Abstract
Radiotherapy alone has several limitations for treating lung cancer. Inhalation, a non-invasive approach for direct delivery of therapeutic agents to the lung, may help to enhance the therapeutic efficacy of radiation. Up-regulating beclin1, known as a tumor suppressor gene that plays a major role in autophagy, may sensitize tumors and lead to tumor regression in lungs of K-rasLA1 lung cancer model mice. To minimize the side-effects of radiotherapy, fractionated exposures (five times, 24-h interval) with low dose (2 Gy) of radiation to the restricted area (thorax, 2 cm) were conducted. After sensitizing the lungs with radiation, beclin1, complexed with a nano-sized biodegradable poly(ester amine), was prepared and delivered into the murine lung via aerosol three times/week for four weeks. In a histopathological analysis, animals treated with beclin1 and radiation showed highly significant tumor regression and low progression to adenocarcinoma. An increase in the number of autophagic vacuoles and secondary lysosomes was detected. Dissociation of beclin1-bcl2 stimulated autophagy activation and showed a synergistic anti-tumor effect by inhibiting the Akt-mTOR pathway, cell proliferation and angiogenesis. The combination of radiation with non-invasive aerosol delivery of beclin1 may provide a prospect for developing novel therapy regimens applicable in clinics.
Keywords: Radiation, Beclin1, aerosol delivery, lung cancer
INTRODUCTION
Lung cancer is one of the most life-threatening diseases. Non-small cell lung cancer (NSCLC) is the most common type of lung cancer, accounting for 75–85%, of which only 15–25% of cases are potentially curable [1]. Many efforts have focused on developing efficient therapeutics for lung cancer; however, morbidity and mortality are still increasing constantly worldwide. Due to its anatomical structure and location, the lung has several limitations for an early diagnosis and surgical approach. Therefore, chemotherapy and/or radiation are compulsory treatments followed by surgical resection. Radiotherapy is the primary choice for inoperable NSCLC (stage I, II) or locally advanced disease (stage III). Various fractionation regimens and/or combination regimens with chemotherapy are on trial due to the poor outcome of high dose radiotherapy [2].
A non-invasive approach is under investigation to minimize side-effects and maximize the efficacy of lung cancer treatment, and aerosol delivery of therapeutic agents directly to the target organ may be one of the most feasible candidates. However, several obstacles must be overcome to design an in vivo gene delivery study, including low delivery/expression efficiency, technical difficulties and organ-specific immune barriers. Previous studies from our group have demonstrated the efficiency of gene delivery when complexed with non-viral or viral vectors through inhalation [3–5], suggesting that aerosol gene delivery is plausible for clinical applications.
Beclin1 is a well-known tumor-suppressor gene, which plays a major role in autophagy, but is usually silenced in various cancers, including breast, cervical, prostate and lung cancer [6, 7]. RNAi against beclin1 increases cell proliferation, and overexpressed beclin1 activates the autophagic death pathway [8]. A recent study reported that beclin1 overexpression may up-regulate chemosensitivity, suggesting that beclin1 is a potent target for cancer gene therapy [7].
Here, we report the synergistic effect of a fractionated regimen of radiotherapy and beclin1 inhalation for tumor regression in the lungs of K-rasLA1 lung cancer model mice. Animals were divided into four groups: control, radiation, beclin1, combination of radiation and beclin1 – and therapeutic efficacies were determined and compared. Mice in the radiation only or beclin1 inhalation groups showed a decrease in tumor progression to some extent, whereas those in the combination treatment group showed a highly significant decrease. Consequently, our findings suggest that radiation with aerosol-delivered beclin1 may induce a synergic anti-tumor effect by controlling autophagic cell death via prolonged activation of autophagy. Our results also suggest that combination gene therapy with radiation may be a good therapeutic strategy applicable in clinics.
MATERIALS AND METHODS
Construction of mTERT-beclin1 and preparation of the poly(ester amine)s/pDNA complex
The mouse TERT promoter sequence (GenBank: AF157502.1) was substituted into pcDNA3.1/CT-GFP-TOPO (Invitrogen, Carlsbad, CA, USA) with mouse beclin1, and the CMV promoter was removed from the BglII/KpnI enzyme sites. Poly(ester amine) was synthesized as described in a previous study, and a weight ratio of 1.3 was chosen [9]. Complexation of poly(ester amine)/pDNA was performed as described earlier and incubated at room temperature for 30 min before use.
Animals
Female K-rasLA1 mice (five mice/group) were obtained from the Human Cancer Consortium, National Cancer Institute Breeding Colony (Frederick, MD, USA) and maintained in a laboratory animal facility with temperature and relative humidity maintained at 23 ± 2°C and 50 ± 20%, respectively, under a 12-h light/dark cycle. All experimental protocols were reviewed and approved by the Animal Care and Use Committee of Seoul National University (SNU-201003-30).
Radiation and inhalation
Animals were anesthetized and immobilized in the treatment position for irradiation. Radiation was delivered at a dose rate of 1.85 Gy/min through a single posterior to anterior collimated 2-cm cobalt-60 beam with a 5-mm bolus placed over the thoracic area. Mice were irradiated with 2-Gy fractions given over five consecutive days. To minimize the side-effects of radiation and to protect the salivary gland from radiation, only the thorax of the K-rasLA1 mice was exposed to 10-Gy radiation, fractionated five times at 24-h intervals [10–12]. Animals were placed into a nose-only exposure chamber for 30 min, and 1 mg plasmid DNA/BECN1 was delivered to the lungs via aerosol each time. Mice were sacrificed after four weeks (12 inhalations; three times/week for four weeks).
Electron microscopy
Lungs were fixed in modified Karnovsky's fixative before post-fixation in 1% osmium tetroxide in 0.05 M sodium cacodylate buffer (pH 7.2). Uranyl acetate (0.5%) was used for en bloc staining. The lungs were dehydrated in an ethanol series and embedded in Spurr's resin. Ultrathin sections were stained with 2% uranyl acetate and Reynolds' lead citrate and observed under transmission electron microscopy (TEM) (LIBRA 120, Carl Zeiss, Oberkochen, Germany).
Western blot analysis
Lungs were homogenized, and protein concentrations were measured with a Bradford kit (Bio-Rad, Hercules, CA, USA). An equal amount of protein (25 µg) was loaded onto a sodium dodecyl sulfate (SDS) gel and separated. Beclin1, p-Akt1 at Ser473, p-Akt1 at Thr308, vascular endothelial growth factor (VEGF), bcl-2, proliferating cell nuclear antigen (PCNA) and β-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA), Atg5 and LC3 were obtained from Abgent (San Diego, CA, USA), Akt1 was purchased from Abfrontier (Seoul, South Korea) and p-mTOR, mTOR, raptor and rictor were purchased from Cell Signaling Technology (Danvers, MA, USA). Secondary antibodies conjugated with horseradish peroxidase (HRP) (Invitrogen) were applied according to the manufacturer's protocols. Bands of interest were obtained with an LAS-3000 luminescent image analyzer (Fujifilm, Tokyo, Japan).
Immunoprecipitation assay
Beclin1 and bcl-2 immunoprecipitation was conducted using Dynabead Protein G (Invitrogen), according to the manufacturer's protocol.
Histopathological examination and immunohistochemistry (IHC)
Lung sections were prepared at a thickness of 3 μm on charged slide glasses (Fisher Scientific, Pittsburgh, PA, USA). Slides were stained with hematoxylin and eosin (H&E) for histopathological analysis. Slides were deparaffinized, rehydrated, antigens were retrieved and endogenous peroxidase was quenched for immunohistochemistry. Primary and secondary antibodies and 3,3-Diaminobenzidine (DAB) were applied accordingly (Vector Laboratories, Burlingame, CA, USA). Slides were reviewed using a light microscope (Carl Zeiss, Thornwood, NY, USA). Staining intensity was assessed by counting the number of positive cells in randomly selected fields viewed with appropriate magnification using In Studio version 3.01 (Pixera, San Jose, CA, USA).
Statistical analysis
Quantification of Western blot analyses was performed with Multi-Gauge version 2.02 program (Fujifilm). All data are given as mean ± SE, and significant differences between groups were determined using Student's t-test (Graphpad Software, San Diego, CA, USA) .
RESULTS
Combination treatment decreased tumor incidence in the lungs of K-rasLA1 mice
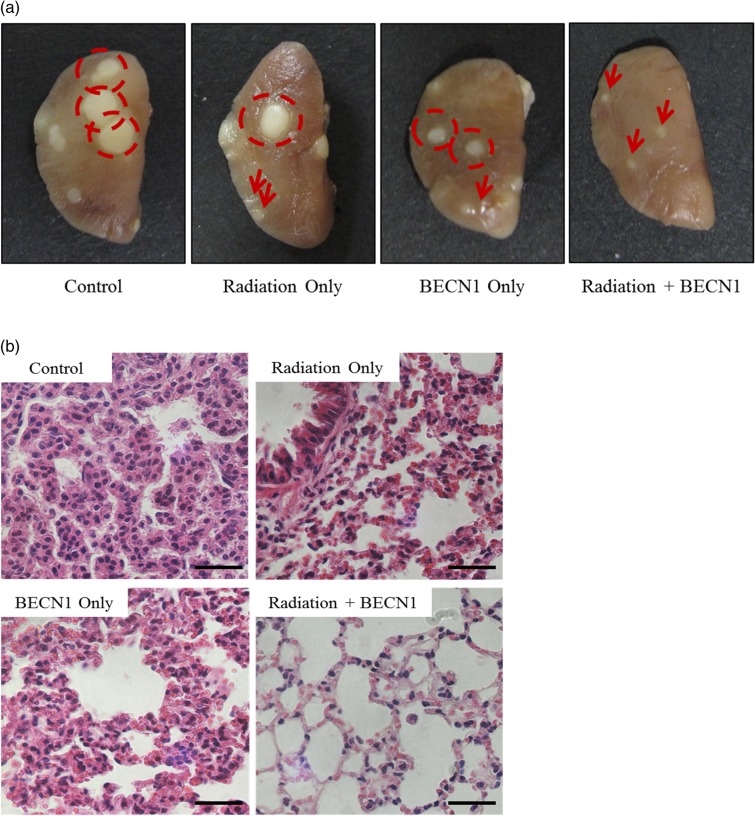
Fractionated radiation alone did not decrease the number or size of tumor nodules compared with those in the control group; however, the changes were significant in the beclin1 and combination treatment groups (Table 1, Fig. 1a). Lungs of the control group showed progressed adenocarcinoma with a dense population of tumor cells on H&E staining. However, adenocarcinomas were alleviated to adenomas in the radiation- and beclin1-treated groups, and near-normal structures of the lungs with a single lining of alveolar walls were observed in the combination treatment group (Fig. 1b). The overall anti-tumor effects of radiation and beclin1 are summarized in Table 1.
Table 1.
Summary of tumor incidence in the lungs of K-rasLA1 mice
| Group | No. of mice | No. of tumors/mouse |
Adenocarcinoma | Adenomaf |
||||
|---|---|---|---|---|---|---|---|---|
| Total | >1 mm | <1 mm | ++ + | ++ | + | |||
| Control | 5 | 17.36 ± 3.04 | 5.89 ± 3.57 | 11.64 ± 0.70 | 3 | 2 | 0 | 0 |
| Radiation only | 5 | 16.44 ± 2.71 | 5.28 ± 2.72 | 10.92 ± 3.83 | 2 | 0 | 1 | 2 |
| Beclin1 only | 5 | 11.19 ± 1.21a,c | 3.28 ± 0.98 | 7.94 ± 1.51 | 1 | 1 | 2 | 0 |
| Radiation + Beclin1 | 5 | 8.97 ± 0.56b,d,e | 2.92 ± 1.10 | 6.06 ± 1.61b | 0 | 0 | 0 | 2 |
Sixteen K-rasLA1 lung cancer model mice were randomly divided into four groups; control, radiation only, beclin1 only and radiation/beclin 1 combination. Animals were 17 weeks old at sacrifice. Lungs were collected, tumor numbers/sizes on the surface of lungs were counted, and fixed in 10% neutral buffered formalin for histological examination. Incidence and multiplicity of lung proliferative lesions were compared (mean ± SE).
aP < 0.05 compared with control group; bP < 0.01 compared with control group; cP < 0.05 compared with radiation only group; dP < 0.01 compared with radiation only group; eP < 0.05 compared with beclin1 only group; fgrades of adenoma: ++ + , severe; ++ , moderate; +, mild.
Fig. 1.
Inhalation of beclin1 resulted in a synergistic anti-tumor effect with radiation in the lungs of K-rasLA1 mice.
(a) The left lobe of the lungs were collected and fixed with 10% neutral formalin for the gross description of the tumors. Tumor nodules were counted, and sizes were measured with automated calipers. Dotted circles represent tumor nodules >1.0 mm. Arrows represent tumor nodules <1.0 mm. (b) Lungs were paraffin-sectioned at 3 µm and slides were stained with H&E for histopathological analysis. Magnification: ×400. Scale bar: 20 µm. Representative figures of five mice per group.
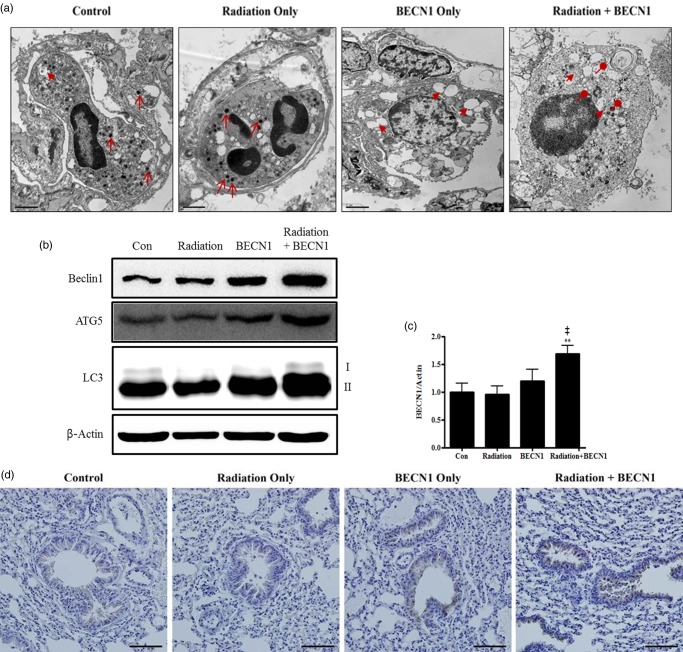
Aerosol delivery of beclin1 increased autophagy in the lungs of K-rasLA1 mice
Activation of the autophagy pathway was considered to examine the correlation between beclin1 and regression of tumor numbers. Intracellular lung tissue structures were observed under TEM. Various sizes of granules, which were considered normal, and primary lysosomes (arrows) were observed in the cytoplasm of cells from the control and radiation groups (Fig. 2a). An increase in the number of vacuoles in the cytoplasm (arrow heads) was distinguishable in the beclin1 group. In particular, the combination treatment group showed many secondary lysosomes (circle heads) indicating autophagosomes fused with lysosomes (Fig. 2a). Western blots were performed to further confirm the activation of autophagy-related proteins. As a result, an increase in beclin1, ATG5 and LC3-II was detected (Fig. 2b), and densitometry analysis of beclin1 supported these findings accordingly (Fig. 2c). Efficiency of beclin1 inhalation was further confirmed with IHC and epithelial cells of the bronchioles showed marked increase, especially in the beclin1- and combination-treated groups (Fig. 2d).
Fig. 2.
Delivery of beclin1 via inhalation was successful and activated the autophagy pathway.
(a) Intracellular lung structures were screened under transmission electron microscopy. Arrows, primary lysosomes; arrow-heads, cytoplasmic vacuoles; circle-heads, secondary lysosomes/autophagolysosomes. Magnification: ×6000. Scale bar: 1 µm. (b) Western blot was performed to monitor the increase in autophagy-related proteins: beclin1 (1:500 dilution), ATG5 (1:1000 dilution) and LC3 (1:1000 dilution). Bands are representative of five individuals from each group. (c) Densitometric analyses reconfirmed the synergistic effect of beclin1 on autophagy. Each bar represents the mean ± SE (n = 5). **P < 0.05 was considered highly significant compared with the control group and ‡P < 0.01 was highly significant compared with the radiation group. (d) Delivery of beclin1 and its synergistic effect in combination group were confirmed with IHC analysis; Beclin1 (1:100 dilution). Magnification: ×200. Scale bar: 20 µm. Representative figures of five mice per group.
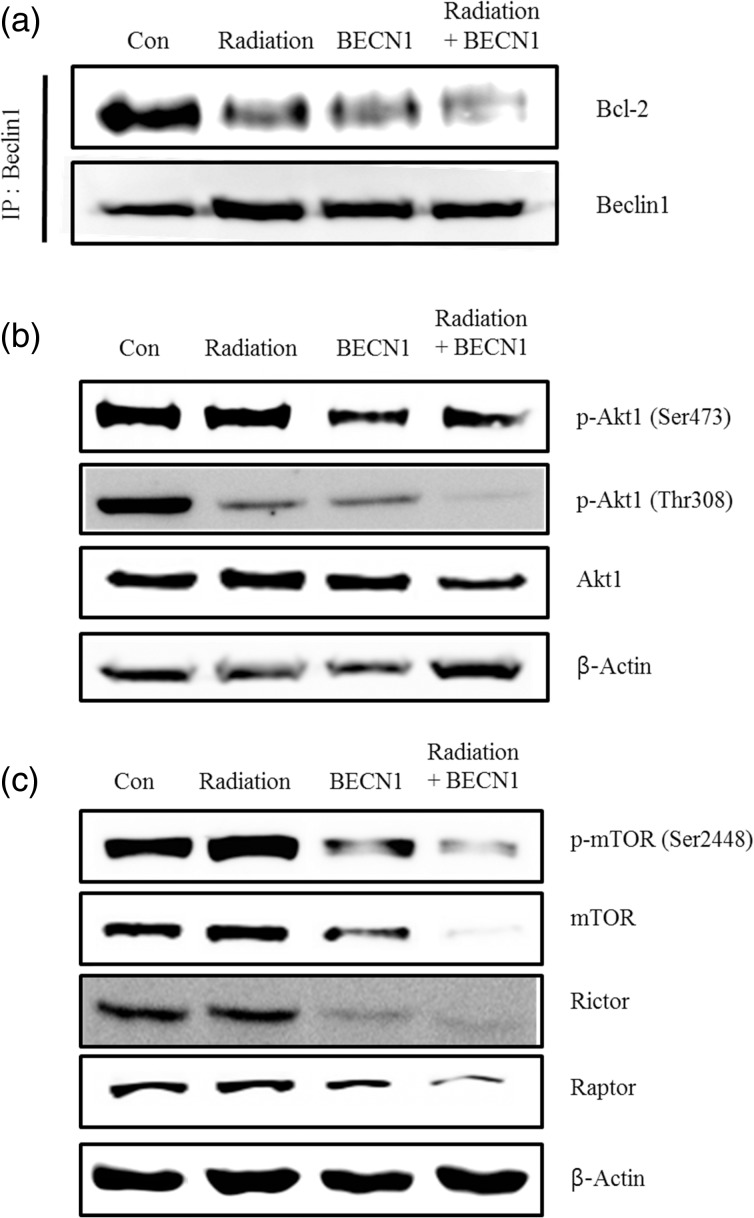
Combination treatment increased the dissociation of the beclin1–bcl2 complex
As beclin1 is a binding partner of bcl2, binding affinity is considered important to maintain the cellular regulation. Dissociation of the beclin1–bcl2 complex is a compulsory event before activating the autophagy pathway [13–16]. An immunoprecipitation assay with beclin1 and bcl2 antibodies was performed to determine the extent of dissociation of this complex. The combination treatment showed a definite decrease on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), confirming less binding affinity of these two proteins (Fig. 3a).
Fig. 3.
Radiation stimulated dissociation of the beclin1-bcl2 complex and downregulated of Akt1-mTOR pathway in the lungs of K-rasLA1 mice.
(a) SDS-PAGE of the beclin1 and bcl2 lung lysate immunoprecipitation. IP beads were bound to the beclin1 antibody and immunoblotted with the bcl2 antibody (1:500 dilution). (b) Western blot analysis using phospho-Akt1 at Thr308 (1:500 dilution), phospho-Akt1 at Ser473 (1:500 dilution) and Akt1 (1:5000 dilution). (c) Western blot analysis with phospho-mTOR at Ser2448, mTOR, raptor and rictor antibodies diluted 1:1000. Bands are representative of five individuals from each group. Representative figures of five mice per group.
Combination treatment down-regulated the Akt–mTOR pathway
Akt1 and mTOR phosphorylation is associated with tumor formation and progression [17], and mTOR has a direct effect on the autophagy pathway [18–21]. Akt1 is a serine/threonine protein kinase that plays a major role in cell growth, proliferation and survival. Therefore, we determined Akt1 phosphorylation status by western blot. The treated groups, particularly the combination treatment group, showed a decrease in both Akt1 phosphorylation sites (Ser473 and Thr308), demonstrating that the anti-tumor effect was activated by suppressing the Akt1 pathway (Fig. 3b). Furthermore, the mTOR and phospho-mTOR expression levels were suppressed. The protein levels of raptor and rictor as a part of mTORC1 and mTORC2, respectively, were also decreased by both the beclin1 and combination treatments (Fig. 3c).
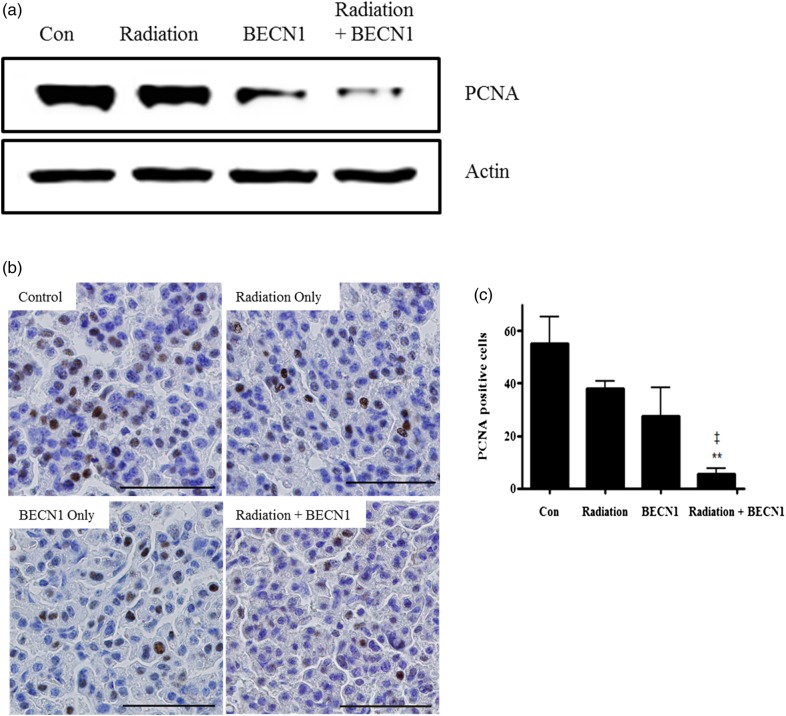
Combination treatment decreased cell proliferation in the lungs of K-rasLA1 mice
PCNA was selected as a marker and a western blot was performed to examine the correlation between tumor regression and cell proliferation. The combination treatment group showed a highly significant decrease in proliferation, whereas beclin1 alone also showed an anti-proliferative effect to some extent, compared with that in the radiation and control groups (Fig. 4a). The IHC analysis further confirmed the anti-proliferative effect of the combination treatment (Fig. 4b and c).
Fig. 4.
Beclin1 with radiation decreased cell proliferation in lungs of K-rasLA1 mice.
(a) Western blot of anti-PCNA (1:5000 dilution) and the lung tissue lysates. Bands are representative of five individuals from each group. (b) The immunohistochemistry analysis showed fewer double stained nuclei with hematoxylin and chromogen on lung tissue slides. Magnification: ×400. Scale bar: 50 µm. (c) Statistical analysis of PCNA positive cells. Nuclei double-stained with DAB and hematoxylin were counted in three different fields from each slide. Each bar represents the mean ± SE (n = 5). **P < 0.01 was considered highly significant compared with the control group. ‡P < 0.01 was considered highly significant compared with the radiation group. Representative figures of five mice per group.
Combination treatment inhibited angiogenesis in lungs of K-rasLA1 mice
Angiogenesis, the ability to build new blood vessels, is a vital process for tumor growth. We selected the pro-angiogenic factor VEGF-A as an angiogenesis marker to determine the extent of angiogenesis at the target site. The radiation group did not show a significant change compared with that in the control; however, a decreased protein level was detected in the beclin1 group and a further decrease was observed in the combination treatment group (Fig. 5a). Decreased VEGF-A expression in the combination treatment group was also demonstrated by IHC (Fig. 5b).
Fig. 5:
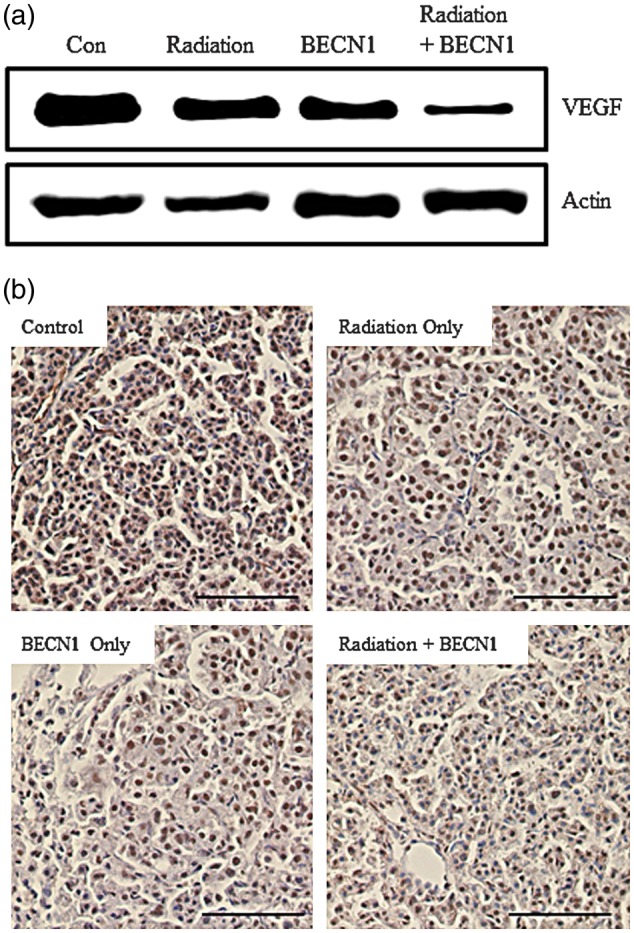
Beclin1 with radiation decreased angiogenic activity in lungs of K-rasLA1 mice.
(a) Western blot analysis using lung tissue lysates and anti-VEGF (1:5000 dilution). Bands are representative of five individuals from each group. (b) The immunohistochemistry analysis showed less chromogen expression in the combination treatment group, confirming (a). Magnification: ×200. Scale bar: 10 µm. Representative figures of five mice per group.
DISCUSSION
Radiation therapy has been applied widely, despite the fact that high dose radiation leads to systemic side-effects. Therefore, in this study, we adopted fractionated radiotherapy to the thorax of K-rasLA1 mice to maximize the therapeutic effects with fewer side-effects. Various kinds of vectors have been developed to deliver specific genes to target organs, and various routes of delivery have been studied [23, 24]. In this study, we used poly(ester)-amine, a modified non-viral vector based on branched polyethyleneimine. Our previous study has shown that this carrier has significantly low cytotoxicity with high transfection efficiency [10]. Fractionated radiation did not result in significant therapeutic effects, as summarized in Fig. 1 and Table 1. Showing no significant difference from the control group, it can be assumed that mice treated with a low dose of radiation have recovered from the autophagic stress by the time of sacrifice. Interestingly, beclin1 alone seemed to have a therapeutic effect to some extent; however, the combination treatment resulted in highly significant tumor regression. Therefore, a combination radiotherapy treatment may allow for more effective outcomes. In fact, our results are supported by recent studies indicating that radiation with chemotherapeutics or other agents increases the effects of radiotherapy alone [25–27].
The autophagy pathway is a catabolic intracellular process that activates the lysosomal degradation pathway. During autophagy, cytoplasmic structures are sequestered into double-membraned or multi-layered autophagosomes and fused with lysosomes to form secondary lysosomes or autophagolysosomes for degradation [28]. In addition to the formation of various density-graded granules in the cytoplasm of lung tissues, an increased number of vacuoles were observed both in the beclin1-delivered and combination treatment groups. Moreover, the beclin1-delivered group showed more secondary lysosomes than primary lysosomes compared with the control and radiation groups, whereas the combination treatment group showed many multi-layered structures and autophago-lysosomes (Fig. 2a). Protein expression levels further supported the TEM analysis in that autophagy-related proteins were overexpressed in the beclin1-delivered group, particularly a highly significant increase was observed in the combination treatment group (Fig. 2b and c). Interestingly, fractionated radiation itself did not show marked autophagy activation in vivo and beclin1 alone activated the autophagy pathway, and the combination therapy further stimulated the beclin1 pathway leading to autophagic cell death. IHC of beclin1 in the lungs confirmed the synergistic effect of beclin1 expression (Fig. 2d). Autophagy is still controversial in that it may promote tumor cell survival, as activated autophagy can be detected under hypoxic or starved conditions of the tumor microenvironment. However, autophagy as a survival mechanism only lasts for a limited time, and prolonged autophagy leads to cell death [29, 30]. Our results are supported by recent studies indicating that activation of the autophagy pathway is correlated with tumor regression [31–33].
As beclin1 is a binding partner of bcl2, binding affinity is considered important to maintain the cellular regulation. According to previous findings, low levels of beclin1 reduce its capacity to activate autophagy [13, 14] and under stress conditions, such as hypoxia or starvation, which activate autophagy, beclin1 dissociates from the complex for activation [15]. Activating autophagy with radiation and beclin1, binding affinity of beclin1-bcl2 decreased significantly (Fig. 3a). A recent study revealed that activating autophagy induces phosphorylation of beclin1 to stimulate dissociation of beclin1-bcl2 complex [16].
Akt1 and mTOR phosphorylation is strongly correlated with tumorigenesis and tumor progression [17]. In particular, mTOR has a direct effect on the autophagy pathway [18–21]. Many studies have been conducted with mTOR inhibitors to induce autophagy to confirm anti-tumor effects, but not with direct targeting of the autophagy pathway. Radiation with beclin1 was a novel approach and proved that it showed similar effects to using mTOR inhibitors (Fig. 3b, c). Akt1 is important in most cellular events [22], and phosphorylation at both serine473 and threonine308 must occur for full Akt1activation [34, 35]. A decrease in phosphorylation at both sites was observed in the beclin1-delivered groups, and the combination treatment group showed a highly significant decrease in phosphorylation at Thr308 and Ser473 (Fig. 3b). Thus, cellular fate induced by beclin1 and/or the combination treatment may be significantly different from that in control cells. In fact, our results are supported by recent studies reporting that different rates of phosphorylation are dependent on the cellular environment [36]. Even the radiation-only group did not show any significant difference in the Akt–mTOR pathway, but interestingly with beclin1 it definitely down-regulated Akt-mTOR signaling. Radiation has been studied to effect mTORC2-Akt, which is upstream of Akt-mTORC1 [37], and combination therapy showed marked suppression of both mTOR complexes. mTOR can be divided into mTORC1 with raptor and mTORC2 with rictor. Delivery of beclin1 affected both complexes, as well as mTOR phosphorylation itself (Fig. 3c), and the combination treatment induced a further decrease. Several lines of evidence have demonstrated that mTORC2 directly phosphorylates Akt1 at Ser473 and facilitates PDK1-mediated Akt1 phosphorylation at Thr308 [38]. Drugs targeting mTOR complexes, such as rapamycin derivatives, potently inhibit Akt activity in cancer cells by suppressing mTORC2 and the mTORC1 pathway followed by down-regulation of p70S6K and 4EPB1 phosphorylation [39]. These findings fit well with our results, because the combination treatment resulted in significant tumor regression by affecting the Akt pathway, leading to mTORC1 and mTORC2 down-regulation.
Cell proliferation and angiogenesis are markers to predict the fate of tumorigenesis [40, 41]. Cancers have a tendency to grow quickly and may metastasize to other organs. Therefore, checkpoints to anticipate the prognosis of tumors are PCNA for proliferation and VEGF-A for angiogenesis [42, 43] in the tumor regions. Overexpression of PCNA and VEGF have been observed in various human tumors; therefore, they are used as biomarkers of invasiveness, vascular density, metastasis and recurrence [44–45]. Our results also demonstrated that a decrease in PCNA and VEGF-A was well correlated with tumor regression concurrent with a decrease in angiogenesis and cell proliferation at the tumor regions (Figs 4 and 5). This result suggests that the combination treatment effectively regressed tumors by inhibiting cancer cell metastasis, proliferation and angiogenesis.
In summary, our results demonstrated that the combination treatment was more effective for treating lung cancer compared with fractionated radiation or beclin1 delivery. Although fractionated radiation itself did not show a therapeutic effect, it seemed to sensitize tumor cells in lung cancer model mice and successfully showed a synergistic anti-tumor effect when beclin1 was delivered to the target organ. We have demonstrated that aerosol delivery of beclin1 enhanced the efficacy of fractionated radiotherapy. Therefore, a combination of radiation with aerosol delivery of a therapeutic gene may provide a prospect for developing novel therapy regimens applicable in clinics.
ACKNOWLEDGEMENTS
This work was supported by a National Research Foundation of Korea Grant funded by the Korean Government (MEST) (NRF-2010-0000784) and partially supported by the Research Institute for Veterinary Science, Seoul National University. The funders had no role in study design, data collection or analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1.RCR. Radiotherapy Dose-Fractionation: Lung Cancer. London: The Royal College of Radiologists; 2006. pp. 43–8. [Google Scholar]
- 2.Brady LW, Heilmann HP, Molls M. Advances in Radiation Oncology in Lung Cancer. Berlin: Springer-Verlag; 2005. [Google Scholar]
- 3.Jin H, Xu C-X, Kim H-W, et al. Urocanic acid-modified chitosan-mediated PTEN delivery via aerosol suppressed lung tumorigenesis in K-ras(LA1) mice. Cancer Gene Ther. 2008;15:275–83. doi: 10.1038/sj.cgt.7701116. [DOI] [PubMed] [Google Scholar]
- 4.Xu C-X, Jere D, Jin H, et al. Poly (ester amine)-mediated, aerosol-delivered Akt1 small interfering RNA suppresses lung tumorigenesis. Am J Respir Crit Care Med. 2008;178:60–73. doi: 10.1164/rccm.200707-1022OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang H-L, Xu C-X, Kim Y-K, et al. The suppression of lung tumorigenesis by aerosol-delivered folate-chitosan-graft-polyethylenimine/Akt1 shRNA complexes through the Akt signaling pathway. Biomaterials. 2009;30:5844–52. doi: 10.1016/j.biomaterials.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 6.Pattingre S, Espert L, Biard-Piechaczyk M, et al. Regulation of macroautophagy by mTOR and Beclin 1 complexes. Biochimie. 2008;90:313–23. doi: 10.1016/j.biochi.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 7.Sun Y, Liu J-H, Jin L, et al. Over-expression of the Beclin1 gene upregulates chemosensitivity to anti-cancer drugs by enhancing therapy-induced apoptosis in cervix squamous carcinoma CaSki cells. Cancer Lett. 2010;294:204–10. doi: 10.1016/j.canlet.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Wang Z-H, Xu L, Duan Z-L, et al. Beclin 1-mediated macroautophagy involves regulation of caspase-9 expression in cervical cancer HeLa cells. Gynecol Oncol. 2007;107:107–13. doi: 10.1016/j.ygyno.2007.05.034. [DOI] [PubMed] [Google Scholar]
- 9.Arote R, Kim T-H, Kim Y-K, et al. A biodegradable poly (ester amine) based on polycaprolactone and polyethylenimine as a gene carrier. Biomaterials. 2007;28:735–44. doi: 10.1016/j.biomaterials.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 10.Albert JM, Cao C, Kim K-W, et al. Inhibition of poly (ADP-ribose) polymerase enhances cell death and improves tumor growth delay in irradiated lung cancer models. Clin Cancer Res. 2007;13:3033–42. doi: 10.1158/1078-0432.CCR-06-2872. [DOI] [PubMed] [Google Scholar]
- 11.Kim K-W, Moretti L, Mitchell LR, et al. Combined Bcl-2/mammalian target of rapamycin inhibition leads to enhanced radiosensitization via induction of apoptosis and autophagy in non-small cell lung tumor xenograft model. Clin Cancer Res. 2009;15:6096–105. doi: 10.1158/1078-0432.CCR-09-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moretti L, Kim K-W, Jung D-K, et al. Radiosensitization of solid tumors by Z-VAD, a pan-caspase inhibitor. Mol Cancer Ther. 2009;8:1270–9. doi: 10.1158/1535-7163.MCT-08-0893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ciechomska IA, Goemans GC, Skepper JN, et al. Bcl-2 complexed with beclin-1 maintains full anti-apoptotic function. Oncogene. 2009;28:2128–41. doi: 10.1038/onc.2009.60. [DOI] [PubMed] [Google Scholar]
- 14.Liang C, Feng P, Ku B, et al. Autophagic and tumour suppressor activity of a novel beclin1-binding protein UVRAG. Nat Cell Biol. 2006;8:688–98. doi: 10.1038/ncb1426. [DOI] [PubMed] [Google Scholar]
- 15.Wei Y, Sinha S, Levine B. Dual role of JNK-mediated phosphorylation of Bcl-2 in autophagy and apoptosis regulation. Autophagy. 2008;4(7):949–51. doi: 10.4161/auto.6788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zalckvar E, Berissi H, Mizrachy L, et al. DAP-kinase-mediated phosphorylation on the BH3 domain of beclin1 promotes dissociation of beclin1 from Bcl-XL and induction of autophagy. EMBO Rep. 2009;10(3):285–92. doi: 10.1038/embor.2008.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choe G, Horvath S, Cloughesy TF, et al. Analysis of the phosphatidylinositol 3′-kinase signaling pathway in glioblastoma patients in vivo. Cancer Res. 2003;63:2742–6. [PubMed] [Google Scholar]
- 18.Maiuri MC, Zalckvar E, Kimchi A, et al. Self-eating and self-killing: Crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8:741–52. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- 19.Jung C-H, Ro S-H, Cao J, et al. mTOR regulation of autophagy. FEBS Lett. 2010;584:1287–95. doi: 10.1016/j.febslet.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jung C-H, Jun C-B, Ro S-H, et al. ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol Biol Cell. 2009;20:1992–2003. doi: 10.1091/mbc.E08-12-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brech A, Ahlquist T, Lothe RA, et al. Autophagy in tumour suppression and promotion. Mol Oncol. 2009;3:366–75. doi: 10.1016/j.molonc.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu C-X, Jin H, Shin J-Y, et al. Roles of protein kinase B/Akt in lung cancer. Front Biosci (Elite edition) 2010;2:1472–84. doi: 10.2741/e206. [DOI] [PubMed] [Google Scholar]
- 23.Dachs GU, Dougherty GJ, Stratford IJ, et al. Targeting gene therapy to cancer: a review. Oncol Res. 1997;9:313–25. [PubMed] [Google Scholar]
- 24.Li S, Huang L. Nonviral gene therapy: Promises and challenges. Gene Ther. 2000;7:31–4. doi: 10.1038/sj.gt.3301110. [DOI] [PubMed] [Google Scholar]
- 25.Adams GE, Ahmed I, Sheldon PW, et al. Radiation sensitization and chemopotentiation: RSU 1069, a compound more efficient than misonidazole in vitro and in vivo. Br J Cancer. 1984;49:571–7. doi: 10.1038/bjc.1984.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lawrence TS, Chang EY, Hahn TM, et al. Radiosensitization of pancreatic cancer cells by 2′, 2′-difluoro-2′-deoxycytidine. Int J Radiat Oncol Biol Phys. 1996;34:867–72. doi: 10.1016/0360-3016(95)02134-5. [DOI] [PubMed] [Google Scholar]
- 27.Kim K-W, Moretti L, Lu B, et al. M867, a novel selective inhibitor of caspase-3 enhances cell death and extends tumor growth delay in irradiated lung cancer models. PLos One. 2008;3(5) doi: 10.1371/journal.pone.0002275. pe2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shintani T, Klionsky DJ. Autophagy in health and disease: A double-edged sword. Science. 2004;306:990–5. doi: 10.1126/science.1099993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang Z-J, Chee C-E, Huang S, et al. The role of autophagy in cancer: therapeutic implications. Mol Cancer Ther. 2011;10:1533–41. doi: 10.1158/1535-7163.MCT-11-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Azad MB, Chen Y, Henson ES, et al. Hypoxia induces autophagic cell death in apoptosis-competent cells through a mechanism involving BNIP3. Autophagy. 2008;4:195–204. doi: 10.4161/auto.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pouyssegur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441:437–43. doi: 10.1038/nature04871. [DOI] [PubMed] [Google Scholar]
- 32.Maiuri MC, Tasdemir E, Criollo A, et al. Control of autophagy by oncogenes and tumor suppressor genes. Cell Death Differ. 2008;16(1):87–93. doi: 10.1038/cdd.2008.131. [DOI] [PubMed] [Google Scholar]
- 33.Gozuacik D, Kimchi A. Autophagy as a cell death tumor suppressor mechanism. Oncogene. 2004;23:2891–906. doi: 10.1038/sj.onc.1207521. [DOI] [PubMed] [Google Scholar]
- 34.Hait WN, Jin S, Yang JM. A matter of life or death (or both): understanding autophagy in cancer. Clin Cancer Res. 2006;12(7):1961–1965. doi: 10.1158/1078-0432.CCR-06-0011. [DOI] [PubMed] [Google Scholar]
- 35.Fujita N, Sato S, Katayama K, et al. Akt-dependent phosphorylation of pp27Kip1 promotes binding to 14-3-3 and cytoplasmic localization. J Biol Chem. 2002;277:28706–13. doi: 10.1074/jbc.M203668200. [DOI] [PubMed] [Google Scholar]
- 36.Chen R, Kim O, Yang J, et al. Regulation of Akt/PKB activation by tyrosine phosphorylation. J Biol Chem. 2001;276:31858–62. doi: 10.1074/jbc.C100271200. [DOI] [PubMed] [Google Scholar]
- 37.Tanaka K, Babic I, Nathanson D, et al. Oncogenic EGFP signaling activates an mTORC2-NFkB pathway that promotes chemotherapy resistance. Cancer Discov. 2011;1(6):524–38. doi: 10.1158/2159-8290.CD-11-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarbassov DD, Guertin DA, Ali SM, et al. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307(5712):1098. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 39.Zeng Z, Sarbassov dos D, Samudio IJ, et al. Rapamycin derivatives reduce mTORC2 signaling and inhibit AKT activation in AML. Blood. 2007;109:3509. doi: 10.1182/blood-2006-06-030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phillips HS, Kharbanda S, Chen R, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9:157–73. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 41.Gasparini G, Harris AL. Clinical importance of the determination of tumor angiogenesis in breast carcinoma: much more than a new prognostic tool. J Clin Oncol. 1995;13:765–82. doi: 10.1200/JCO.1995.13.3.765. [DOI] [PubMed] [Google Scholar]
- 42.Maeda K, Kang S-M, Onoda N, et al. Expression of 53 and vascular endothelial growth factor associated with tumor angiogenesis and prognosis in gastric cancer. Oncology. 2000;55:594–9. doi: 10.1159/000011918. [DOI] [PubMed] [Google Scholar]
- 43.Sui L, Dong Y, Ohno M, et al. Survivin expression and its correlation with cell proliferation and prognosis in epithelial ovarian tumors. Int J Oncol. 2002;21:315. [PubMed] [Google Scholar]
- 44.Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol. 2002;20:4368–80. doi: 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- 45.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25(4):581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]