Abstract
A combination of four-dimensional computed tomography with 18F-fluorodeoxyglucose positron emission tomography (4D CT-FDG PET) was used to delineate gross tumor volume (GTV) in esophageal cancer (EC). Eighteen patients with EC were prospectively enrolled. Using 4D images taken during the respiratory cycle, the average CT image phase was fused with the average FDG PET phase in order to analyze the optimal standardized uptake values (SUV) or threshold. PET-based GTV (GTVPET) was determined with eight different threshold methods using the auto-contouring function on the PET workstation. The difference in volume ratio (VR) and conformality index (CI) between GTVPET and CT-based GTV (GTVCT) was investigated. The image sets via automatic co-registrations of 4D CT-FDG PET were available for 12 patients with 13 GTVCT values. The decision coefficient (R2) of tumor length difference at the threshold levels of SUV 2.5, SUV 20% and SUV 25% were 0.79, 0.65 and 0.54, respectively. The mean volume of GTVCT was 29.41 ± 19.14 ml. The mean VR ranged from 0.30 to 1.48. The optimal VR of 0.98, close to 1, was at SUV 20% or SUV 2.5. The mean CI ranged from 0.28 to 0.58. The best CI was at SUV 20% (0.58) or SUV 2.5 (0.57). The auto-contouring function of the SUV threshold has the potential to assist in contouring the GTV. The SUV threshold setting of SUV 20% or SUV 2.5 achieves the optimal correlation of tumor length, VR, and CI using 4D-PET/CT images.
Keywords: FDG PET/CT, gross tumor volume, radiotherapy, esophageal cancer
INTRODUCTION
The use of 18fluoro-deoxyglucose positron emission tomography (18F-FDG PET) supplements the interdisciplinary process of radiotherapy (RT) by including information on the biological status of tumors, which is complementary to conventional computed tomography (CT) images and may change the tumor volume delineation [1]. RT is an important part of the multidisciplinary approach to treating esophageal cancer (EC), but tumor control and overall survival do not always improve [2]. 18F-FDG PET has been shown to improve the staging of EC [3, 4]. Several studies suggest that overlaying PET images on CT images has some impact on the definition of the gross tumor volume (GTV), decreases inter-observer variability and, thus, changes treatment planning [5–7]. However, when radiation oncologists contour the GTVs on a fused PET and CT image or an integrated PET/CT image, the problem of setting an appropriate threshold for the PET arises.
Because several investigations have found that auto-contouring or manual contouring of PET-based tumor volume results in a change of the GTV compared with CT-based GTV [8–10], standards should be set for PET/CT-based tumor delineation. The published methods are based on a threshold determined by the percentage of the maximal standardized uptake value (SUVmax) using values ranging from 15 to 50% for non-small cell lung cancer (NSCLC) [11–13]. Great variation has been found in the validated standardized methods for EC in setting this threshold [6, 14–16]; these include using mean activity in the liver plus various standard deviations, the various absolute standardized uptake values (SUV) (GTV = SUV of ≥2) or using percentages of the SUVmax (GTV = volume encompassed by ≥25% the SUVmax). During a free breathing cycle, organ or tumor motion always influences the accuracy and quality of CT images in thoracic malignancies including EC. The extent of tumor motion and different spatial tumor positions should be carefully considered when using four-dimensional (4D) CT as described in NSCLC [17, 18]. A recent study reported that EC moved substantially during the respiratory cycle, especially in the cranial–caudal direction of the lower third portion of the esophagus [19]. Although the real benefits of clinical outcomes need further investigation, respiratory 4D-PET/CT techniques are highly useful in targeting volume definitions, which are accurately representative of organ and lesion motion [20]. The feasibility of implementing 4D-PET/CT in determining the GTV for EC is still unknown. Thus, there is a need to perform a pilot study using 4D-PET/CT for contouring.
We hypothesized that some standards could be obtained when defining GTV for EC using the biological target volume from 4D-PET/CT images. We performed this prospective study to evaluate the feasibility of 4D-PET/CT simulations in RT planning for EC. Additionally, the appropriateness of the percentage threshold method was investigated to determine the best volumetric match between PET-based and CT-based GTV when contouring the primary tumor volume of EC.
MATERIALS AND METHODS
Patients
This study was a prospective analysis, approved by the local institutional review board (DMR98-IRB-171), of 4D-PET/CT in RT planning of EC. Patients with histologically approved EC who were scheduled to undergo definitive RT, concurrent chemoradiotherapy or radical surgery, were eligible for this study. Eighteen patients with esophageal squamous cell cancer were enrolled between December 2009 and January 2011. The image data from 12 patients with 13 GTVCT were available for analysis in this study. The median age was 48.5 years (range 38–76 years). All patients were male. Table 1 presents the characteristics of these patients.
Table 1.
Patient characteristics
| Characteristic | Patients (n) |
|---|---|
| Tumor locationa | |
| Upper-middle | 2 |
| Middle | 3 |
| Lower | 8 |
| Clinical stageb | |
| Tumor stage | |
| T1 | 1 |
| T2 | 1 |
| T3 | 10 |
| T4 | 1 |
| Nodal stage | |
| N0 | 3 |
| N1 | 10 |
| Metastasis stage | |
| M0 | 10 |
| Mx | 2c |
| Endoscopic ultrasonography | 9 (75%) |
| CT based tumor length (cm) | 1.75–10.00 (median 5.5) |
| Mean | 5.73 ± 2.40 |
| CT based tumor volume (cm3) | 3.65–70.76 (median 24.95) |
| Mean | 29.41 ± 19.14 |
| SUVmaxd | 13.26 ± 2.78 (median 13.2) |
a One patient with two separate tumors at middle and lower third.
b AJCC cancer staging, 6th, 2002.
c Two patients had small individual hypermetabolic lesions at the left lower lung.
d SUVmax: maximal of standardized uptake value.
PET-CT image acquisition
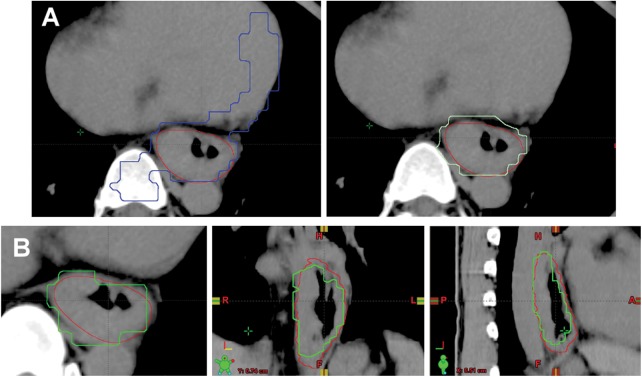
All patients were asked to fast for at least 4 h before 18F-FDG PET/CT imaging. Each of them received 370 MBq (10 mCi) of 18F-FDG intravenously 40 min before scanning and rested in a supine position in a quiet and dimly lit room. All images were acquired with an integrated PET/CT scanner (Discovery STE, GE Medical Systems, Milwaukee, WI, USA). The patient's arms were elevated above their head. First, whole-body PET/CT images were taken according to the standardized protocol. The CT images were reconstructed onto a 512 × 512 matrix and converted to a 128 × 128 matrix, with 511-keV-equivalent attenuation factors for attenuation correction of the corresponding PET emission images. Immediately after finishing the whole-body PET/CT images, patients were repositioned and placed in a simulated RT planning position using the Real-time Position Management (RPM) system respiratory gating hardware (Varian Medical Systems Inc., Palo Alto, CA, USA). 4D-CT images with 2.50-mm slice thickness, and 4D-PET images with two table positions, 7 min per position, were acquired. The respiration cycle was divided into 10 phases. All CT images were automatically sorted using 4D software (Advantage 4D, GE Healthcare). The images were transferred from the PET/CT workstation via DICOM3 to the RTP (Eclipse version 8.6, Varian Medical Systems Inc.) for GTV delineation. All phases of CT images and PET images were automatically fused for this gating study. PET/CT-based GTV of the primary tumor (GTVPET) was defined by the auto-contouring function at the AW workstation (Advantage SimTM 7.6.0, GE Healthcare), either by applying the isodensity volumes and adjusting the different percentages to the maximum threshold levels, or by simply using a fixed value of SUV. The threshold strategies for assessing the optimized SUV for GTV contouring were derived from the results of other studies [6, 7, 15, 21]. Eight different threshold methods were used in this study. They were SUV 15%, SUV 2, SUV 2.5, SUV 20%, SUV 25%, SUV30 %, SUV 40% and SUV 50%. The length of the GTVPET provided by the auto-contouring function was not changed at all. All the artifacts within the GTVPET, including the areas overlaid by the heart, bone and great vessels, were excluded manually in the RTP system (Fig. 1).
Fig. 1.
(A) An example of modification of autocontouring gross tumor volume (GTV) on a standard uptake value of 15% (SUV 15%); (B) Comparison of the GTV on CT (in red) and GTV on SUV 20% (in green) in axial, coronal, and sagittal views.
(A) The red contour represents GTV delineated with computed tomography (CT) and endoscopic ultrasound. The blue contour is the original GTV on SUV 15%. The corrected contour in light green is the blue contour with artifacts, adjacent bone and heart subtracted.
CT-based GTV definition
The temporal resolution of PET is an average of several respiratory cycles. In contrast to helical CT, the temporal resolution of averaged CT (ACT) is comparable with that of PET. Furthermore, Chi et al. [22] demonstrated that respiration artifacts in PET from PET/CT can be minimized using ACT, and ACT is temporally and spatially consistent with PET. On the basis of axial ACT images, contouring of the tumor volume and critical structures was performed without knowing the PET results in an effort to decrease bias. Information about the tumor extent from the contrast CT scan, panendoscopy and endoscopic ultrasonography (EUS) was used when delineating the GTVCT. Excluding the adjacent metastatic lymph nodes, the volume of primary tumors (GTVCT) was contoured as a reference tumor volume. To reduce inter-observer variations, at least two different radiation oncologists carried out the contouring of the tumors for each patient.
Conformality index and volume ratio comparison
After completion of the GTVCT contouring in the RTP system, the radiation oncologists reviewed the consistency of PET/CT images with nuclear medicine physicians. The volume of GTVCT and GTVPET was compared using the conformality index (CI) [23] and volume ratio (VR). The CI is the ratio of the volume of intersection of two volumes (A ∩ B) compared with the volume of union of the two volumes (A ∪ B) under comparison (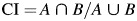 ) [21, 24]. Volume ratio is the ratio of two volumes, and the denominator is the volume of GTVCT. A suitable threshold level could be defined when GTVPET was observed to be the best fit of the length, CI, or VR from the GTVCT.
) [21, 24]. Volume ratio is the ratio of two volumes, and the denominator is the volume of GTVCT. A suitable threshold level could be defined when GTVPET was observed to be the best fit of the length, CI, or VR from the GTVCT.
Statistical analysis
All statistical tests were performed using SPSS 15 (SPSS, Chicago, IL, USA), and each GTV was analyzed by one-way ANOVA with Scheffe's post hoc test. P-values of 0.05 or less were considered statistically significant. Pearson's correlation was performed to assess the correlation between tumor length evaluated by GTVCT with that by GTVPET. Possible values for the decision coefficients (R2) range from 0 to 1, with values closer to 1 indicating a regression line with high correlation.
RESULTS
Of the 18 patients, automatic co-registrations of 4D-PET/CT were successful in 13 tumors from 12 patients. In six patients, the fused images were not available for analysis. One had a small T1 tumor, which was undetectable on PET scans. The SUVmax was 2.96 in another patient with a T1 lesion, and therefore, this was not suitable for further analysis. Because of the different table positions between 4D-PET and 4D-CT, fusion failure occurred in the first patient enrolled in this study. The other two patients excluded had irregular respiratory rhythms, which caused a failure in the image fusion. Diffuse lung and bone metastatic status caused another patient to be excluded. The auto-contouring function for GTVPET was insufficient for primary GTV delineation. Table 1 summarizes the characteristics of the 13 patients with successful automatic co-registrations of 4D-PET/CT. For all of these patients, the histological type was squamous cell carcinoma. The median age was 48.5 years (range, 38–76 years). Eleven lesions (85%) were either T3 or T4 stage. EUS was performed on nine patients (75%). The mean length of GTVCT was 5.73 ± 2.40 cm (range, 1.75–10.00 cm). The mean volume of GTVCT was 29.41 ± 19.14 ml (range, 3.65–70.76 ml). The mean SUVmax was 13.26 ± 2.78 (range, 9.4–16.9).
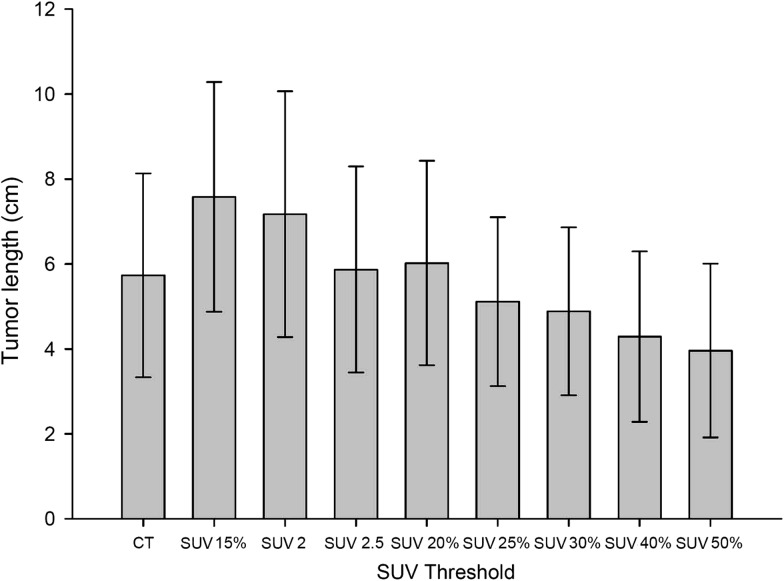
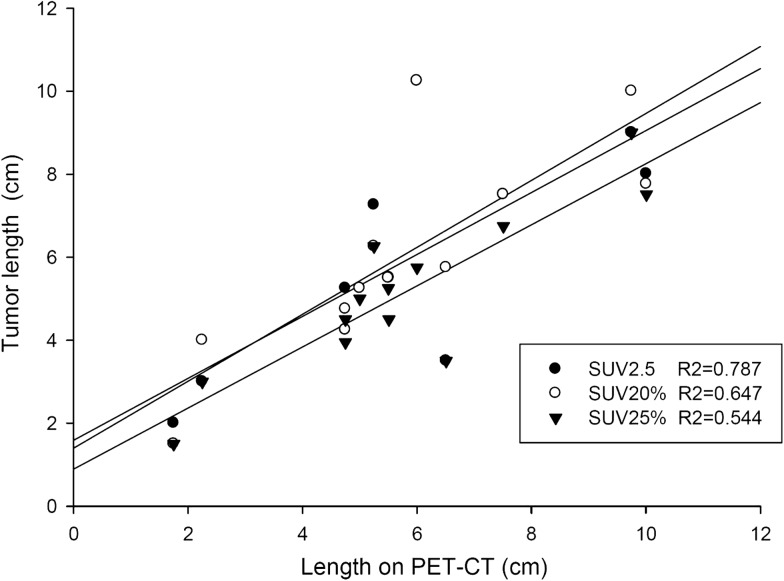
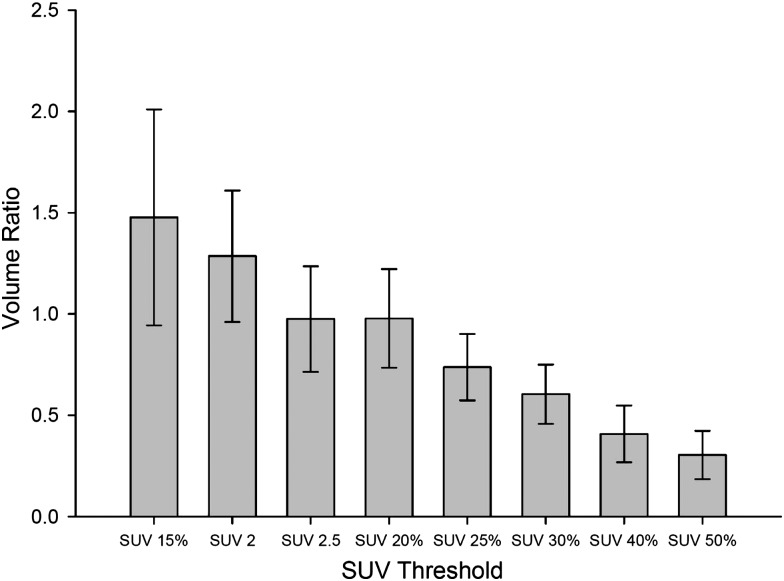
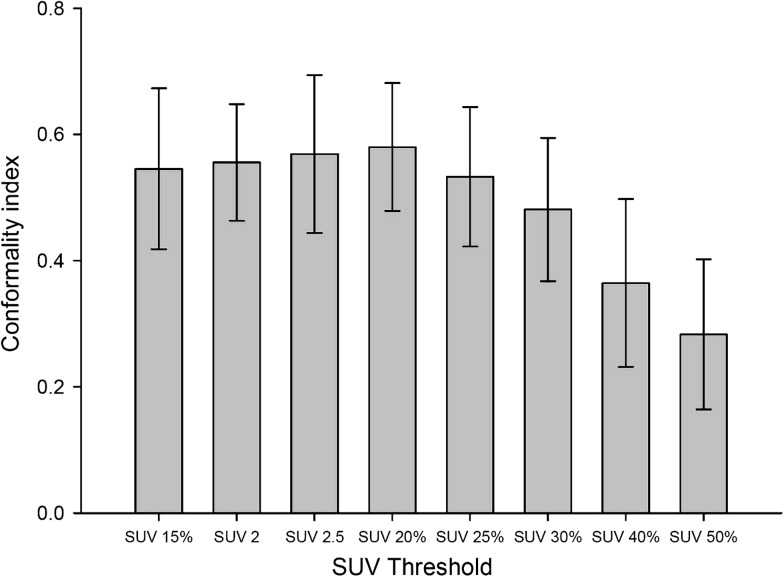
Figure 2 shows the results of the mean tumor lengths on CT (CTlength) and the mean tumor length by different SUV thresholds (PETlength). Figure 3 illustrates the correlation of CTlength compared with PETlength at SUV 2.5, SUV 20% and SUV 25%. The decision coefficients (R2) of tumor length difference at the threshold levels of SUV 2.5, SUV 20% and SUV 25% were 0.79, 0.65, and 0.54, respectively. The mean VR ranged from 0.30 to 1.48 (0.86 ± 0.24) (F = 29.34, P < 0.001). Figure 4 shows the mean VR at different SUV threshold levels. The VR values gradually decreased from SUV 15% to SUV 50%. The best fit for VR was found at SUV 20% (mean SUV 2.65 ± 0.56) and SUV 2.5, which achieved the optimal VR values of 0.98 ± 0.24 and 0.98 ± 0.26, respectively. The VR values at SUV 40% (0.41 ± 0.14) or SUV 50% (0.30 ± 0.12) were not at an ideal threshold level for GTV contouring of EC. The mean CIs ranged from 0.29 to 0.58 (F = 11.34, P < 0.001). Figure 5 displays the mean CI at different SUV threshold levels. The best fit for CI was at SUV 20% (0.58 ± 0.10) or SUV 2.5 (0.57 ± 0.13).
Fig. 2.
Mean tumor length as delineated on average computed tomography (CT) and on positron emission tomography (PET) obtained by eight standard uptake values (SUV) of interest. SUVn = SUV of n; SUV n% = n% of maximum SUV. Error bars indicate standard deviation.
Fig. 3.
Relationship between tumor length on average computed tomography (CT) and on positron emission tomography (PET) of standard uptake value (SUV) 2.5, SUV 20% and SUV 25%.
Fig. 4.
Mean volume ratios of eight SUVs of interest. Error bars indicate standard deviation. All SUV thresholds are compared with SUV 2.5, the P values of SUV 15%, SUV 40%, and SUV 50% are 0.005, 0.001 and 0.000, respectively.
Fig. 5.
Mean conformality index of eight standard uptake values (SUV) of interest. Error bars indicate standard deviation. All SUV thresholds are compared with SUV 20%; the P values of SUV 40% and SUV 50% are 0.004 and 0.000, respectively.
DISCUSSION
This work was a pilot study to investigate the feasibility of 4D-PET/CT when contouring the GTV for EC. By comparing eight different threshold levels, the results revealed that GTVPET using a threshold setting of SUV 20% or SUV 2.5 correlated well with tumor length, VR and CI of GTVCT. The results were comparable with previous studies that investigated the optimal contouring threshold using non-respiratory controlled PET or PET/CT [5, 7, 15, 21]. Zhong et al. [15] found the FDG GTVPET at SUV 2.5 provided the closest estimation of gross tumor length in EC. Han et al. [7] investigated the combined 18F-fluorothymidine (FLT) and FDG PET/CT in assessing the feasibility of GTV delineation in EC and found a threshold setting of SUV 1.4 on FLT PET/CT, and SUV 2.5 on FDG PET/CT correlated well with the pathologic GTV length. When using a threshold level of SUV 40%, the PET-based tumor length was estimated to be smaller than the pathological length [7, 15]. Similarly, on the basis of the results of our study, an SUV threshold setting of ≤30% is not sufficient for auto-contouring.
Panendoscopy, EUS, and CT images were usually used to obtain information regarding the precise tumor location and volume to optimize the GTV contouring for EC. However, neither the panendoscope nor EUS could pass through the obstructive lumen in locally advanced tumors. In this situation, the actual location of the distal margin of the tumor should be assessed using the CT image only. However, the precise tumor length and extent are not always discernible because a CT scan might not visualize mucosal or submucosal lesions. Konski et al. [5] reported that the EUS finding correlated better with PET-based tumor length than with CT scans in a series of 25 patients. The International Atomic Energy Agency expert group concluded that 18F-FDG PET/CT provides the best available imaging for accurate target delineation in RT planning [25]. With the implementation of PET/CT, the risk of geographic miss, underdosing and normal tissue complications may be decreased [9]. Certainly, the use of FDG-PET/CT for GTV delineation in RT planning should be validated on the basis of locoregional control and survival in the future [26].
4D-CT in target volume delineation and motion has been well studied in NSCLC, including by fractionated RT and stereotactic RT [17, 18], but there have been few investigations of esophageal motility during RT. Dieleman et al. [27] reported a retrospective study analyzing 29 patients with nonesophageal cancer, mostly stage 1 lung cancer, using 4D-CT. They suggested that the distal esophagus had the largest motion margins of 9 mm in the mediolateral direction and 8 mm in the dorsoventral direction. Generally, patients undergoing PET are in a free breathing state and are not holding their breath or using respiratory gating techniques, and thus, the PET image is an average obtained during several respiratory cycles. Therefore, FDG quantification, tumor margin definition and detection of smaller tumors can be improved by the application of respiratory gating or 4D-PET [23, 28]. Chi et al. [22] demonstrated that respiratory artifacts in PET from PET/CT could be minimized using ACT, and ACT was temporally and spatially consistent with PET. On the basis of this concept, we assumed that GTV contouring using an average phase of images on 4D-CT would minimize the impact of tumor motion when they were fused with PET.
Several studies have explored the use of a fixed or adaptive threshold setting for accurate measurement of tumor dimensions in thoracic cancer [7, 11, 21], but none of these studies reported comprehensive parameters such as the VR, CI or tumor length when compared with the GTVCT. It is likely that this can be attributed to the complexity of organ motion and the uncertainty of image registration. Hanna et al. [29] investigated the impact of PET/CT simulation for GTV definition in NSCLC using the concordance index (equivalent to CI in our study). The mean concordance index of their study was 0.64 and 0.57 in GTVPET-CT and GTVCT, respectively. This indicated a significant decrease in inter-observer variation by adding PET results to the GTV delineation. In another EC study, Vali et al. [21] compared GTVPET with GTVCT/EUS at the level of the esophageal tumor epicenter, and found that a threshold setting of SUV 2.5 and SUVL4σ (equal to SUV 2.4) resulted in the highest CI value (0.48 and 0.47, respectively). Gondi et al. [24] demonstrated that CI values of NSCLC and EC with the incorporation of FEG-PET and CT were 0.44 and 0.46, respectively. To the best of our knowledge, this is the first study that has compared CI values between GTVPET and GTVCT using 4D-PET/CT in contouring EC and using whole tumor volume. Our results were similar to the studies mentioned above. The CIs of our study implied an overlap of approximately 75% between the two volumes. The CI levels of SUV 40% or 50% were inferior to the other thresholds and were not ideal for auto-contouring the GTVPET. Furthermore, the VR was close to 1 at SUV 20% and SUV 2.5. It was better than the other studies with respect to the partial VR of EC [21] and the VR of NSCLC [11]. When using smaller cutoff values, such as SUV 2 or SUV 15%, for the threshold setting, more adjacent normal tissue would be included within the GTVPET. Thus, the primitive values of CI and VR were changed, but the values improved when some artifacts were corrected. However, these manual procedures were very time consuming.
Our results should be interpreted keeping three limitations in mind. First, the study was based on the comparison of GTVCT and GTVPET without knowledge of the pathological information on tumor length, axial extent or real volume. Certainly, the biological volume could not be definitivelyrelated to the real tumor volume. Similarly, the GTVCT could identify areas without tumor tissue. It seems difficult to perform a direct pathological comparison from the surgical specimen because use of a neoadjuvant or definitive concurrent chemoradiotherapy while treating EC is popular [2, 30]. Second, using the averaged phase of 4D-CT images fused with the averaged PETs to delineate the GTVs is not a flawless system. This approach might provide more accurate functional images supplemental to the CT without increasing the clinical overwork during contouring; however, the impact of tumor motion from maximum intensity projection should be investigated further. In addition, PET images have lower resolution than CT images, which might limit the results of this study. Third, the sample size of this study might lead to some uncertainties. Many more cases involving a variety of tumor sites are required to clarify the association between tumor location and 4D tumor motion [27].
This study demonstrated that 4D-PET/CT is an appropriate method of contouring the GTV in radiation planning for EC. The use of threshold levels of SUV 20% or SUV 2.5 achieves the optimal correlation with tumor length, VR and CI. To assess the final treatment outcome, the benefits of RT planning using 4D-PET/CT need more clinical investigations with a large sample size and different tumor locations.
ACKNOWLEDGEMENTS
This work was supported by study project grants (DMR-100-076 and DMR-101-061) from our institution as well as a grant from the Taiwan Department of Health, Cancer Research Centers for Excellence (DOH101-TD-C-111-005).
REFERENCES
- 1.Gregoire V, Haustermans K, Geets X, et al. PET-based treatment planning in radiotherapy: a new standard? J Nucl Med. 2007;48(Suppl 1):68S–77S. [PubMed] [Google Scholar]
- 2.Berger B, Belka C. Evidence-based radiation oncology oesophagus. Radiother Oncol. 2009;92:276–90. doi: 10.1016/j.radonc.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 3.van Westreenen H-L, Westerterp M, Bossuyt PM, et al. Systematic review of the staging performance of 18F-fluorodeoxyglucose positron emission tomography in esophageal cancer. J Clin Oncol. 2004;22:3805–12. doi: 10.1200/JCO.2004.01.083. [DOI] [PubMed] [Google Scholar]
- 4.Noble F, Bailey D, Tung K, et al. SWCIS Upper Gastrointestinal Tumour Panel. Impact of integrated PET/CT in the staging of oesophageal cancer: a UK population-based cohort study. Clin Radiol. 2009;64:699–705. doi: 10.1016/j.crad.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Konski A, Doss M, Milestone B, et al. The integration of 18-fluoro-deoxy-glucose positron emission tomography and endoscopic ultrasound in the treatment-planning process for esophageal carcinoma. Int J Radiat Oncol Biol Phys. 2005;61:1123–8. doi: 10.1016/j.ijrobp.2004.07.717. [DOI] [PubMed] [Google Scholar]
- 6.Yu W, Fu XL, Zhang YJ, et al. GTV spatial conformity between different delineation methods by 18FDG PET/CT and pathology in esophageal cancer. Radiother Oncol. 2009;93:441–6. doi: 10.1016/j.radonc.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Han D, Yu J, Yu Y, et al. Comparison of (18)F-fluorothymidine and (18)F-fluorodeoxyglucose PET/CT in delineating gross tumor volume by optimal threshold in patients with squamous cell carcinoma of thoracic esophagus. Int J Radiat Oncol Biol Phys. 2010;76:1235–41. doi: 10.1016/j.ijrobp.2009.07.1681. [DOI] [PubMed] [Google Scholar]
- 8.Hong T-S, Killoran JH, Mamede M, et al. Impact of manual and automated interpretation of fused PET/CT data on esophageal target definitions in radiation planning. Int J Radiat Oncol Biol Phys. 2008;72:1612–18. doi: 10.1016/j.ijrobp.2008.07.061. [DOI] [PubMed] [Google Scholar]
- 9.Muijs C-T, Schreurs LM, Busz DM, et al. Consequences of additional use of PET information for target volume delineation and radiotherapy dose distribution for esophageal cancer. Radiother Oncol. 2009;93:447–53. doi: 10.1016/j.radonc.2009.08.030. [DOI] [PubMed] [Google Scholar]
- 10.Moureau-Zabotto L, Touboul E, Lerouge D, et al. Impact of CT and 18F-deoxyglucose positron emission tomography image fusion for conformal radiotherapy in esophageal carcinoma. Int J Radiat Oncol Biol Phys. 2005;63:340–5. doi: 10.1016/j.ijrobp.2005.02.039. [DOI] [PubMed] [Google Scholar]
- 11.Wu K, Ung YC, Hornby J, et al. PET CT thresholds for radiotherapy target definition in non-small-cell lung cancer: how close are we to the pathologic findings? Int J Radiat Oncol Biol Phys. 2010;77:699–706. doi: 10.1016/j.ijrobp.2009.05.028. [DOI] [PubMed] [Google Scholar]
- 12.Biehl K-J, Kong FM, Dehdashti F, et al. 18F-FDG PET definition of gross tumor volume for radiotherapy of non-small cell lung cancer: is a single standardized uptake value threshold approach appropriate? J Nucl Med. 2006;47:1808–12. [PubMed] [Google Scholar]
- 13.Yu J, Li X, Xing L, et al. Comparison of tumor volumes as determined by pathologic examination and FDG-PET/CT images of non-small-cell lung cancer: a pilot study. Int J Radiat Oncol Biol Phys. 2009;75:1468–74. doi: 10.1016/j.ijrobp.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 14.Vesprini D, Ung Y, Dinniwell R, et al. Improving observer variability in target delineation for gastro-oesophageal cancer–the role of (18F)fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography. Clin Oncol (R Coll Radiol) 2008;20:631–8. doi: 10.1016/j.clon.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Zhong X, Yu J, Zhang B, et al. Using 18F-fluorodeoxyglucose positron emission tomography to estimate the length of gross tumor in patients with squamous cell carcinoma of the esophagus. Int J Radiat Oncol Biol Phys. 2009;73:136–41. doi: 10.1016/j.ijrobp.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 16.Mamede M, El Fakhri G, Abreu-e-Lima P, et al. Pre-operative estimation of esophageal tumor metabolic length in FDG-PET images with surgical pathology confirmation. Ann Nucl Med. 2007;21:553–62. doi: 10.1007/s12149-007-0040-0. [DOI] [PubMed] [Google Scholar]
- 17.Hof H, Rhein B, Haering P, et al. 4D-CT-based target volume definition in stereotactic radiotherapy of lung tumours: comparison with a conventional technique using individual margins. Radiother Oncol. 2009;93:419–23. doi: 10.1016/j.radonc.2009.08.040. [DOI] [PubMed] [Google Scholar]
- 18.Rietzel E, Liu AK, Doppke KP, et al. Design of 4D treatment planning target volumes. Int J Radiat Oncol Biol Phys. 2006;66:287–95. doi: 10.1016/j.ijrobp.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 19.Yamashita H, Kida S, Sakumi A, et al. Four-dimensional measurement of the displacement of internal fiducial markers during 320-multislice computed tomography scanning of thoracic esophageal cancer. Int J Radiat Oncol Biol Phys. 2011;79:588–95. doi: 10.1016/j.ijrobp.2010.03.045. [DOI] [PubMed] [Google Scholar]
- 20.Bettinardi V, Picchio M, Di Muzio N, et al. Detection and compensation of organ/lesion motion using 4D-PET/CT respiratory gated acquisition techniques. Radiother Oncol. 2010;96:311–16. doi: 10.1016/j.radonc.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 21.Vali F-S, Nagda S, Hall W, et al. Comparison of standardized uptake value-based positron emission tomography and computed tomography target volumes in esophageal cancer patients undergoing radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78:1057–63. doi: 10.1016/j.ijrobp.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 22.Chi P-C, Mawlawi O, Luo D, et al. Effects of respiration-averaged computed tomography on positron emission tomography/computed tomography quantification and its potential impact on gross tumor volume delineation. Int J Radiat Oncol Biol Phys. 2008;71:890–9. doi: 10.1016/j.ijrobp.2008.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia Vicente AM, Soriano Castrejon AM, Talavera Rubio MP, et al. (18)F-FDG PET-CT respiratory gating in characterization of pulmonary lesions: approximation towards clinical indications. Ann Nucl Med. 2010;24:207–14. doi: 10.1007/s12149-010-0345-2. [DOI] [PubMed] [Google Scholar]
- 24.Gondi V, Bradley K, Mehta M, et al. Impact of hybrid fluorodeoxyglucose positron-emission tomography/computed tomography on radiotherapy planning in esophageal and non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2007;67:187–95. doi: 10.1016/j.ijrobp.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 25.MacManus M, Nestle U, Rosenzweig KE, et al. Use of PET and PET/CT for radiation therapy planning: IAEA expert report 2006-2007. Radiother Oncol. 2009;91:85–94. doi: 10.1016/j.radonc.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 26.Muijs CT, Beukema JC, Pruim J, et al. A systematic review on the role of FDG-PET/CT in tumour delineation and radiotherapy planning in patients with esophageal cancer. Radiother Oncol. 2010;97:165–71. doi: 10.1016/j.radonc.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 27.Dieleman EM, Senan S, Vincent A, et al. Four-dimensional computed tomographic analysis of esophageal mobility during normal respiration. Int J Radiat Oncol Biol Phys. 2007;67:775–80. doi: 10.1016/j.ijrobp.2006.09.054. [DOI] [PubMed] [Google Scholar]
- 28.Lupi A, Zaroccolo M, Salgarello M, et al. The effect of 18F-FDG-PET/CT respiratory gating on detected metabolic activity in lung lesions. Ann Nucl Med. 2009;23:191–6. doi: 10.1007/s12149-008-0225-1. [DOI] [PubMed] [Google Scholar]
- 29.Hanna GG, McAleese J, Carson KJ, et al. (18)F-FDG PET-CT simulation for non-small-cell lung cancer: effect in patients already staged by PET-CT. Int J Radiat Oncol Biol Phys. 2010;77:24–30. doi: 10.1016/j.ijrobp.2009.04.045. [DOI] [PubMed] [Google Scholar]
- 30.Gebski V, Burmeister B, Smithers BM, et al. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis. Lancet Oncol. 2007;8:226–34. doi: 10.1016/S1470-2045(07)70039-6. [DOI] [PubMed] [Google Scholar]