno.1999.5526. [DOI] [PubMed] [Google Scholar]
- 4.Heyer E, Genau F. Vaginal stump recurrence in carcinoma of the corpus uteri. Zentralbl Gynakol. 1977;99(2):81–4. [PubMed] [Google Scholar]
- 5.Kloetzer KH, Gunther R, Wendt T. The vaginal stump recurrence rate in endometrial carcinoma in relation to the target volume of postoperative HDR-afterloading brachytherapy. Strahlenther Onkol. 1997;173(1):13–17. doi: 10.1007/BF03039188. Die Vaginalstumpf-Rezidivrate beim Endometriumkarzinom in Abhangigkeit des Zielvolumens der postoperativen HDR-Afterloading-Brachytherapie. [DOI] [PubMed] [Google Scholar]
- 6.Pötter R, Gerbaulet A, Haie-Meder C. Endometrial cancer. In: Gerbaulet A, Pötter R, Mazeron J, Meertens H, Limbergen E, editors. The GEC ESTRO Handbook of Brachytherapy. ESTRO, Brussels, Belgium, 2007, 365–401. [Google Scholar]
- 7.NCCN. Uterine Neoplasms. NCCN, Fort Washington, PA, 2012, 2. http://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf. (6 March 2012, date last accessed) [Google Scholar]
- 8.Kavanagh BD, Pan CC, Dawson LA, et al. Radiation dose-volume effects in the stomach and small bowel. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl) doi: 10.1016/j.ijrobp.2009.05.071. S101–7. [DOI] [PubMed] [Google Scholar]
- 9.Ito H, Shigematsu N, Kawada T, et al. Radiotherapy for centrally recurrent cervical cancer of the vaginal stump following hysterectomy. Gynecol Oncol. 1997;67(2):154–61. doi: 10.1006/gyno.1997.4855. [DOI] [PubMed] [Google Scholar]
- 10.Ito H, Kumagaya H, Shigematsu N, et al. High dose rate intracavitary brachytherapy for recurrent cervical cancer of the vaginal stump following hysterectomy. Int J Radiat Oncol Biol Phys. 1991;20(5):927–32. doi: 10.1016/0360-3016(91)90187-9. [DOI] [PubMed] [Google Scholar]
- 11.Wakatsuki M, Ohno T, Yoshida D, et al. Intracavitary combined with CT-guided interstitial brachytherapy for locally advanced uterine cervical cancer: introduction of the technique and a case presentation. J Radiat Res (Tokyo) 2011;52(1):54–8. doi: 10.1269/jrr.10091. [DOI] [PubMed] [Google Scholar]
- 12.Kishi K, Adati H, Takada K, (inventors), Kurare Medical Issued in Unexamined Patent Publication Bulletin by Japan Patent Office in 2005 p2005-287728A), the application was in 2004 (P2004-106149). [in Japanese] [Google Scholar]
- 13.Kishi K, Takifuji K, Shirai S, et al. Brachytherapy technique for abdominal wall metastases of colorectal cancer: ultrasound-guided insertion of applicator needle and a skin preservation method. Acta Radiol. 2006;47(2):157–61. doi: 10.1080/02841850500466542. [DOI] [PubMed] [Google Scholar]
- 14.Kishi K, Shirai S, Sato M, et al. Preservation of risk organs in critical brachytherapy by tissue spacing with percutaneous injection. The 5th Japan–US Cancer Therapy Symposium and The 5th S Takahashi Memorial Joint Symposium;8 September 2007, Sendai, Japan. [Google Scholar]
- 15.Kishi K, Shirai S, Sato M, et al. Computer-aided preservation of risk organs in critical brachytherapy by tissue spacing with percutaneous injection of hyaluronic aid solution. Int J Radiat Oncol Biol Phys. 2007;69(3) S568–S9. [Google Scholar]
- 16.Kishi K, Sato M, Sonomura T, et al. Brachytherapy. 2. Vol. 11. Eradicative high dose rate brachytherapy with natural type hyaluronate injection; 2011. Reirradiation of prostate cancer with rectum preservation; pp. 144–8. [DOI] [PubMed] [Google Scholar]
- 17.Kishi K, Sonomura T, Shirai S, et al. Brachytherapy reirradiation with hyaluronate gel injection of paraaortic lymphnode metastasis of pancreatic cancer: paravertebral approach. Journal of Radiation Research. 2011;52(6):840–4. doi: 10.1269/jrr.11141. [DOI] [PubMed] [Google Scholar]
- 18.Viswanathan A, Thomadsen B. American Brachytherapy Society cervical cancer brachytherapy task group. 2007, available from: www.americanbrachytherapy.org/guidelines/cervical_cancer_taskgroup.pdf . [Google Scholar]
- 19.Astrahan M. Some implications of linear-quadratic-linear radiation dose-response with regard to hypofractionation. Med Phys. 2008;35(9):4161–72. doi: 10.1118/1.2969065. Epub 2008/10/10. [DOI] [PubMed] [Google Scholar]
- 20.Potter R, Georg P, Dimopoulos JC, et al. Clinical outcome of protocol based image (MRI) guided adaptive brachytherapy combined with 3D conformal radiotherapy with or without chemotherapy in patients with locally advanced cervical cancer. Radiother Oncol. 2011;100(1):116–23. doi: 10.1016/j.radonc.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Streitparth F, Pech M, Bohmig M, et al. In vivo assessment of the gastric mucosal tolerance dose after single fraction, small volume irradiation of liver malignancies by computed tomography-guided, high-dose-rate brachytherapy. Int J Radiat Oncol Biol Phys. 2006;65(5):1479–86. doi: 10.1016/j.ijrobp.2006.02.052. [DOI] [PubMed] [Google Scholar]
- 22.Lang S, Kirisits C, Dimopoulos J, et al. Treatment planning for MRI assisted brachytherapy of gynecologic malignancies based on total dose constraints. Int J Radiat Oncol Biol Phys. 2007;69(2):619–27. doi: 10.1016/j.ijrobp.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 23.Prada PJ, Fernandez J, Martinez AA, et al. Transperineal injection of hyaluronic acid in anterior perirectal fat to decrease rectal toxicity from radiation delivered with intensity modulated brachytherapy or EBRT for prostate cancer patients. Int J Radiat Oncol Biol Phys. 2007;69(1):95–102. doi: 10.1016/j.ijrobp.2007.02.034. [DOI] [PubMed] [Google Scholar]
- 24.Prada PJ, Gonzalez H, Menendez C, et al. Transperineal injection of hyaluronic acid in the anterior perirectal fat to decrease rectal toxicity from radiation delivered with low-dose-rate brachytherapy for prostate cancer patients. Brachytherapy. 2009;8(2):210–17. doi: 10.1016/j.brachy.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 25.Hamilton R, Strobos J, Adkinson N. Immunogenicity studies of cosmetically administered nonanimal-stabilized hyaluronic acid particles. Dermatol Surg. 2007;33(suppl.2) doi: 10.1111/j.1524-4725.2007.33358.x. s176–s85. [DOI] [PubMed] [Google Scholar]
- 26.Christensen LH. Host tissue interaction, fate, and risks of degradable and nondegradable gel fillers. Dermatol Surg. 2009;35(suppl.2):1612–19. doi: 10.1111/j.1524-4725.2009.01338.x. [DOI] [PubMed] [Google Scholar]