Abstract
The importance of neutralizing antibody in protection against influenza virus is well established, but the role of the early antibody response during the initial stage of infection in affecting the severity of disease is unknown. The 2009 influenza pandemic provided a unique opportunity for study because most patients lacked preexisting neutralizing antibody. In this study, we compared the antibody responses of 52 patients with severe or mild disease, using sera collected at admission. A microneutralization (MN) assay was used to detect neutralizing antibody. We also developed an enzyme-linked immunosorbent assay (ELISA) which detects both neutralizing and nonneutralizing antibodies against viral antigens from a split-virion inactivated monovalent influenza virus vaccine. While the MN titers were not significantly different between the two groups (P = 0.764), the ELISA titer and ELISA/MN titer ratio were significantly higher for patients with severe disease than for those with mild disease (P = 0.004 and P = 0.011, respectively). This finding suggested that in patients with severe disease, a larger proportion of serum antibodies were antibodies with no detectable neutralizing activity. The antibody avidity was also significantly higher in patients with severe disease than in those with mild disease (P < 0.05). Among patients with severe disease, those who required positive pressure ventilation (PPV) had significantly higher ELISA titers than those who did not require PPV (P < 0.05). Multivariate analysis showed that the ELISA titer and antibody avidity were independently associated with severe disease. Higher titers of nonneutralizing antibody with higher avidity at the early stage of influenza virus infection may be associated with worse clinical severity and poorer outcomes.
INTRODUCTION
Epidemiological and virological studies have identified several risk factors for severe influenza virus infection, including host factors such as extremes of age, comorbid illness, pregnancy, and obesity (22, 24, 38) and viral factors such as specific virus strains, including the 1918 H1N1 virus and the A(H5N1) virus subtype (50), and specific mutations of viral proteins, such as the D222G mutation (or D225G with H3 numbering) in the hemagglutinin (HA) of the pandemic H1N1 2009 influenza virus [A(H1N1)pdm09] (7, 8, 40, 53). Immunological studies have linked a lower serum immunoglobulin G2 level with severe disease caused by A(H1N1)pdm09 (5). Though most patients are asymptomatic or develop only mild coryzal symptoms, even if they have multiple risk factors, a significant number of healthy young patients develop respiratory failure or other extrapulmonary life-threatening complications caused by A(H1N1)pdm09 (38, 39, 51). Therefore, unidentified factors that affect the progression and severity of influenza remain to be discovered.
The early innate immune response against influenza virus may be important in controlling viral replication and hence the peak viral load, because most patients who had severe disease were admitted to the hospital within 5 days of symptom onset (38, 47). In addition to the defensive factors mounted by the innate immune system, such as pattern recognition receptors, interferon-related antiviral mechanisms, the complement cascade, and antimicrobial peptides (9, 36), another important immune mechanism operating during this early phase of infection is the presence of cross-reactive antibodies induced by prior influenza virus infection, including preexisting cross-reactive antibodies and the secondary antibody response from memory B cells (45). In this study, we sought to assess the association between the amount of influenza A virus-specific antibodies during the early stage of illness and patient outcome. Furthermore, to understand the contribution of nonneutralizing antibodies, defined here as antibodies that were not detected by the viral microneutralization (MN) assay, we used a split-virion inactivated A(H1N1)pdm09 vaccine as the coating antigen in an enzyme-linked immunosorbent assay (ELISA) and in an avidity assay. Since most patients born after the 1950s had few preexisting cross-reactive neutralizing antibodies against this novel virus (51), the use of the A(H1N1)pdm09 vaccine provided us with a unique opportunity to investigate whether preexisting cross-reactive nonneutralizing antibody against this new virus has a unique role in determining patient outcomes. The relative quantities of these influenza A virus-specific antibodies and neutralizing antibody were analyzed by comparing the ELISA and MN titers. Furthermore, we also compared the quality of the antibodies from severe and mild cases by using antibody avidity assays.
MATERIALS AND METHODS
Patients and clinical characteristics.
Adult patients with laboratory-confirmed A(H1N1)pdm09 infection with available archived serum samples which were obtained within 2 to 4 days after symptom onset were included. Excluded groups were children below 18 years of age and patients without sufficient archived specimens. Clinical data were retrieved from a retrospective review of medical records. Patients were defined as having severe disease if they required respiratory support and/or admission to the intensive care unit or died; those who survived and did not develop oxygen desaturation or require admission to the intensive care unit were defined as having mild disease (41). The study was approved by the institutional review board of the Hospital Authority in Hong Kong.
Composition of the vaccine antigen used for ELISA and avidity assay.
The 5-ml multidose vial of an A(H1N1)pdm09 vaccine (Panenza; Sanofi Pasteur, France) was employed as the coating antigen in the ELISA. This vaccine is a nonadjuvanted, split-virion, inactivated vaccine (31). According to the manufacturer, each 0.5-ml dose of the vaccine contains 15 μg of HA. The same batch of the vaccine was used for all experiments to ensure consistency.
ELISA.
The ELISA method was modified slightly from our published protocols (16, 48). Ninety-six-well immunoplates (Nunc Immuno modules; Nunc, Denmark) were coated with 100 μl of the vaccine at 2 μg HA/ml in 0.05 M NaHCO3 (pH 9.6) overnight at 4°C and then blocked with 1% normal goat serum at 300 μl/well at 37°C for 1 h. After washing 3 times with phosphate-buffered saline containing 0.05% Tween 20 (PBS-T), 100-μl serum samples at 2-fold serial dilutions were added to the wells and incubated at 37°C for 1 h. After 3 washes, 100 μl of PBS was added to each well and incubated at room temperature for 30 min. The plates were washed 6 times, and 100 μl anti-human gamma chain peroxidase (diluted 1:10,000) (Zymax; Invitrogen) was added to each well as a secondary antibody for 1 h at 37°C. The reaction was developed by adding 100 μl diluted 3,3′,5,5′-tetramethylbenzidine single solution (Invitrogen) for 15 min at 37°C and stopped with 100 μl 1 N H2SO4. The optical density (OD) was read at 450 nm. All samples were tested in duplicate, and the mean absorbance was calculated. The ELISA titer was the dilution at which the absorbance was nearest to 1.0.
Determination of avidity.
ELISA was performed as described above, with the following modifications. First, the sera were added at a dilution with an expected absorbance of 1.0 ± 0.2 to reach the linear part of the titration curve. Second, after the sera were incubated for 1 h, 4 M urea was added instead of PBS. This concentration of urea was chosen because our preliminary experiments showed that urea concentrations of >4 M always markedly reduced the OD value even if the urea was added, incubated, and washed before the reaction with the sera. This suggested that such a high urea concentration can cause a falsely low reading by removing the coated vaccine antigen. The avidity index was defined as the ratio of the OD with urea to the OD without urea (26).
MN assay.
The MN assay was performed as described previously (6, 16, 17). Briefly, serial dilutions of serum were mixed with 100 50% tissue culture infective doses (TCID50) of A/HK/415742/2009 virus for 2 h at 37°C before being added to Madin-Darby canine kidney cells. One hour after infection, the virus-serum mixture was removed, and serum-free minimal essential medium with 2 μg/ml of l-1-tosylamide-2-phenylethyl chloromethyl ketone-treated trypsin (TPCK-trypsin; Sigma Immunochemical) was added to each well. Cytopathic effect was observed 3 or 4 days after incubation at 37°C. The highest serum dilution that protected ≥50% of the cells from cytopathology was considered to be the MN titer. All samples were tested in duplicate.
Statistical analysis.
The Mann-Whitney U test was used for continuous variables, whereas the chi-square or Fisher exact test was used for categorical variables. A value of 5 was arbitrarily assigned to all MN titers below the limit of detection. The ratio of ELISA and MN antibody titers (ELISA/MN titer ratio) was used as an estimate of the quantity of nonneutralizing antibody. All statistical calculations involving the geometric mean titer (GMT) were performed with log-transformed titers. Correlations between the ELISA and MN titers were assessed by Spearman's rank correlation coefficient test. Stepwise multiple logistic regression analysis was used to control for confounding variables, including age, sex, and underlying comorbidities. P values of <0.05 were considered to be statistically significant.
RESULTS
A total of 52 patients were included in this study, with a median age of 49.5 years and an age range of 21 to 86 years. Twenty-five patients (48.1%) were female. There were 30 patients with severe disease and 22 with mild disease (Table 1). There was no significant difference between the severe and mild disease groups in the time interval between symptom onset and serum sample collection (P = 0.121). The baseline demographics and underlying predisposing factors between patients with severe and mild disease were well matched. Obesity was not analyzed because the body mass index was not available for some of the patients. Among patients with severe disease, 16 (53.3%) patients required positive pressure ventilation (PPV), 15 (50.0%) had respiratory failure and were admitted to the intensive care unit for close monitoring and respiratory support, and 4 (13.3%) patients succumbed.
Table 1.
Demographics and clinical characteristics of patients
| Parameter | Value for patient group |
P valuea | |
|---|---|---|---|
| Severe disease (n = 30) | Mild disease (n = 22) | ||
| Age (yr) (median [range]) | 46.5 (22–79) | 54.5 (21–86) | 0.136 |
| No. (%) of females | 13 (43.3) | 12 (54.5) | 0.575 |
| No. (%) of patients with underlying predisposing factor | |||
| Heart disease | 4 (13.3) | 3 (13.6) | 1.000 |
| Pulmonary disease | 7 (23.3) | 2 (9.1) | 0.272 |
| Liver disease | 0 (0) | 1 (4.5) | 0.423 |
| Renal disease | 4 (13.3) | 2 (9.1) | 1.000 |
| Hemoglobinopathy | 1 (3.3) | 0 (0) | 1.000 |
| Neurological disease | 2 (6.7) | 6 (27.3) | 0.058 |
| Metabolic disease | 7 (23.3) | 5 (22.7) | 1.000 |
| Malignancy | 6 (20.0) | 3 (13.6) | 0.717 |
| Connective tissue disease | 3 (10.0) | 2 (9.1) | 1.000 |
| Transplant | 3 (10.0) | 2 (9.1) | 1.000 |
| Pregnancy | 0 (0) | 0 (0) | NA |
NA, not applicable.
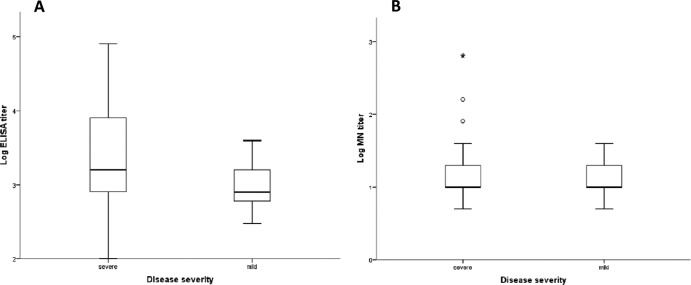
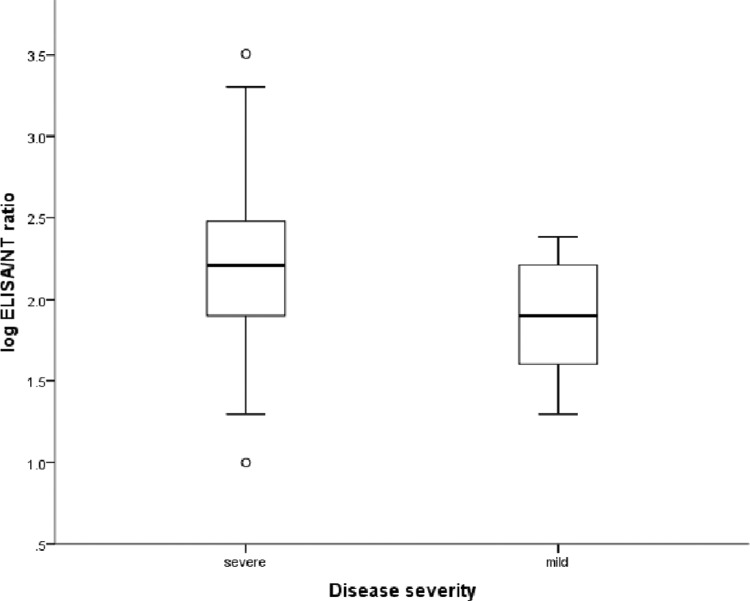
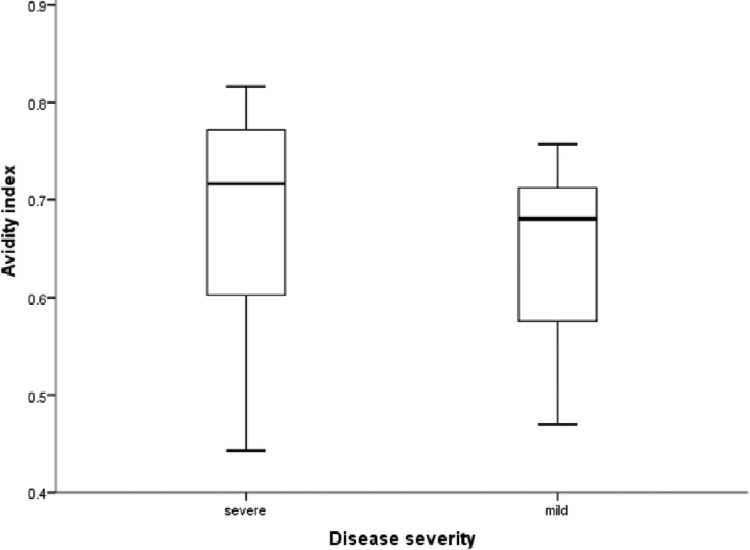
Figure 1 shows a comparison of the antibody titers for patients with severe and mild disease. The ELISA GMT was 3.1 times higher for patients with severe disease than for those with mild disease (P = 0.004). Although the median neutralizing antibody titer determined by MN assay was higher for patients with severe disease than for those with mild disease, the difference did not reach statistical significance (P = 0.764). The ELISA/MN titer ratio was significantly higher for patients with severe disease than for those with mild disease (P = 0.011) (Fig. 2). The antibody avidity, expressed as the avidity index, was significantly higher for patients with severe disease than for those with mild disease (P = 0.045) (Fig. 3). There was no significant correlation between age and ELISA titer, MN titer, ELISA/MN titer ratio, or the antibody avidity index. Among patients with severe disease, those who required PPV had significantly higher ELISA titers than those who did not require PPV, and there was a trend toward higher ELISA/MN titer ratios for patients requiring PPV than for those not requiring PPV, although the difference did not reach statistical significance (Table 2). Multivariate analysis, which adjusted for sex, age, and underlying comorbidities, showed that the ELISA titer (P = 0.003) and avidity index (P = 0.014) were independently associated with severe disease.
Fig 1.
Comparison of antibody titers of patients with severe and mild disease. Medians, quartiles, and ranges are shown. (A) ELISA titers; (B) MN titers.
Fig 2.
Comparison of ELISA/MN titer ratios of patients with severe and mild disease. Medians, quartiles, and ranges are shown. The difference between the groups is significant (Mann-Whitney U test; P = 0.011).
Fig 3.
Differences in antibody avidity between patients with severe disease and those with mild disease. Medians, quartiles, and ranges are shown. The difference between the groups is significant (Mann-Whitney U test; P = 0.045).
Table 2.
Comparison of patients with severe disease who required positive pressure ventilation and those who did not require positive pressure ventilation
| Parametera | Value for patient group |
P value | |
|---|---|---|---|
| Patients requiring PPV (n = 16) | Patients not requiring PPV (n = 14) | ||
| ELISA GMT (95% confidence interval) | 5,077 (2,130–12,102) | 1,585 (724–3,470) | 0.047 |
| MN GMT (95% confidence interval) | 25.9 (10.6–61.4) | 13.5 (6.7–27.0) | 0.247 |
| Median (range) ELISA/MN titer ratio | 200 (20–3,200) | 140 (10–2,000) | 0.116 |
| Median (range) avidity index | 0.71 (0.44–0.80) | 0.72 (0.53–0.82) | 0.589 |
ELISA, enzyme-linked immunosorbent assay; GMT, geometric mean titer; MN, microneutralization. PPV, positive pressure ventilation.
DISCUSSION
Humoral immunity plays an important role in the host defense against influenza virus infection. One of the key functions of antibodies is to neutralize the virus, rendering it noninfective, and these antibodies can be measured by MN assay, which detects antibodies that neutralize viruses, or indirectly by hemagglutination inhibition (HI) assay, which detects antibodies targeting the sialic acid binding sites of the surface HA protein. Antibody titers determined by MN and HI assays usually have good correlation with recent A(H1N1)pdm09 infection (15). MN or HI titers of 40 or more are associated with protection from influenza virus infection and are often used as markers of successful immunization in influenza vaccine trials (23, 30). During the first few days of an infection, the neutralizing antibody titers are often low (34). However, the magnitude of nonneutralizing antibodies during this period has not been studied. We hypothesized that the nonneutralizing antibodies in the early stage of infection may play a role in the outcome of an infection. To detect nonneutralizing antibodies, we used an ELISA plate coated with a split-virion inactivated A(H1N1)pdm09 virus vaccine as antigen, which could detect both neutralizing and nonneutralizing antibodies against the vaccine proteins. We showed that within 2 to 4 days after symptom onset, significantly higher ELISA titers were found for patients with severe disease than for those with mild disease, but there was no significant difference in their MN titers. The ELISA/MN titer ratio, which is a surrogate marker for the amount of nonneutralizing antibody, was higher for patients with severe disease than for those with mild disease. Patients requiring PPV, representing those who had the most severe respiratory disease, had higher ELISA titers than those who did not require PPV. The results from our study suggested that an exaggerated nonneutralizing antibody response during the early stage of infection was associated with severe disease.
The nonneutralizing antibody present in patients during the early stage of infection was likely to be preexisting or was the result of a secondary heterotypic antibody response against conserved epitopes, which may be found outside the receptor binding pocket of HA or in the highly conserved nucleoprotein, matrix proteins, and polymerase proteins, or even the less conserved NS proteins (11). This early IgG response can happen within a few days after infection because of immune priming by previous exposure to similar viral epitopes. In our ELISA, we used a split-virion inactivated A(H1N1)pdm09 vaccine as the coating antigen, and it contained mainly HA but also other proteins of the A(H1N1)pdm09 virus, including the neuraminidase, the matrix protein, and the nucleoprotein (4). The matrix proteins and nucleoprotein have conserved amino acid sequences, and therefore antibodies against these proteins from prior seasonal influenza virus infection or vaccination could be induced. Upon infection with the A(H1N1)pdm09 virus, memory B cells can proliferate rapidly and generate a large amount of these high-avidity nonneutralizing antibodies, especially in patients with severe disease. This is consistent with the observation that the number of peripheral blood B cells is higher in patients with severe disease than in those with mild disease during the early stage of infection (14).
Nonneutralizing antibodies against influenza virus can have either protective, neutral nonprotective, or detrimental effects. Antibodies against the M2 ectodomain and the nucleoprotein have been shown to be protective in animal models (2, 10, 19, 21). On the other hand, antibodies against NS1 have been shown to delay viral clearance (21). This is consistent with the previous finding that a delay in viral clearance rather than a high initial viral load in the respiratory tract is associated with severe disease (37, 38). Antibodies against PB1-F2 and M1 can be detected in patients with influenza virus infection, but the functional significance of these antibodies is unknown (20, 52). Other potential effects of nonneutralizing antibody include the activation of the complement cascade and antibody-dependent cellular cytotoxicity, which can be protective or detrimental if excessively proinflammatory (1, 27). Besides the uncertainty of these effects on the clinical outcome, the avidity of such nonneutralizing antibodies for complement activation and antibody-dependent cytotoxicity is also unknown. If these effects turn out to be detrimental, it is conceivable that such nonneutralizing antibodies with high avidity would produce more damage to patients than antibodies with low avidity. Though the notion that prior seasonal influenza vaccination is associated with worse outcomes for patients with subsequent A(H1N1)pdm09 virus infection has been disputed, nonneutralizing antibodies may play a role if this is indeed the case (33). Detrimental effects of nonneutralizing antibody have been speculated to be associated with poor outcomes of dengue virus, respiratory syncytial virus, and measles virus infections via antibody-dependent enhancement (42). Though the laboratory phenomenon of antibody-dependent enhancement has also been shown for influenza virus, no clinical study has ever been performed to ascertain the link (35). The pro- or anti-inflammatory effects of nonneutralizing antibody in the pathogenesis and outcome of influenza should be investigated further (3).
Antibody avidity was assessed in the current study by comparing the ELISA OD values with and without urea, which is a dissociating agent that can disrupt the interaction between the antibody and the coating antigen. If an antibody binds to the antigen weakly, then the addition of urea will disrupt the binding, resulting in a lower OD value. We have shown that higher antibody avidities are independently associated with severe disease. This is consistent with our finding that patients with severe disease have higher levels of secondary antibody production, presumably from memory B cells. Our finding appears to contradict a study by Monsalvo et al., who showed that patients with severe A(H1N1)pdm09 virus infection had lower antibody avidity than those with mild disease (28). Several groups have also examined the relationship between age and antibody avidity in patients with natural infection, and they have shown that antibody avidity is higher in older than in younger age groups (28, 44). For antibody responses after influenza vaccination, one study showed a higher antibody avidity in the elderly than in younger vaccine recipients, while another study did not find any differences (18, 32). In our study, we did not find any correlation between age and antibody avidity. There are several important differences between other studies and ours which may explain the discrepancy of the results. First, we examined the serum antibodies that were present shortly after a natural infection, which are probably different from the antibodies arising in the convalescent phase after natural infection or vaccination. Second, we used a split-virion influenza vaccine, which contains HA and other viral proteins, while other studies used pure recombinant HA without other viral proteins. There may be differences in avidity for antibodies directed against HA but not for those against other viral proteins. Finally, a sampling bias may have occurred in the severe cases, because for those patients who died early on, no sera could be collected in the convalescent phase.
In this study, we employed a split-virion inactivated vaccine. This approach has several advantages over using recombinant antigens. First, by using the vaccine as the coating antigen, we could detect the sum of a wide range of IgGs against different viral components instead of a limited IgG response specific to a particular protein. Since it has been demonstrated that antibodies may have synergistic effects, our study is deemed more relevant by examining the antibody response against a range of different viral proteins instead of just the antibody response against a single viral protein (43). Second, recombinant proteins may assume an altered conformation, and conformation-dependent antibodies may not recognize the altered antigen structure (49). The use of a vaccine also differs from the use of inactivated virus, because zonal centrifugation will remove nonviral host materials which may lead to nonspecific reactions (13).
There were several limitations to our study. We did not test the antibody titers in serum samples collected on day 0 and day 1 after symptom onset because most patients did not attend the hospital during this early stage of illness. Except for HA, the exact quantity of each protein present in our batch of the vaccine was not known. However, we used the same batch of vaccine across all experiments to ensure consistency of the antigen content. Another limitation of our assay was that the high ELISA titers may have been related to antibodies that could neutralize the virus in vivo but could not be detected with the current MN assay (25). Finally, although the high ELISA titers were likely related to the rapid production of nonneutralizing antibody targeting conserved epitopes, we do not know the quantity of preexisting antibody, as preinfection serum samples were not available.
Our study is the first to look at the early antibody response (both neutralizing and nonneutralizing antibodies) against current vaccine components and the association between these antibodies and patient outcomes. The excessive nonneutralizing antibody response during early infection may have contributed to the dysregulated inflammation in severe infection (38). It is important that even an HI titer of 40, which is considered to be a protective titer reflecting mainly a neutralizing antibody response in vaccine studies (12), was associated with a protective efficacy of only 70% in an exposed population (30). Moreover, some vaccinated patients with very high HI titers (up to 2,048) developed disease after exposure to influenza virus (29). This protection is therefore not absolute and can be overcome by a larger dose of virus (3). Once the virus enters cells and the viral life cycle is started, every infected cell will produce millions of infectious virions, which can easily overcome the neutralizing antibody in the interstitial fluid to infect adjacent cells as far away as where the balance occurs between the dilution of virus and the neutralization effect of antibody. Thus, at the anatomical site where the viral load cannot be controlled by the initial innate immune response, the concomitant innate inflammatory response and the subsequently mounted adaptive immune response with nonneutralizing cross-reactive antibody may just fuel inflammatory damage. This is a significant issue in the elderly and those with severe comorbidities, whose respiratory reserve is marginal and tissue regenerative power is poor. In addition, inflammatory cytokines spilled over into the circulation may precipitate acute catastrophes such as stroke or heart attack (46). Further studies should be conducted to ascertain the role of nonneutralizing antibody in the pathogenesis of severe influenza.
ACKNOWLEDGMENTS
This work was supported by the National Science and Technology Major Project of China (grant 2009ZX10004-306), the Providence Foundation Limited (in memory of the late Lui Hac Minh), and the Consultancy Service for Enhancing Laboratory Surveillance of Emerging Infectious Disease for the HKSAR Department of Health.
Footnotes
Published ahead of print 9 May 2012
REFERENCES
- 1. Beebe DP, Schreiber RD, Cooper NR. 1983. Neutralization of influenza virus by normal human sera: mechanisms involving antibody and complement. J. Immunol. 130: 1317–1322 [PubMed] [Google Scholar]
- 2. Carragher DM, Kaminski DA, Moquin A, Hartson L, Randall TD. 2008. A novel role for non-neutralizing antibodies against nucleoprotein in facilitating resistance to influenza virus. J. Immunol. 181: 4168–4176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Casadevall A, Pirofski LA. 2011. A new synthesis for antibody-mediated immunity. Nat. Immunol. 13: 21–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chaloupka I, Schuler A, Marschall M, Meier-Ewert H. 1996. Comparative analysis of six European influenza vaccines. Eur. J. Clin. Microbiol. Infect. Dis. 15: 121–127 [DOI] [PubMed] [Google Scholar]
- 5. Chan JF, et al. 2011. The lower serum immunoglobulin G2 level in severe cases than in mild cases of pandemic H1N1 2009 influenza is associated with cytokine dysregulation. Clin. Vaccine Immunol. 18: 305–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chan KH, et al. 2011. Differences in antibody responses of individuals with natural infection and those vaccinated against pandemic H1N1 2009 influenza. Clin. Vaccine Immunol. 18: 867–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chan KH, et al. 2010. Wild type and mutant 2009 pandemic influenza A (H1N1) viruses cause more severe disease and higher mortality in pregnant BALB/c mice. PLoS One 5: e13757 doi: 10.1371/journal.pone.0013757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen H, et al. 2010. Quasispecies of the D225G substitution in the hemagglutinin of pandemic influenza A(H1N1) 2009 virus from patients with severe disease in Hong Kong, China. J. Infect. Dis. 201: 1517–1521 [DOI] [PubMed] [Google Scholar]
- 9. Ehrhardt C, et al. 2010. Interplay between influenza A virus and the innate immune signaling. Microbes Infect. 12: 81–87 [DOI] [PubMed] [Google Scholar]
- 10. El Bakkouri K, et al. 2011. Universal vaccine based on ectodomain of matrix protein 2 of influenza A: Fc receptors and alveolar macrophages mediate protection. J. Immunol. 186: 1022–1031 [DOI] [PubMed] [Google Scholar]
- 11. ElHefnawi M, et al. 2011. Identification of novel conserved functional motifs across most influenza A viral strains. Virol. J. 8: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.European Agency for the Evaluation of Medicinal Products (EMEA) Committee for Proprietary Medicinal Products (CPMP) 1997. Note for guidance on harmonization of requirements for influenza vaccines. EMEA, London, United Kingdom [Google Scholar]
- 13. Gerin JL, Anderson NG. 1969. Purification of influenza virus in the K-II zonal centrifuge. Nature 221: 1255–1256 [DOI] [PubMed] [Google Scholar]
- 14. Guo X, et al. 2011. Dynamic variations in the peripheral blood lymphocyte subgroups of patients with 2009 pandemic H1N1 swine-origin influenza A virus infection. Virol. J. 8: 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hancock K, et al. 2009. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N. Engl. J. Med. 361: 1945–1952 [DOI] [PubMed] [Google Scholar]
- 16. Hung IF, Levin Y, To KK. 2012. Quantitative and qualitative analysis of antibody response after dose sparing intradermal 2009 H1N1 vaccination. Vaccine 30: 2707–2708 [DOI] [PubMed] [Google Scholar]
- 17. Hung IF, et al. 2010. Effect of clinical and virological parameters on the level of neutralizing antibody against pandemic influenza A virus H1N1 2009. Clin. Infect. Dis. 51: 274–279 [DOI] [PubMed] [Google Scholar]
- 18. Khurana S, Verma N, Talaat KR, Karron RA, Golding H. 2012. Immune response following H1N1pdm09 vaccination: differences in antibody repertoire and avidity in young adults and elderly populations stratified by age and gender. J. Infect. Dis. 205: 610–620 [DOI] [PubMed] [Google Scholar]
- 19. Kiraly J, Vareckova E, Mucha V, Kostolansky F. 2011. Evaluation of anti-influenza efficiency of polyclonal IgG antibodies specific to the ectodomain of M2 protein of influenza A virus by passive immunization of mice. Acta Virol. 55: 261–265 [DOI] [PubMed] [Google Scholar]
- 20. Krejnusova I, et al. 2009. Antibodies to PB1-F2 protein are induced in response to influenza A virus infection. Arch. Virol. 154: 1599–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. LaMere MW, et al. 2011. Contributions of antinucleoprotein IgG to heterosubtypic immunity against influenza virus. J. Immunol. 186: 4331–4339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lapinsky SE. 2010. Critical illness as a result of influenza A/H1N1 infection in pregnancy. BMJ 340: c1235 doi: 10.1136/bmj.c1235 [DOI] [PubMed] [Google Scholar]
- 23. Liu W, et al. 2010. Clinical and immunological characteristics of patients with 2009 pandemic influenza A (H1N1) virus infection after vaccination. Clin. Infect. Dis. 51: 1028–1032 [DOI] [PubMed] [Google Scholar]
- 24. Louie JK, et al. 2011. A novel risk factor for a novel virus: obesity and 2009 pandemic influenza A (H1N1). Clin. Infect. Dis. 52: 301–312 [DOI] [PubMed] [Google Scholar]
- 25. Lynch GW, Selleck P, Church WB, Sullivan JS. 2012. Seasoned adaptive antibody immunity for highly pathogenic pandemic influenza in humans. Immunol. Cell Biol. 90: 149–158 [DOI] [PubMed] [Google Scholar]
- 26. Mathew A, et al. 2011. B-cell responses during primary and secondary dengue virus infections in humans. J. Infect. Dis. 204: 1514–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mochizuki Y, et al. 1990. Protection of mice against Sendai virus pneumonia by non-neutralizing anti-F monoclonal antibodies. Microbiol. Immunol. 34: 171–183 [DOI] [PubMed] [Google Scholar]
- 28. Monsalvo AC, et al. 2011. Severe pandemic 2009 H1N1 influenza disease due to pathogenic immune complexes. Nat. Med. 17: 195–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ohmit SE, Petrie JG, Cross RT, Johnson E, Monto AS. 2011. Influenza hemagglutination-inhibition antibody titer as a correlate of vaccine-induced protection. J. Infect. Dis. 204: 1879–1885 [DOI] [PubMed] [Google Scholar]
- 30. Potter CW, Oxford JS. 1979. Determinants of immunity to influenza infection in man. Br. Med. Bull. 35: 69–75 [DOI] [PubMed] [Google Scholar]
- 31. Sanofi Pasteur. 2009. Panenza package insert. Sanofi Pasteur, Lyon, France [Google Scholar]
- 32. Sasaki S, et al. 2011. Limited efficacy of inactivated influenza vaccine in elderly individuals is associated with decreased production of vaccine-specific antibodies. J. Clin. Invest. 121: 3109–3119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Skowronski DM, et al. 2010. Association between the 2008-09 seasonal influenza vaccine and pandemic H1N1 illness during spring-summer 2009: four observational studies from Canada. PLoS Med. 7: e1000258 doi: 10.1371/journal.pmed.1000258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sun Y, et al. 2010. Immune protection induced on day 10 following administration of the 2009 A/H1N1 pandemic influenza vaccine. PLoS One 5: e14270 doi: 10.1371/journal.pone.0014270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tamura M, Webster RG, Ennis FA. 1994. Subtype cross-reactive, infection-enhancing antibody responses to influenza A viruses. J. Virol. 68: 3499–3504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tecle T, Tripathi S, Hartshorn KL. 2010. Defensins and cathelicidins in lung immunity. Innate Immun. 16: 151–159 [DOI] [PubMed] [Google Scholar]
- 37. To KK, et al. 2010. Viral load in patients infected with pandemic H1N1 2009 influenza A virus. J. Med. Virol. 82: 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. To KK, et al. 2010. Delayed clearance of viral load and marked cytokine activation in severe cases of pandemic H1N1 2009 influenza virus infection. Clin. Infect. Dis. 50: 850–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. To KK, et al. 2010. Concurrent comparison of epidemiology, clinical presentation and outcome between adult patients suffering from the pandemic influenza A (H1N1) 2009 virus and the seasonal influenza A virus infection. Postgrad. Med. J. 86: 515–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tse H, et al. 2011. Structural basis and sequence co-evolution analysis of the hemagglutinin protein of pandemic influenza A/H1N1 (2009) virus. Exp. Biol. Med. (Maywood) 236: 915–925 [DOI] [PubMed] [Google Scholar]
- 41. Tse H, et al. 2011. Clinical and virological factors associated with viremia in pandemic influenza A/H1N1/2009 virus infection. PLoS One 6: e22534 doi: 10.1371/journal.pone.0022534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ubol S, Halstead SB. 2010. How innate immune mechanisms contribute to antibody-enhanced viral infections. Clin. Vaccine Immunol. 17: 1829–1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Varshney AK, et al. 2011. Generation, characterization, and epitope mapping of neutralizing and protective monoclonal antibodies against staphylococcal enterotoxin B-induced lethal shock. J. Biol. Chem. 286: 9737–9747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Verma N, et al. 2012. Influenza virus H1N1pdm09 infections in the young and old: evidence of greater antibody diversity and affinity for the hemagglutinin globular head domain (HA1 domain) in the elderly than in young adults and children. J. Virol. 86: 5515–5522 doi: 10.1128/JVI.07085-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Waffarn EE, Baumgarth N. 2011. Protective B cell responses to flu—no fluke! J. Immunol. 186: 3823–3829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ward JR, Wilson HL, Francis SE, Crossman DC, Sabroe I. 2009. Translational mini-review series on immunology of vascular disease: inflammation, infections and Toll-like receptors in cardiovascular disease. Clin. Exp. Immunol. 156: 386–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Webb SA, et al. 2009. Critical care services and 2009 H1N1 influenza in Australia and New Zealand. N. Engl. J. Med. 361: 1925–1934 [DOI] [PubMed] [Google Scholar]
- 48. Woo PC, et al. 2004. Relative rates of non-pneumonic SARS coronavirus infection and SARS coronavirus pneumonia. Lancet 363: 841–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yewdell JW. 2010. Monoclonal antibodies specific for discontinuous epitopes direct refolding of influenza A virus hemagglutinin. Mol. Immunol. 47: 1132–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yuen KY, et al. 1998. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet 351: 467–471 [DOI] [PubMed] [Google Scholar]
- 51. Zhang AJ, et al. 2011. High incidence of severe influenza among individuals over 50 years of age. Clin. Vaccine Immunol. 18: 1918–1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhang R, Rong X, Pan W, Peng T. 2011. Determination of serum neutralization antibodies against seasonal influenza A strain H3N2 and the emerging strains 2009 H1N1 and avian H5N1. Scand. J. Infect. Dis. 43: 216–220 [DOI] [PubMed] [Google Scholar]
- 53. Zheng B, et al. 2010. D225G mutation in hemagglutinin of pandemic influenza H1N1 (2009) virus enhances virulence in mice. Exp. Biol. Med. (Maywood) 235: 981–988 [DOI] [PubMed] [Google Scholar]