Abstract
Previously, we demonstrated that Lactobacillus jensenii TL2937 attenuates the inflammatory response triggered by activation of Toll-like receptor 4 (TLR-4) in porcine intestinal epithelial cells. In view of the critical importance of antigen-presenting cell (APC) polarization in immunoregulation, the objective of the present study was to examine the effect of strain TL2937 on the activation patterns of APCs from swine Peyer's patches (PPs). We demonstrated that direct exposure of porcine APCs to L. jensenii in the absence of inflammatory signals increased expression of interleukin-10 (IL-10) and transforming growth factor β in CD172a+ APCs and caused them to display tolerogenic properties. In addition, pretreatment of CD172a+ APCs with L. jensenii resulted in differential modulation of the production of pro- and anti-inflammatory cytokines in response to TLR4 activation. The immunomodulatory effect of strain TL2937 was not related to a downregulation of TLR4 but was related to an upregulation of the expression of three negative regulators of TLRs: single immunoglobulin IL-1-related receptor (SIGIRR), A20, and interleukin-1 receptor-associated kinase M (IRAK-M). Our results also indicated that TLR2 has an important role in the anti-inflammatory activity of L. jensenii TL2937, since anti-TLR2 antibodies blocked the upregulation of SIGIRR and IRAK-M in CD172a+ APCs and the production of IL-10 in response to TLR4 activation. We performed, for the first time, a precise functional characterization of porcine APCs from PPs, and we demonstrated that CD172a+ cells were tolerogenic. Our findings demonstrate that adherent cells and isolated CD172a+ cells harvested from swine PPs were useful for in vitro study of the inflammatory responses in the porcine gut and the immunomodulatory effects of immunobiotic microorganisms.
The gut of vertebrates is rich in antigen-presenting cells (APCs), such as macrophages and dendritic cells (DCs). These APCs reside underneath the epithelial cell layer in an immature state and are prepared to recognize foreign antigens or invading pathogens (21). In addition, APCs in gut-associated lymphoid tissues (GALT) persist both in the subepithelial dome region and in the interfollicular regions of Peyer's patches (PPs). Under steady-state conditions, APCs, together with intestinal epithelial cells, create a tolerogenic environment in response to food antigens and commensal bacteria. However, in the presence of pathogenic microorganisms, APCs undergo a maturation process and the development of adaptive immune responses is initiated (21). These functions of intestinal APCs—specifically, to distinguish between the diverse elements of the intestinal flora and to respond to invading pathogens—are principally determined by pattern recognition receptors (PRRs). Toll-like receptors (TLRs) are an important class of PRRs in innate immunity and play a critical role in pathogen recognition and host defense. However, inappropriate TLR signaling can contribute to loss of tolerance and result in tissue injury (1, 18); for example, the inflammatory response triggered by the interaction between lipopolysaccharide (LPS) and TLR4 can cause serious intestinal damage. LPS present in the outer membrane of Gram-negative bacteria triggers the production of proinflammatory mediators that can contribute to intestinal inflammation and damage during infection. Thus, while TLR4 recognition of LPS is required for clearance of Gram-negative organisms, it is believed that excessive and/or prolonged proinflammatory cytokine secretion can be harmful to the host (1, 18).
Lactic acid bacteria (LAB) able to modulate the immune system (immunobiotics) (9) are known to play a beneficial role in the prevention and therapy of a variety of intestinal inflammatory disorders, including atopic and inflammatory bowel diseases (9). In this sense, we have demonstrated that Lactobacillus jensenii TL2937 attenuates the expression of proinflammatory cytokines and chemokines triggered by enterotoxigenic Escherichia coli (ETEC) or by LPS (28). L. jensenii TL2937 attenuates proinflammatory responses in a porcine intestinal epitheliocyte (PIE) cell line by downregulating TLR4-dependent NF-κB and mitogen-activated protein kinase (MAPK) activation. Furthermore, we demonstrated that L. jensenii TL2937 stimulation of PIE cells results in upregulation of three negative regulators of TLRs, A20, B-cell lymphoma 3-encoded protein (Bcl-3), and mitogen-activated protein kinase 1 (MPK-1), and that these effects are partially dependent on the activation of TLR2 (28).
Studies on the precise mechanisms of probiotic action indicate that the immunoregulatory mechanisms behind the positive effects of immunobiotics are related to the modulation of immune cells, such as APCs (7, 17, 34). Moreover, different probiotic strains affect APC maturation in different ways since cytokine and surface marker expression in APCs varies with the probiotic strains used (8). L. jensenii TL2937 may be capable of inducing tolerance to LPS in APCs; therefore, studying the effects of this probiotic strain on porcine APCs is important. Most studies addressing the effect of probiotics on APCs have been conducted in mice, and very few studies have investigated these effects in commercially important livestock animals, such as pigs. Recent advances in the characterization of the porcine immune system, particularly in porcine macrophages and DCs, have permitted the use of the porcine model for many immunological studies (2, 38). Although the library of reagents for such studies is still small compared to that for mouse studies, knowledge of porcine immunology is advancing rapidly. Moreover, the porcine immune system has been the focus of increased interest in recent years because of its potential as a suitable model for the study of the human intestinal immune system, since the porcine gastrointestinal tract has many structural aspects that are more similar to those of the human tract than those of the rodent tract are.
In view of the critical importance of APC polarization in immunoregulation, the objective of the present study was to examine the effect of L. jensenii TL2937 on activation patterns of APCs from swine PPs. Therefore, in this study, we characterized APCs that were harvested from swine PPs, developed in vitro systems that allowed the evaluation of APC activity, and evaluated the functional consequences of direct exposure of APCs to L. jensenii TL2937 under both inflammatory and noninflammatory conditions.
MATERIALS AND METHODS
Microorganisms.
ETEC strain 987 was kindly provided by M. Nakazawa at the National Institute of Animal Health (Tsukuba, Japan) (28). ETEC cells were grown in tryptic soy broth (TSB; Becton Dickinson and Company, San Jose, CA) for 24 h at 37°C with shaking. After overnight incubation, bacteria from subcultures were centrifuged at 5,000 × g for 10 min at 4°C, washed with phosphate-buffered saline (PBS), and heat killed (100°C, 30 min). Cultures of each of three Lactobacillus strains (L. jensenii TL2937, L. casei MEP221115, and L. casei MEP221104) were grown in Man-Rogosa-Sharpe (MRS) medium (Difco, Detroit, MI) for 16 h at 37°C, washed with PBS, and heat killed (56°C, 30 min). These bacterial samples were resuspended in Dulbecco's modified Eagle medium (DMEM), enumerated using a microscope and a Petroff-Hausser counting chamber, and stored at −80°C until use (28).
Isolation of immunocompetent cells from swine Peyer's patches.
Suspensions of porcine PP immunocompetent cells were prepared from the ilea of adult swine as previously described (16, 31). All procedures were conducted in accordance with the Guidelines for Animal Experimentation of Tohoku University, Sendai, Japan. Briefly, PPs were cut into small fragments; the fragments were then gently pressed through a nylon mesh and washed three times in complete RPMI 1640 medium (Sigma, St. Louis, MO) supplemented with 10% fetal calf serum (FCS; Sigma). Residual erythrocytes were lysed by resuspension in hypotonic salt solution (0.2% NaCl). Next, harvested PP cells were subjected to hypertonic rescue in an equal volume of 1.5% NaCl. Finally, immune cells were fractionated using Lympholyte-mammal (Cedarlane, Hornby, Ontario, Canada) density gradient centrifugation, and the isolated immune cells were suspended in complete DMEM (Invitrogen, Tokyo, Japan) supplemented with 10% FCS (Sigma), 50 μg/ml penicillin-streptomycin, and 50 μg/ml gentamicin (Nacalai Tesque, Kyoto, Japan).
Isolation of adherent population from swine Peyer's patches.
APCs (macrophages and DCs) from PPs were isolated by taking advantage of their capacity to adhere to glass. After mononuclear cells were isolated from swine PP samples as described above, cell suspensions were adjusted to a concentration of 5 × 107 cells/ml. Cell suspensions (1 ml/well) were placed into 2-well glass plates (Iwaki, Tokyo, Japan) and incubated for 2 h at 37°C (5% CO2 atmosphere) to allow cells to adhere to the glass surface. Subsequently, these glass plates were washed gently with complete RPMI 1640 medium (Sigma) to remove nonadherent cells.
Isolation of CD172a+ cells from swine Peyer's patches.
CD172a+ cells were isolated from swine PPs using anti-porcine CD172a-biotin-conjugated SWC3a IgG1 (Southern Biotech, Birmingham, AL), streptavidin microbeads, and 25 LS+ MACS separation columns from a magnetically activated cell sorting (MACS) separation system (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). All procedures were conducted according to the manufacturer's instructions.
Immunohistochemical analysis.
Immunohistochemical analysis of swine PPs and adherent cells was conducted as described in our previous reports (29, 32). Fresh PPs were obtained from adults pigs, washed with PBS, cut into small pieces (5 mm by 10 mm), and fixed in Zamboni fixative (0.2% saturated picric acid and 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4) for 16 h at 4°C. The fixed tissues were washed once for 24 h with 1% arabic gum in 0.1 M phosphate buffer containing 8% sucrose and then once again for 24 h with the same solution containing 16% rather than 8% sucrose. The washed samples were then immersed in Tissue-Tek OCT compound (Sakura Finetek, Tokyo, Japan) and quickly frozen in a dry ice-acetone bath. Cryostat sections (10 μm thick) were prepared from the frozen tissue; these sections were then mounted on poly-l-lysine-coated glass slides and washed in PBS. The mounted sections were incubated for 20 min with 1% bovine serum albumin and 2% normal goat serum (Vector Laboratories, Burlingame, CA) in PBS at room temperature to block nonspecific binding sites. After removal of the blocking solution, mounted sections were incubated for 16 h at 4°C in a humidified chamber with one of the following primary antibodies: anti-porcine CD172a-phycoerythrin (PE) SWC3 IgG1 (Southern Biotech), anti-porcine CD172a-fluorescein isothiocyanate (FITC) SWC3 IgG1 (Southern Biotech), anti-porcine CD11R1-unlabeled mouse IgG1 (AbD Serotec, Kidlington, Oxford, United Kingdom), or anti-porcine major histocompatibility complex class II (MHC-II)-unlabeled mouse IgG2a (VMRD, Pullman, WA). Mounted sections were washed in PBS and then incubated with one of the following secondary antibodies: anti-mouse IgG2a-FITC (AbD Serotec) or anti-mouse IgG-FITC (AbD Serotec).
Immunomodulatory effect of lactobacilli.
Evaluation of the immunomodulatory activity of L. jensenii TL2937, L. casei MEP221115, or L. casei MEP221104 was performed using mononuclear cells from PPs, adherent cells, or isolated CD172a+ cells prepared as described above. In each case, cells were plated at a density of 1.5 × 106 cells/well in 12-well type I collagen-coated plates (Iwaki) or in 2-well glass plates (Iwaki). Lactobacilli were added to each well at a concentration of 5 × 108 cells/ml. Porcine cells were incubated with lactobacilli for 16 h; each well was then washed vigorously with medium at least three times to eliminate bacterial strains; finally, the porcine cells were stimulated with ETEC (5 × 107 cells/ml) or LPS (1,000 ng/ml; from E. coli O55:B5, prepared by phenol extraction followed by gel filtration chromatography; Sigma) for 12 h. Changes in expression of costimulatory molecules and of cytokines in the cultured porcine APCs were evaluated by flow cytometry and real-time PCR using the methods described below. In some experiments, a TLR2 agonist, Pam3CSK4 (200 ng/ml; EMC Microcollection, Tubingen, Germany), was used to stimulate mononuclear PP cells, adherent cells, or isolated CD172a+ cells for 16 h. In addition, unlabeled anti-porcine TLR2-rabbit IgG (Santa Cruz, Santa Cruz, CA) was used in blocking experiments. Cultured porcine cells were incubated with the unlabeled anti-TLR2 antibodies for 12 h before stimulation with L. jensenii TL2937.
Flow cytometric analysis.
Flow cytometry was used to assess expression of MHC-II, TLR2, TLR4, and several cytokine proteins in CD172a+ CD11R1−, CD172a+ CD11R1high, and CD172a− CD11R1low cells from PPs, as well as in adherent cells and CD172a+ PP cells. Cells were isolated as described above and labeled with primary antibodies: anti-porcine CD172a-PE SWC3 IgG1 (Southern Biotech), anti-porcine CD11R1-unlabeled IgG1 (AbD Serotec), anti-porcine MHC-II-unlabeled IgG2a (VMRD), anti-porcine CD4-FITC IgG2b (Southern Biotech), anti-porcine CD14-FITC IgG2b (AbD Serotec), anti-porcine TLR2-unlabeled rabbit IgG (Santa Cruz), anti-porcine TLR4-unlabeled rabbit IgG (Santa Cruz), anti-porcine gamma interferon (IFN-γ)-unlabeled IgG2b (R&D Systems, Minneapolis, MN), anti-porcine interleukin-10 (IL-10)-unlabeled IgG2b (R&D Systems), anti-porcine IL-1β/IL-1F2-unlabeled IgG1 (R&D Systems), anti-porcine IL-6-unlabeled IgG2b (R&D Systems), and anti-porcine transforming growth factor β2 (TGF-β2)-unlabeled IgG (R&D Systems). The binding of unlabeled monoclonal antibodies was visualized using the following secondary antibodies: anti-mouse IgG1-peridinin chlorophyll protein (PerCP)/Cy5.5 (Bio Legend, San Diego, CA), anti-mouse IgG2a-FITC (AbD Serotec), anti-rabbit IgG-Alexa Fluor 489 (Santa Cruz), anti-mouse IgG2b-FITC (AbD Serotec), and anti-mouse IgG-FITC (AbD Serotec). In addition, expression levels of CD80/86 proteins were evaluated using a human CD152 (cytotoxic-T-lymphocyte-associated antigen 4) Ig/FITC fusion protein (Ancell, Bayport, MN). Cells stained with irrelevant mouse IgG-FITC, IgG2b-FITC, IgG2a-PerCP, IgG2b-PE, IgG2a-PE, or IgG1-PE antibodies (eBioscience, San Diego, CA) were included as isotype controls. Analysis of the stained cells was performed using a FACSCalibur apparatus (BD, Franklin Lakes, NJ), which was equipped with Cell-Quest software. Data analysis was performed using FlowJo software (Tree Star, Ashland, OR).
Quantitative expression analysis using real-time PCR.
Two-step real-time quantitative PCR (qPCR) was used to characterize the expression of specific mRNAs in immune cells (13, 28). Total RNA was isolated from individual samples of porcine APCs using TRIzol reagent. All cDNAs were synthesized using a Quantitect reverse transcription (RT) kit (Qiagen, Tokyo, Japan) according to the manufacturer's recommendations. Real-time quantitative PCR was carried out using a 7300 real-time PCR system (Applied Biosystems, Warrington, United Kingdom) and Platinum SYBR green qPCR SuperMix UDG with carboxy-X-rhodamine (Invitrogen). The primers used for the analysis of proinflammatory (IL-1β, IL-6, and tumor necrosis factor alpha [TNF-α]), Th1 (IL-2, IL-12 and IFN-γ), Th2 (IL-4), and immunomodulatory (TGF-β and IL-10) cytokines were described previously (23). The primers used to assess expression of six negative regulators of TLR signaling (single immunoglobulin IL-1-related receptor [SIGIRR], Toll-interacting protein [Tollip], interleukin-1 receptor-associated kinase M [IRAK-M], A20, Bcl-3, and MKP-1) are described by Shimazu et al. (28). PCR cycling conditions were 2 min at 50°C, followed by 2 min at 95°C and then 40 cycles of 15 s at 95°C, 30 s at 60°C, and 30 s at 72°C. The reaction mixtures each contained 5 μl of the sample cDNA and 15 μl of the master mix, which included the appropriate sense and antisense primers. Expression of β-actin in each sample was assessed, and the β-actin data were used as an internal control to normalize differences between samples and to calculate relative expression levels.
Statistical analysis.
Statistical analyses were performed using the GLM and REG procedures available in the SAS computer program (SAS, 1994). Comparisons between mean values were carried out using one-way analysis of variance and Fisher's least-significant-difference (LSD) test. For these analyses, P values of <0.05 were considered significant.
RESULTS
Phenotypes and distribution of APCs in swine PPs.
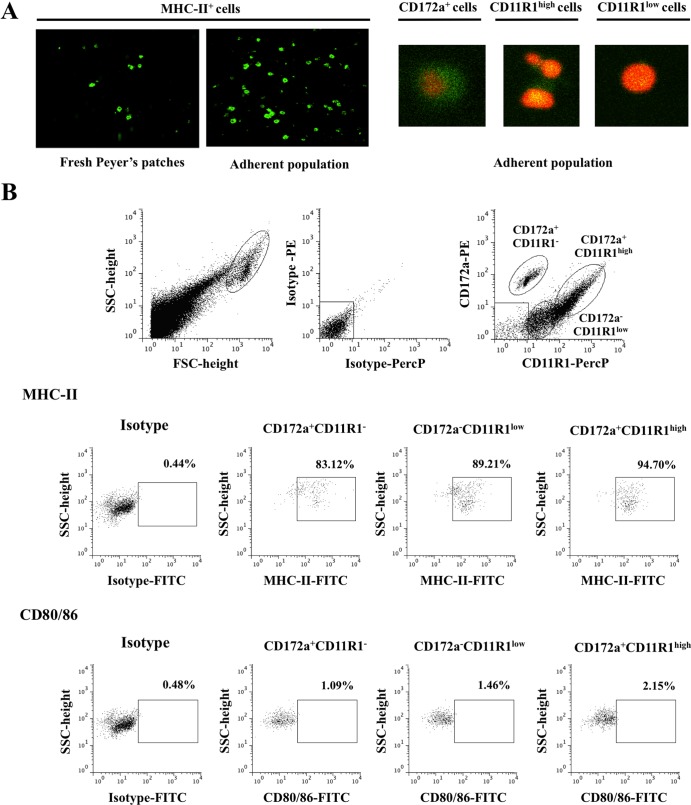
The expression of two markers, CD172a and CD11R1, in tissue sections of swine ileal PPs was analyzed. We found CD172a+ CD11R1−, CD172a+ CD11R1+, and CD172a− CD11R1+ cells in both the subepithelial and the interfollicular regions of PPs (Fig. 1A). CD172a− CD11R1+ cells were more abundant in the interfollicular region, whereas the CD172a+ CD11R1+ cells were apparently more uniformly distributed than the CD172a− CD11R1+ cells. Interestingly, there were fewer CD172a+ CD11R1− cells than either CD172a+ CD11R1+ cells or CD172a+ CD11R1− cells, and most CD172a+ CD11R1− cells were observed in the subepithelial region of PPs. The strong expression of MHC-II has been used as a working definition of porcine APCs (3). Therefore, we aimed to examine the expression of MHC-II in mononuclear cells harvested from swine PPs. We found that almost 60% of these cells exhibit strong expression of MHC-II (Fig. 1B). These cells include not only macrophages and DCs but also T and B cells, as we have demonstrated previously (11, 12). Using anti-CD172a and anti-CD11R1 antibodies, we were able to define three populations of MHC-II+ cells in fresh PPs: CD172a+ CD11R1−, CD172a+ CD11R1high, and CD172a− CD11R1low (Fig. 1B). These three populations of cells all exhibited strong expression of MHC-II (Fig. 1C). In addition, we examined the expression of other molecules in order to characterize each cell population. We found that only 1 to 2% of PP cells expressed CD80/86 molecules, and no expression of CD4 was detected (Fig. 1C). Cells from PPs expressed variable levels of CD14: CD172a+ CD11R1− cells expressed the highest levels of CD14 (8.1 ± 2.5%), and CD172a− CD11R1low cells did not express detectable levels of CD14 (Fig. 1C). In addition, we evaluated the expression of TLR2 and TLR4. APCs from PPs expressed variable levels of TLR2. CD172a+ CD11R1high cells exhibited the strongest TLR2 expression (58.5% ± 3.1%); these cells were followed by CD172a+ CD11R1− (35.1% ± 4.2%) and CD172a− CD11R1low (30.3% ± 3.7%) (Fig. 1C). In addition, low levels of TLR4 expression were evident in APCs (Fig. 1C).
Fig 1.
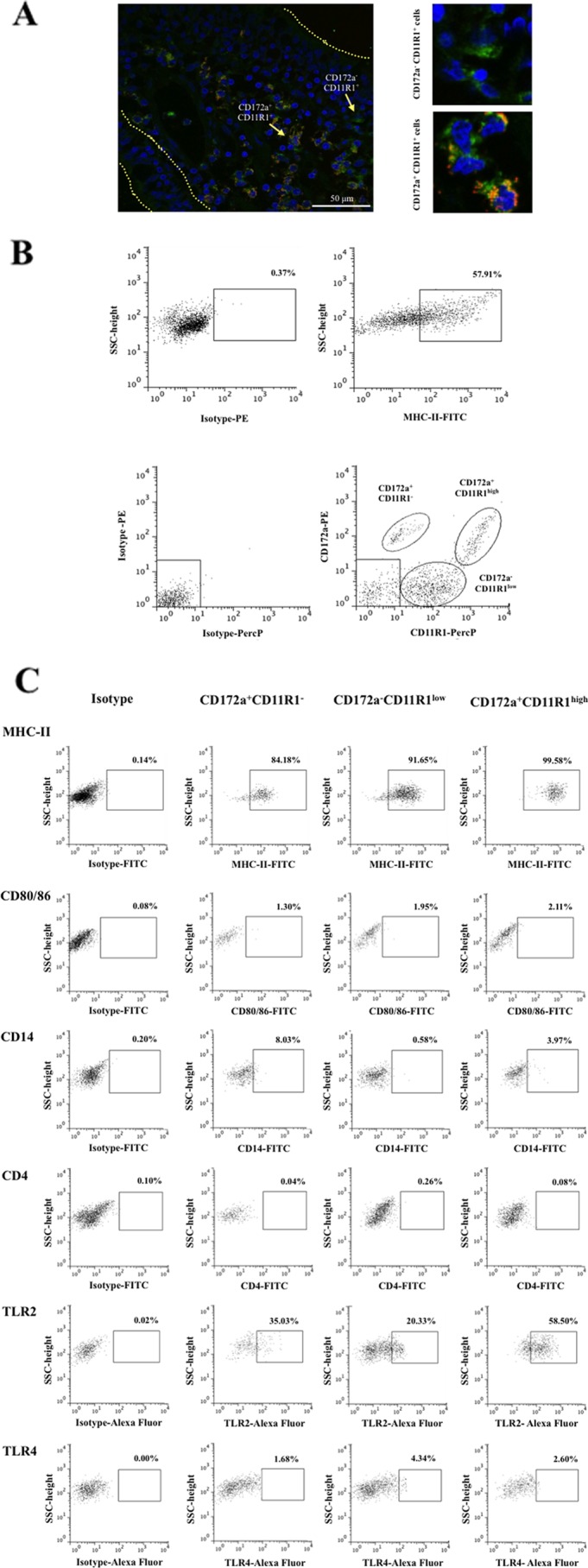
Phenotypes and distribution of APCs in swine PPs. (A) Immunohistochemical analysis of APCs from ileal PPs. Tissue sections were stained with antibodies against CD172a and CD11R1, and the distribution of CD172a+ CD11R1−, CD172a+ CD11R1+, and CD172a− CD11R1+ cells was examined. (B) Flow cytometric analysis of APCs in suspensions of mononuclear cells isolated from adult swine PPs and incubated with antibodies against MHC-II, CD172a, and CD11R1. Strong expression of MHC-II was used as a working definition of porcine APCs. Three populations in MHC-II+ cells were defined: CD172a+ CD11R1−, CD172a+ CD11R1high, and CD172a− CD11R1low. SSC, side scatter. (C) Flow cytometric analysis of the expression of MHC-II, CD80/86, CD14, CD4, TLR2, and TLR4 in CD172a+ CD11R1−, CD172a+ CD11R1high, and CD172a− CD11R1low APCs from swine PPs. The results represent data from three independent experiments using ileal PPs from at least three different swine.
Phenotype and distribution of adherent cells isolated from swine PPs.
Next, we isolated APCs (DCs and macrophages) from porcine PP tissue samples by culturing the mononuclear cells from these samples on glass plates and selecting the adherent cells. Here, these cells are referred to as adherent cells. After 2 h of incubation and subsequent removal of the nonadherent cells, we were able to obtain a cell population enriched in MHC-II-positive (MHC-II+) cells (Fig. 2A). Expression of CD172a and CD11R1 was variable among this population of cells (Fig. 2A). Flow cytometric analysis of these adherent cells showed that it was possible to identify the three populations of APCs detected in mononuclear cells isolated from fresh PPs (Fig. 2B). This method of APC isolation did not completely eliminate CD172a− CD11R1− cells (which include T and B cells) from the cultures; however, it did allow us to harvest samples with a high proportion of APCs. To assess whether the in vitro culture of adherent cells itself induced the activation of these cells, we evaluated the expression MHC-II and CD80/86. There were no apparent differences in the expression of MHC-II or CD80/86 in any of the populations of isolated APCs (Fig. 2B) when expression was compared with that in cells of fresh PPs (Fig. 1C).
Fig 2.
Phenotype and distribution of adherent population in swine PPs. Antigen-presenting cells (macrophages and dendritic cells) from PPs were obtained by taking advantage of their capacity to adhere to glass. (A) Immunohistochemical analysis of populations of adherent cells isolated from swine PPs. Cells were stained with antibodies for MHC-II, CD172a, and CD11R1. (B) Flow cytometric analysis of the adherent cell population. Cell suspensions were incubated with antibodies against CD172a and CD11R1. Three cell populations were defined: CD172a+ CD11R1−, CD172a+ CD11R1high, and CD172a− CD11R1low. In addition, expression of MHC-II and CD80/86 in CD172a+ CD11R1−, CD172a+ CD11R1high, and CD172a− CD11R1low adherent cells was examined. The results represent data from three independent experiments using ileal PPs from at least three different swine. FSC, forward scatter.
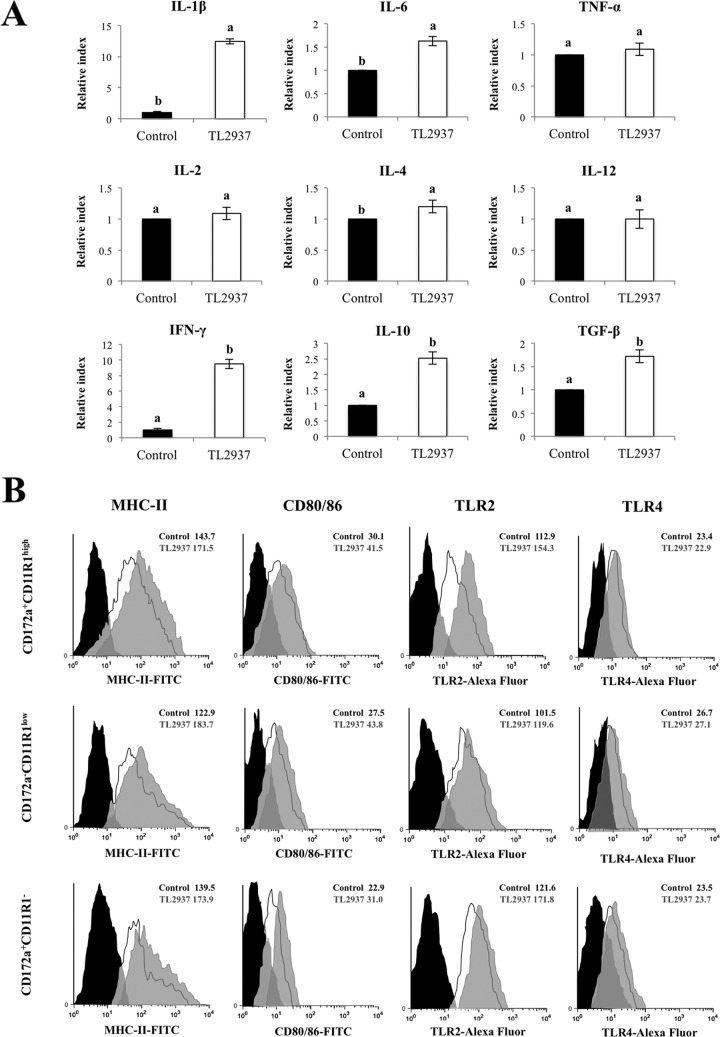
Effect of L. jensenii TL2937 on adherent cells isolated from swine PPs.
The stimulation of adherent cells from swine PPs with L. jensenii TL2937 did not induce significant changes in expression of TNF-α, IL-2, IL-4, or IL-12 (Fig. 3A). However, in adherent cells from PPs treated with L. jensenii TL2937, expression of IL-1β, IFN-γ, IL-6, TGF-β, and IL-10 did increase: 12.44, 9.5, 1.63, 1.72, and 2.52 times, respectively (Fig. 3A). We also observed that stimulation with L. jensenii TL2937 increased the expression of MHC-II, CD80/86, and TLR2 in all three populations of APCs, but there was no apparent difference in the expression of TLR4 between L. jensenii TL2937-treated and -untreated cells (Fig. 3B).
Fig 3.
Effect of Lactobacillus jensenii TL2937 on adherent population from swine PPs. Antigen-presenting cells (macrophages and dendritic cells) from PPs were obtained by taking advantage of their capacity to adhere to glass. Adherent cells were treated with L. jensenii TL2937 for 16 h. Untreated adherent cells were used as controls. (A) Expression of IL-1β, IL-6, TNF-α, IL-2, IL-4, IL-12, IFN-γ, TGF-β, and IL-10 mRNAs was examined using RT-PCR. Values for bars with different letters were significantly different (P < 0.05). (B) Expression of MHC-II, CD80/86, TLR2, and TLR4 was studied in CD172a+ CD11R1−, CD172a+ CD11R1high, and CD172a− CD11R1low adherent cells by flow cytometric analysis. Histograms represent data from flow cytometric analysis, as follows: adherent cells treated with L. jensenii TL2937 (gray histograms), untreated control cells (white histograms), and isotype controls (black histograms). Values of mean fluorescence intensity (MFI) are shown for treated and control groups. The results represent data from three independent experiments.
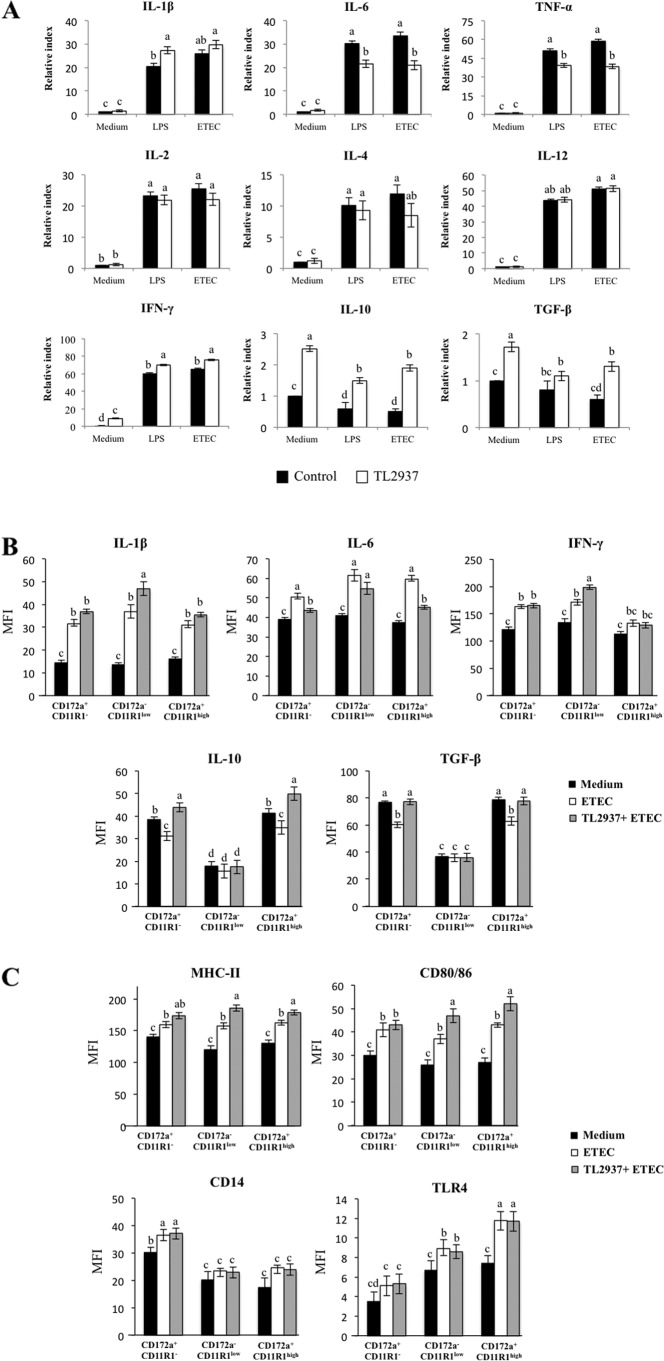
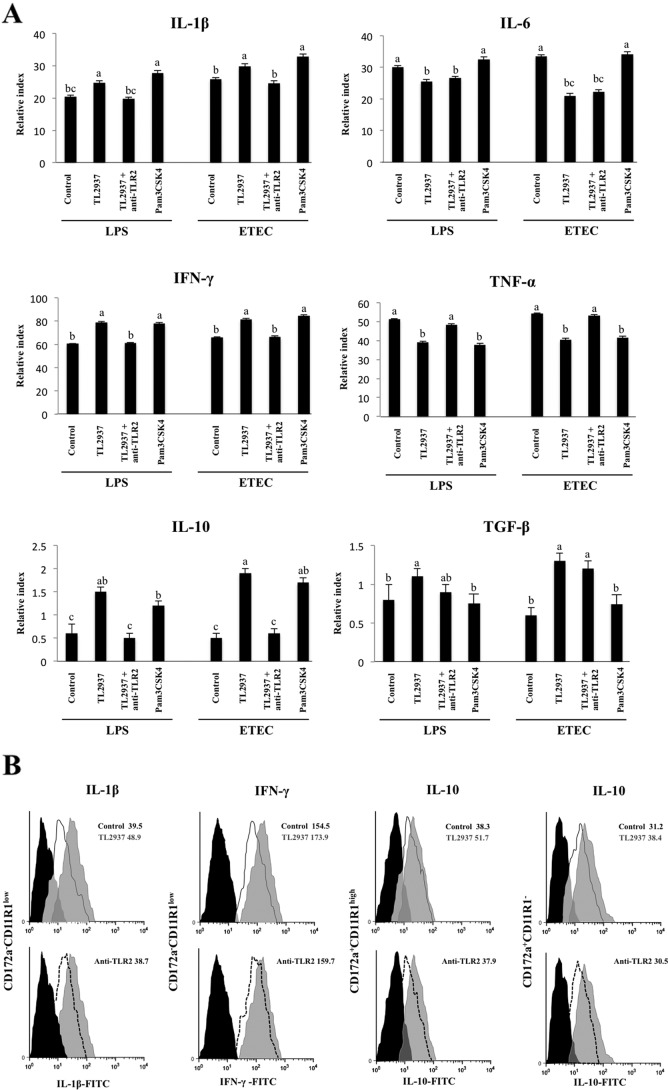
In order to evaluate the effect of L. jensenii TL2937 on APCs under inflammatory conditions, adherent cells were treated with the Lactobacillus strain for 16 h and then challenged with LPS or ETEC. The mRNA levels of various cytokines were assessed 12 h after the challenge with LPS or ETEC (Fig. 4A). The challenge with either LPS or ETEC resulted in an increase in the expression of proinflammatory (IL-1β, IL-6, and TNF-α), Th1 (IL-2, IL-12, and IFN-γ), and Th2 (IL-4) cytokines; in contrast, either challenge resulted in a decrease in the expression of the immunomodulatory cytokines TGF-β and IL-10 (Fig. 4A). Pretreatment of adherent cells with L. jensenii TL2937 did not induce significant changes in the levels of IL-2, IL-4, or IL-12 after the challenge with ETEC or LPS compared with those for the respective controls. However, the TL2937 strain did induce higher levels of IFN-γ, IL-1β, and IL-10 and lower levels of IL-6 and TNF-α in these challenged adherent cells (Fig. 4A). In addition, L. jensenii TL2937 prevented the decrease of TGF-β in response to the challenge with ETEC or LPS (Fig. 4A).
Fig 4.
Effect of Lactobacillus jensenii TL2937 on adherent population from swine PPs under inflammatory conditions. Antigen-presenting cells (macrophages and dendritic cells) from PPs were obtained by taking advantage of their capacity to adhere to glass. Adherent cells were treated with L. jensenii TL2937 for 16 h and then challenge with ETEC (5 × 107 cells/ml), LPS (1,000 ng/ml), or sterile medium for 12 h. Adherent cells not treated with L. jensenii TL2937 and challenged with ETEC, LPS, or sterile medium were used as controls. (A) Expression of IL-1β, IL-6, TNF-α, IL-2, IL-4, IL-12, IFN-γ, TGF-β, and IL-10 mRNAs was examined using RT-PCR. Expression of IL-1β, IL-6, IFN-γ, TGF-β, and IL-10 (B) and MHC-II, CD80/86, TLR2, and TLR4 (C) proteins was studied in CD172a+ CD11R1−, CD172a+ CD11R1high, and CD172a− CD11R1low adherent cells using flow cytometric analysis. Values of mean fluorescence intensity (MFI) are shown for each group. The results represent data from three independent experiments. Values for bars with different letters were significantly different (P < 0.05).
We next aimed to determine whether the changes in the expression of cytokines, as measured by RT-PCR, correlated with changes in the expression of the respective proteins. Moreover, we aimed to determine which APC populations were involved in the production of cytokines after L. jensenii TL2937 treatment. Therefore, we evaluated the expression of IL-1β, IL-6, IFN-γ, TGF-β, and IL-10 in the three populations of APCs. As observed in the RT-PCR analysis, flow cytometric studies showed that challenge of APCs with ETEC resulted in increased levels of IL-1β, IL-6, and IFN-γ, while it resulted in reductions of TGF-β and IL-10 (Fig. 4B). Moreover, the levels of IL-1β and IFN-γ were significantly higher in L. jensenii TL2937-treated CD172a− CD11R1low cells. Additionally, CD172a+ CD11R1− and CD172a+ CD11R1high cells treated with the TL2937 strain showed lower levels of IL-6 than the controls (Fig. 4B). The stimulation of APCs with L. jensenii TL2937 did not prevent the decrease in IL-10 or TGF-β levels in CD172a− CD11R1low cells. In contrast, pretreatment with the TL2937 strain significantly increased the levels of IL-10 in CD172a+ CD11R1high and CD172a+ CD11R1− cells, which showed higher values than unchallenged control cells (Fig. 4B). Interestingly, L. jensenii TL2937 prevented the decrease of TGF-β in CD172a+ CD11R1high and CD172a+ CD11R1− populations (Fig. 4B).
Challenge of APCs with ETEC resulted in significant increases in the expression of MHC-II and CD80/86 in all populations of APCs. However, in CD172a− CD11R1low and CD172a+ CD11R1high cells pretreated with L. jensenii TL2937, the levels of MHC-II and CD80/86 were higher than those observed in control cells (Fig. 4C). Challenge of APCs with ETEC also increased the levels of CD14 and TLR4, but no significant differences were observed between cells treated with the lactic acid bacterium and controls (Fig. 4C).
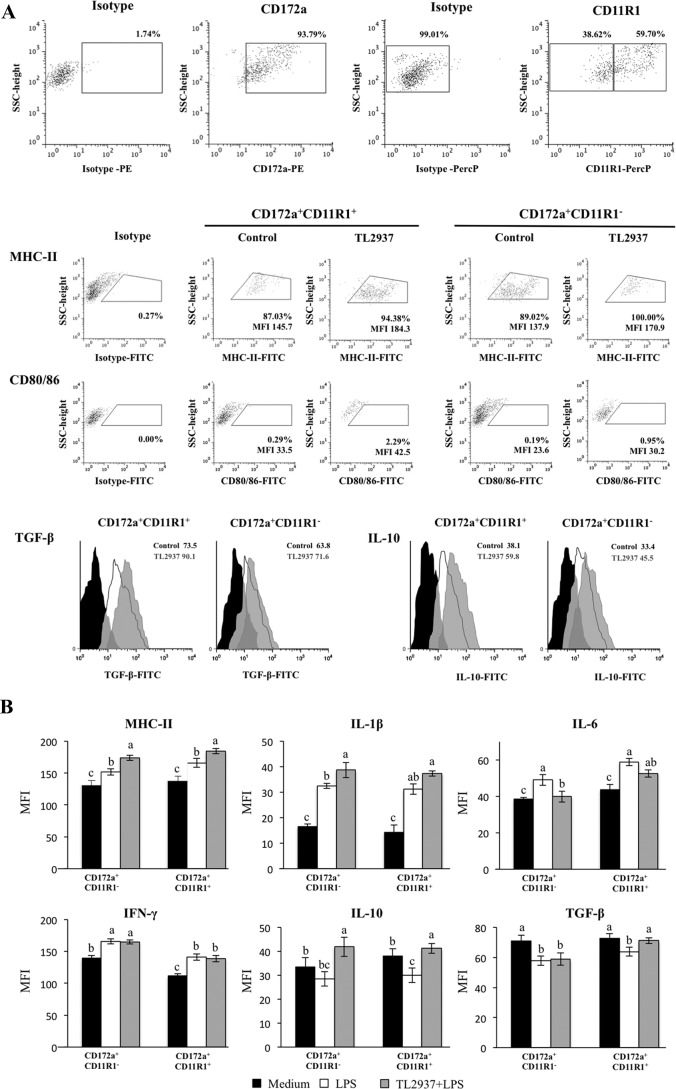
Effect of L. jensenii TL2937 on isolated CD172a+ cells.
We next aimed to assess the immunomodulatory effect of L. jensenii TL2937 in isolated CD172a+ cells obtained using the MACS cell separation system (magnetic cell labeling). Isolated CD172a+ cells from PPs can be divided into CD11R1+ and CD11R1− cells (Fig. 5A). When we evaluated the effects of the lactic acid bacterium on isolated CD172a+ cells, we found that treatment with L. jensenii TL2937 increased the expression of MHC-II and CD80/86 in both cell populations, confirming our previous findings. Moreover, treatment of isolated CD172a+ cells with the TL2937 strain resulted in increases of IL-10 in both CD172a+ CD11R1+ and CD172a+ CD11R1− cell populations, but TGF-β production was upregulated only in CD172a+ CD11R1+ cells (Fig. 5B). The effect of L. jensenii TL2937 was also evaluated under inflammatory conditions. CD172a+ cells were stimulated with L. jensenii TL2937 for 16 h and then challenged with LPS (Fig. 5C). As observed in the previous experiments, the inflammatory challenge induced an increase in the expression of MHC-II and proinflammatory cytokines, along with a decrease of immunomodulatory cytokines. However, L. jensenii TL2937-treated cells showed significantly higher levels of MHC-II, IL-1β, and IL-10 than control cells. In addition, we observed that treatment with the TL2937 strain resulted in upregulation of the production of TGF-β in CD172a+ CD11R1+ cells (Fig. 5C). The Lactobacillus strain did not induce any changes in the levels of IFN-γ, while it induced decreased levels of IL-6 in both populations of APCs (Fig. 5C). Similar results were observed when the effect of L. jensenii TL2937 in CD172a+ cells challenged with ETEC was studied (data not shown).
Fig 5.
Effect of Lactobacillus jensenii TL2937 on isolated CD172a+ cells from swine PPs. CD172a+ cells from swine PPs were obtained by a MACS cell separation system (magnetic cell labeling). Isolated CD172a+ cells can be divided into CD11R1+ and CD11R1− cells. (A) CD172a+ cells were treated with L. jensenii TL2937 for 16 h. Untreated CD172a+ cells were used as controls. Expression of MHC-II, CD80/86, TGF-β, and IL-10 proteins was examined in CD172a+ CD11R1− and CD172a+ CD11R1+ cells using flow cytometric analysis. Histograms represent data from the flow cytometric analysis, as follows: CD172a+ cells treated with L. jensenii TL2937 (gray histograms), untreated control cells (white histograms), and isotype controls (black histograms). Values of mean fluorescence intensity (MFI) are shown for each group. (B) CD172a+ cells were treated with L. jensenii TL2937 for 16 h and then challenged with LPS (1,000 ng/ml) or sterile medium for 12 h. CD172a+ cells not treated with L. jensenii TL2937 and challenged with LPS or sterile medium were used as controls. Values of MFI are shown for each group. The results represent data from three independent experiments. Values for bars with different letters were significantly different (P < 0.05).
Role of TLR2 in the immunomodulatory effect of L. jensenii TL2937.
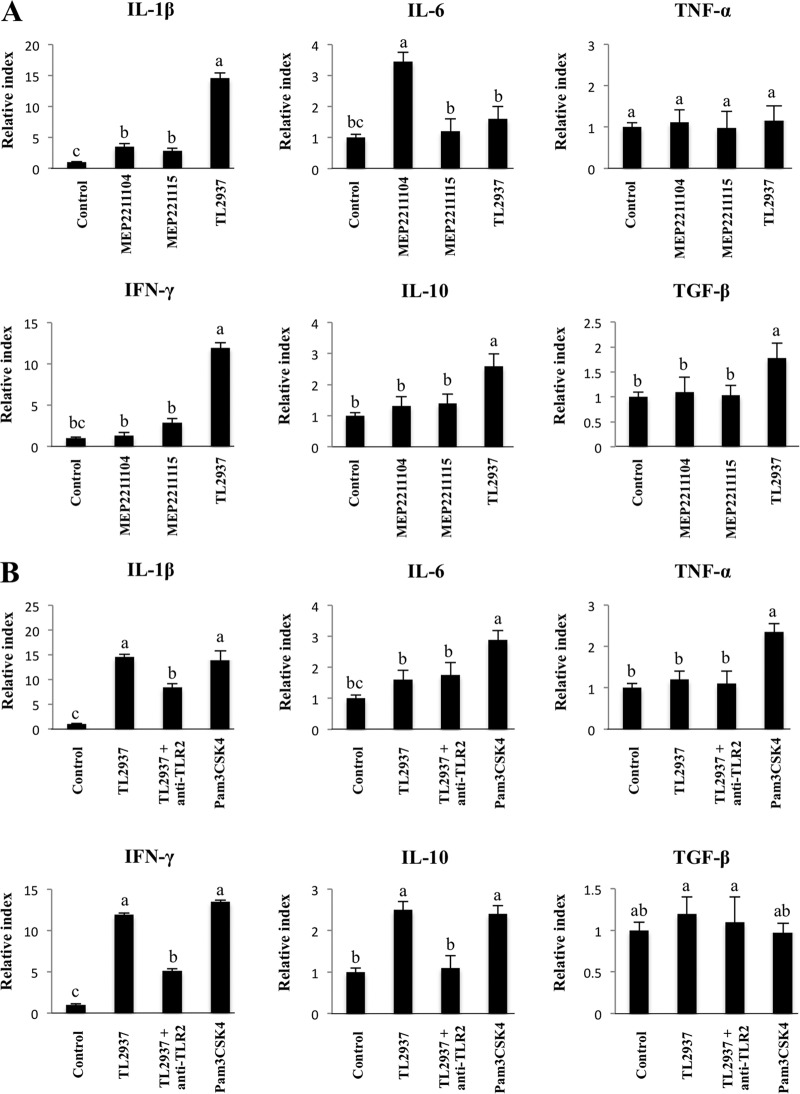
We next evaluated the role of TLR2 in the immunomodulatory effect of L. jensenii TL2937 on porcine APCs. We previously established a line of pTLR2-transfected cells (HEKpTLR2) using lipofection and a pcDNA-FLAG vector containing pTLR2 (33). Using RT-PCR, confocal laser microscopy, and two-dimensional microscopy of HEKpTLR2 cells treated with FITC-zymosan, we confirmed that pTLR2 was expressed and functional in HEKpTLR2 cells. Moreover, TLR2-mediated immune activation via a wide range of LAB strains was evaluated using these pTLR2-expressing cells. We found that different Lactobacillus strains have different capacities to induce the NF-κB-dependent promoter that drives expression of luciferase. These strains can be classified into three functional groups: strains with (i) high HEKpTLR2 activity (strain TL2937), (ii) moderate HEKpTLR2 activity (strain MEP221115), or (iii) no HEKpTLR2 activity (strain MEP221104) (33). Thus, L. jensenii TL2937, L. casei MEP221115, and L. casei MEP221104 were selected for subsequent experiments. Adherent cells were previously stimulated with one of the LAB strains for 16 h, and then the levels of six mRNAs (IL-1β, IL-6, TNF-α, IFN-γ, TGF-β, and IL-10) were evaluated (Fig. 6A). None of the strains studied induced alterations in the levels of TNF-α. In contrast, stimulation with each strain resulted in upregulation of the expression of IL-1β, IL-6, and IFN-γ (Fig. 6A). The levels of IL-6 were significantly higher in APCs treated with L. casei MEP221104 than in untreated cells or in cells treated with either of the other strains, but the highest levels of IL-1β and IFN-γ were induced by L. jensenii TL2937. We also observed that, unlike L. jensenii TL2937, the MEP221115 and MEP221104 strains did not induce significant changes in the expression of TGF-β or IL-10 (Fig. 6A). To study the role of TLR2 in the immunomodulatory effect of L. jensenii TL2937, we next performed comparative studies with the TLR2 agonist Pam3CSK4 and with anti-TLR2 antibodies. The use of anti-TLR2 antibodies did not induce any modification in the levels of IL-6 in porcine APCs treated with L. jensenii TL2937 (Fig. 6B). In contrast, treatment with Pam3CSK4 resulted in a significant enhancement in the production of IL-6 in APCs. Pam3CSK4 also induced upregulation of IL-1β, IFN-γ, and IL-10, and these effects were similar to those induced by L. jensenii TL2937. Moreover, levels of IL-1β, IFN-γ, and IL-10 were reduced by half when APCs were pretreated with anti-TLR2 antibodies (Fig. 6B). However, anti-TLR2 antibodies did not influence the effect of L. jensenii TL2937 on the expression of TGF-β in these APCs.
Fig 6.
Role of TLR2 in the immunomodulatory effect of Lactobacillus jensenii TL2937 on adherent cells from swine PPs. (A) Transfected cells that stably expressed porcine TLR2 (designated HEKpTLR2 cells) were isolated as previously described (33). The capacity of several lactobacillus strains to activate TLR2 was evaluated using this immune assay and stimulating HEKpTLR2 cells with the different lactobacillus strains for 24 h. Strains were classified into three functional groups according to the relative index (RI) values, as follow: high TLR2 activation (relative index, >5; Lactobacillus jensenii TL2937), moderate TLR2 activation (relative index, between 1 and 5; Lactobacillus casei MEP221115), and negative TLR2 activation (relative index, <1; Lactobacillus casei MEP221104). Adherent cells were treated with L. jensenii TL2937, L. casei MEP221115, or L. casei MEP221104 for 16 h. Untreated adherent cells were used as controls. Expression of IL-1β, IL-6, TNF-α, IFN-γ, TGF-β, and IL-10 mRNAs was examined using RT-PCR. (B) Adherent cells were treated with L. jensenii TL2937, with L. jensenii TL2937 plus anti-TLR2 antibodies, or with Pam3CSK4. Untreated adherent cells were used as controls. Expression of IL-1β, IL-6, TNF-α, IFN-γ, TGF-β, and IL-10 mRNAs was examined using RT-PCR. The results represent data from three independent experiments. Values for bars with different letters were significantly different (P < 0.05).
We also evaluated the role of TLR2 in the immunomodulatory effect of L. jensenii TL2937 under inflammatory conditions (Fig. 7A). Treatment of porcine APCs with Pam3CSK4 did not affect the levels of IL-6 or TGF-β after the challenge with ETEC or LPS. However, Pam3CSK4 did induce changes in IL-1β, TNF-α, IFN-γ, and IL-10 levels that were similar to those observed in cells treated with L. jensenii TL2937 (Fig. 7A). In addition, the capacity of L. jensenii TL2937 to increase IL-1β, IFN-γ, and IL-10 levels and to reduce TNF-α levels was inhibited by treatment with anti-TLR2 antibodies (Fig. 7A). In contrast, treatment of APCs with anti-TLR2 antibodies did not modify the reduction of IL-6 or the enhancement of TGF-β induced by L. jensenii TL2937 following challenge with LPS or ETEC (Fig. 7A). In order to confirm the effect of anti-TLR2 antibodies on cytokine production, we evaluated the expression of IL-1β, IFN-γ, and IL-10 proteins in the three populations of porcine APCs. Treatment of adherent cells with anti-TLR2 antibodies inhibited the enhancement of IL-1β and IFN-γ expression in CD172a− CD11R1low cells and the enhancement of IL-10 expression in CD172a+ CD11R1− and CD172a+ CD11R1high cells (Fig. 7B).
Fig 7.
Role of TLR2 in the immunomodulatory effect of Lactobacillus jensenii TL2937 on adherent cells from swine PPs under inflammatory conditions. Antigen-presenting cells (macrophages and dendritic cells) from PPs were obtained by taking advantage of their capacity to adhere to glass. Adherent cells were treated with L. jensenii TL2937, with L. jensenii TL2937 plus anti-TLR2 antibodies, or with Pam3CSK4. Untreated adherent cells challenged with ETEC or LPS were used as controls. (A) Expression of IL-1β, IL-6, TNF-α, IFN-γ, TGF-β, and IL-10 mRNAs was examined using RT-PCR. (B) Expression of IL-1β and IFN-γ in CD172a− CD11R1low cells and IL-10 in CD172a+ CD11R1− and CD172a+ CD11R1high cells was examined using flow cytometric analysis. Histograms represent data from flow cytometric analysis, as follows: adherent cells treated with L. jensenii TL2937 (gray histograms) or L. jensenii TL2937 plus anti-TLR2 antibodies (dotted lines), untreated control cells (white histograms), and isotype controls (black histograms). Values of mean fluorescence intensity (MFI) are shown for each group. The results represent data from three independent experiments. Values for bars with different letters were significantly different (P < 0.05).
Effect of L. jensenii TL2937 on negative regulators of the TLR signaling pathway in APCs.
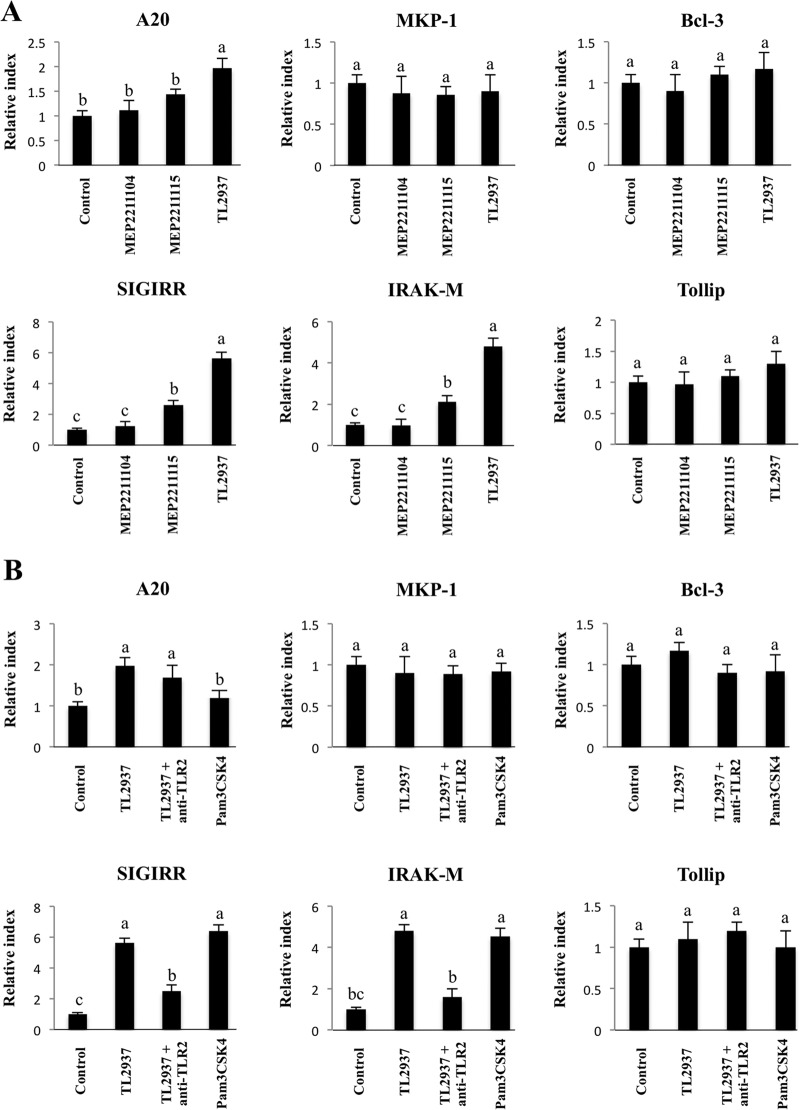
We also studied regulators that inhibit the TLR signaling pathway by evaluating the expression of SIGIRR, Tollip, A20, Bcl-3, MKP-1, and IRAK-M mRNAs in PP-derived adherent cells. APCs were stimulated for 16 h with L. jensenii TL2937, L. casei MEP221115, or L. casei MEP221104, and the expression of the negative regulators of TLRs was determined using real-time PCR (Fig. 8). Treatment with L. jensenii TL2937 resulted in upregulation of the expression of SIGIRR, IRAK-M, and A20, while treatment with L. casei MEP221115 induced upregulation of SIGIRR and IRAK-M (Fig. 8A). No modifications in the expression of MKP-1, Bcl-3, or Tollip were observed following treatment with any of the strains studied (Fig. 8A). There were also no changes in expression of any of these negative regulators of TLRs in the studies in which we employed anti-TLR2 antibodies or Pam3CSK4 (Fig. 8B). However, the treatment of APCs with Pam3CSK4 alone resulted in upregulation of the expression of SIGIRR and IRAK-M, and these changes were similar to those induced by L. jensenii TL2937 (Fig. 8B). We also observed that the enhancement of SIGIRR and IRAK-M expression induced by L. jensenii TL2937 was blocked by pretreatment with anti-TLR2 antibodies. No modifications in the levels of A20 were induced with anti-TLR2 antibodies or Pam3CSK4 (Fig. 8B). The upregulation of SIGIRR, IRAK-M, and A20 induced by L. jensenii TL2937 was confirmed in isolated CD172a+ cells (data not shown).
Fig 8.
Effect of Lactobacillus jensenii TL2937 on negative regulators of the TLR signaling pathway in adherent cells from swine PPs. (A) Transfected cells stably expressing porcine TLR2 (designated HEKpTLR2 cells) were isolated as previously reported (33). The capacity of several lactobacillus strains to activate TLR2 was evaluated using this immune assay. Strains were classified into three functional groups according to the relative index (RI) values, as follows: high TLR2 activation (relative index, >5; Lactobacillus jensenii TL2937), moderate TLR2 activation (relative index, between 1 and 5; Lactobacillus casei MEP221115), and negative TLR2 activation (relative index, <1; Lactobacillus casei MEP221104). Adherent cells were treated with L. jensenii TL2937, L. casei MEP221115, or L. casei MEP221104 for 16 h. Untreated adherent cells were used as controls. Expression of SIGIRR, Tollip, A20, Bcl-3, MKP-1, and IRAK-M mRNAs was measured using RT-PCR. (B) Adherent cells were treated with L. jensenii TL2937, with L. jensenii TL2937 plus anti-TLR2 antibodies, or with Pam3CSK4. Untreated adherent cells were used as controls. Expression of SIGIRR, Tollip, A20, Bcl-3, MKP-1, and IRAK-M mRNAs was examined using RT-PCR. The results represent data from three independent experiments. Values for bars with different letters were significantly different (P < 0.05).
DISCUSSION
In swine, no single specific marker is known to allow definitive identification of APCs, although monocytes, macrophages, and DCs have been studied in various tissues. The most frequent marker expressed on porcine macrophages and DCs is CD172a. Additionally, CD11R1 is considered to be a marker specifically and differentially expressed on porcine DCs but not on macrophages (30). The CD11R1 molecule is frequently expressed in mucosal DCs; moreover, swine DCs emigrating from the intestine via lymphatic vessels have been identified to be CD172a+ CD11R1+ and CD172a− CD11R1+ cells (3). Thus, in the present study, we used CD172a and CD11R1, together with MHC-II, to define APCs in swine PPs. Three different populations of APCs in swine PPs were defined using CD172a and CD11R1 as markers: CD172a+ CD11R1high, CD172a− CD11R1low, and CD172a+ CD11R1− cells. According to our present studies of CD4 and CD14 expression and previously published work (3, 39, 42), CD172a+ CD11R1high and CD172a− CD11R1low cells could be DCs and CD172a+ CD11R1− cells could be macrophages; however, functional studies are needed to accurately define each population. Therefore, in this study, we refer to each of the three populations as APCs.
We demonstrated that direct exposure of porcine APCs to L. jensenii TL2937 in the absence of inflammatory signals activated CD172a+ APCs and caused them to become phenotypically and functionally mature and to display tolerogenic properties. Our findings show both similarities to and differences from previous studies with lactobacilli and APCs from different species. For example, human myeloid DCs exposed to lactobacilli increased expression of MHC-II and costimulatory molecules (7, 17, 34). Moreover, similar to our current work, previous studies by Drakes et al. (11) revealed that probiotic lactobacilli could induce upregulation of IL-10 production and cell surface markers of maturation and activation in DCs (11). However, other studies found that coculture of lactobacilli with human myeloid DCs induced activation and maturation of these APCs and increased bioactive IL-12 production but not IL-10 production (22). The differences in the findings from these studies may result from one or more causes, including differences in the Lactobacillus species and strains investigated and the timing of sampling to assay cytokine production. Moreover, the pattern of cytokine expression in bone marrow-derived murine DCs is affected by exposure to various Lactobacillus strains, and the changes in the pattern are dependent on the concentration of the bacteria in the treatment (8). Specifically, low concentrations of L. casei yielded maximal levels of IL-6, IL-12, and TNF-α but virtually no IL-10 induction. At higher concentrations of L. casei, IL-10 production increased radically, but there were no changes in the levels of other cytokines (8). Another factor influencing the differential immunomodulatory effect of lactobacilli is thought to be the type of APCs interacting with the bacteria. The treatment with L. jensenii TL2937 increased the expression of IL-10 and TGF-β in CD172a+ CD11R1high and CD172a+ CD11R1− cells, while the treatment with this bacterium was associated with increased levels of IFN-γ in CD172a− CD11R1low cells. One possible explanation for the differential immunomodulatory effect of TL2937 could be the levels of expression of TLR2 in distinct APCs. CD172a+ CD11R1high, CD172a+ CD11R1−, and CD172a− CD11R1low cells differ with regard to TLR2 expression; therefore, they are likely to differ in the degree to which they interact with L. jensenii.
We also demonstrated that pretreatment of APCs with L. jensenii TL2937 resulted in differential modulation of the production of pro- and anti-inflammatory cytokines in response to ETEC or LPS challenge. The differential effects of the TL2937 strain in each APC population persisted because increased production of IFN-γ was observed in CD172a− CD11R1low cells. Moreover, IL-6 was downregulated and IL-10 was upregulated in CD172a+ CD11R1high and CD172a+ CD11R1− cells. This capacity of L. jensenii TL2937 to stimulate both tolerogenic and effector APCs could have a beneficial effect in vivo because the preventive administration of this strain may result in an improvement in the adaptive immune response and protection from unproductive inflammation. In fact, evidence from in vivo trials of some strains indicates that immunobiotics can simultaneously improve both resistance against infection and protection from inflammatory tissue damage (26, 36, 37). It will be of interest to examine whether L. jensenii TL2937 is able to improve resistance to ETEC challenge and to protect against inflammatory damage using in vivo experiments on pigs. Our laboratory is currently conducting studies to test this hypothesis.
Immunological tolerance can operate via deletion of reactive cells, downregulation of one or more receptors, blockade of signaling, suppression via cytokines, or some combination of these (4, 20). Therefore, one possibility to explain the immunomodulatory effects of L. jensenii TL2937 is that they occur through the downregulation of TLR4 in APCs and the blocking of signal transduction at the receptor level, as described by Nomura et al. (24). We have addressed this point, but we did not detect any changes in TLR4 expression in APCs treated with L. jensenii TL2937 (data not shown). We also evaluated the effect of the strain on the expression of negative regulators of TLRs in APCs. Of the six regulators tested, SIGIRR, A20, and IRAK-M mRNA expression was upregulated in CD172a+ cells stimulated with L. jensenii TL2937. Notably, IRAK-M-deficient cells stimulated by TLR ligands or bacteria produce elevated amounts of proinflammatory cytokines, such as IL-12, IL-6, or TNF-α, and these increases are accompanied by an increase in NF-κB and MAPK activation (10). IRAK-M expression is induced upon LPS stimulation, and endotoxin tolerance is diminished in IRAK-M-deficient cells; these observations indicate that IRAK-M plays a critical role in regulating innate immunity through a negative-feedback loop (12). Moreover, Biswas et al. (5) showed that expression of IRAK-M is largely dependent on the presence of commensal bacteria. The expression of IRAK-M is greatly reduced in mice under germfree conditions, and this expression is restored by colonization of a single strain of commensal bacteria, either a Gram-positive L. plantarum strain or a Gram-negative E. coli strain (5). In addition, overexpression of SIGIRR inhibits TLR-induced NF-κB activation and attenuates the production of inflammatory cytokines in vitro (25), and the LPS-induced inflammatory response is enhanced in SIGIRR-deficient mice (19). A20 also has an essential role in the termination of NF-κB signaling in response to microbial products such as LPS (6, 40). Reportedly, A20 deficiency in enterocytes renders mice sensitive to TNF-α-induced lethal inflammation, and this deficiency leads to disruption of the epithelial barrier and infiltration of commensal bacteria that initiate a systemic inflammatory response (35). Therefore, induction of these three negative regulators by L. jensenii TL2937 in CD172a+ APCs from swine PPs may be important for establishing tolerance against LPS and ETEC.
TLR2 is required for some probiotics to exert their immunomodulatory effects. In vitro and ex vivo studies demonstrated that some immunostimulatory lactobacilli interact with intestinal epithelial cells and induce release of IL-6 through an interaction with TLR2 (14). Moreover, immunoenhancing lactobacilli are able to increase the expression of TLR2 in DCs and macrophages isolated from PPs in mice (14) and in human myeloid DCs (22). Notably, TLR2 also seems to play an important regulatory role in the recognition of probiotic bacteria, which possess immunoinhibitory effects in both human and murine DCs (15, 41). Immunobiotics can inhibit IL-12 production by macrophages (27) and DCs (41) via recognition of lipoproteins and insoluble cell walls by TLR2. In addition, lipoteichoic acid isolated from L. plantarum reduces LPS-induced TNF-α production in human THP-1 monocyte-like cells in a TLR2-dependent manner (18). These studies demonstrate that TLR2 not only is involved in producing an inflammatory response but also can be involved in producing regulatory DCs. Our results indicated that TLR2 may have an important role in the anti-inflammatory activity of L. jensenii TL2937. Comparative experiments using strains with moderate or no capacity to stimulate TLR2, anti-TLR2 antibodies, or Pam3CSK4 demonstrated that TLR2 was involved in the upregulation of SIGIRR and IRAK-M in CD172a+ APCs and the elevated production of IL-10 in response to ETEC or LPS challenge. In addition, our studies showed that TLR2 was involved in the stimulation of CD172a− CD11R1low cells and the elevated production of IFN-γ in response to TLR4 activation. We also observed that L. jensenii TL2937, but not Pam3CSK4, induced increased expression of A20 and TGF-β and that these increases were not abolished by the treatment with anti-TLR2 antibodies. Therefore, our results indicated that some other PRR(s), in addition to TLR2, might be involved in the anti-inflammatory effect of L. jensenii TL2937 on CD172a+ APCs. Identification of this unknown receptor will be an important focus of future investigations.
In conclusion, we have performed, for the first time, a functional characterization of porcine APCs from PPs, and we demonstrated that CD172a+ cells were tolerogenic, while CD172a− CD11R1low cells were involved in the generation of specific immune responses. These findings represent an important advance in understanding the gut immune system in pigs. Moreover, the present study demonstrated that adherent cells and isolated CD172a+ cells harvested from swine PPs were useful for in vitro study of the inflammatory responses in the porcine gut.
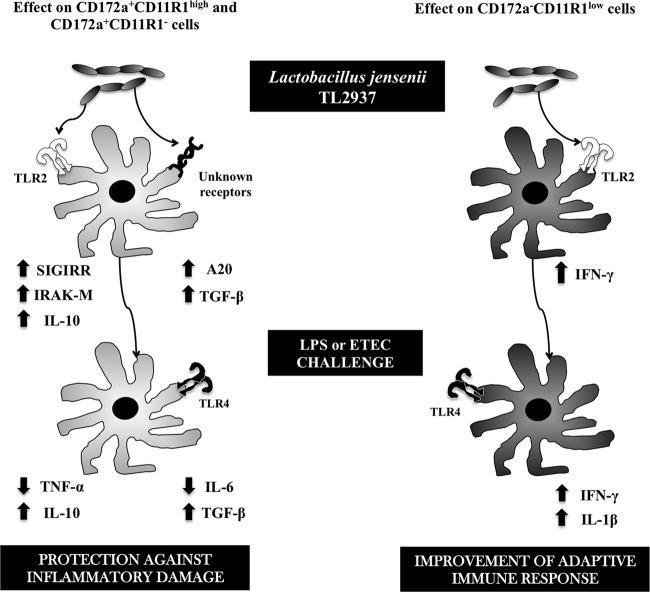
Our results also showed that porcine APCs could be used for studying the mechanisms involved in the immunomodulatory effects of probiotic microorganisms, such as their protective activity against pathogen-induced inflammatory damage. Research using these porcine APCs may provide useful information for the development of new immunologically functional foods and feeds that might be used to prevent intestinal disorders. In fact, using this adherent cell population and isolated CD172a+ cells from swine PPs, we were able to demonstrate that L. jensenii TL2937 functionally modulated APCs to induce tolerance against LPS challenge via the upregulation of TLR-negative regulators and immunomodulatory cytokines. Moreover, these in vitro systems allowed us to demonstrate that this effect was partially mediated by the activation of TLR2 (Fig. 9).
Fig 9.
Proposed mechanism for the immunomodulatory effect of Lactobacillus jensenii TL2937 on antigen-presenting cells from swine Peyer's patches.
Our studies contribute to knowledge of animal immunology and the use of immunobiotics in animals. Moreover, they can potentially contribute to an understanding of the mechanisms of action of immunobiotics and selection of new immunomodulatory strains for application in humans. For this reason, there has been growing interest in the porcine immune system because it has substantial potential as a low-cost model for the study of the human immune system. The porcine gastrointestinal tract has many structural aspects that are more similar to the human gastrointestinal tract than the structural aspects of the rodent tract are. Pigs have several advantages as experimental animals; for example, pigs are subject to naturally occurring diseases. Moreover, tissue culture protocols and pig breeding and maintenance practices allow researchers to conduct manipulations of the mucosal immune system in relatively large populations in vivo, as well as in detailed molecular studies in vitro (2, 38).
ACKNOWLEDGMENTS
This study was supported by a Grant-in-Aid for Scientific Research (B)(2) (no. 21380164 and 24380146) and a Grant-in-Aid for Exploratory Research (no. 23658216) from the Japan Society for the Promotion of Science (JSPS), a grant from the Japan Racing Association to H. Kitazawa, and a Grant-in-Aid for JSPS fellows (no. 21-09335) to J. Villena, who is a JSPS postdoctoral fellow.
Footnotes
Published ahead of print 9 May 2012
REFERENCES
- 1. Abreu MT, Fukata M, Arditi M. 2005. TLR signaling in the gut in health and disease. J. Immunol. 174: 4453–4460 [DOI] [PubMed] [Google Scholar]
- 2. Bailey M. 2009. The mucosal immune system: recent developments and future directions in the pig. Dev. Comp. Immunol. 33: 375–383 [DOI] [PubMed] [Google Scholar]
- 3. Bimczok D, Sowa EN, Faber-Zuschratter H, Pabst R, Rothkötter HJ. 2005. Site-specific expression of CD11b and SIRPalpha (CD172a) on dendritic cells: implications for their migration patterns in the gut immune system. Eur. J. Immunol. 35: 1418–1427 [DOI] [PubMed] [Google Scholar]
- 4. Biswas SK, Lopez-Collazo E. 2009. Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol. 30: 475–487 [DOI] [PubMed] [Google Scholar]
- 5. Biswas A, Wilmanski J, Forsman H, Hrncir T, Hao LH. 2011. Negative regulation of Toll-like receptor signaling plays an essential role in homeostasis of the intestine. Eur. J. Immunol. 41: 182–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boone DL, et al. 2004. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat. Immunol. 5: 1052–1060 [DOI] [PubMed] [Google Scholar]
- 7. Braat H, et al. 2004. Dichotomy between Lactobacillus rhamnosus and Klebsiella pneumoniae on dendritic cell phenotype and function. J. Mol. Med. 82: 197–205 [DOI] [PubMed] [Google Scholar]
- 8. Christensen HR, Frøkiaer H, Pestka JJ. 2002. Lactobacilli differentially modulate expression of cytokines and maturation surface markers in murine dendritic cells. J. Immunol. 168: 171–178 [DOI] [PubMed] [Google Scholar]
- 9. Clancy R. 2003. Immunobiotics and the probiotic evolution. FEMS Immunol. Med. Microbiol. 38: 9–12 [DOI] [PubMed] [Google Scholar]
- 10. Deng JC, et al. 2006. Sepsis-induced suppression of lung innate immunity is mediated by IRAK-M. J. Clin. Invest. 116: 2532–2542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Drakes M, Blanchard T, Czinn S. 2004. Bacterial probiotic modulation of dendritic cells. Infect. Immun. 72: 3299–3309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Escoll P, et al. 2003. Rapid up-regulation of IRAK-M expression following a second endotoxin challenge in human monocytes and in monocytes isolated from septic patients. Biochem. Biophys. Res. Commun. 311: 465–472 [DOI] [PubMed] [Google Scholar]
- 13. Fujie H, et al. 2011. Toll-like receptor-2 activating bifidobacteria strains differentially regulate inflammatory cytokines in porcine intestinal epithelial cell culture system: finding new anti-inflammatory immunobiotics. FEMS Immunol. Med. Microbiol. 63: 129–139 [DOI] [PubMed] [Google Scholar]
- 14. Galdeano CM, Perdigón G. 2006. The probiotic bacterium Lactobacillus casei induces activation of the gut mucosal immune system through innate immunity. Clin. Vaccine Immunol. 13: 219–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hoarau C, Lagaraine C, Martin L, Velge-Roussel F, Lebranchu Y. 2006. Supernatant of Bifidobacterium breve induces dendritic cell maturation, activation, and survival through a Toll-like receptor 2 pathway. J. Allergy Clin. Immunol. 117: 696–702 [DOI] [PubMed] [Google Scholar]
- 16. Hosoya S, et al. 2011. Immunobiotic lactic acid bacteria beneficially regulate immune response triggered by poly(I:C) in porcine intestinal epithelial cells. Vet. Res. 42: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Karlsson H, Larsson P, Wold AE, Rudin A. 2004. Pattern of cytokine responses to gram-positive and gram-negative commensal bacteria is profoundly changed when monocytes differentiate into dendritic cells. Infect. Immun. 72: 2671–2678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kessel A, et al. 2008. Treatment with glutamine is associated with down-regulation of Toll-like receptor-4 and myeloid differentiation factor 88 expression and decrease in intestinal mucosal injury caused by lipopolysaccharide endotoxaemia in a rat. Clin. Exp. Immunol. 151: 341–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lech M, et al. 2007. Different roles of TiR8/Sigirr on Toll-like receptor signaling in intrarenal antigen-presenting cells and tubular epithelial cells. Kidney Int. 72: 182–192 [DOI] [PubMed] [Google Scholar]
- 20. Liew FY, Xu DM, Brint EK, O'Neill LAJ. 2005. Negative regulation of Toll-like receptor-mediated immune responses. Nat. Rev. Immunol. 5: 446–458 [DOI] [PubMed] [Google Scholar]
- 21. Macdonald TT, Monteleone G. 2005. Immunity, inflammation and allergy in the gut. Science 307: 1920–1925 [DOI] [PubMed] [Google Scholar]
- 22. Mohamadzadeh M, et al. 2005. Lactobacilli activate human dendritic cells that skew T cells toward T helper 1 polarization. Proc. Natl. Acad. Sci. U. S. A. 102: 2880–2885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moue M, et al. 2008. Toll-like receptor 4 and cytokine expression involved in functional immune response in an originally established porcine intestinal epitheliocyte cell line. Biochim. Biophys. Acta 1780: 134–144 [DOI] [PubMed] [Google Scholar]
- 24. Nomura F, et al. 2000. Cutting edge: endotoxin tolerance in mouse peritoneal macrophages correlates with down-regulation of surface Toll-like receptor 4 expression. J. Immunol. 164: 3476–3479 [DOI] [PubMed] [Google Scholar]
- 25. Qin J, Qian Y, Yao J, Grace C, Li X. 2005. SIGIRR inhibits interleukin-1 receptor- and Toll-like receptor 4-mediated signaling through different mechanisms. J. Biol. Chem. 280: 25233–25241 [DOI] [PubMed] [Google Scholar]
- 26. Salva S, Villena J, Alvarez S. 2010. Differential immunomodulatory activity of Lactobacillus rhamnosus strains isolated from goat milk: impact on intestinal and respiratory infections. Int. J. Food Microbiol. 141: 82–89 [DOI] [PubMed] [Google Scholar]
- 27. Shida K, Kiyoshima-Shibata J, Kaji R, Nagaoka M, Nanno M. 2009. Peptidoglycan from lactobacilli inhibits interleukin-12 production by macrophages induced by Lactobacillus casei through Toll-like receptor 2-dependent and independent mechanisms. Immunology 128: e858–e869 doi: 10.1111/j.1365-2567.2009.03095.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shimazu T, et al. 2012. Immunobiotic Lactobacillus jensenii elicit anti-inflammatory activity in porcine intestinal epithelial cells by modulating negative regulators of the Toll-like receptor signaling pathway. Infect. Immun. 80: 276–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shimosato T, et al. 2005. Toll-like receptor 9 is expressed on follicle-associated epithelia containing M cells in swine Peyer's patches. Immunol. Lett. 98: 83–89 [DOI] [PubMed] [Google Scholar]
- 30. Summerfield A, McCullough KC. 2009. The porcine dendritic cell family. Dev. Comp. Immunol. 33: 299–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tohno M, et al. 2005. Toll-like receptor 2 is expressed on the intestinal M cells in swine. Biochem. Biophys. Res. Commun. 330: 547–554 [DOI] [PubMed] [Google Scholar]
- 32. Tohno M, et al. 2006. Toll-like receptor 2 and 9 are expressed and functional in gut-associated lymphoid tissues of presuckling newborn swine. Vet. Res. 37: 791–812 [DOI] [PubMed] [Google Scholar]
- 33. Tohno M, et al. 2007. Advanced molecular immunoassay system for immunobiotic lactic acid bacteria using a transfectant of Toll-like receptor 2. Anim. Sci. J. 78: 195–205 [Google Scholar]
- 34. Veckman V, et al. 2004. Streptococcus pyogenes and Lactobacillus rhamnosus differentially induce maturation and production of Th1-type cytokines and chemokines in human monocyte-derived dendritic cells. J. Leukoc. Biol. 75: 764–771 [DOI] [PubMed] [Google Scholar]
- 35. Vereecke L, et al. 2010. Enterocyte-specific A20 deficiency sensitizes to tumor necrosis factor-induced toxicity and experimental colitis. J. Exp. Med. 207: 1513–1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Villena J, et al. 2005. Lactobacillus casei improves resistance to pneumococcal respiratory infection in malnourished mice. J. Nutr. 135: 1462–1469 [DOI] [PubMed] [Google Scholar]
- 37. Villena J, Medina M, Vintiñi E, Alvarez S. 2008. Stimulation of respiratory immunity by oral administration of Lactococcus lactis. Can. J. Microbiol. 54: 630–638 [DOI] [PubMed] [Google Scholar]
- 38. Villena J, Aso H, Alvarez S, Kitazawa H. Porcine Toll-like receptors and their crosstalk with immunobiotics: impact in the regulation of gut inflammatory immunity. In Smith A, Jones CL. (ed.), Probiotics: sources, types and health benefits, in press. NOVA Science Publishers, Hauppauge, NY [Google Scholar]
- 39. Wen K, et al. 2009. Toll-like receptor and innate cytokine responses induced by lactobacilli colonization and human rotavirus infection in gnotobiotic pigs. Vet. Immunol. Immunopathol. 127: 304–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Werner SL, et al. 2008. Encoding NF-kappa B temporal control in response to TNF: distinct roles for the negative regulators I kappa B alpha and A20. Genes Dev. 22: 2093–2101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zeuthen LH, Fink LN, Frokiaer H. 2008. Toll-like receptor 2 and nucleotide-binding oligomerization domain-2 play divergent roles in the recognition of gut-derived lactobacilli and bifidobacteria in dendritic cells. Immunology 124: 489–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang W, et al. 2008. Lactic acid bacterial colonization and human rotavirus infection influence distribution and frequencies of monocytes/macrophages and dendritic cells in neonatal gnotobiotic pigs. Vet. Immunol. Immunopathol. 121: 222–231 [DOI] [PMC free article] [PubMed] [Google Scholar]