Abstract
Many bacterial components selectively activate immune and nonhematopoietic target cells via Toll-like receptor (TLR) signaling; modulation of such host responses defines the immune adjuvant properties of these bacterial products. For example, the outer membrane protein porins from Neisseria, Salmonella, and Shigella are known TLR2 agonists with established systemic and mucosal immune adjuvanticity. Early work indicated that the FomA porin from Fusobacterium nucleatum has immune adjuvant activity in mice. Using a purified recombinant FomA, we have verified its immune stimulatory properties and have defined a role for TLR2 signaling in its in vitro and in vivo activity. FomA induces interleukin 8 (IL-8) secretion and NF-κB-dependent luciferase activity in HEK cells expressing TLR2, IL-6 secretion, and cell surface upregulation of CD86 and major histocompatibility complex (MHC) II in primary B cells from wild-type mice, but it fails to activate cells from TLR2 knockout mice. Accordingly, the immune adjuvant activity of FomA is also TLR2 dependent. In a mouse model of immunization with ovalbumin (OVA), FomA induces enhanced production of OVA-specific IgM and IgG, including IgG1 and IgG2b antibodies, as well as enhanced secretion of IL-10 and IL-6, consistent with a Th2-type adjuvant effect. We also observe a moderate production of anti-FomA antibodies, suggesting that FomA is also immunogenic, a quality that is also TLR2 dependent. Therefore, modulation of host immune responses by FomA may be effective for targeting general host immunity not only to pathogens (as a novel TLR2 adjuvant) but also to F. nucleatum itself (as an antigen), expanding its use as a self-adjuvanted antigen in an immunization strategy against polymicrobial infections, including those by F. nucleatum.
INTRODUCTION
In the past 2 decades, modulation of host innate and adaptive immunity by bacterial products has been explained in vitro and in vivo by signaling via Toll-like receptors (TLRs), cell surface and intracellular receptors that recognize microbial products (pathogen-associated molecular patterns [PAMPs]) (1, 48). Upon TLR engagement, activation of NF-κB nuclear translocation and mitogen-activated protein (MAP) kinases leads to secretion of inflammatory mediators, expression of costimulatory ligands, and major histocompatibility complex (MHC) molecules, ultimately enhancing host humoral and cellular immune response. Numerous bacterial components have been shown to activate diverse TLRs; for example, lipopolysaccharide (LPS) is the ligand for TLR4 (in complex with MD2) (15, 60), flagellin activates TLR5 (25), and CpG DNA engages TLR9 (7). TLR2 has the broadest ligand repertoire, due to its hetero-dimerization with TLR1 or TLR6; these different TLR2 complexes recognize lipopeptides (19, 31), peptidoglycans (4), and porins from numerous microorganisms (13, 21, 45, 47, 57). Immune cell activation via TLR signaling is the basis for the observed immune adjuvant effect of these bacterial components (30). Among the known bacterial TLR2 ligands with adjuvant activity, the immune stimulatory effect of neisserial porin PorB (14, 20, 41, 65) and porins from Shigella, Salmonella, and Haemophilus influenzae (2, 56, 57), as well as the Escherichia coli heat-labile enterotoxin B subunits [LT-IIa-B(5) and LT-IIb-B(5)], has been extensively characterized (24, 39).
FomA (∼41 kDa) is a major outer membrane protein from Fusobacterium nucleatum, a human oral pathogen (9). It exhibits the functional properties of a general trimeric diffusion porin, although it has no sequence similarity to other porins (6, 10). According to the proposed porin topology model, most porin monomers have a β-barrel conformation and 16 transmembrane antiparallel β-strands (64). The β-strands are connected by short loops at the periplasmic side, while longer loops are exposed to the outside with eight surface-exposed loops. The third surface-exposed loop from the N terminus (loop 3) of most porins, the eyelet loop, folds back into the pore lumen and plays a major role in the permeability of the porin. In contrast, FomA monomers have a 14-strand β-barrel fold with six surface-exposed, variable hydrophilic loops (53) and loop 6 actually functions as the eyelet loop (37, 54). Both native FomA and recombinant FomA expressed in E. coli have been purified (18, 23, 28, 52). Proper refolding of recombinant FomA has been demonstrated, and its structural and functional features have been extensively described, including trimer formation, insertion in lipid bilayers, and pore-forming functions (3, 35, 36, 52).
Previous studies in the late 1980s, antecedent to identification of TLRs, reported immune adjuvant activity of FomA in a mouse model of immunization with sheep red blood cells (SRBCs) (62). This work suggested that the effect of purified native FomA was independent of an abundant F. nucleatum LPS contamination of such preparation. So far, this assumption has not been validated in the context of TLR-dependent signaling induced by different bacterial products. Other in vitro effects attributed to FomA included induction of polyclonal B cell activation and mitogenicity of murine splenocytes, stimulation of guinea pig peritoneal macrophages, and enhancement of human blood monocyte migration (62). More recently, indirect evidence has suggested a role for TLR2 in the activity of FomA. For example, FomA-containing bacterial sonicates induced TLR2-dependent interleukin 8 (IL-8) production (27, 33) but failed to induce reactive-oxygen species (ROS) production by macrophages from TLR2 knockout (KO) mice compared to LPS-sensitive and LPS-nonresponder mice (43). FomA-containing outer membrane preparations also induced expression of antimicrobial compounds, human β-defensins (hBD-2 and hBD-3), in human gingival epithelial cells via TLR2-dependent MAPK/p38 and MAPK/JNK signaling. This was prevented by heat treatment of F. nucleatum and was not due to Fusobacterium LPS (29, 38, 51).
While no further studies on the immune stimulatory effect of FomA have been carried out to date, evidence of its immunogenicity and of the protective effect of anti-FomA antibodies on F. nucleatum infection has been reported. For example, immunization of mice with whole FomA-expressing E. coli organisms or with recombinant FomA and the adjuvant cholera toxin (CT) (a non-TLR adjuvant [26]) induced high levels of circulating and salivary anti-FomA IgG and IgA antibodies protective against F. nucleatum infection (40, 50).
Based on the structural and functional similarities between FomA and other bacterial porins with well-established TLR2-dependent immune stimulatory properties, in this work the potential TLR2 adjuvant nature of FomA was reevaluated. Here, the molecular mechanism of cell activation by purified, LPS-free FomA is examined in vitro and its immune adjuvant activity is evaluated in vivo in a mouse model of immunization with the prototype antigen, ovalbumin (OVA).
MATERIALS AND METHODS
Bacterial strains and growth conditions.
E. coli strain PC2889 harboring the plasmid pET-10953 expressing recombinant FomA protein (52) was a kind gift from H. Kleinschmidt (University of Konstanz, Germany). The organisms were grown aerobically for 12 to 15 h at 37°C in LB medium supplemented with 100 μg/ml ampicillin. Expression of FomA was induced with 0.1 M IPTG for an additional 3 h. The cells were harvested by centrifugation at 4°C, and FomA was purified from inclusion bodies as described below.
FomA purification.
Recombinant FomA was purified by adapting the methods described for neisserial PorB purification (46, 55). FomA-containing E. coli inclusion bodies were suspended in 8 M urea and 5% Zwittergent 3-14 to extract the total protein content and were subjected to protein chromatography purification (46). First, a DEAE-Sepharose column was used and FomA was eluted with buffer containing 50 mM Tris, pH 8.0, 10 mM EDTA, 0.05% Zwittergent 3-14, 0.02% NaN3, and 0.2 M NaCl. The FomA-containing fractions, identified by SDS-PAGE and Coomassie staining, were precipitated with 80% (vol/vol) ethanol and separated by size exclusion chromatography on a Sephacryl-300 column with 100 mM Tris, pH 8.0, 10 mM EDTA, 0.2 M NaCl, 0.05% Zwittergent 3-14, and 0.02% NaN3 (46). FomA-enriched fractions, identified and pooled as described above, were concentrated and further separated on a Matrex Cellufine sulfate column eluted with a 0.2 M to 0.5 M NaCl gradient in 50 mM Tris, pH 7.5, 10 mM EDTA, 0.05% Zwittergent 3-14, and 0.02% NaN3. Concentrated purified FomA fractions were resuspended in 10% d-octyl-glucoside (DOG) in 10 mM HEPES, pH 7.5, followed by extensive dialysis in phosphate-buffered saline (PBS) containing 0.02% NaN3 for formation of detergent-free protein micelles (proteosomes). The protein concentration was determined using the bicinchoninic acid (BCA) assay.
Gel electrophoresis and Western blotting.
Equal amounts of protein fractions were analyzed by Coomassie brilliant blue staining with 12% SDS-PAGE or silver staining with 15% SDS-PAGE. For silver staining, 2 μg total of purified FomA and of E. coli LPS, as a positive control, was used. Prior to electrophoresis, the samples were dissolved in 4× sample buffer (400 mM dithiothreitol [DTT], 40% glycerol, 8% SDS, 200 mM Tris, pH 6.8) and heated for 5 min at 100°C. Western blot analyses were performed using 1 μg total of purified FomA. Blots were blocked for 1 h with 5% nonfat dry milk in Tris-buffered saline (TBS), pH 7.6, 0.1% Tween 20 (TBS-T) and incubated with sera from mice immunized with FomA as described below (1:1,000 dilution) in 5% bovine serum albumin (BSA) in TBS-T overnight at 4°C followed by horseradish peroxidase (HRP)-labeled anti-mouse secondary antibody (Cell Signaling). The immunoreactive bands were detected by enhanced chemiluminescence (ECL) (Amersham).
Cell culture and stimulation.
Stably transfected HEK cells overexpressing TLR2 and TLR4 or an empty vector (pcDNA) (15, 47) were cultured in Dulbecco modified Eagle medium (DMEM) containing 5% fetal bovine serum (FBS), 2 mM l-glutamine, and 10 μg/ml of ciprofloxacin. Murine B cells were isolated from spleens of C57BL/6 mice and TLR2 knockout mice as previously described (66) and grown in RPMI (Mediatech) containing 10% FBS, 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 10 mM HEPES, and 10 mM β-mercaptoethanol (β-ME). HEK cells (105 cells/ml) and B cells (106 cells/ml) were incubated with purified FomA (10 μg/ml), Pam3CSK4 (100 ng/ml) (InvivoGen), lipoprotein-free E. coli LPS (100 ng/ml) (Sigma), or recombinant human tumor necrosis factor alpha (TNF-α) (20 ng/ml) (eBioscience) in triplicate wells for 18 h at 37°C.
NF-κB luciferase reporter assay.
HEK cells were transiently transfected with an NF-κB luciferase reporter vector as previously described (15, 47). Transfected cells were left to adhere overnight in 24-well cell culture plates and stimulated the next day as described above. Luciferase activity was measured using commercial reagents (Promega) per the manufacturer's protocol, and luminescence was assessed using a Wallac Victor2 luminometer.
Cytokine ELISA.
IL-8 and IL-6 secretion was measured by enzyme-linked immunosorbent assay (ELISA) of supernatants from HEK cell cultures and murine B cell cultures stimulated as described above using Opt-EIA kits (BD Biosciences) per the manufacturer's protocol. Results are expressed as pg/ml ± standard deviation (SD). Gamma interferon (IFN-γ), IL-10, IL-6 (R&D System ELISA kit), and TNF-α (eBiosciences ELISA kit) were measured by ELISA of sera from mice immunized as described below. Preimmune sera from each group of mice were pooled and used as a negative control. Results are expressed as pg/ml normalized to preimmune sera ± SD.
Flow cytometry.
Purified mouse B lymphocytes (106/ml) stimulated as described above were collected and examined for upregulation of cell surface activation markers using anti-CD86 and anti MHC-class II murine fluorescein isothiocyanate (FITC)-labeled antibodies (PharMingen, San Diego, CA) (45). As a negative control, anti-rat IgG FITC-labeled antibody was used. Labeled cells were analyzed by flow cytometry on a FACScan flow cytometer using CellQuest acquisition and analysis software (Becton, Dickinson, Mountain View, CA). Gating was used to exclude cellular debris. All the histograms shown are representative of three separate experiments.
Immunization of animals.
Animals were housed and cared for in accordance with National Institutes of Health (NIH) and Boston University protocols for the care and use of laboratory animals. Six-week-old female C57BL/6 mice (Jackson Laboratory) and TLR2 knockout mice (63) (n = 4) were immunized subcutaneously three times at 2-week intervals with 10 μg of ovalbumin (OVA) combined with either 10 μg of FomA in a 1:1 mixture (based on previous experience with neisserial PorB [41, 42]) or 200 μg of alum (Sigma) as adjuvants in a total volume of 100 μl of sterile PBS. Additional groups were immunized with OVA alone and with FomA alone in the absence of other adjuvants. Preimmune sera were collected prior to the first immunization and 2 weeks after each subsequent immunization (1st bleed at week 2, 2nd bleed at week 4, and 3rd bleed at week 6). Sera were aliquoted and kept at −20°C until further analysis. Ovalbumin was obtained from commercial chicken egg whites by freeze-drying followed by lyophilization and resuspension of the total egg white proteins in sterile PBS (61). Ovalbumin obtained with this method represents ∼65% of the total protein content and is LPS-free.
Serum antibody ELISA.
Levels of OVA-specific total IgG and IgM in the sera of immunized mice were determined by ELISA. Immulon 96-well plates were coated overnight with 5 μg of OVA in carbonate buffer, pH 9.6, at 4°C, washed three times with PBS-0.05% Tween 20, and blocked for 2 h with 5% BSA in PBS at room temperature. Serial dilutions of individual mouse sera were added, and the plates were incubated at 4°C for 24 h. Plates were washed and incubated with secondary anti-mouse IgG or IgM alkaline phosphatase (AP)-conjugated antibodies (Sigma) for 2 h at room temperature followed by detection with 1-step PNPP substrate (Pierce) as specified by the manufacturer. Anti-FomA IgG and IgM antibodies were measured as described above using plates coated with 2 μg/ml of purified FomA. The absorbance was measured as the optical density at 405 nm (OD405), and antibody quantification was performed using IgG and IgM standard curves (41). Results are expressed in μg/ml of specific antibodies. For detection of IgG antibody subclasses to OVA and to FomA, plates were coated as described above and incubated with mouse sera (1:100 dilution) followed by goat anti-mouse specific anti-IgG1, -IgG2b, and -IgG2c AP-conjugated secondary antibodies (Southern Biotech). Results are expressed as anti-OVA- and anti-FomA-specific IgG subclass titers at OD405 normalized to those from the preimmune groups.
Statistical analysis.
Statistical analyses were calculated using GraphPad PRISM software. P values were calculated by unpaired t test and by one-way analysis of variance (ANOVA) with Dunnett's multiple comparison posttest, with 99.9% confidence intervals as indicated in the text.
RESULTS
Purification of FomA.
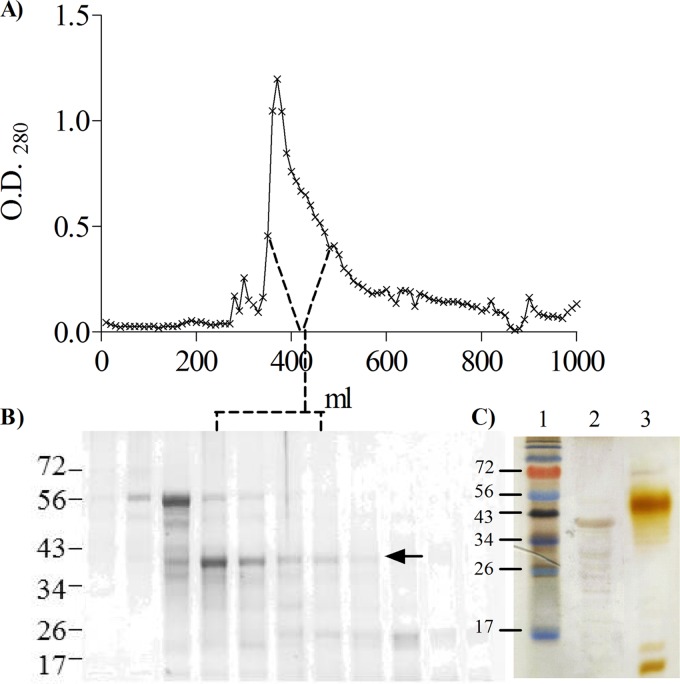
Recombinant FomA (28, 52) was purified following the method that our group has developed for purification of LPS-free and lipoprotein-free neisserial porins (41, 46). E. coli FomA-containing inclusion bodies were solubilized by adding the zwitterionic detergent Zwittergent 3-14, and the protein purification was carried out in the presence of detergent. A three-step process was applied, consisting of ion exchange, size exclusion, and affinity chromatography. Following ion-exchange chromatography on DEAE resin, the majority of FomA was recovered in the column flowthrough, as detected by SDS-PAGE and Coomassie staining (not shown). The FomA-containing material was concentrated and applied to a Sephacryl 300 column, granting separation from protein contaminants of different molecular sizes, as shown in Fig. 1A. Fractions from the observed peaks were examined by SDS-PAGE and Coomassie staining; a band with a molecular size of approximately 40 kDa was identified in the major elution peak (Fig. 1B, arrow), along with minor contaminant bands of smaller molecular size. Enriched FomA-containing fractions were pooled and applied to a Matrex cellulose column for removal of contaminating endotoxin and lipopeptide (46). Purified FomA fractions, identified by SDS-PAGE and Coomassie staining (not shown), were concentrated, resuspended in 10% d-octyl-glucoside (DOG), and dialyzed extensively against PBS to allow formation of detergent-free protein micelles (termed proteosomes). The absence of E. coli LPS contamination was assessed by SDS-PAGE and silver staining, shown in Fig. 1C, and also confirmed by functional assays described below.
Fig 1.
FomA purification. (A) Representative chromatogram of FomA elution from gel filtration chromatography on Sephacryl S300 column. (B) SDS-PAGE and Coomassie staining of representative fractions from panel A. The FomA-containing fractions are indicated by the arrow. FomA was identified as a band of approximately 40 kDa in the major elution peak. (C) SDS-PAGE and silver staining of final FomA preparation. Lane 1, molecular weight standard. Lane 2, FomA, 2 μg total. Lane 3, E. coli LPS, 2 μg total.
FomA induces TLR2-dependent HEK cell activation.
Purified recombinant, LPS-free FomA (10 μg/ml) was used to stimulate HEK cells overexpressing TLR2 (TLR2-HEK cells) or transfected with an empty vector, pcDNA-HEK cells, as a negative control. Induction of NF-κB-driven luciferase activity was measured after overnight incubation (15, 47) and expressed in arbitrary units ± SD normalized to nonstimulated cells. In these cells, FomA induced high luciferase activity only when TLR2 was overexpressed, similar to the TLR2 agonist Pam3CSK4 (100 ng/ml) (Fig. 2A, black bars and white bars) (**, P < 0.0023 by unpaired t test with Welch's correction; n = 12). As a non-TLR2-dependent control, cells were also incubated with recombinant human TNF-α (20 ng/ml), which induced high luciferase activity in both cell types. As another indicator of TLR2-dependent cell activation, secretion of IL-8 was measured by ELISA of cell supernatants and expressed as pg/ml ± SD. Both FomA and Pam3CSK4 induced high levels of IL-8 in TLR2-HEK cells but not in pcDNA-HEK cells (Fig. 2C, black bars and white bars, respectively) (***, P = 0.0002; ****, P < 0.0001; n = 12), whereas both cell types were activated by TNF-α.
Fig 2.
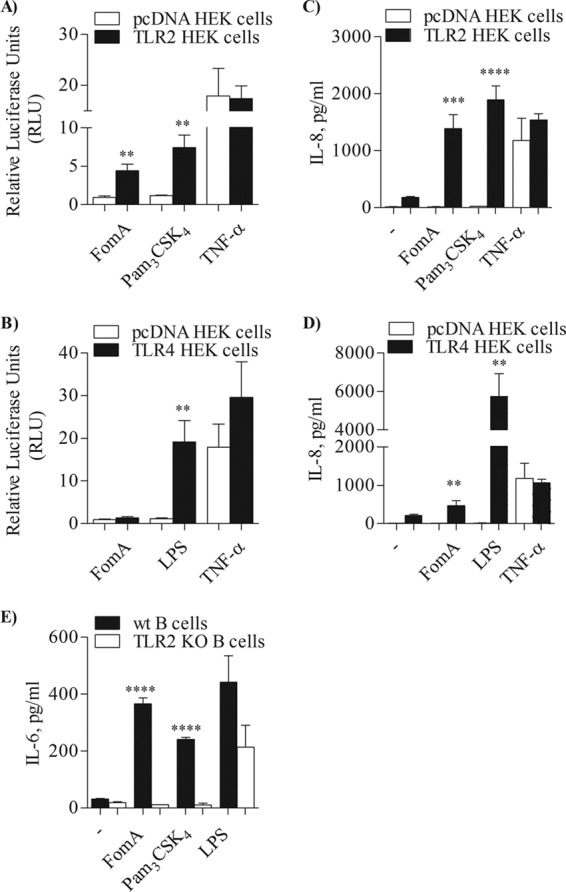
TLR2-dependent cell activation by FomA. (A and B) TLR2-HEK cells and pcDNA-HEK cells (A) or TLR4-HEK cells and pcDNA-HEK cells (B) incubated with purified FomA (10 μg/ml), Pam3CSK4 (100 ng/ml), E. coli LPS (100 ng/ml), and TNF-α (20 ng/ml) for 18 h. NF-κB-dependent luciferase activity was measured and is expressed in arbitrary units ± SD normalized to nonstimulated cells. **, P < 0.0023 (for panel A) and P = 0.0075 (for panel B). (C and D) IL-8 secretion measured by ELISA of supernatants of TLR2-HEK cells and pcDNA-HEK cells (C) and TLR4-HEK cells and pcDNA-HEK cells (D) incubated as described above. IL-8 is expressed as pg/ml ± SD. ***, P = 0.0002; ****, P < 0.0001 (for panel C). **, P = 0.0014; ** P = 0.008 (for panel D). (E) IL-6 measured by ELISA of supernatant from purified splenic B cells from C57BL/6 mice (wt) and TLR2 KO mice stimulated with FomA, Pam3CSK4, and LPS as described above. IL-6 is expressed as pg/ml ± SD. ****, P < 0.0001.
In contrast, while E. coli LPS (100 ng/ml) induced luciferase activity and IL-8 secretion in HEK cells overexpressing TLR4 (TLR4-HEK cells), these cells were not activated by FomA, consistent with the absence of endotoxin contamination observed by biochemical analysis of purified FomA. In fact, luciferase activity induced by LPS in TLR4-HEK cells was significantly higher than that induced in pcDNA cells (Fig. 2B, black bars and white bars) (**, P = 0.0075; n = 9), as was IL-8 secretion (Fig. 2D, black bars and white bars) (**, P = 0.0014; n = 9), while the effects of FomA were comparable in TLR4-HEK cells and pcDNA-HEK cells (Fig. 2B and D). In addition, TLR4-HEK cell activation induced by LPS was significantly higher than that induced by FomA (**, P = 0.008). As expected, only TNF-α induced luciferase activity and IL-8 secretion in pcDNA-HEK cells (Fig. 2B and 2D, respectively, white bars).
FomA induces TLR2-dependent mouse B cell activation in vitro.
Having demonstrated that purified recombinant FomA activates cells via TLR2 signaling and that it is free of endotoxin contamination, its ability to induce immune cell activation was examined using primary murine B cells. Splenic B cells were isolated from wild-type C57BL/6 mice (wt mice) and from TLR2 knockout mice (TLR2 KO mice) and incubated for 18 h with FomA (10 μg/ml), Pam3CSK4 (100 ng/ml), and E. coli LPS (100 ng/ml). Induction of IL-6 secretion was measured by ELISA of cell supernatants, shown in Fig. 2E. IL-6 induced by FomA was comparable to that of Pam3CSK4 in B cells from wt mice (Fig. 2E, black bars) but was significantly lower in B cells from TLR2 KO mice (Fig. 2E, white bars) (****, P < 0.001 by unpaired t test). As expected, IL-6 induced by LPS was comparable in B cells from wt mice and TLR2 KO mice (Fig. 2E).
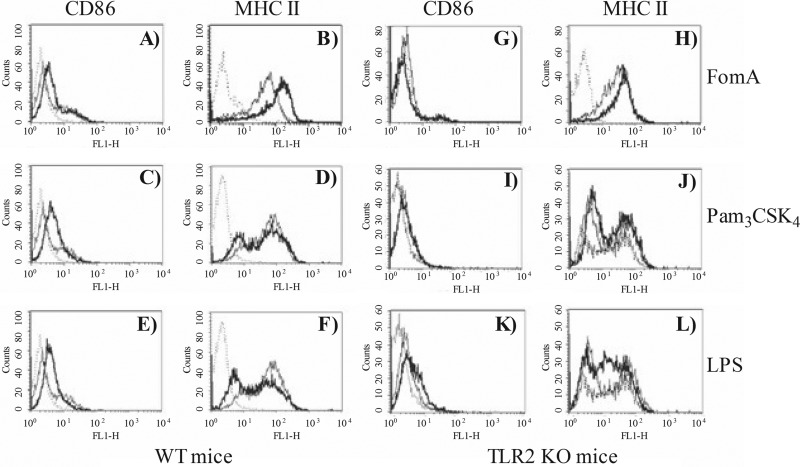
Next, the ability of FomA to induce upregulation of surface markers indicative of B cell activation was examined by flow cytometry. Following incubation as described above, B cells were collected and stained with FITC-labeled mouse anti-CD86 and anti-MHC II antibodies. In all the histograms in Fig. 3, the isotype control is shown by the dotted line, the thin line represents cells incubated with medium alone, and the thick line represents stimulated cells. FomA, Pam3CSK4, and LPS upregulated surface expression of CD86 on the surface of B cells from wt mice (Fig. 3A, C, and E, histogram shift [thick line] versus baseline expression [thin line]). FomA also induced upregulation of MHC II surface expression (Fig. 3B); by contrast, in this experiment, the effect of Pam3CSK4 and LPS appeared less substantial (Fig. 3D and F). In B cells from TLR2 KO mice, FomA and Pam3CSK4 failed to induce CD86 and MHC II surface upregulation (Fig. 3G, H, J, and I). Stimulation with LPS was not affected by the lack of TLR2 expression (Fig. 3K and L).
Fig 3.
Murine B cell activation in vitro. Purified splenic B cells from wt mice (A to F) and TLR2 KO mice (G to L) were incubated with 10 μg/ml of FomA (A, B, G, and H), 100 ng/ml of Pam3CSK4 (C, D, J, and I) or 100 ng/ml of E. coli LPS (E, F, K, and L) for 18 h. Surface upregulation of CD86 and MHC II was measured by flow cytometry. In all the histograms, the isotype control is indicated by the dotted line, cells incubated with medium alone are represented by the thin line, and stimulated cells are represented by the thick line.
FomA induces TLR2-dependent antigen-specific antibody secretion in vivo.
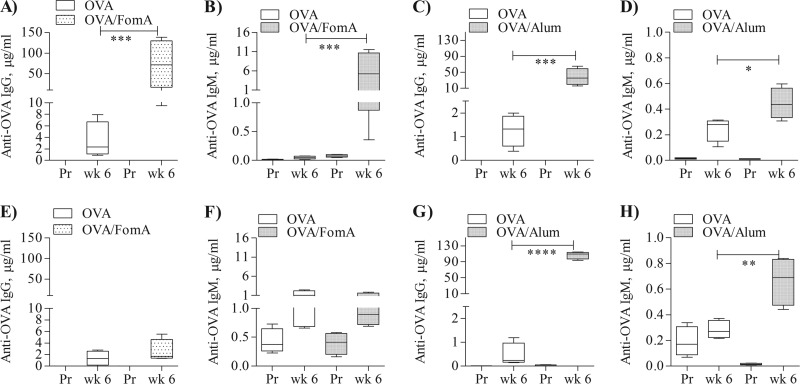
To reevaluate the immune adjuvant activity of FomA and to rule out the potential contribution of LPS, mouse immunizations were performed using our LPS-free FomA and ovalbumin (OVA) (41). Wild-type C57BL/6 mice were immunized subcutaneously with OVA (10 μg/mouse) alone and combined with LPS-free FomA (10 μg/mouse) or with the non-TLR adjuvant, alum (200 μg/mouse). The animals received three immunizations 2 weeks apart, and aliquots of preimmune sera and sera 2 weeks after each immunization (week 2, week 4, and week 6) were collected. Levels of serum anti-OVA specific antibodies were measured by ELISA and expressed in μg/ml ± SD (41). Both anti-OVA IgG and IgM induced by immunization with OVA-FomA were significantly higher than those induced by OVA alone (Fig. 4A and B, dotted bars and white bars, respectively) (***, P = 0.0005 and 0.0001, respectively, by unpaired t test). Similarly, both anti-OVA IgG and IgM induced by immunization with OVA-alum were significantly higher than those induced by OVA alone (Fig. 4C and D, dotted bars and white bars, respectively) (***, P = 0.0001; *, P = 0.04).
Fig 4.
Enhancement of antigen-specific antibody production by FomA. (A to D) wt mice immunized with ovalbumin (OVA) (10 μg) alone and in combination with FomA (10 μg/ml) or alum (200 μg). Preimmune sera (Pr) and sera after the final immunization (week 6) were examined by ELISA for quantification of OVA-specific total IgG and IgM levels. (A and B) Anti-OVA IgG (A) and anti-OVA IgM (B), expressed as μg/ml ± SD relative to mice immunized with OVA alone and OVA-FomA. ***, P = 0.0005 and 0.0001 by unpaired t test. (C and D) Anti-OVA IgG (C) and anti-OVA IgM (D), expressed as μg/ml ± SD relative to mice immunized with OVA alone and OVA-alum. ***, P = 0.0001; *, P = 0.04. (E to H) TLR2 KO mice were immunized as described above and OVA-specific IgG (E and G) and IgM (F and H) were quantified by ELISA. ****, P < 0.0001; **, P = 0.009.
To examine whether the effect of FomA correlated with its observed TLR2-dependent in vitro activity, TLR2 KO mice were also immunized. As shown in Fig. 4E and F, low levels of specific anti-OVA IgG and IgM were detected in the sera of TLR2 KO mice immunized with OVA-FomA compared to sera of wt mice (dotted bars and white bars, respectively). As expected, anti-OVA IgG and IgM levels in TLR2 KO mice immunized with OVA-alum remained high, comparable to those induced in wt mice (Fig. 4G and H) (****, P < 0.0001; **, P = 0.009).
Analysis of FomA-induced IgG subclasses and Th1/Th2 cytokine response.
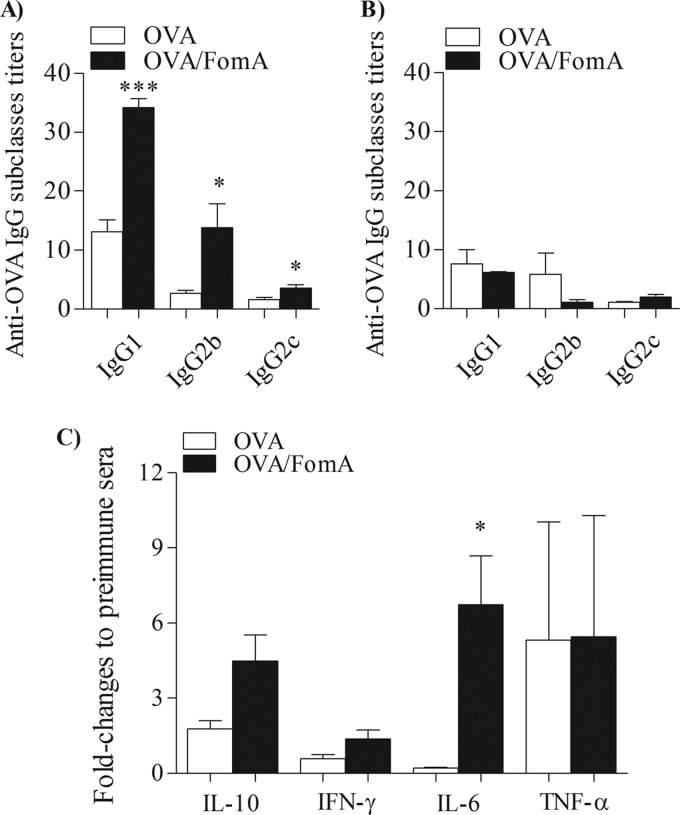
Next, levels of IgG subclasses and Th1-type and/or Th2-type cytokines were analyzed in the sera of immunized mice. IgG subclasses were measured by ELISA and expressed as titers normalized to preimmune sera. In wt mice, immunization with OVA-FomA induced higher titers of anti-OVA-specific IgG1 than immunization with OVA alone (Fig. 5A, black bars and white bars, respectively) (***, P = 0.0002 by unpaired t test). FomA also induced increased levels of OVA-specific IgG2b and, although to a minor extent, IgG2c antibodies (IgG2c is the isotype present in C57BL/6 mice [44]) compared to OVA alone (Fig. 5A; *, P = 0.03). As expected, alum induced a prevalent IgG1 response (not shown) (41). Levels of OVA-specific IgG subclasses induced by immunization of TLR2 KO mice with OVA-FomA or OVA alone were comparable (Fig. 5B, black bars and white bars, respectively).
Fig 5.
Induction of antigen-specific IgG subclasses and cytokines by FomA. OVA-specific IgG subclasses measured in the sera of wt mice (A) and TLR2 KO mice (B) immunized with OVA alone or OVA-FomA by ELISA normalized to preimmune sera and expressed as mean titers ± SD. *, P = 0.03; ***, P = 0.0002. Sera were used at a 1:100 dilution. (C) Cytokine levels in sera from wt mice immunized with OVA alone or OVA-FomA measured by ELISA. The values were normalized to pooled preimmune sera and expressed as fold change ± SD. *, P = 0.015.
The cytokines IL-10 and IFN-γ were chosen to evaluate the effect of FomA on the Th2 arm and the Th1 arm, respectively. A trend of increased levels of IL-10 was observed in the sera from wt mice immunized with OVA-FomA compared to sera of mice immunized with OVA alone (Fig. 5C, black bars and white bars, respectively) (P = 0.0506). Although a slight increase in IFN-γ secretion was measured in wt mice immunized with OVA-FomA compared to mice immunized with OVA alone, this was also nonstatistically significant and of overall low magnitude (Fig. 5C). The cytokine IL-6, which promotes Th2 differentiation and simultaneously inhibits Th1 polarization (17), was significantly increased in the sera of OVA-FomA-immunized mice compared to those immunized with OVA alone (Fig. 5C; *, P = 0.015). No difference in serum levels of TNF-α was observed among the two mouse groups (Fig. 5C). Results are expressed as fold change of titers ± SD normalized to pooled preimmune sera. In TLR2 KO mice, immunization with OVA-FomA did not increase cytokine secretion compared to immunization with OVA alone (not shown).
TLR2-dependent immunogenicity of FomA.
Since FomA is known to be immunogenic (40, 50, 53), induction of specific anti-FomA antibodies was examined in the sera from mice immunized with FomA in the absence of other adjuvants. Sera were tested by ELISA using plates coated with FomA (2 μg/ml), and IgG and IgM levels were expressed as μg/ml ± SD.
As shown in Fig. 6A, anti-FomA total IgG production in wt mice increased in a dose-response fashion throughout the immunization and was significantly higher after the last boost (****, P < 0.0001 by one-way ANOVA with Dunnett's multiple comparison posttest and 99.9% confidence intervals). A similar statistically significant difference within the sera from TLR2 KO mice immunized with FomA was observed (Fig. 6B) (****, P < 0.0001), but when the amounts of anti-FomA IgG produced by wt mice and by TLR2 KO were compared, it was found that anti-FomA IgG levels in wt mice were significantly higher than those elicited in TLR2 KO mice (compare Fig. 6A and B; **, P = 0.004 by unpaired t test).
Fig 6.
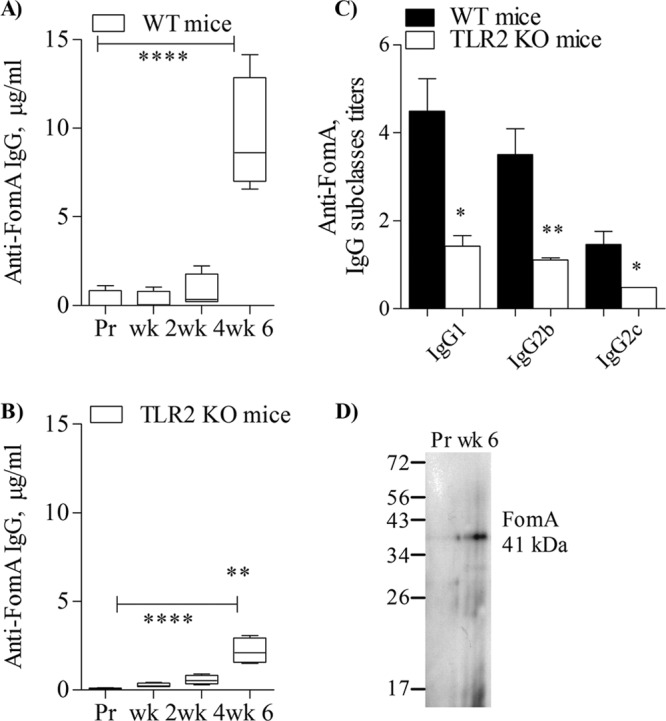
Immunogenicity of FomA. (A) Anti-FomA total IgG from wt mice immunized with FomA in the absence of additional adjuvants measured by ELISA. Preimmune sera (Pr) and sera after each boost (week 2, week 4, and week 6) are shown. Results are expressed in μg/ml ± SD. ****, P < 0.0001. (B) Anti-FomA total IgG from TLR2 KO mice as in panel A. ****, P < 0.0001. **, P = 0.004 for comparison of week 6 results in panels A and B. (C) Anti-FomA IgG subclasses in the sera from wt mice and TLR2 KO mice. Sera were used at a 1:100 dilution. Results are expressed as titers normalized to preimmune sera ± SD. *, P = 0.02; **, P = 0.006; *, P = 0.01 (left to right, respectively). (D) Western blot of FomA, detected as a band of ∼40 kDa by sera from a representative FomA-immunized mouse (week 6, 1:1,000 dilution).
Analysis of anti-FomA IgG subclass titers revealed that IgG1and IgG2b were the most prominent subclasses in wt mice and that both were significantly higher in these mice than in TLR2 KO mice (Fig. 6C, black bars and white bars, respectively) (*, P = 0.02; **, P = 0.006 by unpaired t test). A relatively small, but statistically significant increase of IgG2c was also detected in wt mice compared to TLR2 KO mice (Fig. 6C) (*, P = 0.01). IgG subclasses are expressed as titers normalized to preimmune sera ± SD.
Anti-FomA sera also recognized FomA as a band of approximately 40 kDa in Western blotting experiments (Fig. 6D, representative wt mouse serum, week 6, 1:1,000 dilution).
DISCUSSION
Bacterial porins are well-established TLR2 agonists, in combination with the coreceptors TLR1 and TLR6, and their molecular mechanism of immune adjuvant activity has been explained via TLR2 signaling (8, 12, 47, 58). Another example of bacterial product with TLR2-dependent adjuvanticity is the E. coli heat-labile enterotoxin subunits [LT-IIa-B(5) and LT-IIb-B(5)] (16, 39). A strong in vitro immunobiological activity was reported for the FomA porin from F. nucleatum (62) by immune cell activation in vitro and adjuvanticity in vivo. Although a copious contamination (>12%) by F. nucleatum endotoxin (a classical TLR4 agonist [67]) was acknowledged in the native FomA preparation used in this study, FomA activated cells from both LPS-sensitive and LPS-nonresponder mice. It was thus concluded that its effect was not due to F. nucleatum LPS. However, following discovery of TLR signaling in response to specific bacterial components, such ample LPS contamination poses a challenge for interpretation of the immune adjuvanticity of FomA. To date, no further in vitro or in vivo studies have been carried out to elucidate the mechanism of action of FomA.
More recent in vitro and in vivo evidence has indirectly suggested a role for TLR2 in the activity of FomA. This was indicated by TLR2-dependent IL-8 secretion, ROS production, and expression of antimicrobial peptides in response to FomA-containing bacterial fractions in a variety of cell systems (cell lines, gingival epithelial cells, and primary murine macrophages) (27, 29, 33, 43, 51). None of these responses have been attributed to F. nucleatum LPS or peptidoglycan (a classical TLR2/TLR6 agonist [29, 38, 51]), while little is known about Fusobacterium lipoproteins, except their predicted existence (5, 32).
Thus, the immune stimulatory properties of FomA were examined using a recombinant, LPS-free FomA obtained using a method that warrants removal of LPS and lipoprotein contaminants (41, 46). Our results show that this FomA induced TLR2-dependent cell activation in a human nonhematopoietic cell model of TLR expression (HEK cells) and in purified splenic B cells from wild-type and TLR2 KO mice. In HEK cells, FomA induced IL-8 secretion and NF-κB-dependent luciferase activity only in cells in which TLR2 was overexpressed, whereas it did not activate HEK cells overexpressing TLR4. This observation indicates that FomA signals via TLR2 and confirms the absence of LPS contamination (E. coli LPS is a known TLR4 ligand [15]). FomA also induced IL-6 secretion and cell surface upregulation of the costimulatory molecule CD86 and of MHC II in vitro in B cells from wild-type mice but failed to activate B cells from TLR2 KO mice, similar to the TLR2 agonist Pam3CSK4. Our studies thus provide the first direct evidence of TLR2-dependent activity for FomA.
The adjuvanticity of FomA was also reevaluated in vivo, and FomA was shown to enhance antibody production to OVA when used in immunization of wt mice, similar to the effect of the adjuvant alum. However, in TLR2 KO mice, FomA, but not alum, failed to improve the specific response to OVA.
It is generally accepted that, in vaccinations, the adjuvant's nature contributes to modify local inflammatory responses and infection outcomes based on its effect on T-cell polarity. High levels of antibodies and isotype class switch toward IgG1 are considered features of the Th2-type immune response, along with anti-inflammatory cytokines IL-6, IL-4, and IL-10 (49). Such responses are classically induced by alum (11) and by TLR2 adjuvants (30). In contrast, production of IgG2a, IgG2b, IgG2c, and IgG3 (depending on the mouse strain [44]), as well as proinflammatory cytokines IFN-γ, IL-2 and IL-12, indicates induction of Th1-type immune responses, favored by TLR4 and TLR9 adjuvants. Remarkably, in the past few years, increasing evidence has demonstrated that immune cell activation via TLR2 is involved in shaping both types of immune responses (22, 59). In wild-type mice, both alum and FomA induced OVA-specific IgG1 antibodies (important for complement-independent pathogen neutralization). However, FomA also induced increased titers of anti-OVA IgG2b (important for complement fixation and both complement- and Fc-mediated bacterial opsonophagocytosis). FomA also induced TLR2-dependent production of IL-10, in the serum from mice immunized with FomA/OVA, and of IL-6, which plays a dual role in controlling Th1/Th2 differentiation by promoting Th2 responses and inhibiting Th1 differentiation (17). Our results strongly indicated that FomA is a TLR2 agonist with a classical TLR2-dependent immune adjuvant activity.
The immunogenicity of FomA was also assessed. Previously, production of circulating and salivary anti-FomA IgG and IgA antibodies was demonstrated following intranasal immunization of mice with whole E. coli transfected to express FomA or with purified FomA plus cholera toxin (CT) (a strong mucosal non-TLR-dependent adjuvant) (26, 34, 40, 50). In our subcutaneous immunization of mice with purified FomA in the absence of additional adjuvants, production of anti-FomA IgG antibodies was also found to be dependent on TLR2 expression and was detected only in wt mice, in agreement with the notion that FomA has TLR2-dependent activity. High levels of FomA-specific IgG1 and IgG2b were observed. The immune mouse sera also recognized linear epitopes of FomA by Western blotting, in addition to conformational epitopes by ELISA.
Enhancement of systemic responses is crucial for development of protective immunity to infections, but immunization with soluble antigens requires the addition of adjuvants to be effective. Our studies demonstrate that FomA is a novel potential TLR2 adjuvant. In addition, FomA is moderately immunogenic. Given the significantly high enhancement of antigen-specific immune responses induced by FomA in vivo and its observed moderate immunogenicity, FomA could represent a valuable self-adjuvanted element in a multicomponent vaccine against mixed infections caused by F. nucleatum and other oral pathogens.
ACKNOWLEDGMENTS
This work was supported by the Basic Research Transitional Program Award from the Boston University School of Medicine, Section of Infectious Diseases.
We thank J. H. Kleinschmidt, University of Konstanz, Germany, for the FomA-expressing E. coli strain.
Footnotes
Published ahead of print 23 May 2012
REFERENCES
- 1. Akira S, Takeda K, Kaisho T. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2: 675–680 [DOI] [PubMed] [Google Scholar]
- 2. Alurkar V, Kamat R. 1997. Immunomodulatory properties of porins of some members of the family Enterobacteriaceae. Infect. Immun. 65: 2382–2388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anbazhagan V, Vijay N, Kleinschmidt JH, Marsh D. 2008. Protein-lipid interactions with Fusobacterium nucleatum major outer membrane protein FomA: spin-label EPR and polarized infrared spectroscopy. Biochemistry 47: 8414–8423 [DOI] [PubMed] [Google Scholar]
- 4. Asong J, Wolfert MA, Maiti KK, Miller D, Boons GJ. 2009. Binding and cellular activation studies reveal that Toll-like receptor 2 can differentially recognize peptidoglycan from Gram-positive and Gram-negative bacteria. J. Biol. Chem. 284: 8643–8653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Babu MM, et al. 2006. A database of bacterial lipoproteins (DOLOP) with functional assignments to predicted lipoproteins. J. Bacteriol. 188: 2761–2773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bakken V, Aaro S, Jensen HB. 1989. Purification and partial characterization of a major outer-membrane protein of Fusobacterium nucleatum. J. Gen. Microbiol. 135: 3253–3262 [DOI] [PubMed] [Google Scholar]
- 7. Bauer S, et al. 2001. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc. Natl. Acad. Sci. U. S. A. 98: 9237–9242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Biswas A, Banerjee P, Biswas T. 2009. Porin of Shigella dysenteriae directly promotes toll-like receptor 2-mediated CD4+ T cell survival and effector function. Mol. Immunol. 46: 3076–3085 [DOI] [PubMed] [Google Scholar]
- 9. Bolstad AI, Jensen HB, Bakken V. 1996. Taxonomy, biology, and periodontal aspects of Fusobacterium nucleatum. Clin. Microbiol. Rev. 9: 55–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bolstad AI, Tommassen J, Jensen HB. 1994. Sequence variability of the 40-kDa outer membrane proteins of Fusobacterium nucleatum strains and a model for the topology of the proteins. Mol. Gen. Genet. 244: 104–110 [DOI] [PubMed] [Google Scholar]
- 11. Brewer JM, et al. 1999. Aluminium hydroxide adjuvant initiates strong antigen-specific Th2 responses in the absence of IL-4- or IL-13-mediated signaling. J. Immunol. 163: 6448–6454 [PubMed] [Google Scholar]
- 12. Burke JM, Ganley-Leal LM, Khatri A, Wetzler LM. 2007. Neisseria meningitidis PorB, a TLR2 ligand, induces an antigen-specific eosinophil recall response: potential adjuvant for helminth vaccines? J. Immunol. 179: 3222–3230 [DOI] [PubMed] [Google Scholar]
- 13. Cervantes-Barragan L, et al. 2009. TLR2 and TLR4 signaling shapes specific antibody responses to Salmonella typhi antigens. Eur. J. Immunol. 39: 126–135 [DOI] [PubMed] [Google Scholar]
- 14. Chiavolini D, Weir S, Murphy JR, Wetzler LM. 2008. Neisseria meningitidis PorB, a Toll-like receptor 2 ligand, improves the capacity of Francisella tularensis lipopolysaccharide to protect mice against experimental tularemia. Clin. Vaccine Immunol. 15: 1322–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F. 1999. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J. Biol. Chem. 274: 10689–10692 [DOI] [PubMed] [Google Scholar]
- 16. Connell TD, Metzger D, Sfintescu C, Evans RT. 1998. Immunostimulatory activity of LT-IIa, a type II heat-labile enterotoxin of Escherichia coli. Immunol. Lett. 62: 117–120 [DOI] [PubMed] [Google Scholar]
- 17. Diehl S, Rincon M. 2002. The two faces of IL-6 on Th1/Th2 differentiation. Mol. Immunol. 39: 531–536 [DOI] [PubMed] [Google Scholar]
- 18. DiRienzo JM, Rosan B. 1984. Isolation of a major cell envelope protein from Fusobacterium nucleatum. Infect. Immun. 44: 386–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fisette PL, Ram S, Andersen JM, Guo W, Ingalls RR. 2003. The Lip lipoprotein from Neisseria gonorrhoeae stimulates cytokine release and NF-kappaB activation in epithelial cells in a Toll-like receptor 2-dependent manner. J. Biol. Chem. 278: 46252–46260 [DOI] [PubMed] [Google Scholar]
- 20. Fusco PC, Michon F, Tai JY, Blake MS. 1997. Preclinical evaluation of a novel group B meningococcal conjugate vaccine that elicits bactericidal activity in both mice and nonhuman primates. J. Infect. Dis. 175: 364–372 [DOI] [PubMed] [Google Scholar]
- 21. Galdiero M, et al. 2004. Haemophilus influenzae porin induces Toll-like receptor 2-mediated cytokine production in human monocytes and mouse macrophages. Infect. Immun. 72: 1204–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goriely S, Neurath MF, Goldman M. 2008. How microorganisms tip the balance between interleukin-12 family members. Nat. Rev. Immunol. 8: 81–86 [DOI] [PubMed] [Google Scholar]
- 23. Haake SK, Wang X. 1997. Cloning and expression of FomA, the major outer-membrane protein gene from Fusobacterium nucleatum T18. Arch. Oral Biol. 42: 19–24 [DOI] [PubMed] [Google Scholar]
- 24. Hajishengallis G, et al. 2005. Toll-like receptor 2 mediates cellular activation by the B subunits of type II heat-labile enterotoxins. Infect. Immun. 73: 1343–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hayashi F, et al. 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410: 1099–1103 [DOI] [PubMed] [Google Scholar]
- 26. Holmgren J, et al. 2005. Mucosal adjuvants and anti-infection and anti-immunopathology vaccines based on cholera toxin, cholera toxin B subunit and CpG DNA. Immunol. Lett. 97: 181–188 [DOI] [PubMed] [Google Scholar]
- 27. Huang GT, Zhang HB, Dang HN, Haake SK. 2004. Differential regulation of cytokine genes in gingival epithelial cells challenged by Fusobacterium nucleatum and Porphyromonas gingivalis. Microb. Pathog. 37: 303–312 [DOI] [PubMed] [Google Scholar]
- 28. Jensen HB, et al. 1996. Cloning of the fomA gene, encoding the major outer membrane porin of Fusobacterium nucleatum ATCC10953. Microb. Pathog. 21: 331–342 [DOI] [PubMed] [Google Scholar]
- 29. Ji S, et al. 2009. Toll-like receptor 2 and NALP2 mediate induction of human beta-defensins by Fusobacterium nucleatum in gingival epithelial cells. Infect. Immun. 77: 1044–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kaisho T, Akira S. 2002. Toll-like receptors as adjuvant receptors. Biochim. Biophys. Acta 1589: 1–13 [DOI] [PubMed] [Google Scholar]
- 31. Kang JY, et al. 2009. Recognition of lipopeptide patterns by Toll-like receptor 2-Toll-like receptor 6 heterodimer. Immunity 31: 873–884 [DOI] [PubMed] [Google Scholar]
- 32. Karpathy SE, et al. 2007. Genome sequence of Fusobacterium nucleatum subspecies polymorphum - a genetically tractable fusobacterium. PLoS One 2: e659 doi:10.1371/journal.pone.0000659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kikkert R, Laine ML, Aarden LA, van Winkelhoff AJ. 2007. Activation of toll-like receptors 2 and 4 by gram-negative periodontal bacteria. Oral Microbiol. Immunol. 22: 145–151 [DOI] [PubMed] [Google Scholar]
- 34. Kim TG, et al. 2009. Immunogenicity of a cholera toxin B subunit Porphyromonas gingivalis fimbrial antigen fusion protein expressed in E. coli. Mol. Biotechnol. 41: 157–164 [DOI] [PubMed] [Google Scholar]
- 35. Kleinschmidt JH. 2006. Folding kinetics of the outer membrane proteins OmpA and FomA into phospholipid bilayers. Chem. Phys. Lipids 141: 30–47 [DOI] [PubMed] [Google Scholar]
- 36. Kleivdal H, Benz R, Tommassen J, Jensen HB. 1999. Identification of positively charged residues of FomA porin of Fusobacterium nucleatum which are important for pore function. Eur. J. Biochem. 260: 818–824 [DOI] [PubMed] [Google Scholar]
- 37. Kleivdal H, Puntervoll P, Jensen HB. 2001. Topological investigations of the FomA porin from Fusobacterium nucleatum and identification of the constriction loop L6. Microbiology 147: 1059–1067 [DOI] [PubMed] [Google Scholar]
- 38. Krisanaprakornkit S, et al. 2000. Inducible expression of human beta-defensin 2 by Fusobacterium nucleatum in oral epithelial cells: multiple signaling pathways and role of commensal bacteria in innate immunity and the epithelial barrier. Infect. Immun. 68: 2907–2915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee CH, Masso-Welch P, Hajishengallis G, Connell TD. 2011. TLR2-dependent modulation of dendritic cells by LT-IIa-B5, a novel mucosal adjuvant derived from a type II heat-labile enterotoxin. J. Leukoc. Biol. 90: 911–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu PF, et al. 2010. Vaccination targeting surface FomA of Fusobacterium nucleatum against bacterial co-aggregation: Implication for treatment of periodontal infection and halitosis. Vaccine 28: 3496–3505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu X, Wetzler LM, Massari P. 2008. The PorB porin from commensal Neisseria lactamica induces Th1 and Th2 immune responses to ovalbumin in mice and is a potential immune adjuvant. Vaccine 26: 786–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mackinnon FG, et al. 1999. The role of B/T costimulatory signals in the immunopotentiating activity of neisserial porin. J. Infect. Dis. 180: 755–761 [DOI] [PubMed] [Google Scholar]
- 43. Marcato LG, et al. 2008. The role of Toll-like receptors 2 and 4 on reactive oxygen species and nitric oxide production by macrophage cells stimulated with root canal pathogens. Oral Microbiol. Immunol. 23: 353–359 [DOI] [PubMed] [Google Scholar]
- 44. Martin RM, Brady JL, Lew AM. 1998. The need for IgG2c specific antiserum when isotyping antibodies from C57BL/6 and NOD mice. J. Immunol. Methods 212: 187–192 [DOI] [PubMed] [Google Scholar]
- 45. Massari P, et al. 2002. Cutting edge: immune stimulation by neisserial porins is toll-like receptor 2 and MyD88 dependent. J. Immunol. 168: 1533–1537 [DOI] [PubMed] [Google Scholar]
- 46. Massari P, King CA, Macleod H, Wetzler LM. 2005. Improved purification of native meningococcal porin PorB and studies on its structure/function. Protein Expr. Purif. 44: 136–146 [DOI] [PubMed] [Google Scholar]
- 47. Massari P, et al. 2006. Meningococcal porin PorB binds to TLR2 and requires TLR1 for signaling. J. Immunol. 176: 2373–2380 [DOI] [PubMed] [Google Scholar]
- 48. Medzhitov R, Janeway C., Jr 2000. The Toll receptor family and microbial recognition. Trends Microbiol. 8: 452–456 [DOI] [PubMed] [Google Scholar]
- 49. Mosmann TR, Coffman RL. 1989. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 7: 145–173 [DOI] [PubMed] [Google Scholar]
- 50. Nakagaki H, et al. 2010. Fusobacterium nucleatum envelope protein FomA is immunogenic and binds to the salivary statherin-derived peptide. Infect. Immun. 78: 1185–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Peyret-Lacombe A, Brunel G, Watts M, Charveron M, Duplan H. 2009. TLR2 sensing of F. nucleatum and S. sanguinis distinctly triggered gingival innate response. Cytokine 46: 201–210 [DOI] [PubMed] [Google Scholar]
- 52. Pocanschi CL, et al. 2006. The major outer membrane protein of Fusobacterium nucleatum (FomA) folds and inserts into lipid bilayers via parallel folding pathways. J. Mol. Biol. 355: 548–561 [DOI] [PubMed] [Google Scholar]
- 53. Puntervoll P, et al. 2000. The Fusobacterium nucleatum porin FomA possesses the general topology of the non-specific porins. Microbiology 146(Pt 6): 1437–1445 [DOI] [PubMed] [Google Scholar]
- 54. Puntervoll P, et al. 2002. Structural characterization of the fusobacterial non-specific porin FomA suggests a 14-stranded topology, unlike the classical porins. Microbiology 148: 3395–3403 [DOI] [PubMed] [Google Scholar]
- 55. Qi HL, Tai JY, Blake MS. 1994. Expression of large amounts of neisserial porin proteins in Escherichia coli and refolding of the proteins into native trimers. Infect. Immun. 62: 2432–2439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rao J, Seshagiri PB, Shetty G, Ramesh G, Adiga PR. 2000. Active immunization against riboflavin carrier protein results in peri-implantation embryonic loss leading to pregnancy termination in rats: use of alternate adjuvants. Indian J. Exp. Biol. 38: 863–872 [PubMed] [Google Scholar]
- 57. Ray A, Biswas T. 2005. Porin of Shigella dysenteriae enhances Toll-like receptors 2 and 6 of mouse peritoneal B-2 cells and induces the expression of immunoglobulin M, immunoglobulin G2a and immunoglobulin A. Immunology 114: 94–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ray A, Karmakar P, Biswas T. 2004. Up-regulation of CD80-CD86 and IgA on mouse peritoneal B-1 cells by porin of Shigella dysenteriae is Toll-like receptors 2 and 6 dependent. Mol. Immunol. 41: 1167–1175 [DOI] [PubMed] [Google Scholar]
- 59. Re F, Strominger JL. 2001. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human dendritic cells. J. Biol. Chem. 276: 37692–37699 [DOI] [PubMed] [Google Scholar]
- 60. Shimazu R, et al. 1999. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J. Exp. Med. 189: 1777–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Singleton TE, Massari P, Wetzler LM. 2005. Neisserial porin-induced dendritic cell activation is MyD88 and TLR2 dependent. J. Immunol. 174: 3545–3550 [DOI] [PubMed] [Google Scholar]
- 62. Takada H, et al. 1988. Immunobiological activities of a porin fraction isolated from Fusobacterium nucleatum ATCC 10953. Infect. Immun. 56: 855–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Takeuchi O, et al. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 11: 443–451 [DOI] [PubMed] [Google Scholar]
- 64. van der Ley P, Heckels JE, Virji M, Hoogerhout P, Poolman JT. 1991. Topology of outer membrane porins in pathogenic Neisseria spp. Infect. Immun. 59: 2963–2971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wetzler LM. 2010. Innate immune function of the neisserial porins and the relationship to vaccine adjuvant activity. Future Microbiol. 5: 749–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wetzler LM, Ho Y, Reiser H. 1996. Neisserial porins induce B lymphocytes to express costimulatory B7-2 molecules and to proliferate. J. Exp. Med. 183: 1151–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yoshimura A, Kaneko T, Kato Y, Golenbock DT, Hara Y. 2002. Lipopolysaccharides from periodontopathic bacteria Porphyromonas gingivalis and Capnocytophaga ochracea are antagonists for human toll-like receptor 4. Infect. Immun. 70: 218–225 [DOI] [PMC free article] [PubMed] [Google Scholar]