Abstract
Intensity-modulated radiation therapy (IMRT) has recently become popular in Japan. Prostate cancer is indisputably one of the main targets of IMRT. However, the current status and interfacility differences in dose-prescription policies for prostate IMRT are unknown. Therefore, a nationwide survey of 43 institutions that had implemented prostate IMRT was conducted by sending a questionnaire regarding the above-mentioned issues. Thirty-three institutions (77%) had responded to the questionnaire by the end of October 2010. A total of 5245 patients with localized prostate cancer had been treated with IMRT by the end of 2009. Regular multileaf collimator-based techniques were the most common beam delivery method. Dose-prescription policies were divided into four major categories: isocenter-based (@isocenter), dose delivered to 95% of the planning target volume (PTV) (D95)-based (D95@PTV), mean dose to the PTV-based (Mean@PTV), and mean dose to the clinical target volume (CTV)-based (@CTV). The mean doses of the CTV and PTV, and the volume of the PTV receiving 95% of the dose (V95) were significantly higher with the D95@PTV policy than with the other prescription policies. Low-dose areas and hot spots were observed within the PTV in plans with @isocenter and @CTV policies. In conclusion, there are currently considerable differences among institutions in Japan regarding target doses for prostate IMRT. The D95@PTV prescription policy resulted in significant dose escalation compared with the other policies. These differences should be taken into consideration when interpreting treatment outcomes and creating multi-institutional protocols in the future.
Keywords: intensity-modulated radiation therapy, prostate cancer; target dose prescription
INTRODUCTION
Intensity-modulated radiation therapy (IMRT) is a highly sophisticated irradiation technique representing high-precision radiotherapy, which realizes higher radiation dose conformity to the target, as well as significant dose sparing of organs at risk compared with conventional three-dimensional conformal radiation therapy (3D-CRT). As a result of the dosimetric advantages, IMRT has become widely used in patients with prostate, head and neck, and central nervous system (CNS) tumors in the United States [1–6]. Currently, IMRT has spread to many radiotherapy departments worldwide and is now applied to many sites for a wide range of indications [7, 8].
In Japan, Chiba Cancer Center and Kyoto University initiated the clinical application of IMRT in patients with prostate cancer in 2000. Since then, IMRT has been used clinically only at selected university hospitals and cancer centers, because it was not covered by national health insurance in Japan at that time. As of 2006, only 30 institutions in Japan had clinically implemented IMRT, according to a survey conducted by the Japan Conformal External-beam Radiotherapy Group. However, IMRT was more rapidly adopted after 2008, when the Japanese government approved insurance coverage for IMRT as a definitive treatment for prostate cancer, head and neck cancer, and CNS tumors. Insurance coverage was extended to curative applications for all solid cancers by April 2010.
Prostate cancer is indisputably a good indication for the use of IMRT [9], and it seems likely that many Japanese institutions are conducting IMRT at present. However, there are no data regarding the current status of prostate IMRT in Japan. In addition, approaches to designing treatment plans have not been standardized and vary greatly among institutions. An understanding of the differences in dose-prescription policy is especially important for interpreting outcomes from different institutions and designing IMRT protocols for future multi-institutional studies.
The present study was conducted to evaluate interfacility differences in dose-prescription policies for prostate IMRT plans in Japan, using a questionnaire survey. The current status of prostate IMRT in Japan and the differences among dose-prescription policies for definitive IMRT plans for prostate cancer are reported.
METHODS
A questionnaire regarding the current status of prostate IMRT and the details of treatment designs, with a special interest in dose-prescription policy, was created and mailed to 43 institutions in Japan that reported having experience with the clinical application of IMRT for prostate cancer, according to a 2008 nationwide survey regarding IMRT conducted by the 3D Conformal External-beam Radiotherapy Group. The questionnaire gathered information on four major topics: the state of implementation, the details of treatment delivery systems, the methods for designing treatment plans and dose-volume data for cases receiving IMRT for prostate cancer. A brief summary of the questionnaire content is given in Table 1.
Table 1.
Summary of the contents of the questionnaire for prostate IMRT
1. State of implementation of prostate IMRT
|
2. Equipment and beam delivery methods
|
3. Treatment planning
|
4. Dose-volume data of five typical cases
|
IMRT, intensity-modulated radiation therapy; CTV, clinical target volume; PTV, planning target volume; D95, the percentage of the prescribed dose covering 95% of the volume; V90, the percentage of the volume covered by 90% of the prescribed dose.
All statistical analyses were performed with commercial statistical software (StatView 5.0; SAS Institute Inc., Cary, NC, USA). The Mann–Whitney U test was used to detect the significance of differences between two sets of dose-volume data.
RESULTS
Thirty-three institutions (77%) had responded to the questionnaire by the end of October 2010.
State of implementation of IMRT for prostate cancer
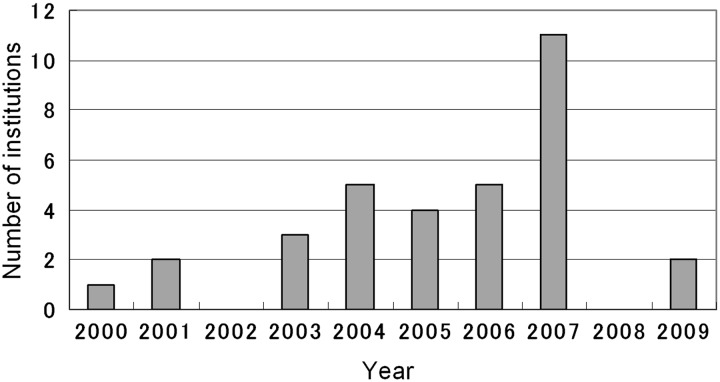
The distribution of commencement years, between 2000 and 2009, of definitive IMRT for prostate cancer at the responding institutions is shown in Fig. 1. Eleven institutions launched prostate IMRT in 2007. The numbers of treated patients by institution ranged from nine to 509. By the end of 2009, a total of 5245 patients with localized prostate cancer had received IMRT, with 1589 cases (1–227 cases per institution) being treated in 2009 alone.
Figure 1.
Number of institutions that started prostate IMRT each year from 2000 to 2009.
The numbers of institutions using IMRT according to clinical stages T1N0M0, T2aN0M0, T2bN0M0, T2cN0M0, T3aN0M0, T3bN0M0, T4N0M0 and TanyN1M0 were 33, 30, 31, 31, 30, 26, 14 and 8, respectively. Thirty-two institutions used IMRT throughout the treatment period, and one used IMRT as a boost therapy following conventional 3D-CRT.
Equipment and beam delivery methods
The radiation therapy equipments, treatment planning systems and beam delivery methods used for IMRT in the 33 institutions are listed in Table 2. With respect to radiation therapy equipments for beam delivery, linear accelerators distributed by Varian Medical Systems (Palo Alto, CA, USA) represented the majority of responses. Eclipse (Varian Medical Systems) and -XiO (Elekta AB, Stockholm, Sweden) were the main manufacturers of treatment planning systems.
Table 2.
Summary of beam delivery methods and X-ray energies
| Method/energy | Number of institutions |
|---|---|
| Beam delivery method | |
| SMLC | 17 |
| DMLC | 13 |
| Tomotherapy | 3 |
| VMAT | 1 |
| X-ray energy | |
| 4 MV | 1 |
| 6 MV | 8 |
| 10 MV | 22 |
| 15 MV | 3 |
| 18 MV | 1 |
| 20 MV | 1 |
SMLC, segmental multileaf collimator; DMLC, dynamic multileaf collimator; VMAT, volumetric-modulated arc therapy.
The segmental multileaf collimator (MLC) technique was the delivery method used at almost half of the institutions (n = 17), followed by the dynamic MLC technique (n = 13). The X-ray energy ranged from 4 to 20 MV, with the majority of the institutions adopting 10-MV (n = 22) or 6-MV (n = 8) X-rays (Table 2).
Twenty-nine institutions irradiated patients in the supine position and four in the prone position. Twenty-nine institutions used fixation devices such as a vacuum pillow or thermoplastic shell, while four institutions did not use any fixation devices for IMRT delivery.
With respect to an error reduction strategy, 16 institutions conducted bony structure-based error reduction, 11 applied a prostate-based image-guided radiotherapy (IGRT) approach and six used a mixed approach of bony structure- and prostate-based IGRT. In the mixed approach, daily set-up was done based on the bony structures, and prostate-based IGRT using techniques such as cone-beam computed tomography (CT) to locate the target was typically combined once weekly. Various modalities were used for prostate-based image guidance at 11 institutions (Table 3).
Table 3:
Summary of error reduction strategies
| Error reduction strategies (33 institutions) | ||||
| Bony structure-based | Prostate-based | Mixed | ||
| Number of institutions | 16 | 11 | 6 | |
| Prostate-based IGRT approaches (11 institutions) | ||||
| MV-CT-based | kV-CT-based | Implanted marker-based | US-based | |
| Number of institutions | 3 | 2 | 3 | 3 |
IGRT, image-guided radiotherapy; MV-CT, megavoltage computed tomography; kV-CT, kilovoltage computed tomography; US, ultrasound.
Treatment planning
In principle, the clinical target volume (CTV) at all institutions consisted of the prostate with or without some or all seminal vesicles. The margins that were added to the CTV to create the planning target volume (PTV) are summarized in Table 4. Thirty-two institutions three-dimensionally added margins; only one institution reported two-dimensional margins. When looking at the margins to create the PTV from the CTV according to error reduction strategies, the mean values of the added margins were smallest with the prostate-based IGRT approach and largest with the bony structure-based error reduction strategy (Table 5).
Table 4.
Summary of the CTV to PTV margins at 33 institutions
| Margin size range | R–L (lateral) (mm) | Ventral (mm) | Dorsal (mm) | Cranial (mm) | Caudal (mm) |
|---|---|---|---|---|---|
| Maximum | 10 | 10 | 7 | 10 | 10 |
| Minimum | 5 | 5 | 3 | 5 | 5 |
| Average | 7.7 | 8.0 | 5.3 | 8.0 | 8.1 |
R–L, right/left.
Table 5.
Summary of the margins used to create the PTV from the CTV according to error reduction strategies
| Margin | Bony structure-based (mm) | Mixed (mm) | Prostate-based (mm) |
|---|---|---|---|
| Mean PTV margin, except posteriorly | 9.2 | 7.1 | 6.5 |
| Mean PTV margin, posteriorly | 5.8 | 5.3 | 4.8 |
CTV, clinical target volume; PTV, planning target volume.
Dose-prescription policies of IMRT plans were based on one of four different measurement categories: isocenter (center of the prostate or CTV) (@isocenter); D95 (the percentage of the prescribed dose covering 95% of the volume) of the PTV or the PTV minus the organs at risk (D95@PTV); mean dose or multiple constraints of the PTV (Mean@PTV); and D95, D100 or mean dose of the CTV (@CTV) (Table 6). Prescribed doses ranged from 66 Gy (in 3-Gy fractions) to 80 Gy (in 2-Gy fractions), with a mean dose of 75.4 Gy. Most institutions (n = 30) adopted a conventional fraction size of 2 Gy (Table 6).
Table 6.
Dose-prescription methods and prescribed doses
| Prescription policy | @isocenter | D95@PTV | Mean@PTV | @CTV |
|---|---|---|---|---|
| Number of institutions | 8 | 13 | 7 | 5 |
| Prescribed dose (Gy) | ≤70 | >70 and ≤74 | >74 and ≤78 | >78 |
| Number of institutions | 3 | 16 | 25 | 1 |
| Fraction size (Gy) | 2 | 2.08 − 2.2 | 3 | |
| Number of institutions | 30 | 2 | 1 | |
Dose-volume statistics of five typical cases
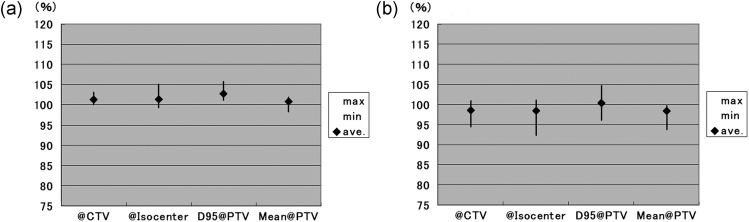
Dose statistics for the CTV and PTV in five randomly selected typical cases treated at each institution are indicated in Table 7. Large variations were observed for each dose characteristic for both the CTV and PTV. Dose-volume data for the CTVs with the different dose-prescription policies are presented in Fig. 2 and Table 8. The mean dose of the CTV was significantly higher with the D95@PTV policy than with the @isocenter (P = 0.045) or Mean@PTV (P = 0.027) policies.
Table 7.
Dose statistics of the CTV and PTV at 33 institutions
| CTV | D95 (%) | Mean dose (%) | Maximum dose (%) | |
|---|---|---|---|---|
| Minimum | 92.5 | 98.3 | 101.6 | |
| Maximum | 104.6 | 105.7 | 115.0 | |
| Mean | 99.2 | 101.8 | 105.6 | |
| PTV | D95 (%) | Mean dose (%) | Maximum dose (%) | V90 (%) |
| Minimum | 77.0 | 97.4 | 101.6 | 89.0 |
| Maximum | 101.6 | 104.8 | 116.0 | 101.1 |
| Mean | 95.2 | 100.8 | 106.1 | 98.6 |
CTV, clinical target volume; PTV, planning target volume; D95, the percentage of the prescribed dose covering 95% of the volume; V90, the percentage of the volume covered by 90% of the prescribed dose.
Figure 2.
Mean dose (a) and D95 (b) of CTV according to different dose-prescription policies.
Error bar indicates the range of each data point.
Table 8.
Differences in mean delivery dose among four major dose-prescription policies
| Policy | Mean dose (%) of CTV | Mean dose (%) of PTV | D95 (%) of PTV | V90 (%) of PTV |
|---|---|---|---|---|
| @isocenter | 101.4 | 100.2 | 92.9 | 97.2 |
| @CTV | 101.4 | 99.7 | 91.8 | 98.0 |
| Mean@PTV | 100.7 | 99.6 | 94.3 | 98.6 |
| D95@PTV | 102.8* | 102.3** | 98.6** | 100.2* |
*Significantly higher than those of @isocenter and Mean@PTV; **significantly higher than all others.
CTV, clinical target volume; PTV, planning target volume; D95, the percentage of the prescribed dose covering 95% of the volume; V90, the percentage of the volume covered by 90% of the prescribed dose.
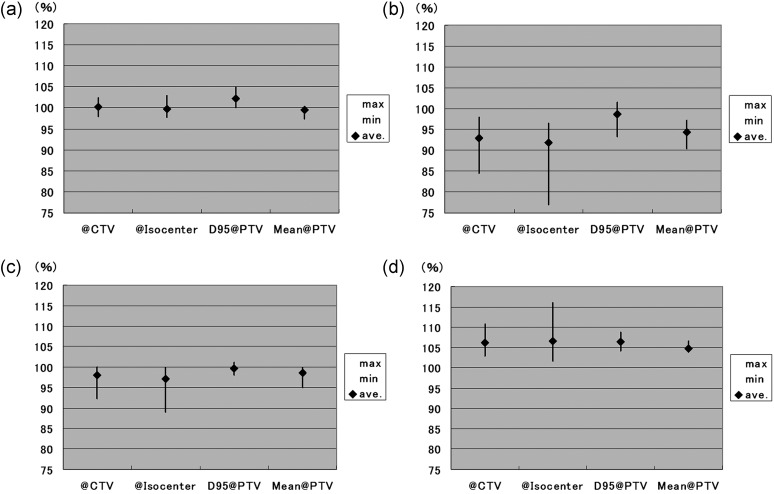
Dose-volume data of the PTV according to the different dose-prescription policies are shown in Fig. 3 and Table 8. The mean dose of the PTV was significantly higher with the D95@PTV policy than with any other prescription policy, with P values of 0.016, 0.0053 and 0.0004 for the @CTV, @isocenter, and Mean@PTV policies, respectively. D95 of the PTV was also significantly higher with the D95@PTV policy than with any other policy (P values of 0.076, 0.0023 and 0.0062 for the @CTV, @isocenter and Mean@PTV policies, respectively). In addition, the percentage of the volume covered by 90% of the prescribed dose (V90) of the PTV was significantly higher with the D95@PTV policy than with the @isocenter (P = 0.045) or Mean@PTV (P = 0.041) policy.
Figure 3.
Mean dose (a), D95 (b), V90 (c) and maximum dose (d) of PTV according to the different dose-prescription policies.
Error bar indicates the range of each data point.
DISCUSSION
Since its first clinical application at Chiba Cancer Center in 2000, IMRT has slowly become widespread in Japan, mainly because of the shortage of medical specialists in radiation oncology. The widespread adoption of IMRT occurred more rapidly in the late 2000s, as the Japanese government approved national health insurance coverage for IMRT in April 2008. At that time, coverage was limited to a definitive application for prostate, CNS, and head and neck tumors. However, insurance coverage for IMRT was extended to all types of localized solid cancers in April 2010.
According to the current survey, more than 5000 patients with localized prostate cancer had been treated with IMRT by the end of 2009. The actual number of prostate cancer patients that have been treated with IMRT in Japan is almost certainly much higher, as not all institutions responded to this survey and some institutions that have implemented prostate IMRT do not belong to the Japan Conformal External-beam Radiotherapy Group.
The majority of the institutions applied IMRT to patients with localized or locally advanced prostate cancer throughout the whole treatment period. Although the radiation therapy equipment and treatment planning systems, treatment devices and X-ray energy varied among institutions, most of the institutions implemented conventional MLC-based IMRT with 6- or 10-MV X-rays.
With respect to the error reduction strategies, about half of the institutions conducted IMRT with a bony structure-based approach, but a prostate-based IGRT approach was implemented at a significant number of institutions. The PTV margins were largest at the institutions using bony structure-based approaches, smallest at the institutions using prostate-based approaches and somewhere in the middle at the institutions using a mixed approach. However, the adequacy of the PTV margins for the corresponding error reduction strategies should be carefully validated through clinical outcomes. For example, Engels reported that by reducing the PTV margin from 10 mm (6 mm in the lateral direction) with bony structure-based correction to 5 mm (3 mm in the lateral direction) with prostate-based IGRT (using implanted markers in 3D-CRT) the 5-year biochemical failure-free survival rate decreased significantly, from 91% to 58% [10], even though the margins applied were theoretically adequate with reference to the International Commission on Radiation Units and Measurements (ICRU) definition of the PTV [11].
Four major types of dose-prescription policies were identified in our survey results: @isocenter, D95@PTV, Mean@PTV and @CTV. In addition, there was considerable variation among institutions and dose-prescription policies with respect to both the dose coverage of the PTV and the actual dose delivered to the target (mean doses for the CTV and PTV). It is expected that these differences would have a significant impact on clinical outcomes. Therefore, in addition to the prescribed dose value, detailed dosimetric data should be reported in order to properly evaluate outcomes in the future [12, 13]. Among the four dose-prescription policies, non-negligible low-dose and high-dose areas within the PTV were formed readily from dose-prescription data for both the @CTV and @isocenter policies. Therefore, these prescription policies should probably be replaced by D95@PTV or Mean@PTV policies in the future.
Although D95@PTV achieved the best PTV coverage among the four dose-prescription policies, there are several issues that should be addressed. First, although the dose was prescribed according to D95 of the PTV and therefore D95 should have been 100%, D95 of the PTV was not always 100%, but rather slightly lower (mean value: 98.6%) in actual clinical plans. This most likely occurred because most of the institutions placed higher priority on the dose constraints of organs at risk (OAR) over the target dose in order to reduce the risk for late radiation toxicity. Second, even though the PTV coverage was compromised, the D95@PTV policy still resulted in significant dose escalations of 102.8% (range: 101.2–105.7%) for the CTV and 102.3% (range: 100.1–104.8%) for the PTV. It is notable that at one institution, the mean doses delivered to the targets were 5–6% higher than the nominal dose. This potential dose escalation should be taken into account for dose-prescriptions with the D95@PTV policy and outcome evaluation in the future.
The Mean@PTV policy seemed to be well balanced in terms of the target and OAR doses, although V90 was generally lower than that with the D95@PTV policy. ICRU Report 83 recommends that the median absorbed dose, specified by D50%, should be reported because it is considered to correspond best with the previously defined dose at the ICRU reference point [14]. Report 83 also describes that the median (D50%) and mean absorbed doses are nearly identical because a typical differential dose-volume histogram for the PTV is often symmetric and unimodal [14]. Dose-volume data suggested that the Mean@PTV policy was reasonable in terms of avoiding excess dose escalation and avoiding non-negligible low-dose and high-dose areas as detected in plans made under @CTV and @isocenter policies. Nevertheless, it would be necessary to put adequate dose constraints on the PTV to avoid the appearance of an excessive number of low-dose areas within the PTV.
The current survey is helpful for creating treatment planning protocols for multi-institutional studies of prostate IMRT in the future. Creating plans of uniform quality among different institutions is difficult, even when the planning goals are indicated [15]. In the present study, although there was wide variation among the optimization parameters, even when using the same planning system, the resulting PTV doses were similar. However, there was considerable variation in the dose distribution of OARs such as the rectum, bladder and femoral heads. Therefore, it would be necessary to conduct cold runs and discuss a common planning policy to ensure planning homogeneity among institutions joining a specific study.
In conclusion, we established the current status of prostate IMRT in Japan and showed that there is considerable variation in the dose-prescription policy of IMRT for prostate cancer. The D95@PTV dose-prescription policy resulted in significant dose escalation at the target. @CTV and @isocenter policies may have to be replaced by other policies because of the risk of excessively low-dose and high-dose regions within the PTV. The nominal dose is an inadequate representation of the actual dose delivered to the target. Detailed dosimetric statistical evaluations of the dose-volume data are needed to interpret clinical outcomes and to create new planning protocols for prostate IMRT.
FUNDING
This study was supported by a Grant-in-aid for research on radiation oncology, from JASTRO, 2009–2010.
ACKNOWLEDGEMENTS
The authors are deeply grateful to the following institutions that participated in this survey: Aichi Cancer Center Hospital and Research Institute, Asahikawa Medical University, Ise Municipal General Hospital, Edogawa Hospital, NTT Osaka Hospital, Osaka Medical Center for Cancer and Cardiovascular Diseases, Japanese Red Cross Otsu Hospital, Okinawa Prefectural Nannbu Medical Center & Children's Medical Center, Cancer Institute Hospital, Kizawa Memorial Hospital, Kyushu University Hospital, Kyoto University Hospital, Kinki University Hospital, Kyorin University Hospital, Kurashiki Central Hospital, Tokyo Metropolitan Cancer and Infectious Diseases Center Komagome Hospital, Saitama Medical University International Medical Center, Saitama Prefectural Cancer Center, Shikoku Cancer Center, Nagasaki Prefecture Shimabara Hospital, Juntendo University Hospital, Tsuruoka Municipal Shonai Hospital, Institute of Biomedical Research and Innovation Hospital, Chiba Cancer Center, Tenri Hospital, Tokyo Women's Medical University Hospital, The University of Tokyo Hospital, Tohoku University Hospital, Tochigi Cancer Center, Nagoya City University Hospital, Hirosaki University School of Medicine and Hospital, Fukui Prefectural Hospital, and Yokohama City University Hospital.
This work was presented at the 24th Annual Meeting of the Japanese Society for Therapeutic Radiology and Oncology, 17–19 November 2011, Tokyo, Japan.
REFERENCES
- 1.Burman C, Chui CS, Kutcher G, et al. Planning, delivery, and quality assurance of intensity-modulated radiotherapy using dynamic multileaf collimator: a strategy for large-scale implementation for the treatment of carcinoma of the prostate. Int J Radiat Oncol, Biol, Phys. 1997;39:863–73. doi: 10.1016/s0360-3016(97)00458-6. [DOI] [PubMed] [Google Scholar]
- 2.Kavanagh BD, Schefter TE, Wu Q, et al. Clinical application of intensity-modulated radiotherapy for locally advanced cervical cancer. Sem Radiat Oncol. 2002;12:260–71. doi: 10.1053/srao.2002.32471. [DOI] [PubMed] [Google Scholar]
- 3.Chao KS, Low DA, Perez CA, et al. Intensity-modulated radiation therapy in head and neck cancers: The Mallinckrodt experience. Int J Cancer. 2000;90:92–103. doi: 10.1002/(sici)1097-0215(20000420)90:2<92::aid-ijc5>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 4.Eisbruch A. Clinical aspects of IMRT for head-and-neck cancer. Med Dosim. 2002;27:99–104. doi: 10.1016/s0958-3947(02)00091-2. [DOI] [PubMed] [Google Scholar]
- 5.Zagar TM, Willett CG, Czito BG. Intensity-modulated radiation therapy for anal cancer: Toxicity versus outcomes. Oncology. 2010;24 815–23, 828. [PubMed] [Google Scholar]
- 6.Hatano K, Araki H, Sakai M, et al. Current status of intensity-modulated radiation therapy (IMRT) International Journal of Clinical Oncology/Japan Society of Clinical Oncology. 2007;12:408–15. doi: 10.1007/s10147-007-0703-9. [DOI] [PubMed] [Google Scholar]
- 7.Bortfeld T. IMRT: A review and preview. Physics in Medicine and Biology. 2006;51:R363–79. doi: 10.1088/0031-9155/51/13/R21. [DOI] [PubMed] [Google Scholar]
- 8.Veldeman L, Madani I, Hulstaert F, et al. Evidence behind use of intensity-modulated radiotherapy: A systematic review of comparative clinical studies. Lancet Oncol. 2008;9:367–75. doi: 10.1016/S1470-2045(08)70098-6. [DOI] [PubMed] [Google Scholar]
- 9.Cahlon O, Hunt M, Zelefsky MJ. Intensity-modulated radiation therapy: Supportive data for prostate cancer. Sem Radiat Oncol. 2008;18:48–57. doi: 10.1016/j.semradonc.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Engels B, Soete G, Verellen D, et al. Conformal arc radiotherapy for prostate cancer: Increased biochemical failure in patients with distended rectum on the planning computed tomogram despite image guidance by implanted markers. Int J Radiat Oncol Biol Phys. 2009;74:388–91. doi: 10.1016/j.ijrobp.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 11.Skarsgard D, Cadman P, El-Gayed A, et al. Planning target volume margins for prostate radiotherapy using daily electronic portal imaging and implanted fiducial markers. Radiat Oncol. 2010;5:52. doi: 10.1186/1748-717X-5-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Norihisa Y, Mizowaki T, Takayama K, et al. Detailed dosimetric evaluation of intensity-modulated radiation therapy plans created for stage C prostate cancer based on a planning protocol. Int J Clin Oncol. 2011 doi: 10.1007/s10147-011-0324-1. in press. [DOI] [PubMed] [Google Scholar]
- 13.Zhu S, Mizowaki T, Nagata Y, et al. Comparison of three radiotherapy treatment planning protocols of definitive external-beam radiation for localized prostate cancer. Int J Clin Oncol. 2005;10:398–404. doi: 10.1007/s10147-005-0519-4. [DOI] [PubMed] [Google Scholar]
- 14.DeLuca P, Jones D, Gahbauer R, et al. Prescribing, recording, and reporting photon-beam intensity-modulated radiation therapy (IMRT) J ICRU. 2010;10:1–106. Report 83. [Google Scholar]
- 15.Skala M, Holloway L, Bailey M, et al. Australia-wide comparison of intensity modulated radiation therapy prostate plans. Australas Radiol. 2005;49:222–9. doi: 10.1111/j.1440-1673.2005.01419.x. [DOI] [PubMed] [Google Scholar]