Abstract
This study was undertaken to assess the diagnostic value of 2-[18F]-fluoro-2-deoxy-D-glucose positron emission tomography with computed tomography ([18F]-FDG-PET/CT) in the detection of radiation toxicity in normal bone marrow using Tibet minipigs as a model. Eighteen Tibet minipigs were caged in aseptic rooms and randomly divided into six groups. Five groups (n = 3/group) were irradiated with single doses of 2, 5, 8, 11 and 14 Gy of total body irradiation (TBI) using an 8-MV X-ray linear accelerator. These pigs were evaluated with [18F]-FDG-PET/CT, and their marrow nucleated cells were counted. The data were initially collected at 6, 24 and 72 h after treatment and were then collected on Days 5–60 post-TBI at 5-day intervals. At 24 and 72 h post-TBI, marrow standardized uptake value (SUV) data showed a dose-dependent decrease in the radiation dose range from 2–8 Gy. Upon long-term observation, SUV and marrow nucleated cell number in the 11-Gy and 14-Gy groups showed a continuous and marked reduction throughout the entire time course, while Kaplan–Meier curves of survival showed low survival. In contrast, the SUVs in the 2-, 5- and 8-Gy groups showed early transient increases followed by a decline from approximately 72 h through Days 5–15 and then normalized or maintained low levels through the endpoint; marrow nucleated cell number and survival curves showed approximately the same trend and higher survival, respectively. Our findings suggest that [18F]-FDG-PET/CT may be helpful in quickly assessing the absorbed doses and predicting the prognosis in patients.
Keywords: [18F]-FDG-PET/CT, total body irradiation, bone marrow, Tibet minipigs
INTRODUCTION
The hematopoietic system is considered to be the system that is most sensitive to radiation exposure due to its rapid differentiation, proliferation and cellular cycle [1]. Accidental or therapeutic radiation exposure can lead to hematopoietic suppression, and the severity of this suppression is dose dependent [2]. When the body is exposed to a dose of 6 Gy, the resultant reduction in leukocytes and platelets may lead to infection and hemorrhage, respectively, and death can occur within 30 days of exposure. Exposure to high doses (≥10.4 Gy) of penetrating radiation over a short period of time increases an individual's morbidity and mortality, and may result in irreversible hematopoietic and gastrointestinal injury [3].
The absorbed radiation dose can be determined at any time by analyzing the electron spin resonance of tooth enamel or with conventional thermoluminescent dosimeters [4]. However, simply determining the absorbed dose is not sufficient to guide the diagnosis, determine the prognosis and decide on a therapeutic approach. Instead, according to the guidelines published by the Strategic National Stockpile Radiation Working Group in 2004 [5], a sensitive, timely and accurate assay to determine the severity of the radiation dosage effect on or the damage sustained by the critical organ systems is essential for determining an appropriate medical intervention [6]. The current clinical monitoring approaches, such as symptom observation (e.g. nausea and/or vomiting), peripheral lymphocyte count dynamics and scoring of unstable chromosomal-type aberrations in mitogen-stimulated peripheral blood cannot correlate damage with the functional capacity of critical organ systems or the general health status of the individual [7, 8]. Therefore, safe, fast and sensitive diagnostic methods to assess radiation-induced organ damage are urgently needed for patients subjected to different doses of radiation exposure.
2-[18F]-Fluoro-2-deoxy-D-glucose positron emission tomography with computed tomography ([18F]-FDG-PET/CT, henceforth FDG-PET/CT) has been well-established in tumor diagnosis and treatment response monitoring. Furthermore, its application in an increasing number of non-tumor disorders (e.g. autoimmune and inflammatory disorders, fever of undetermined origin and chronic osteomyelitis) has been a topic of interest for some time [9–12]. There have been a few reports examining the application of FDG-PET/CT in radiation-induced hematopoietic injury [13]. Therefore, we used a range of indices, such as marrow FDG uptake and bone marrow-nucleated cell number, to investigate the diagnostic value of FDG-PET/CT in radiation-induced hematopoietic injury.
MATERIALS AND METHODS
Experimental design and radiation protocol
A total of 18 adult male (8–15 months) Tibet minipigs were used for total body irradiation (TBI) (purchased from the Laboratory Animal Center of Southern Medical University of China). The average weight and height of the pigs were 22.36 ± 7.74 kg and 82.88 ± 9.13 cm, respectively. The pigs were caged in aseptic rooms and were randomly divided into six groups. They were anesthetized with ketamine (0.05 ml/kg i.v.) before radiation exposure. One control group (n = 3) was not exposed to radiation. Five treatment groups (n = 3 for each group) were irradiated with single doses of 2, 5, 8, 11 or 14 Gy TBI using an 8-MV X-ray linear accelerator (Elekta Synergy Platform, ELEKTA Ltd, Sweden) at a dose rate of 255 cGy/min for all experimental groups. The linear accelerator was balanced by a CRS-3D tank system (MED-TEC, USA) before radiation. The radiation field was calculated as 2ab/a + b (a: length, b: width). The source–axis distance was 100 cm, and the source–surface distance (SSD) was 85 cm (the thickness of the minipigs was presumed to be 30 cm). The conversion formula for the given dose and the machine output was as follows: Dm = DT/TPR × SADF × Sc.P (TPR: tissue phantom ratio; SADF: source–axis distance factor; Sc,P: total scatter calibration factor; Dm: monitor unity (MU) dose; DT: tumor dose). The standardized uptake value (SUV) and the marrow nucleated cell number data were initially collected at 6, 24 and 72 h and were then collected on Days 5–60 post-TBI at 5-day intervals. The experimental protocol and ethical considerations for using the animals were reviewed and approved by the Institutional Animal Care and Use Committee of the Southern Medical University of China.
FDG-PET/CT scan
The minipigs were scanned with FDG-PET/CT (Discovery-LS PET/CT, GE, USA). All of these images and data were processed with a Xeleris workstation system (GE, USA). The quantitation of FDG uptake was analyzed based on regions of interest (ROIs). Based on the ROIs placed on the femurs (vertical section), the FDG uptake was calculated as a percentage of the injected dose of radioactivity (% ID). The normalization and correction of the data were automatically completed by a computer and presented in terms of the SUV. The color of the picture was determined based on the SUV and represented by a color bar. The following measures were obtained to ensure the objectivity and accuracy of the SUVs and imaging: (i) we injected FDG tracer at a fixed dose range (0.11–0.13 millicurie/kg/pig) at 5, 23 and 71 h after TBI; (ii) all pigs were fasted for at least 8 h before each PET/CT scan; (iii) the bone marrow of each pig was scanned in three neighboring layers, and the maximum SUV in each layer was collected for the statistical analysis; and (iv) the minipigs were scanned 1 h after FDG injection.
Marrow nucleated cell numbering
The 18 minipigs were prepared for marrow nucleated cell numbering. Three marrow specimens (0.5 ml) collected from the left femurs (irradiated and non-irradiated) were examined per minipig. A 15% ethylenediamine tetraacetic acid-K2 solution (20 μl) was added to the marrow samples (0.5 ml) to prevent marrow coagulation. Then, the anticoagulated marrow (20 μl) was added to 0.1 Mol/l hydrochloric acid (0.38 ml) and homogenized. Finally, the mixture was slowly infused into a cell numbering chamber, and the number of nucleated cells per square was counted.
Statistical analyses
Data are presented as the mean ± standard deviation (SD) as indicated. The repeated measures ANOVA test was used for the statistical analysis of SUV and marrow nucleated cell numbers. The simple-effect was analyzed while the P value of the crossover effect was less than 0.05. Post hoc multiple comparisons within each group were measured with the least significant difference (LSD) test or the Games–Howell test as determined by a homogeneity of variance test. P values less than 0.05 were considered to be statistically significant (two-tailed). The survival rate was analyzed based on Kaplan–Meier curves. All data were processed by Statistical Product and Service Solutions 13.0 (SPSS 13.0) software.
RESULTS
Survival test
The Kaplan–Meier survival curves (Fig. 1) show that 100% of the control animals and those in the 2- and 5-Gy TBI groups were alive after 1 month compared with only 10% (1/9) in the 8-, 11- and 14-Gy groups. After 2 months, the minipigs in the control, 2- and 5-Gy groups were still alive (6/6) whereas all the pigs in the high dose groups had died.
Fig. 1.
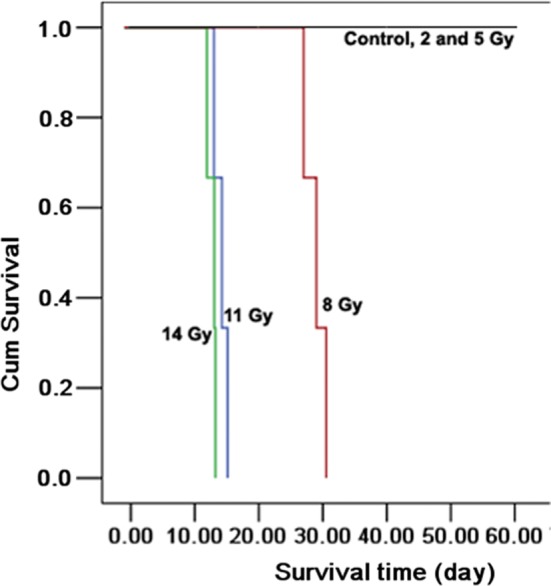
The survival rate for different radiation doses. According to the results, deaths in the 8-, 11- and 14-Gy groups (100%) were greater than those observed in other groups (0%) within 60 days. Differently colored lines indicate the different radiation doses. The same survival rate in the control, 2- and 5-Gy groups leads to a coincidence of the three lines and visually appears as one black line in Fig. 1.
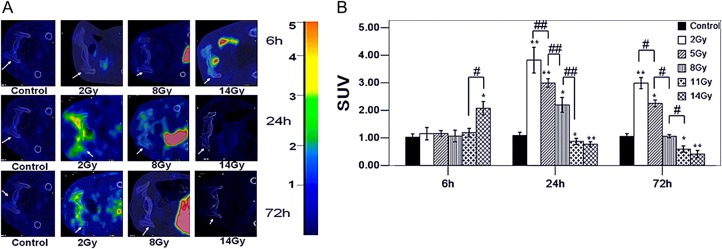
FDG uptake with different radiation doses at 6, 24 and 72 h post-TBI
The factors of radiation dose and dosage time-point had significant effects on marrow SUV (Fig. 2A and B). The P values of crossover effect, main effect of dose and time were less than 0.05. In terms of the radiation dose, at 6 h, the SUVs in the 2-Gy (1.16 ± 0.22), 5-Gy (1.16 ± 0.11), 8-Gy (1.07 ± 0.21) and 11-Gy (1.19 ± 0.16) groups showed no significant differences from the control group (1.02 ± 0.12) (2, 5, 8- and 11 Gy vs. control, all P values > 0.05), while the SUVs in the 14-Gy (2.08 ± 0.25) group were higher than those in the control group (14 Gy vs. control, P < 0.05). At 24 h post-TBI, the SUVs in the 2-Gy (4.22 ± 0.40), 5-Gy (3.73 ± 0.29) and 8-Gy (2.12 ± 0.23) groups were higher than those in the control group (1.09 ± 0.11) (2, 5 and 8 Gy vs. control, all P values < 0.05), whereas the SUVs in the 11-Gy (0.62 ± 0.10) and 14-Gy (0.72 ± 0.08) groups were lower than those in the control group (11 Gy and 14 Gy vs. control, all P values < 0.05). Furthermore, the SUVs between the 2–11-Gy groups were significantly different and decreased with increased radiation doses (2 Gy vs. 5 Gy, 5 Gy vs. 8 Gy and 8 Gy vs. 11 Gy, all P values < 0.01). However, there was no significant difference between the 11-Gy group and the 14-Gy group (11 Gy vs. 14 Gy, P > 0.05). At 72 h post-TBI, the SUVs in the 2-Gy (2.99 ± 0.20) and 5-Gy (2.25 ± 0.13) groups were higher than those in the control group (1.06 ± 0.10) (2 Gy and 5 Gy vs. control group, all P values < 0.05), whereas the SUVs in the 11-Gy (0.60 ± 0.11) and 14-Gy (0.41 ± 0.13) groups were lower than those in the control group (11 Gy and 14 Gy vs. control group, all P values < 0.05). However, there was no significant difference in the SUVs between the 11-Gy and 14-Gy groups (11 Gy vs. 14 Gy, P > 0.05).
Fig. 2.
(A) Shows FDG-PET/CT images of the bone marrow at 6, 24 and 72 h after TBI. The right-side color scale indicates FDG uptake and is determined by SUV, and the scale of brown to blue represents SUVs from 5 to <1. The white arrows indicate the femur (vertical section). A large contrast of FDG uptake in these irradiated groups is shown. (B) shows the trend of the marrow SUVs based on the radiation doses at 6, 24 and 72 h post-TBI. Error bars indicate the SD. Differently patterned bars indicate different observation times.* P < 0.05, ** P < 0.01 (versus control); # P < 0.05, ## P < 0.01 (versus each other, analyzed by post hoc multiple comparisons).
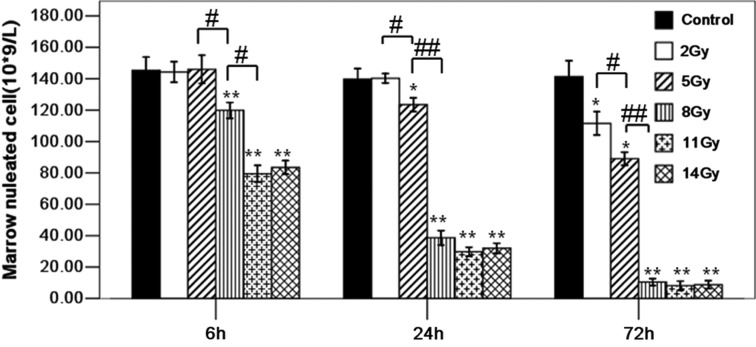
Marrow nucleated cell number with different doses at 6, 24 and 72 h post-TBI
As with the SUVs, the radiation dose and dosage time-point differences had significant effects on the marrow nucleated cell number. The P values of the crossover effect, main effect of radiation dose and time were less than 0.05 (Fig. 3). At 6 h post-TBI, the marrow nucleated cell numbers in the 2-Gy (144.27 ± 6.60) and 5-Gy (145.94 ± 9.02) groups showed no significant differences from those in the control group (145.33 ± 8.41) (2 and 5 Gy vs. control, P > 0.05) and those in the 11-Gy (79.44 ± 5.29) and 14-Gy (83.49 ± 4.29) groups were lower than those in the control group (11 Gy and 14 Gy vs. control, P < 0.05). There was no significant difference in the marrow nucleated cell number between the 11-Gy and 14-Gy groups (11 Gy vs. 14 Gy, P < 0.05). At 24 h post-TBI, there was no significant difference in the marrow nucleated cell number between the control group (139.67 ± 6.71) and the 2-Gy group (140.28 ± 2.94) (control vs. 2 Gy, P > 0.05). The marrow nucleated cell numbers in the 5-Gy (123.47 ± 4.38), 8-Gy (38.67 ± 4.82), 11-Gy (29.80 ± 2.93) and 14-Gy (32.01 ± 3.23) groups were lower than those in the control group (5, 8, 11 and 14 Gy vs. control, P < 0.05). However, the marrow nucleated cell number showed no differences between the 8-, 11- and 14-Gy groups (8 Gy vs. 11 Gy, 11 Gy vs. 14 Gy, P > 0.05). At 72 h post-TBI, the marrow nucleated cell number in the 2-Gy (111.52 ± 7.42), 5-Gy (89.04 ± 4.16), 8-Gy (10.47 ± 2.14), 11-Gy (8.16 ± 2.88) and 14-Gy (8.48 ± 2.71) groups were lower than those in the control group (141.32 ± 10.15) (2, 5, 8, 11 and 14 Gy vs. control, all P values < 0.05). The marrow nucleated cell number sequentially decreased in the dose range from 2 to 8 Gy (2 Gy vs. 5 Gy and 5 Gy vs. 8 Gy, P < 0.05). However, there was no difference between the 8-, 11- and 14-Gy groups (8 Gy vs. 11 Gy, 11 Gy vs. 14 Gy, P > 0.05) with regard to the marrow nucleated cell number.
Fig. 3.
Marrow nucleated cell number with different doses and time points. Differently patterned bars indicate different radiation doses and observation times. *P < 0.05, **P < 0.01 (versus control); #P < 0.05, ##P < 0.01 (versus each other, analyzed by post hoc multiple comparisons).
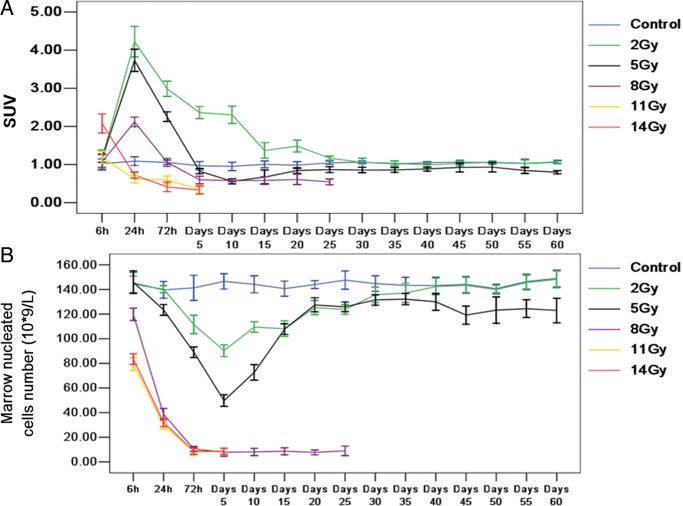
The long-term observation of FDG uptake and marrow nucleated cell number within 60 days
The long-term observation of FDG uptake and marrow nucleated cell number were analyzed with data collected every 5 days until Day 60 after TBI (Fig. 4A and B). The SUVs in the 2-Gy group significantly increased at 24 h and gradually returned to control levels on Day 25 (1.07 ± 0.15); the SUVs then stayed at control levels until Day 60 post-TBI (1.06 ± 0.03). The SUVs in the 5-Gy group increased at 24 h, gradually decreased below control levels on Day 10 (0.56 ± 0.07), gradually returned to control levels on Day 20 (0.80 ± 0.12) and then stayed at control levels until Day 60 (0.90 ± 0.34). The SUVs in the 8-Gy group increased at 24 h, gradually decreased below the control level until Day 5 (0.60 ± 0.10) and then stayed at a low level until Day 25 (0.55 ± 0.07). The SUVs in the 11-Gy and 14-Gy groups decreased with time until the endpoints. The marrow nucleated cell numbers in the 8-, 11- and 14-Gy groups showed marked and continuous reductions starting around Day 5 through to the endpoint, whereas those in the 2-Gy and 5-Gy groups decreased progressively from 72 h to Day 5 and then returned to control levels around Day 10 through to Day 60 post-TBI.
Fig. 4.
A and B show the trends of the SUVs and marrow nucleated cell numbers over the entire time course. Error bars indicate the SD. Differently colored lines indicate different radiation doses.
DISCUSSION
The initial question that motivated our study was whether molecular imaging with [18F]-FDG-PET/CT would prove useful in diagnosing radiation-induced hematopoietic injury. It is important to note that before our study, McGuire et al. [14] used 3′-deoxy-3′-[18F]fluorothymidine PET/CT to image bone marrow response to radiation dose. In contrast to that study, our approach was based on the use of a Food and Drug Administration-approved PET probe, [18F]-FDG, which is routinely used in clinical practice.
[18F]-FDG-PET/CT May be used to quickly assess the radiation dose in the dose range of 2–8 Gy
This study clearly demonstrated that the factors of radiation dose and time significantly affected marrow FDG uptake. Our results in this experimental model showed that TBI of normal marrow with a single dose of 2, 5, 8, 11 or 14 Gy caused considerable changes in FDG uptake in the early period after irradiation (≤72 h). Furthermore, our study also sequentially observed FDG uptake on the time course from 6 h to Day 60 after various doses of TBI. Thus, for the first time, the time-point and radiation dose factors were analyzed comprehensively to assess the diagnostic value of [18F]-FDG-PET/CT in radiation-induced hematopoietic toxicity.
The marrow FDG uptake exhibited markedly different patterns at 6, 24 and 72 h post-TBI. First, based on the relative levels of marrow FDG uptake, FDG levels only increased in the 14-Gy group at 6 h post-TBI. Although a previous study proved that chemoradiation therapy itself did not change FDG uptake of bone marrow, at least initially [15], our data revealed that high-dose radiation exposure caused enhanced FDG uptake of bone marrow at 6 h post-TBI. Second, FDG uptake transiently increased in the 2-, 5- and 8-Gy groups, which is partly consistent with the prior observation that normal marrow uptake of FDG transiently increased after external beam irradiation [16]. However, the dose-dependent decreases of FDG uptake at 24 and 72 h post-TBI and early significant decreases of FDG uptake at the 24 and 72 h post-TBI time-points in high radiation doses (11 and 14 Gy) were not described in other reports. Third, although FDG also showed radiation dose-dependent decreases at 72 h post-TBI, there was no significant difference between the 8-Gy group and the control group. Therefore, marrow FDG uptake at the time-points prior to 24 h post-TBI could possibly be used to quickly assess the absorbed radiation dose in the dose range of 2–8 Gy.
However, our results also showed that the decrease of FDG uptake was saturated at higher radiation doses (>11 Gy) at 24 and 72 h post-TBI. Therefore, compared with other approaches for assessing radiation dose, FDG-PET/CT did not present an advantage in terms of the radiation dose range but did present an advantage in terms of diagnostic time. Some concerns may be raised, including the following: (i) in a population that has been involved in terrorism or a nuclear accident, PET/CT may not be a practical method for assessing the radiation doses due to the complexity of the process and time constraints; and (ii) other current approaches are better than PET/CT in terms of the diagnostic dose range, the diagnostic time and practicality [17–21]. In fact, the diagnostic value of PET/CT is not restricted by these factors.
FDG PET/CT has the potential for early prognostic assessment in hematopoietic radiation toxicity
Our results demonstrate that FDG-PET/CT imaging can also be used for the early prognostic assessment of hematopoietic consequences and overall survival. As demonstrated in the 18 minipigs that underwent long-term observation, the continuous and marked reductions of marrow FDG uptake over the entire time course in the 11- and 14-Gy groups were associated with low survival and severe irreversible hematopoietic suppression that could be confirmed based on the marrow total nucleated cell numbers and the survival analysis. The continuous and marked decrease of myeloproliferative activity in the 11- and 14-Gy groups is generally consistent with previous studies in humans; in those studies, a whole body exposure of ≥12–13 Gy induced irreversible hematopoietic suppression, gastrointestinal syndrome and 100% mortality within 10–15 days and there were no medical interventions that were able to halt or reverse the clinical effects [22–25]. Therefore, the abnormal decreases in FDG uptake in irradiated bone marrow in the early period post-TBI (24 and 72 h) suggest the life-threatening irreversible clinical course.
In contrast, the FDG uptake in minipigs exposed to 2 Gy and 5 Gy TBI transiently increased at 24 h post-TBI, declined approximately 72 h through Days 5–15 and then normalized. The FDG uptake in the 8-Gy group also transiently rose at 24 h post-TBI, then decreased and reached a nadir on Day 10 and stayed at a low level until the endpoint. Combining the results of survival observations and marrow cellularity changes, minipigs in these doses showed longer survival than those in the 11- Gy and 14-Gy groups, and marrow nucleated cell numbers also showed approximately the same trend as FDG uptake from Day 5 to the endpoint (Day 60 for the 2-Gy and 5-Gy groups and Day 30 for the 8-Gy group). In our study, the LD50/30 value in the Tibet minipigs seems to be approximately 5–8 Gy. This result is similar to the findings of previous studies in humans in which hematopoietic syndrome can occur after TBI at a dose of 6 Gy and death can occur within 30 days of exposure [26]. In addition, several prior studies demonstrated that hematopoietic syndrome (2–8 Gy total-body dose) in humans is associated with lethality at 30 days and is defined by the radiation dose that can be rescued by hematopoietic stem cell transplantation (HSCT) [5, 24, 25]. Therefore, based on our results and those in the literature, the transient increase of FDG uptake at 24 h post-TBI indicates a better prognosis. In addition, this type of transient increase of FDG uptake in the 2-, 5- and 8 Gy groups at 24 h post-TBI also seems to suggest that victims at least have the chance of being successfully treated with HSCT, in contrast to the abnormal reduction in the 11- and 14-Gy groups, which have no successful medical interventions.
However, the marrow FDG uptake observed in the control group is seemingly inconsistent with the results of a previous study. Higashi et al.. [16] reported that the unirradiated marrow of rodents also showed a transient increase of FDG uptake on Day 18, and this phenomenon could increase the difficulty of FDG PET diagnosis or confuse the evaluation of the treatment effect if the FDG PET was performed as an early follow-up after irradiation. However, they did not explain the increased FDG uptake observed on Day 18 in the unirradiated marrow. In contrast to their results, our present work shows a stable level of FDG uptake throughout the entire time course in the control group. We believe that this disagreement may result from the experimental operation and other confounding factors. In general, physiological FDG uptake by the bone marrow is often mild and homogeneous. Factors such as blood glucose level, uptake time and the use of contrast agents during CT attenuation correction can affect FDG uptake [27]. In our study, the minipigs were caged in aseptic rooms, and these potential interfering factors were seriously controlled to ensure the objectivity of our results (described in the Materials and Methods section). Therefore, knowledge of this early radiation effect on bone marrow FDG uptake may be helpful for the early assessment of the prognosis of overall survival and the selection of an appropriate medical intervention after irradiation.
The mechanism of TBI-induced marrow FDG uptake change
We assume that the early transient increase in marrow FDG uptake in the 2-, 5- and 8-Gy groups is due to infiltration by inflammatory cells. Although our results showed that marrow nucleated cell number decreased at 24 h and 72 h after TBI, other studies have reported that the percentage of mature neutrophils observed in the irradiated marrow was 45% of the total marrow cells on Day 1, which is at least two-fold higher than that of the non-irradiated group [16]. Kallfass et al. and Vos et al. [28, 29] also reported inflammatory cell migration to irradiated regions within 8 h after irradiation; these cells disappeared from those regions within 2–10 days. Palestro et al. [30] reported that an increased uptake of 111In-labeled leukocytes was observed in the irradiated bone marrow 1 day after irradiation. Therefore, the early transient increase in marrow FDG uptake in the 2-, 5- and 8-Gy groups may be explained by the trade-off between the infiltration of the inflammatory cells and the relatively decreased cellularity.
The early reduction of marrow FDG uptake in the 11-Gy and 14-Gy groups can be explained by the above reason as well, although a loss of cellularity might be more important. Combining the results of the FDG uptake and the marrow nucleated cell numbers, FDG uptake seemed to be closely related to the number of nucleated cells. This finding is consistent with prior in vitro investigations that used cancer cells; these studies showed that FDG uptake was not strongly related to proliferative activity but was more closely related to the number of viable tumor cells [31]. Thus, the changes in marrow FDG uptake on Day 5 through to the endpoint can also be easily explained by the marrow nucleated cell number. First, the marked reduction of FDG uptake on Day 5 in the 11-Gy and 14-Gy groups was also accompanied by a severe overall cellular loss. Second, the marrow FDG uptake in the 2-, 5- and 8-Gy groups also showed approximately the same fluctuation as the marrow nucleated cell number on Day 5 through to the endpoint. Therefore, FDG uptake is at least partly correlated with the marrow nucleated cell number.
The potential uses of PET/CT in radiation-induced organ injury
In patients, a timely and accurate assessment of organ radiation toxicity and absorbed radiation dose, whether the exposure to ionizing radiation is accidental or therapeutic, would be helpful in designing an effective individual treatment plan. [18F]-PET/CT undoubtedly has some of these advantages. Although organ tolerances to guide radiation therapy planning are well established, organ dose response values have proven to be more elusive, partly due to the lack of accurate and specific approaches [14]. Therefore, our results in the minipig model suggest the potential uses of [18F]-FDG-PET/CT in the diagnosis of hematopoietic radiation toxicity. More generally, our results also suggest the use of PET/CT to evaluate other organs as a safe and noninvasive approach because multi-organ monitoring is the unique advantage of PET/CT compared with other approaches for radiation-induced injury [32]. Several PET/CT imaging studies on radiation-induced organ injury have been published during the last 10 years [33–35]. The diagnostic value of FDG-PET/CT in radiation-induced organ responses is receiving increasing attention by clinicians and radiologists. At our institution, the same techniques used in the present study have been employed to investigate the radiation-induced FDG uptake change in other organs using the Tibet minipig as a model. These results will be published soon. It is therefore likely that FDG-PET/CT could greatly expand our ability to noninvasively and quickly assess and monitor radiation-induced organ toxicity.
FUNDING
This study was supported by ‘The Third Phase Project of Chinese National 211 Leading Academic Discipline’ (Project Number: C1030380).
ACKNOWLEDGEMENTS
The authors would like to thank Dr Guang-Jin Lu for critical reading of the manuscript and Jin-Jun Xue for technical assistance with the irradiation protocol.
REFERENCES
- 1.Woodward WA, Strom EA, McNeese MD., et al. Cardiovascular death and second non-breast cancer malignancy after postmastectomy radiation and doxorubicin-based chemotherapy. Int J Radiat Oncol Biol Phys. 2003;57:327–35. doi: 10.1016/s0360-3016(03)00594-7. [DOI] [PubMed] [Google Scholar]
- 2.Dainiak N. Hematologic consequences of exposure to ionizing radiation. Exp Hematol. 2002;30:513–28. doi: 10.1016/s0301-472x(02)00802-0. [DOI] [PubMed] [Google Scholar]
- 3.Chambers KA, Harrington NP, Ross WM, et al. Relative alterations in blood mononuclear cell populations reflect radiation injury in mice. Cytometry. 1998;31:45–52. [PubMed] [Google Scholar]
- 4.Kirsch DG, Santiago PM, di Tomaso E, et al. P53 controls radiation-induced gastrointestinal syndrome in mice independent of apoptosis. Science. 2010;327:593–596. doi: 10.1126/science.1166202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sevan'kaev AV, Lloyd DC, Edwards AA, et al. Cytogenic investigations of serious overexposures to an industrial gamma radiography source. Radiat Prot Dosimetry. 2002;102:201–6. doi: 10.1093/oxfordjournals.rpd.a006090. [DOI] [PubMed] [Google Scholar]
- 6.Waselenko JK, MacVittie TJ, Blakely WF, et al. Medical management of the acute radiation syndrome: recommendations of the Strategic National Stockpile Radiation Working Group. Ann Intern Med. 2004;140:1037–1051. doi: 10.7326/0003-4819-140-12-200406150-00015. [DOI] [PubMed] [Google Scholar]
- 7.Donnelly EH, Nemhauser JB, Smith JM, et al. Acute radiation syndrome: assessment and management. South Med J. 2010;103:541–6. doi: 10.1097/SMJ.0b013e3181ddd571. [DOI] [PubMed] [Google Scholar]
- 8.Egami MI, Segreto C, Kerbauy J, et al. Effects of whole-body X-irradiation on the peripheral blood of primate Cebus apella. Braz J Med Biol Res. 1991;122:271–4. [PubMed] [Google Scholar]
- 9.Feyer PC, Maranzano E, Molassiotis A, et al. Radiotherapy-induced nausea and vomiting (RINV): MASCC/ESMO guideline for antiemetics in radiotherapy: update 2009. Support Care Cancer. 2011;19(Suppl 1):5–14. doi: 10.1007/s00520-010-0950-6. [DOI] [PubMed] [Google Scholar]
- 10.Radu CG, Shu CJ, Shelly SM, et al. Positron emission tomography with computed tomography imaging of neuroinflammation in experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2007;104:1937–42. doi: 10.1073/pnas.0610544104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng MF, Wu YW, Liu KL, et al. Diagnostic value of 18F-FDG-PET/CT in indeterminate infiltrative hepatic lesions in an endemic area of viral hepatitis. Nucl Med Commun. 2011;32:252–9. doi: 10.1097/MNM.0b013e32834368bf. [DOI] [PubMed] [Google Scholar]
- 12.Tian J, Yang X, Yu L, et al. A multicenter clinical trial on the diagnostic value of dual-tracer PET/CT in pulmonary lesions using 3'-deoxy-3'-18F-fluorothymidine and 18F-FDG. J Nucl Med. 2008;49:186–194. doi: 10.2967/jnumed.107.044966. [DOI] [PubMed] [Google Scholar]
- 13.Hartmann A, Eid K, Dora C, et al. Diagnostic value of 18F-FDG PET/CT in trauma patients with suspected chronic osteomyelitis. Eur J Nucl Med Mol Imaging. 2007;34:704–14. doi: 10.1007/s00259-006-0290-4. [DOI] [PubMed] [Google Scholar]
- 14.Kesner AL, Lau VK, Speiser M, et al. Time-course of effects of external beam radiation on [18F]FDG uptake in healthy tissue and bone marrow. J Appl Clin Med Phys. 2008;9:2747. doi: 10.1120/jacmp.v9i3.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGuire SM, Menda Y, Boles Ponto LL, et al. 3'-deoxy-3'-[(1)F]fluorothymidine PET quantification of bone marrow response to radiation dose. Int J Radiat Oncol Biol Phys. 2011;81:888–93. doi: 10.1016/j.ijrobp.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sugawara Y, Fisher SJ, Zasadny KR, et al. Preclinical and clinical studies of bone marrow uptake of fluorine-1-fluorodeoxyglucose with or without granulocyte colony-stimulating factor during chemotherapy. J Clin Oncol. 1998;16:173–80. doi: 10.1200/JCO.1998.16.1.173. [DOI] [PubMed] [Google Scholar]
- 17.Higashi T, Fisher SJ, Brown RS, et al. Evaluation of the early effect of local irradiation on normal rodent bone marrow metabolism using FDG: preclinical PET studies. J Nucl Med. 2000;41:2026–35. [PubMed] [Google Scholar]
- 18.Pinto MM, Santos NF, Amaral A. Current status of biodosimetry based on standard cytogenetic methods. Radiat Environ Biophys. 2010;49:567–581. doi: 10.1007/s00411-010-0311-3. [DOI] [PubMed] [Google Scholar]
- 19.Liu Q, Cao J, Liu Y, et al. Follow-up study by chromosome aberration analysis and micronucleus assays in victims accidentally exposed to 60Co radiation. Health Phys. 2010;98:885–8. doi: 10.1097/HP.0b013e3181c4b9c1. [DOI] [PubMed] [Google Scholar]
- 20.Hsu WH, Braby LA, Reece WD. Detection system built from commercial integrated circuits for real-time measurement of radiation dose and quality using the variance method. Radiat Prot Dosimetry. 2008;128:5–11. doi: 10.1093/rpd/ncm231. [DOI] [PubMed] [Google Scholar]
- 21.Goldberg Z, Rocke DM, Schwietert C, et al. Human in vivo dose-response to controlled, low-dose low linear energy transfer ionizing radiation exposure. Clin Cancer Res. 2006;12:3723–9. doi: 10.1158/1078-0432.CCR-05-2625. [DOI] [PubMed] [Google Scholar]
- 22.Siegel JA, Yeldell D, Goldenberg DM, et al. Red marrow radiation dose adjustment using plasma FLT3-L cytokine levels: improved correlations between hematologic toxicity and bone marrow dose for radioimmunotherapy patients. J Nucl Med. 2003;44:67–76. [PubMed] [Google Scholar]
- 23.Bhanja P, Saha S, Kabarriti R, et al. Protective role of R-spondin1, an intestinal stem cell growth factor, against radiation-induced gastrointestinal syndrome in mice. PLOS ONE. 2009;4:1–10. doi: 10.1371/journal.pone.0008014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hempelmann LH, Lisco H, Hoffman JG, et al. The acute radiation syndrome: a study of nine cases and a review of the problem. Ann Intern Med. 1952;36:279–510. doi: 10.7326/0003-4819-36-2-279. [DOI] [PubMed] [Google Scholar]
- 25.Stone HB, Moulder JE, Coleman CN, et al. Models for evaluating agents intended for the prophylaxis, mitigation and treatment of radiation injuries. Report of an NCI Workshop, December 3–4. Radiat Res. 2004;162:711–28. doi: 10.1667/rr3276. [DOI] [PubMed] [Google Scholar]
- 26.Dainiak N, Waselenko JK, Armitage JO, et al. The hematologist and radiation casualties. Hematology Am Soc Hematol Educ Program. 2003;11:473–96. doi: 10.1182/asheducation-2003.1.473. [DOI] [PubMed] [Google Scholar]
- 27.Mettler FA, Jr, Voelz GL. Major radiation exposure—what to expect and how to respond. N Engl J Med. 2002;346:1554–61. doi: 10.1056/NEJMra000365. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Ghesani NV, Zuckier LS. Physiology and pathophysiology of incidental findings detected on FDG-PET scintigraphy. Semin Nucl Med. 2010;40:294–315. doi: 10.1053/j.semnuclmed.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 29.Kallfass E, Kramling HJ, Schultz-Hector S. Early inflammatory reaction of the rabbit coeliac artery wall after combined intraoperative (IORT) and external (ERT) irradiation. Radiother Oncol. 1996;39:167–78. doi: 10.1016/0167-8140(96)01708-2. [DOI] [PubMed] [Google Scholar]
- 30.Vos J, Aarnoudse MW, Dijk F, et al. On the cellular origin and development of atheromatous plaques. A light and electron microscopic study of combined X-ray and hypercholesterolemia-induced atheromatosis in the carotid artery of the rabbit. Virchows Arch B Cell Pathol Incl Mol Pathol. 1983;43:1–16. doi: 10.1007/BF02932938. [DOI] [PubMed] [Google Scholar]
- 31.Palestro CJ, Kim CK, Vega A, et al. Acute effects of radiation therapy on indium-111-labeled leukocyte uptake in bone marrow. J Nucl Med. 1989;30:1889–91. [PubMed] [Google Scholar]
- 32.Higashi K, Clavo AC, Wahl RL. Does FDG uptake measure proliferative activity of human cancer cells? In vitro comparison with DNA flow cytometry and tritiated thymidine uptake. J Nucl Med. 1993;34:414–19. [PubMed] [Google Scholar]
- 33.Kapoor V, McCook BM, Torok FS. An introduction to PET-CT imaging. Radiographics. 2004;24:523–43. doi: 10.1148/rg.242025724. [DOI] [PubMed] [Google Scholar]
- 34.Antoch G, Kaiser GM, Mueller AB, et al. Intraoperative radiation therapy in liver tissue in a pig model: monitoring with dual-modality PET/CT. Radiology. 2004;230:753–760. doi: 10.1148/radiol.2303021183. [DOI] [PubMed] [Google Scholar]
- 35.Ford EC, Herman J, Yorke E, et al. 18F-FDG PET/CT for image-guided and intensity-modulated radiotherapy. J Nucl Med. 2009;50:1655–65. doi: 10.2967/jnumed.108.055780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carson KJ, Young VA, Cosgrove VP, et al. Personnel radiation dose considerations in the use of an integrated PET-CT scanner for radiotherapy treatment planning. Br J Radiol. 2009;82:946–9. doi: 10.1259/bjr/73200201. [DOI] [PubMed] [Google Scholar]