Abstract
The purpose of this retrospective study is to investigate the impact of concurrent chemotherapy on definitive radiotherapy for the International Federation of Gynecology and Obstetrics (FIGO) IIIb cervical cancer. Between 2000 and 2009, 131 women with FIGO IIIb cervical cancer were treated by definitive radiotherapy (i.e. whole pelvic external beam radiotherapy for 40–60 Gy in 20–30 fractions with or without center shielding and concomitant high-dose rate intracavitary brachytherapy with 192-iridium remote after loading system for 6 Gy to point A of the Manchester method). The concurrent chemotherapy regimen was cisplatin (40 mg/m2/week). After a median follow-up period of 44.0 months (range 4.2–114.9 months) and 62.1 months for live patients, the five-year overall survival (OS), loco-regional control (LRC) and distant metastasis-free survival (DMFS) rates were 52.4, 80.1 and 59.9%, respectively. Univariate and multivariate analyses revealed that lack of concurrent chemotherapy was the most significant factor leading to poor prognosis for OS (HR = 2.53; 95% CI 1.44–4.47; P = 0.001) and DMFS (HR = 2.53; 95% CI 1.39–4.61; P = 0.002), but not for LRC (HR = 1.57; 95% CI 0.64–3.88; P = 0.322). The cumulative incidence rates of late rectal complications after definitive radiotherapy were not significantly different with or without concurrent chemotherapy (any grade at five years 23.9 vs 21.7%; P = 0.669). In conclusion, concurrent chemotherapy is valuable in definitive radiotherapy for Japanese women with FIGO IIIb cervical cancer.
Keywords: cervical cancer, IIIb, chemotherapy, radiotherapy, HDR
INTRODUCTION
External beam radiotherapy (EBRT) combined with intracavitary brachytherapy (ICBT) is the standard treatment for women with cervical cancer [1–3]. A combination of EBRT plus high-dose rate (HDR) ICBT for Japanese women with cervical cancer has provided acceptable outcomes and late complication rates despite the lower dose prescription in Japan than in the US [4–9]. In 2000s concurrent chemoradiotherapy (CCRT) became standard after the National Cancer Institute (NCI) announcement recommending concurrent chemotherapy in 1999 [10], however, the benefits of concurrent chemotherapy on definitive radiotherapy might not be applicable to concomitant EBRT plus HDR-ICBT and are not clear yet in Japan and other Asian countries [9]. We therefore performed a retrospective analysis in a mono-institutional group with newly diagnosed International Federation of Gynecology and Obstetrics (FIGO) IIIb cervical cancer treated by definitive radiotherapy, the purpose of this study being to investigate the impact of concurrent chemotherapy on definitive radiotherapy for Japanese women.
MATERIALS AND METHODS
Patients
We reviewed our database looking for women with newly diagnosed FIGO IIIb uterine cervical cancers with a maximum diameter over 4 cm treated with definitive radiotherapy at the National Cancer Center Hospital between 2000 and 2009. Patients who received palliative EBRT alone, postoperative radiotherapy, interstitial brachytherapy or an experimental regimen of concurrent chemotherapy were excluded. A total of 131 women treated with EBRT plus HDR-ICBT were admitted to this retrospective analysis. All patients underwent pelvic examination, cystoscope, urography, computed tomography (CT), magnetic resonance imaging (MRI), ultrasound (US) and blood tests. Maximum tumor diameters were measured based on the MRI findings and/or US. FIGO staging was allocated for tumor boards of gynecological, medical and radiation oncologists. The pathological diagnosis was carried out with a central pathology review at our pathological division.
Treatment
Treatment selection was determined by the gynecological cancer board, our treatment policy for FIGO IIIb cervical cancer is CCRT to aim for loco-regional control (LRC) even if distant metastasis is not ruled out. Neoadjuvant chemotherapy was prohibited. The concurrent chemotherapy regimen was cisplatin (40 mg/m2/week). Supportive treatments such as blood transfusions were encouraged during radiotherapy.
Radiotherapy
The radiotherapy field selected was the whole pelvis but exceptions were as follows: para-aortic node (PAN) area irradiation was acceptable in cases with suspicions of PAN metastasis, bilateral inguinal node area irradiation was acceptable in cases with vaginal involvement of more than two-thirds of total vaginal length. Radiotherapy doses of 40–60 Gy in 20–30 fractions were carried out with a 4-field box or the anterior–posterior technique. Center shield radiotherapy (CS) was performed for a shorter overall treatment time (OTT) reducing organ at risk (OAR) exposure depending on tumor shrinkage. CS was carried out 3–4 days/week, and HDR-ICBT 1–2 days/week, but both therapies were not carried out on the same day. All patients underwent EBRT with 10-, 15- and 20-MV X-rays from linear accelerators (Clinac IX, Varian, Palo Alto, CA, USA). Two-dimensional conventional radiotherapy (2DCRT) was employed between 2000 and 2005, and three-dimensional conformal radiotherapy (3DCRT) was used between 2005 and 2010. All patients underwent HDR-ICBT with 192-iridium remote after loading system (RALS, Microselectron). The point A dose prescription for 6 Gy using the Manchester method was performed with the ICBT planning system (Plato®, Nucletron). Image-guided optimization was not applicable even in the case of CT-based ICBT planning. A tandem-cylinder was used only in cases with vaginal involvement of more than one-third of total vaginal length or of an extraordinarily narrow vagina.
Follow-up
All patients were evaluated weekly for toxicity during radiotherapy through physical examinations and blood tests. CT and/or MRI scans and cytology were performed 1–3 months after radiotherapy for initial response, physical examination and blood tests were performed regularly every 1–6 months. Disease progression was defined by the response evaluation criteria in solid tumours (RECIST) version 1.1, new clinical symptoms or observable pelvic deficits.
Statistical analysis
Patient and treatment characteristics were compared using the Mann-Whitney U test and Pearson's chi-square test. OS was estimated from the beginning of radiotherapy to the date of death considered as an event, and censored at the time of last follow-up. LRC rate was estimated from the beginning of radiotherapy to the date of LRC failure including both central and lateral pelvic relapse considered as an event, and censored at the time of death or last follow-up. DMFS rate was estimated from the beginning of radiotherapy to the date of distant metastasis considered as an event, and censored at the time of death or last follow-up. The cumulative incidence rate of late rectal complication was estimated from the beginning of radiotherapy to the date of any grade rectal hemorrhage according to common terminology criteria for adverse events (CTCAE) version 4.0. [11] OS, LRC and DMFS, and the cumulative incidence rates of late rectal complication were calculated using the Kaplan–Meier method [12].
As a measure of radiotherapeutic intensity to point A, we used the equivalent dose in 2-Gy fractions (EQD2) calculated from total irradiated dose (D) and each dose (d) with α/β for 10 Gy and potential doubling time (Tpot) defined as five days' subtraction from EQD2 with correction for tumor proliferation associated with OTT (EQD2T) as shown in the following formula:
TK is the kick-off time of accelerated repopulation and was defined as 21 days, and 0.3 for α [13]. These parameters are not well estimated for cervical cancer so we used those for head and neck squamous cell carcinoma (SCC) and extrapolated them. The survival curves were compared using the log-rank test and Cox's proportional hazards model. In order to carry out univariate and/or multivariate analysis comparing OS, LRC and DMFS rates, patients were categorized as follows: age (<60 vs ≥60), tumor bulk (<55 vs ≥55 mm), OTT (<6 vs ≥6 weeks), hemoglobin (Hb) before (<11.9 vs ≥11.9 mg/dl) and concurrent chemotherapy. We added univariate and multivariate analysis to assess the impact of concurrent chemotherapy on OS, LRC and DMFS after stratified analysis for age and tumor bulk. All statistical analyses were performed using PASW statistics (Version 18.0, SPSS Japan Inc., an IBM company, Chicago, IL, USA). A P value of <0.05 was considered significant.
RESULTS
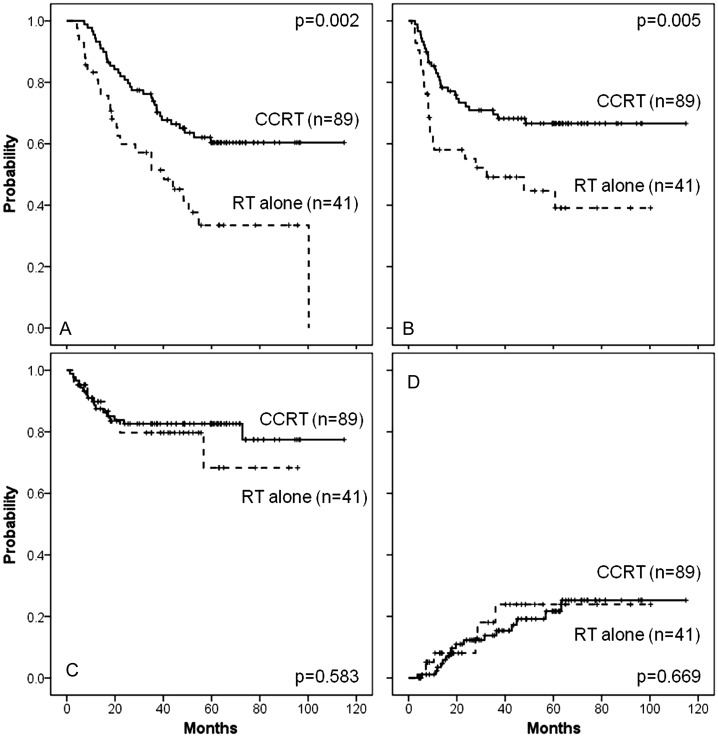
Patient and treatment characteristics are shown in Table 1. There were differences in age and Hb level after treatment between the radiotherapy alone and CCRT groups. After a median follow-up period of 44.0 months (range 4.2–114.9 months) collectively and 62.1 months for live patients, five-year OS, LRC and DMFS rates were 52.4, 80.1 and 59.9%, respectively. Univariate and multivariate analyses revealed that default of concurrent chemotherapy was the most significant factor leading to poor prognosis for OS (HR = 2.53; 95% CI 1.44–4.47; P = 0.001) and DMFS (HR = 2.53; 95% CI 1.39–4.61; P = 0.002), but not for LRC (HR = 1.57; 95% CI 0.64–3.88; P = 0.322). (Table 2). The cumulative incidence rates of late rectal complications after definitive radiotherapy were not significantly different with or without concurrent chemotherapy (any grade at five years 23.9 vs 21.7%; P = 0.669) (Fig. 1). After stratifying 131 patients for age and tumor bulk, subgroup analysis with or without concurrent chemotherapy revealed that non-elderly women (HR = 2.78; 95% CI 1.25–6.18; P = 0.012) with even bulky length (HR = 2.53; 95% CI 1.26–5.07; P = 0.009) clearly benefit from concurrent chemotherapy (Table 3).
Table 1.
Patient and treatment characteristics for RT alone and CCRT
| RT alone (n = 42) | CCRT (n = 89) | P | ||
|---|---|---|---|---|
| Age | Median (range) | 66 (36–85) | 55 (29–73) | 0.000 |
| Tumor bulk | mm | 55 (45–87) | 55 (40–95) | 0.302 |
| Pathology | SCC | 37 (88.1%) | 82 (92.1%) | 0.454 |
| non-SCC | 5 (11.9%) | 7 (7.9%) | ||
| Hb before RT | mg/dl | 11.9 (6.4–14.2) | 11.9 (7.1–14.5) | 0.653 |
| Hb after RT | mg/dl | 11.3 (7.6–14.4) | 10.3 (6.9–12.3) | 0.002 |
| OTT | days | 42 (30–69) | 42 (36–62) | 0.217 |
| EQD2 | Gy | 56.4 (44.0–74.0) | 54.0 (52.2–74.0) | 0.128 |
| EQD2T | Gy | 50.0 (40.9–66.2) | 48.2 (39.2–61.2) | 0.177 |
| wCDDP courses | 1 | 0 | 5 ( 5.6%) | 0.000 |
| 2 | 0 | 6 ( 6.8%) | ||
| 3 | 0 | 12 (13.5%) | ||
| 4 | 0 | 23 (25.8%) | ||
| 5 | 0 | 30 (33.7%) | ||
| 6 | 0 | 13 (14.6%) | ||
| Reason for RT alone | Advanced age | 17 (40.4%) | 0 | 0.000 |
| PAN irradiation | 8 (19.0%) | 0 | ||
| No consent | 5 (11.9%) | 0 | ||
| Renal function | 3 (7.2%) | 0 | ||
| Hepatitis | 2 (4.8%) | 0 | ||
| Others | 7 (16.7%) | 0 | ||
| Follow-up | months | 30.7 (4.2–100.3) | 48.8 (7.3–114.9) | 0.001 |
RT = radiotherapy, CCRT = concurrent chemoradiotherapy, FIGO = International Federation of Gynecology and Obstetrics, SCC = squamous cell carcinoma, Hb = hemoglobin, OTT = overall treatment time, EQD2 = the equivalent dose in 2-Gy fractions, EQD2T = EQD2 with correction for tumor proliferation associated with OTT, wCDDP = weekly cisplatin, ns = not significant.
Table 2.
Univariate and multivariate analyses on OS, LRC and DMFS
| OS |
LRC |
DMFS |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variants | n | Five years | uni | multi | Five years | uni | multi | Five years | uni | multi | |
| Age | <60 | 72 | 51.4 | 0.631 | 0.121 | 73.3 | 0.129 | 0.076 | 56.0 | 0.173 | 0.033 |
| ≥60 | 59 | 53.7 | 89.2 | 64.8 | |||||||
| Tumor bulk | <55 mm | 54 | 59.8 | 0.358 | 0.486 | 79.5 | 0.768 | 0.856 | 74.4 | 0.010 | 0.027 |
| ≥55 mm | 77 | 47.6 | 80.6 | 50.2 | |||||||
| OTT | <6 weeks | 75 | 53.1 | 0.789 | 0.639 | 78.5 | 0.532 | 0.258 | 63.5 | 0.626 | 0.918 |
| ≥6 weeks | 56 | 50.8 | 82.6 | 56.0 | |||||||
| Hb before RT | <11.9 mg/dl | 62 | 53.1 | 0.627 | 0.934 | 74.5 | 0.380 | 0.599 | 59.3 | 0.527 | 0.988 |
| ≥11.9 mg/dl | 69 | 52.2 | 84.8 | 60.6 | |||||||
| Concurrent chemotherapy | Yes | 89 | 60.4 | 0.002 | 0.001 | 82.6 | 0.583 | 0.322 | 66.6 | 0.005 | 0.002 |
| No | 42 | 33.5 | 68.3 | 44.7 | |||||||
OS = overall survival, LRC = loco-regional control, DFMS = distant metastasis free survival, uni = univariate analysis, multi = multivariate analysis, OTT = overall treatment time, Hb = hemoglobin, ns = not significant.
Fig. 1.
OS (A), DMFS (B), LRC (C) and the cumulative incidence rates of late rectal complication (D) of women with FIGO IIIb cervical cancer after definitive radiotherapy with or without concurrent chemotherapy.
Solid line for CCRT, dashed line for RT alone. OS = overall survival, DMFS = distant metastasis free survival, LRC = loco-regional control, CCRT = concurrent chemoradiotherapy, RT = radiotherapy.
Table 3.
Impact of concurrent chemotherapy on OS, LRC and DMFS in the stratified analysis
| OS |
LRC |
DMFS |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Log-rank | Cox's |
Log-rank | Cox's |
Log-rank | Cox's |
|||||
| Variates | P | HR (95%CI) | P | P | HR (95%CI) | P | P | HR (95%CI) | P | |
| Age | <60 | 0.005 | 2.78 (1.25–6.18) | 0.012 | 0.145 | 2.31 (0.76–6.96) | 0.136 | 0.001 | 2.83 (1.32–6.05) | 0.007 |
| ≥60 | 0.023 | 2.55 (1.10–5.89) | 0.028 | 0.942 | 1.05 (0.23–4.85) | 0.942 | 0.079 | 2.29 (0.88–5.94) | 0.087 | |
| Tumor bulk | <55 mm | 0.118 | 2.36 (0.85–6.52) | 0.096 | 0.108 | 5.87 (1.27–27.0) | 0.023 | 0.043 | 3.46 (1.01–11.9) | 0.049 |
| ≥55 mm | 0.018 | 2.53 (1.26–5.07) | 0.009 | 0.587 | 0.75 (0.22–2.49) | 0.645 | 0.085 | 2.23 (1.12–4.44) | 0.021 | |
OS = overall survival, DMFS = distant metastasis free survival, ns = not significant.
DISCUSSION
Various predictors such as treatment duration and anemia had been reported in the last decade before CCRT [14–18]. Concomitant EBRT with HDR-ICBT, which requires shorter treatment duration, was originally the mainstream treatment for women with cervical cancer in Japan [5]. Treatment durations of gross tumor irradiation had a median of 42 days, and were mostly 6 weeks, which is much shorter than the 8 weeks recommended by the American brachytherapy society (ABS) [14]. Concurrent chemotherapy has the potential hazard of treatment interruption associated with acute toxicities, however OTT was not significantly different between radiotherapy alone and CCRT (42 (30–69) vs 42 (36–62) days; P = 0.217). In this situation, OTT is no longer a prognostic factor [17]. Similarly, a low Hb value before radiotherapy has no impact on survival, and is no longer a prognostic factor if anemia has been actively corrected using blood transfusion during radiotherapy [18].
Randomized trials have shown survival benefits of CCRT for cervical cancer [19–23]. Incorporating concurrent chemotherapy contributed to improvement in both LRC and DMFS [19–23]. This impact is less in stages III–IV than in stages I–II [20–23]. Our study also supported this impact on OS and DMFS even in cases of FIGO IIIb, but not on LRC (Table 2). The cumulative incidence rates of late rectal complications after definitive radiotherapy were not significantly different with or without chemotherapy (any grade at five years 23.9 vs 21.7%; P = 0.669) and reached a plateau (Fig. 1), though limited by the short follow-up period for late radiation-induced complications of other organs such as bladder or small intestine [7].
There were important limitations on this retrospective analysis: the advantage of concurrent chemotherapy might merely indicate that the reasons for not undergoing concurrent chemotherapy were associated with poor prognosis. Forty-two women with FIGO IIIb cervical cancer did not undergo concurrent chemotherapy in our study because of advanced age (77 (72–85) years) for 17 patients (40.4%), and the other half (53 (36–70)) had the following reasons for not undergoing concurrent chemotherapy:PAN irradiation for eight patients (19.0%), renal failure for three patients (7.2%), lack of patient's consent for five patients (11.9%), chronic hepatitis for two patients (4.8%), active pyometra, uncontrolled anemia, synchronous double cancer, hypertrophic cardiomyopathy, low white blood cell counts and sequential chemotherapy for one patient each (2.4%). These reasons not to perform concurrent chemotherapy seem to be clinically ordinary and acceptable, but could indicate a potential selection bias that modified the impact of concurrent chemotherapy. Our study revealed that concurrent chemotherapy is the most significant predictor of definitive radiotherapy, thus we conclude that concurrent chemotherapy combined with definitive radiotherapy for FIGO IIIb cervical cancer is advantageous for survival improvement.
Development of the optimal chemotherapy regimen and schedule to increase chemotherapeutic intensity as a cytotoxic agent but not a radiosensitizer seems to be warranted because our results indicated concurrent chemotherapy has impacts on DMFS but not on LRC. It is not reasonable for Japanese women with cervical cancer to undergo increased intensity of dose-dense concurrent chemotherapy due to a lack of relevant feasibility [24]. There is no evidence that platinum-doublet is superior to platinum-alone as concurrent chemotherapy for cervical cancer [22–23]. Therefore, devising the best form of concurrent chemotherapy is considered to be a limitation. The efficacy of adjuvant chemotherapy after definitive CCRT is unclear but worth testing as it is a feasible method [25].
In conclusion, though limited to a mono-institutional retrospective analysis, this study revealed that concurrent chemotherapy is valuable in definitive radiotherapy for Japanese women with FIGO IIIb cervical cancer. A randomized controlled trial is needed to establish the optimal chemotherapy combined with definitive radiotherapy for women with advanced cervical cancer.
ACKNOWLEDGEMENTS
We declare no conflict of interest. This work was partly supported by a Grant-in-Aid from the Tohoku Cancer EBM project of Yamagata University Faculity of Medicine, Cancer Research Development Fund, National Cancer Center.
REFERENCES
- 1.Thoms WW, Jr, Eifel PJ, Smith TL, et al. Bulky endocervical carcinoma: a 23-year experience. Int J Radiat Oncol Biol Phys. 1992;23:491–9. doi: 10.1016/0360-3016(92)90003-z. [DOI] [PubMed] [Google Scholar]
- 2.Perez CA, Grigsby PW, Nene SM, et al. Effect of tumor size on the prognosis of carcinoma of the uterine cervix treated with irradiation alone. Cancer. 1992;69:2796–806. doi: 10.1002/1097-0142(19920601)69:11<2796::aid-cncr2820691127>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 3.Landoni F, Maneo A, Colombo A, et al. Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer Lancet. 1997;350:535–40. doi: 10.1016/S0140-6736(97)02250-2. [DOI] [PubMed] [Google Scholar]
- 4.Eifel PJ, Moughan J, Erickson B, et al. Patterns of radiotherapy practice for patients with carcinoma of the uterine cervix: a patterns of care study. Int J Radiat Oncol Biol Phys. 2004;60:1144–53. doi: 10.1016/j.ijrobp.2004.04.063. [DOI] [PubMed] [Google Scholar]
- 5.Toita T, Kodaira T, Shinoda A, et al. Patterns of radiotherapy practice for patients with cervical cancer (1999–2001): patterns of care study in Japan. Int J Radiat Oncol Biol Phys 2008. 70:788–94. doi: 10.1016/j.ijrobp.2007.10.045. [DOI] [PubMed] [Google Scholar]
- 6.Toita T, Kato S, Niibe Y, et al. Prospective multi-institutional study of definitive radiotherapy with high-dose-rate intracavitary brachytherapy in patients with nonbulky (<4-cm) stage i and ii uterine cervical cancer (JAROG0401/JROSG04-2) Int J Radiat Oncol Biol Phys. 2012;82:49–56. doi: 10.1016/j.ijrobp.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 7.Nakano T, Kato S, Ohno T, et al. Long-term results of high-dose rate intracavitary brachytherapy for squamous cell carcinoma of the uterine cervix. Cancer. 2005;103:92–101. doi: 10.1002/cncr.20734. [DOI] [PubMed] [Google Scholar]
- 8.Toita T, Moromizato H, Ogawa K, et al. Concurrent chemoradiotherapy using high-dose-rate intracavitary brachytherapy for uterine cervical cancer. Gynecol Oncol. 2005;96:665–70. doi: 10.1016/j.ygyno.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 9.Nagase S, Inoue Y, Umesaki N, et al. Evidence-based guidelines for treatment of cervical cancer in Japan: Japan Society of Gynecologic Oncology (JSGO) 2007 edition. Int J Clin Oncol. 2010;15:117–24. doi: 10.1007/s10147-010-0061-x. [DOI] [PubMed] [Google Scholar]
- 10.NCI Issues Clinical Announcement on Cervical Cancer: Chemotherapy Plus Radiation Improves Survival. http://www.nih.gov/news/pr/feb99/nci-22.htm. (11 April 2012, date last accessed) [Google Scholar]
- 11.National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.03, 2010. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. (10 April 2012, date last accessed) [Google Scholar]
- 12.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am StatAssoc. 1958;53:457–81. [Google Scholar]
- 13.Hall EJ, Giaccia AJ. Radiobiology for the Radiologist: Time, Dose, and Fractionation in Radiotherapy, 6th edn. Philadelphia: Lippincott Williams & Wilkins; 2006. pp. 378–97. [Google Scholar]
- 14.Nag S, Erickson B, Thomadsen B, et al. The American Brachytherapy Society recommendations for high-dose-rate brachytherapy for carcinoma of the cervix. Int J Radiat Oncol Biol Phys. 2000;48:201–11. doi: 10.1016/s0360-3016(00)00497-1. [DOI] [PubMed] [Google Scholar]
- 15.Lanciano RM, Martz K, Coia LR, et al. Tumor and treatment factors improving outcome in stage III-B cervix cancer. Int J Radiat Oncol Biol Phys. 1991;20:95–100. doi: 10.1016/0360-3016(91)90143-r. [DOI] [PubMed] [Google Scholar]
- 16.Toita T, Kakinohana Y, Ogawa K, et al. Combination external beam radiotherapy and high-dose-rate intracavitary brachytherapy for uterine cervical cancer: analysis of dose and fractionation schedule. Int J Radiat Oncol Biol Phys. 2003;56:1344–53. doi: 10.1016/s0360-3016(03)00288-8. [DOI] [PubMed] [Google Scholar]
- 17.Chatani M, Matayoshi Y, Masaki N, et al. High-dose rate intracavitary irradiation for carcinoma of the uterine cervix. The adverse effect of treatment prolongation. Strahlenther Onkol. 1997;173:379–84. doi: 10.1007/BF03038241. [DOI] [PubMed] [Google Scholar]
- 18.Grogan M, Thomas GM, Melamed I, et al. The importance of hemoglobin levels during radiotherapy for carcinoma of the cervix. Cancer. 1999;86:1528–36. doi: 10.1002/(sici)1097-0142(19991015)86:8<1528::aid-cncr20>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 19.Rose PG, Bundy BN, Watkins EB, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999;340:1144–53. doi: 10.1056/NEJM199904153401502. [DOI] [PubMed] [Google Scholar]
- 20.Morris M, Eifel PJ, Lu J, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999;340:1137–43. doi: 10.1056/NEJM199904153401501. [DOI] [PubMed] [Google Scholar]
- 21.Eifel PJ, Winter K, Morris M, et al. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: an update of radiation therapy oncology group trial (RTOG) 90-01. J Clin Oncol. 2004;22:872–80. doi: 10.1200/JCO.2004.07.197. [DOI] [PubMed] [Google Scholar]
- 22.Green JA, Kirwan JM, Tierney JF, et al. Survival and recurrence after concomitant chemotherapy and radiotherapy for cancer of the uterine cervix: a systematic review and meta-analysis. Lancet. 2001;358:781–6. doi: 10.1016/S0140-6736(01)05965-7. [DOI] [PubMed] [Google Scholar]
- 23.Chemoradiotherapy for Cervical Cancer Meta-Analysis Collaboration. Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: a systematic review and meta-analysis of individual patient data from 18 randomized trials. J Clin Oncol. 2008;26:5802–12. doi: 10.1200/JCO.2008.16.4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watanabe Y, Nakai H, Shimaoka M, et al. Feasibility of concurrent cisplatin use during primary and adjuvant chemoradiation therapy: a phase I study in Japanese patients with cancer of the uterine cervix. Int J Clin Oncol. 2006;11:309–13. doi: 10.1007/s10147-006-0567-4. [DOI] [PubMed] [Google Scholar]
- 25.Dueñas-González A, Zarbá JJ, Patel F, et al. Phase III, open-label, randomized study comparing concurrent gemcitabine plus cisplatin and radiation followed by adjuvant gemcitabine and cisplatin versus concurrent cisplatin and radiation in patients with stage IIB to IVA carcinoma of the cervix. J Clin Oncol. 2011;29:1678–85. doi: 10.1200/JCO.2009.25.9663. [DOI] [PubMed] [Google Scholar]