Abstract
Human papillomavirus (HPV) is able to inhibit the secretion of gamma interferon (IFN-γ) and the expression of some immune innate cell receptors. Immunoglobulin-like transcript 2 (ILT2) is a regulatory receptor that seems to participate in the pathogenesis of viral infections. We have studied the expression and function of ILT2 and the expression of other NK cell receptors in 23 healthy women before and after immunization with the quadrivalent HPV (type 6/11/16/18) vaccine (Gardasil). Receptor expression was analyzed by flow cytometry in freshly isolated peripheral blood mononuclear cells as well as after in vitro stimulation with the quadrivalent HPV (type 6/11/16/18) vaccine. In addition, the regulatory function of ILT2 on cell proliferation and IFN-γ production was analyzed. We found a significant increase in the expression of ILT2 by NK and CD3+ CD56+ lymphocytes and monocytes after quadrivalent HPV (type 6/11/16/18) vaccine immunization. In addition, the in vitro stimulation with the quadrivalent HPV (type 6/11/16/18) vaccine also increased the proportion of CD3− CD56+ ILT2+ NK cells. Although the inhibitory function of ILT2 on cell proliferation was enhanced after HPV immunization, the in vitro engagement of this receptor did not affect the synthesis of IFN-γ induced by HPV. Finally, a significant increase in the expression of NKG2D, NKp30, and NKp46 by NK and CD3+ CD56+ lymphocytes was detected after quadrivalent HPV (type 6/11/16/18) vaccine immunization. Our data indicate that HPV immunization is associated with significant changes in the expression and function of different innate immune receptors, including ILT2, which may participate in the protective effect of HPV vaccines.
INTRODUCTION
Cervical cancer is the second cause of mortality in women worldwide, and 98% of these cases are associated with infection by human papillomavirus (HPV) (4). HPV infection prevalence is reported to be between 2 and 44% worldwide, but this percentage increases up to 70% in sexually active women. However, only 10 to 25% of infected women develop cervical lesions induced by HPV. In this regard, 90% of the women that acquire the HPV will eliminate the virus in less than 3 years (24).
The immune response elicited by HPV includes the production of specific antibodies as well as a cell-mediated response, with activation of Th1 lymphocytes (21). The humoral immune response is observed in approximately 50% of infected women, and only 20 to 25% of them will have significant antibody titers 10 years after the infection. On the other hand, the cellular immune response includes the activation of CD4+ Th1 cells and CD8+ cytotoxic lymphocytes and the synthesis and release of proinflammatory cytokines such as interleukin-12 (IL-12), gamma interferon (IFN-γ), and tumor necrosis factor alpha (TNF-α) (6, 21). In this regard, it has been reported that L1 and L2 capsid viral proteins are immunogenic, and it is widely recognized that immunization with these antigens confers effective protection against HPV infection (25). However, HPV has different immune evasion mechanisms, including its nonlytic effect and its episomal state in the initial period of the infection (11, 29). In addition, HPV is able to decrease the secretion of the chemokine IL-8 as well as to diminish the synthesis of IFN-γ (12). Furthermore, it has been reported that HPV infection is associated with a reduction in the expression of different innate immune receptors, including Toll-like receptor 9 (TLR9) (9), NKp30, NKp46, and NKG2D, diminishing the cytotoxic activity NK cells (2, 7). Finally, HPV is also able to inhibit the expression of the zeta chain of T cell receptor complex (CD247) and to induce the release of IL-10, interfering with the activation of Th1 lymphocytes (27, 28).
Immunoglobulin-like transcript 2 (ILT2 or LILRB1/LIR1/CD85j) is a negative regulatory receptor expressed by different leukocyte subsets (16). This receptor binds to different HLA class I molecules and the human cytomegalovirus (HCMV) UL18 protein (1, 18). ILT2 has a close relationship with the killer inhibitory receptors (KIR) and exhibits cytoplasmic immunoreceptor tyrosine-based inhibitory motifs, which are able to recruit the SHP-2 phosphatase, thus inhibiting T cell activation (20). ILT2 is expressed by myeloid cells, mainly phagocytic and antigen-presenting cells (monocytes, B lymphocytes, dendritic cells, and macrophages). This receptor is also detected in a significant fraction of NK cells and in 4 to 20% of T lymphocytes, mainly CD8+ cells (14).
Different studies have shown that the expression and function of ILT2 are increased in HCMV infection, phenomena that could contribute to the pathogenesis of disorders caused by this virus (8, 15). In addition, it has been reported that ILT2 may also participate in the pathogenesis of HIV infection, tuberculosis, and some malignant conditions (13). However, the possible role of this receptor in HPV infection has not been described.
In this work, we analyzed different immune parameters in healthy volunteers before and after immunization with the quadrivalent HPV (type 6/11/16/18) vaccine (Gardasil). Thus, we have analyzed the expression and function of ILT2 as well as the expression of other NK cell receptors (NKG2A, NKG2D, NKp30, and NKp46) which seem to participate in the innate immune response against different viruses (8, 15). We have found that HPV immunization is associated with significant changes in the expression and function of ILT2, the number of IFN-γ-producing cells, and an increase in the expression of activation receptors by NK and CD3+ CD56+ cells.
MATERIALS AND METHODS
Patients and samples.
Twenty-three healthy volunteers were included in the study. All individuals were female, ranging in age from 14 to 34 years (mean, 25.8 years), and no evidence of HPV infection was detected in any of them. Immunization against HPV with the quadrivalent HPV (type 6/11/16/18) vaccine was performed according to the manufacturer's instructions. No side effects of immunization were registered in any case. Informed consent was obtained in writing from all volunteers, and this study was done in accordance with the principles set out in the Declaration of Helsinki.
Peripheral blood samples were obtained before immunization (first sample, time zero [T0]) and 15 days after the administration of the first and third doses of the quadrivalent HPV (type 6/11/16/18) vaccine (days 15 and 195; designated T1 and T2 samples).
Cells.
Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque (Sigma Chemical Co., St. Louis, MO) gradient centrifugation. Cells were suspended in RPMI 1640 culture medium (HyClone, Logan, UT), supplemented with 10% fetal bovine serum (HyClone), 2.0 mM glutamine, 100 IU/ml penicillin, and 100 μg/ml streptomycin (Sigma).
Antibodies.
The following monoclonal antibodies (MAbs) were employed: anti-CD4, anti-CD8, anti-CD19, anti-CD56, and anti-CD14, all conjugated with phycoerythrin (PE) (eBioscience, San Diego, CA). An anti-CD3-peridinin chlorophyll protein (PerCP) MAb was purchased from BD Biosciences (San Jose, CA), and the ILT2 receptor was analyzed with a biotinylated MAb (R&D Systems, Minneapolis, MN) and streptavidin conjugated with fluorescein isothiocyanate (FITC) (Sigma). For the analysis of expression of NK cell receptors, anti-CD3-PerCP, anti-CD56-allophycocyanin (APC), and anti-NKG2A, anti-NKG2D, anti-NKp30, and anti-NKp46 labeled with PE were employed (R&D Systems).
Flow cytometry analysis.
Cells were first immunostained with the biotinylated anti-ILT2 MAb, followed by incubation with streptavidin-FITC. Then, the cells were incubated with the antibody indicated in the figures for 20 min at 4°C in darkness. Cells were analyzed in a FACSCalibur flow cytometer (Becton, Dickinson, San Jose, CA), using CellQuest software (Becton, Dickinson).
For the analysis of the NK cell repertoire receptors, three-color flow cytometry analysis was performed by labeling with the anti-CD56-APC and anti-CD3-PerCp MAbs. Then, samples were washed and incubated with the anti-NKp30, anti-NKp46, anti-NKG2A, and anti-NKG2D labeled with PE. Finally, cells were washed with phosphate-buffered saline (PBS) with 2% fetal bovine serum and fixed in 1% paraformaldehyde. Cells were analyzed in a FACSAria flow cytometer (Becton, Dickinson), using FACSDiva software (Becton, Dickinson).
Inhibition of cell proliferation assays.
PBMCs (1 × 106) were loaded with 0.5 mM carboxyfluorescein succinimidyl ester (CFSE) and incubated for 72 or 96 h under standard conditions (with 5% CO2 at 37°C and 100% humidity) in the presence and absence of a mixture of anti-CD3 and anti-CD28 MAbs (10 μg/ml; Immunotech, Marseille, France) and with or without the addition of 2 μg/ml of an agonistic anti-ILT2 MAb (HP-F1; BioLegend, San Diego, CA) plus a cross-linker antibody (4.0 μg/ml rabbit anti-mouse IgG; Sigma-Aldrich). After incubation, cells were washed, and the proportion of divided cells (cells in which the CFSE was diluted during mitosis and that therefore show diminished fluorescence emission) was determined by flow cytometry analysis (FACSCalibur flow cytometer). Results are expressed as the percentage of divided cells.
Cell cycle analysis.
Cell cycle analysis was performed by a DNA content assay by using propidium iodide staining and flow cytometry. PBMCs (1 × 106 poured in 48-well flat bottom plates) were cultured for 72 hours in the presence of two different mixtures of L1 protein from the quadrivalent HPV (type 6/11/16/18) vaccine 10 ng/ml of HPV-6 and -18 and 20 ng/ml of HPV-11 and -16, or 20 ng/ml of HPV-6 and -18 and 40 ng/ml of HPV-11 and -16. Then, cells were harvested and incubated with a staining buffer (30 mg/ml propidium iodide, 0.5 mg/ml RNase, and 1.0% Triton X-100 [all from Sigma-Aldrich]) for 30 min at 4°C and analyzed in a FACSCalibur flow cytometer. Results were expressed as the percentage of cell nuclei in the S, G2, and M phases of cell cycle.
Cytokine production assays.
Supernatants from the different culture conditions were collected, and the concentrations of IL-10 were determined by enzyme-linked immunosorbent assay ([ELISA] BD OptEIA; BD Biosciences-Pharmingen, San Jose, CA), according to the manufacturer's instructions.
For enzyme-linked immunosorbent spot (ELISPOT) assays, PBMCs (2 × 105) were stimulated with the quadrivalent HPV (type 6/11/16/18) vaccine and cultured for 48 h in the presence and absence of the HP-F1 anti-ILT2 MAb plus a cross-linker antibody. Then, the number of IFN-γ-producing cells was determined by using a Mabtech ELISpot kit (Stockholm, Sweden) according to the manufacturer's recommendations. Finally, the plates were read in a StereoZoom SZ-4 stereomicroscope (Leica, Wetzlar, Germany) with a 30× objective.
Statistical analysis.
Results are represented as the arithmetic mean ± standard deviation (SD). Statistical analysis was made as indicated in each figure legend. A P value of <0.05 was considered significant. The statistical analysis was performed using GraphPad Prism, version 4.0, or GraphPad InStat, version 3, software (GraphPad Software, San Diego, CA).
RESULTS
Expression of immune innate receptors by PBMCs.
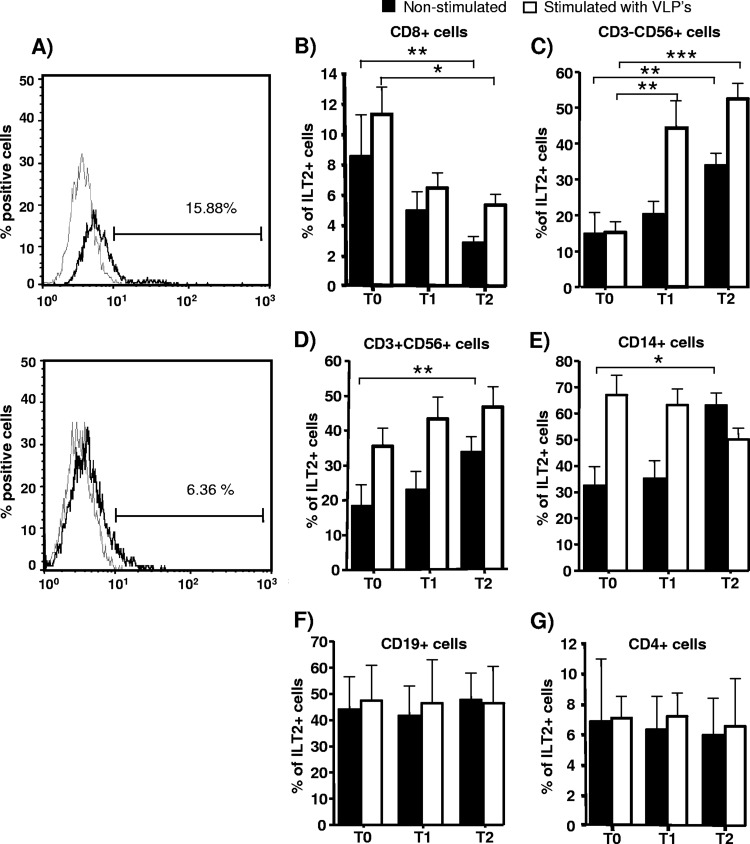
We first explored the expression of ILT2 by different leukocyte subsets before and after quadrivalent HPV (type 6/11/16/18) vaccine immunization and stimulated in vitro or not with virus-like particles (VLPs) from this vaccine. We found that the expression of ILT2 (percentage of positive cells) by CD8+ cells diminished after quadrivalent HPV (type 6/11/16/18) vaccine immunization (P < 0.01) (Fig. 1A and B, filled bars). In contrast, the expression of this regulatory receptor by NK (CD3− CD56+) and NKT (CD3+ CD56+) lymphocytes and monocytes (CD14+) was significantly increased after HPV immunization (P < 0.05 in all cases) (Fig. 1C, D, and E, filled bars). Although the expression of ILT2 by CD4+ lymphocytes tended to diminish after immunization, no significant differences were detected, and in the case of CD19+ B cells, no apparent differences in ILT2 expression were detected (Fig. 1E and G). In addition, when PBMCs were stimulated in vitro with the quadrivalent HPV (type 6/11/16/18) vaccine, we found that the percentage of CD8+ cells expressing ILT2 diminished after the administration of the HPV vaccine (P < 0.05) (Fig. 1A, empty bars). In contrast, the percentage of ILT2-positive (ILT2+) cells into the NK and CD3+ CD56+ lymphocyte subsets increased upon in vitro stimulation after immunization (Fig. 1C and D, empty bars), with no significant differences in the case of B and CD4+ lymphocytes and monocytes (Fig. 1E to G, empty bars).
Fig 1.
Expression of ILT2 after HPV immunization. The percentage of ILT2+ cells in different leukocyte subsets of PBMCs was determined by flow cytometry in 23 healthy volunteers before (T0) and 15 days after the first (T1, day 15) and third (T2, day 195) quadrivalent HPV (type 6/11/16/18) vaccine doses. (A) Flow cytometry histograms of ILT2 expression by CD8+ lymphocytes at T0 (upper panel) and T2 (lower panel) of quadrivalent HPV (type 6/11/16/18) vaccine immunization. Data from a representative individual are shown. The thick lines correspond to cells stained with the anti-ILT2 MAb, and thin lines correspond to cells incubated with an isotype-matched MAb. (B to G) Expression levels of ILT2 by the indicated subsets of PBMCs are shown as the arithmetic mean and SD of the percentage of positive cells in nonstimulated cells and cells stimulated in vitro for 72 h with HPV (a mixture of 10 ng/ml of L1 protein of HPV-6 and -18 and 20 ng/ml of HPV-11 and -16 from the Gardasil vaccine). *, P < 0.05; **, P < 0.01; ***, P < 0.001 (two-way repeated measures analysis of variance).
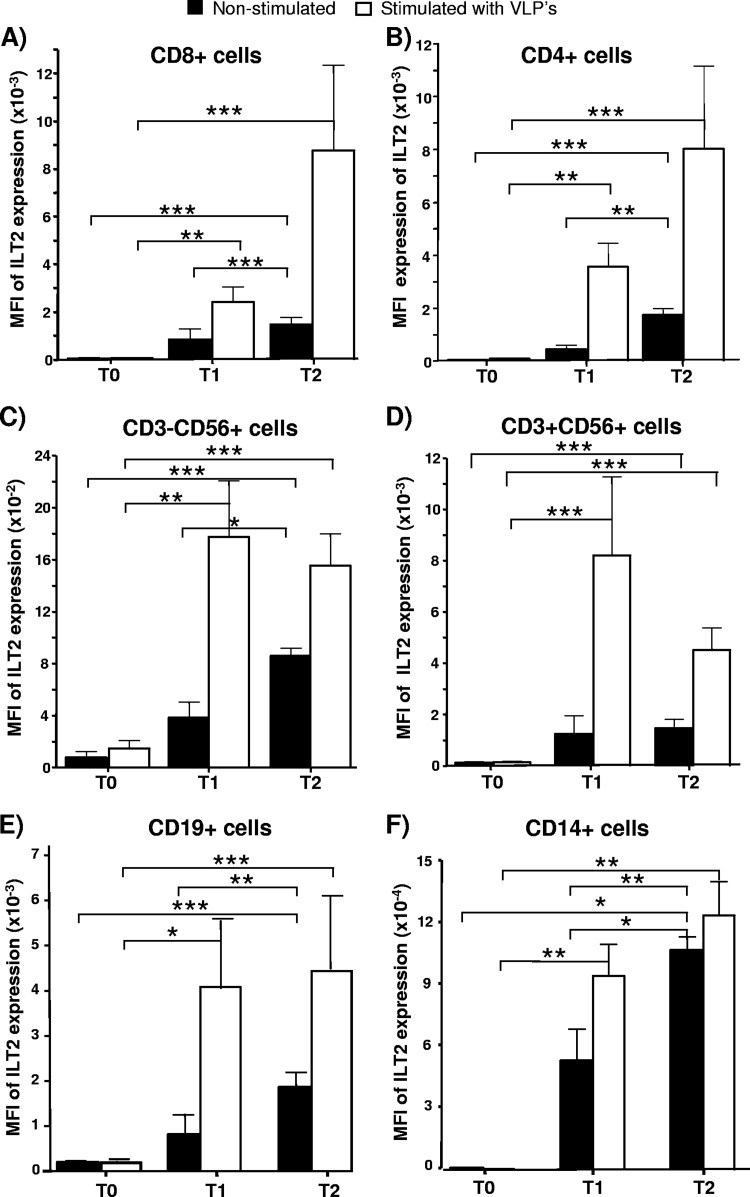
When the mean fluorescence intensity (MFI) of ILT2 expression was analyzed, we detected a significant increase of this parameter upon HPV immunization in the CD4+, CD8+, CD19+, CD14+, NK, and CD3+ CD56+ cell subsets (P < 0.05 in all cases) (Fig. 2, filled bars). Similar results were obtained when PBMCs were stimulated in vitro with the quadrivalent HPV (type 6/11/16/18) vaccine (P < 0.05) (Fig. 2, empty bars).
Fig 2.
Level of expression of ILT2 in different leukocyte subsets of PBMCs after HPV immunization. The MFI of ILT2 expression in different subsets of PBMCs was determined by flow cytometry in 23 healthy volunteers before (T0) and 15 days after the first (T1, day 15) and third (T2, day 195) quadrivalent HPV (type 6/11/16/18) vaccine doses. Panels show the MFIs of ILT2 expression in CD8+ lymphocytes (A), CD4+ cells (B), NK (CD3− CD56+) cells (C), CD3+ CD56+ cells (D), B cells (CD19+) (E), and monocytes (CD14+) (F). Cells were stimulated in vitro for 72 h with the quadrivalent HPV (type 6/11/16/18) vaccine (10 ng/ml of L1 protein of HPV-6 and -18 and 20 ng/ml of HPV-11 and -16). Data correspond to the arithmetic mean and SD. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (two-way repeated measures analysis of variance).
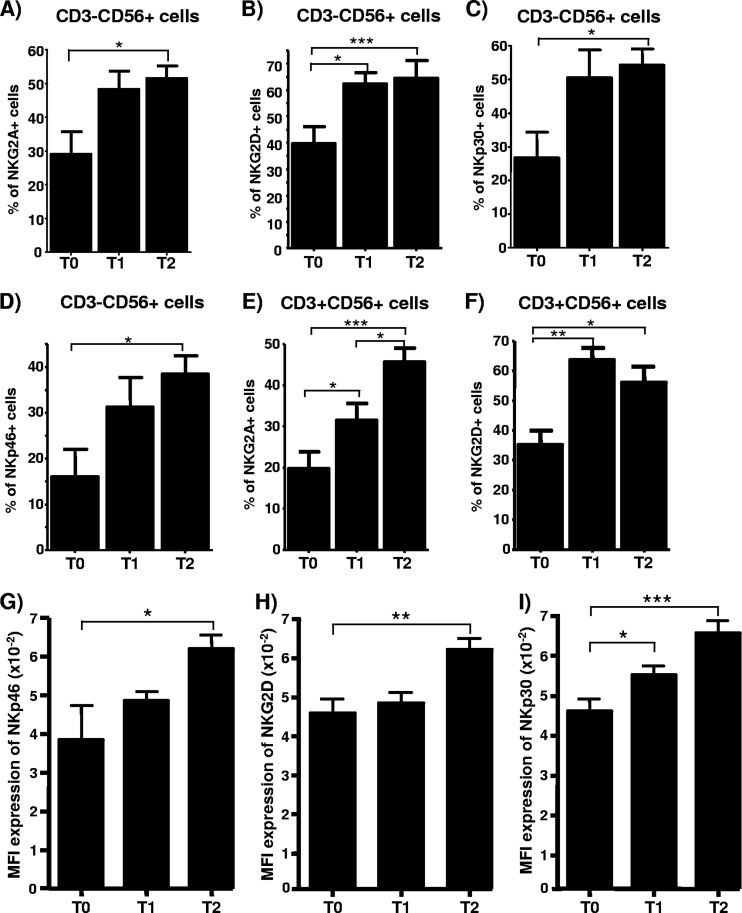
We also assessed the possible effect of quadrivalent HPV (type 6/11/16/18) vaccine immunization on the expression of other immune innate receptors, including the inhibitory receptor NKG2A (which is associated with CD94) and the stimulatory receptors NKG2D, NKp30 (which interacts with viral and endogenous proteins), and NKp46 (which interacts with the hemagglutinin of influenza virus). We found that quadrivalent HPV (type 6/11/16/18) vaccine administration was associated with a significant increase in the percentage of NK cells expressing the four receptors that were tested (P < 0.05 in all cases) (Fig. 3A to D). Similar results were found in the case of CD3+ CD56+ lymphocytes (Fig. 3E and F). These results were also associated with a significant increase in the density (MFI) of NKG2D, NKp30, and NKp46 molecule expression by NK cells (P < 0.05 in all cases) (Fig. 3G to I). However, CD3+ CD56+ cells did not show significant variations in the level of expression of the four receptors studied (data not shown). Finally, it is important to mention that we did not observe significant variations in the proportion of CD3− CD56+ or CD3+ CD56+ cells in the three samples obtained during the study.
Fig 3.
Analysis of expression of NK cell receptors after HPV immunization. (A to F) PBMCs from 23 healthy volunteers were obtained before (T0) and 15 days after the first (T1, day 15) and third (T2, day 195) quadrivalent HPV (type 6/11/16/18) vaccine doses. These cells were immunostained with anti-CD3, -CD56, and the indicated MAbs, and the percentage of positive cells was analyzed by flow cytometry. Data correspond to the expression of the indicated antigens in CD3− CD56+ or CD3+ CD56+ cells. (G to I) MFI of expression of the indicated receptors by NK cells (CD3− CD56+) in PBMCs from the same individual whose data are reflected in panels A to F. Data correspond to the arithmetic mean and SD. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (one-way repeated measures analysis of variance).
Functional assays.
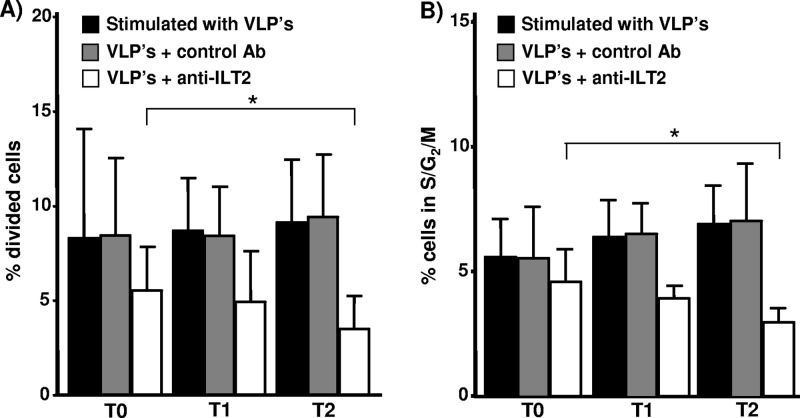
We then studied the function of ILT2 before and after quadrivalent HPV (type 6/11/16/18) vaccine immunization through an assay of inhibition of cell proliferation by using CFSE labeling and flow cytometry analysis. As shown in Fig. 4A and B, a significant reduction in the cell proliferation induced by the quadrivalent HPV (type 6/11/16/18) vaccine was observed when PBMCs were incubated in the presence of an agonistic anti-ILT2 MAb. In addition, an enhanced inhibitory function of ILT2 after quadrivalent HPV (type 6/11/16/18) vaccine administration was detected in both CFSE and DNA content analysis (P < 0.05 in both cases) (Fig. 4A and B, empty bars, and C).
Fig 4.
Regulatory function of ILT2 after HPV immunization. (A) PBMCs from 23 healthy volunteers were obtained before (T0) and 15 days after the first (T1, day 15) and third (T2, day 195) quadrivalent HPV (type 6/11/16/18) vaccine doses. Cells were loaded with CFSE and incubated for 72 h in the presence of the quadrivalent HPV (type 6/11/16/18) vaccine and with the addition (empty bars) or not (black bars) of the agonistic HP-F1 anti-ILT2 or an isotype-matched MAb (gray bars). Then, the percentage of divided cells was determined by flow cytometry analysis, as stated in Materials and Methods. Data correspond to the arithmetic mean and SD. *, P < 0.05, two-way repeated measures analysis of variance. (B) PBMCs from the same individuals whose data are represented in panel A were incubated for 72 h in the presence of quadrivalent HPV (type 6/11/16/18) vaccine and with the addition (empty bars) or not (black bars) of the agonistic HP-F1 anti-ILT2 or an isotype-matched MAb (gray bars). Then, cell nuclei were stained with propidium iodide and analyzed for DNA content by flow cytometry. Data correspond to the arithmetic mean and SD. *, P < 0.05, two-way repeated measures analysis of variance.
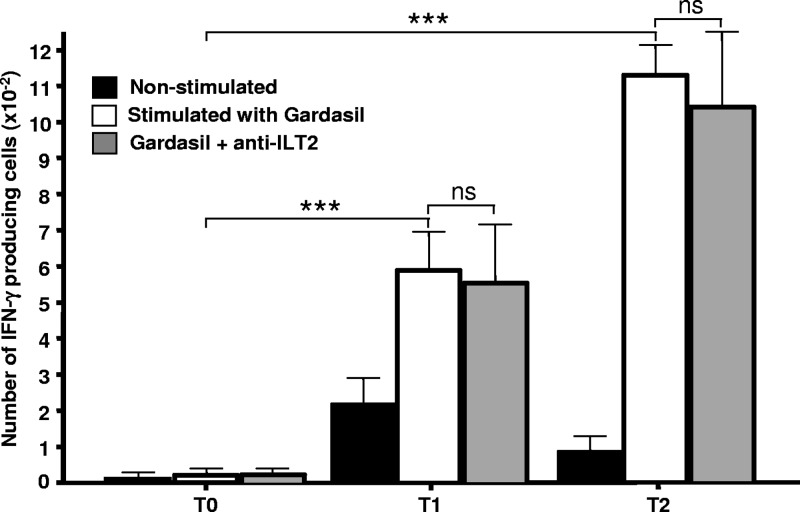
Additional functional assays of cytokine synthesis showed that the number of IFN-γ-producing lymphocytes tended to increase upon quadrivalent HPV (type 6/11/16/18) vaccine administration (Fig. 5, black bars). Furthermore, when cells were stimulated in vitro with the quadrivalent HPV (type 6/11/16/18) vaccine, we detected a significant increase in the number of cells synthesizing IFN-γ (Fig. 5, empty bars). Additional experiments indicated that CD56+ lymphocytes were the main source of IFN-γ synthesis in these assays (data not shown). However, when these cells were simultaneously stimulated through ILT2 (with the agonistic HP-F1 MAb plus a cross-linker antibody), no significant inhibition of IFN-γ production was detected (Fig. 5, gray bars).
Fig 5.
Analysis of IFN-γ release after HPV immunization. PBMCs from 23 healthy volunteers were obtained before (T0) and 15 days after the first (T1, day 15) and third (T2, day 195) quadrivalent HPV (type 6/11/16/18) vaccine doses. Cells were stimulated (empty bars) or not (black bars) with the quadrivalent HPV (type 6/11/16/18) vaccine for 72 h, in the presence or absence of an agonistic anti-ILT2 antibody, and the number of IFN-γ-producing cells was analyzed by an ELISPOT assay. Data correspond to the arithmetic mean and SD. ***, P < 0.001 (two-way analysis of variance); ns, nonsignificant.
When the production of IL-10 was analyzed, we detected a significant reduction in the release of this cytokine when cells were incubated in the presence of the quadrivalent HPV (type 6/11/16/18) vaccine and an agonistic anti-ILT2 MAb (P < 0.05) (data not shown). However, the biological consequences of these variations may not be significant due to the small reduction in concentration (approximately 2 pg/ml) (data not shown).
DISCUSSION
Cervical cancer affects approximately 500,000 women each year, and almost all cases are associated with HPV infection (4). The immune response elicited by HPV includes the production of specific antibodies as well as a cell-mediated response. However, persistent HPV infection occurs in 10 to 20% of infected individuals due to a series of immune evasion mechanisms (24). Although HPV infects basal keratinocytes, high-level viral protein expression and viral assembly are usually limited to the upper layers of epithelia, thus avoiding contact with cells of the immune system, such as Langerhans cells. In addition, assembly of HPV virions or self-assembly of L1 into VLPs is required for efficient production of L1-specific IgG1 and IgG2 antibodies (25, 29). Thus, HPV-specific antibodies are not detected in a large proportion of infected individuals. Nevertheless, clearance of HPV is accompanied by antibody production directed against L1 antigen, and passive transfer of specific antibodies in animal models has been shown to confer immunity against infection (25). Likewise, the protection against HPV induced by the L1 vaccine is associated with production of antibodies specific for this antigen (24). On the other hand, cell-mediated immunity also has a relevant role in the control of HPV infection. Thus, the development of an immune response mediated by CD4+ Th1 lymphocytes has been associated with regression of ano-genital warts (21). In addition, the increased frequency of cervical cancer in patients with altered CD4+ cells highlights the importance of this cell population in the control of HPV infection (23).
Evasion of the innate immune system is thought to play a key role in the ability of HPV to cause persistent infection. This is reflected in the absence of an inflammatory response to the virus, as well as downregulation of interferon release (12). In addition, several studies have suggested that evasion of NK cell activity may play a role in the development of cervical cancer (3, 7, 21, 27). In this regard, Carrington et al. found that the presence of the KIR3DS1 gene is a risk factor for developing cervical cancer (3). In addition, a reduction in the expression of NKp30, NKp46, and NKG2D by NK cells of women with cervical cancer has been reported (2, 7). It has also been shown that HPV E6 and E7 proteins interfere with the binding of IL-18 to its receptor, resulting in a reduction in IFN-γ production by NK cells (12). Furthermore, in different malignant tumors the expression of HLA-G, which interacts with the inhibitory receptors ILT2, ILT4, and KIR2DL4, has been detected, and this has been suggested as a mechanism leading to evasion of NK cell activity (22). In this regard, increased expression of HLA-G in patients with cervical malignancies has been reported (30) as well as reduced synthesis of some activating ligands for NK cell receptors (26). Altogether, these studies suggest that modulation of the expression of NK receptors and their ligands affects the immune response to HPV. However, the precise role of different NK cell receptors in this context has not been fully defined. Furthermore, there is little information regarding the response of NK cells to HPV vaccination.
In this study, we have detected increased expression of NKG2A, NKG2D, NKp30, and NKp46 by NK cells and of ILT2 by monocytes, NK, and CD3+ CD56+ lymphocytes after HPV vaccination. In addition, an increase in the density of expression (MFI) of ILT2, NKG2D, NKp30, and NKp36 was also observed. ILT2 is a negative regulatory receptor that has been shown to participate in the immunopathogenesis of several autoimmune diseases (5, 14). Furthermore, an increase in the expression of this receptor by NK and T lymphocytes has been shown in association with HCMV infection (8, 15). Although the precise role of ILT2 under this condition has not been fully elucidated, it is very feasible that increased expression of this receptor may contribute to downregulating the immune response against HCMV. In this regard, it has been reported that cytotoxic T cells may express ILT2 and that this receptor modulates their lytic activity (10, 13). Thus, our data showing an increase in the expression and function of ILT2 after quadrivalent HPV (type 6/11/16/18) vaccine immunization strongly suggest that this receptor may also have a relevant role in the modulation of the innate and adaptive immune responses against HPV. Interestingly, we have observed that the enhanced synthesis of IFN-γ observed in vitro upon quadrivalent HPV (type 6/11/16/18) vaccine immunization is not affected by ILT2 engagement, indicating that the regulatory effect of this receptor is not exerted on all immune parameters that are relevant in the response to HPV. In this regard, it has been recently reported that NK cells exposed to HPV VLPs show increased cytotoxic activity and cytokine production (including IFN-γ) (19). Therefore, it is possible that the enhanced production of IFN-γ observed by us upon HPV immunization could be related to the increased expression of activating receptors by NK cells and to a lack of a regulatory effect mediated by ILT2 on this immune parameter. In this regard, the possible effect of quadrivalent HPV (type 6/11/16/18) vaccine immunization on the cytotoxic activity of NK lymphocytes remains an interesting point to be addressed.
Interestingly, we have observed that while the proportion of CD8+ lymphocytes that expressed ILT2 decreased after immunization, there was an increase in the density (MFI) of this receptor in these cells. Although the underlying mechanism for this apparent paradoxical effect remains to be determined, our data suggest that in the fraction of CD8+ cells that remain ILT2 positive after quadrivalent HPV (type 6/11/16/18) vaccine immunization, the enhanced density of this receptor may exert an increased regulatory effect.
The in vitro effect of the quadrivalent HPV (type 6/11/16/18) vaccine on the expression of ILT2 by NK cells after HPV immunization is of interest. It is feasible that the induction of ILT2 expression by quadrivalent HPV (type 6/11/16/18) vaccine in these cells observed by us corresponds to the memory-like phenomenon described under different circumstances for NK lymphocytes (17). However, it is also possible that this induction is mediated by cytokines released by T cells. Finally, the adjuvant contained in the vaccine (aluminum) might also contribute to the induction of ILT2.
Overall, the profile of NK receptors displayed by NK cells after immunization differs significantly from changes observed in association with persistent HPV infection and cervical cancer (7). This may reflect differences in the route of antigen exposure, an effect produced by the combination of antigen and adjuvant contained in the vaccine, the absence of the effects produced by HPV-E6 and −E7, or genetic host-related factors.
In summary, our observations indicate that HPV immunization is associated with significant changes in the expression and function of immune innate and regulatory receptors, phenomena that may contribute to the protective effect of this vaccine.
ACKNOWLEDGMENT
This work was supported by grant UASLP-CA-44 from PROMEP-SEP, Mexico.
Footnotes
Published ahead of print 9 May 2012
REFERENCES
- 1. Antrobus RD, et al. 2005. Virus-specific cytotoxic T lymphocytes differentially express cell-surface leukocyte immunoglobulin-like receptor-1, an inhibitory receptor for class I major histocompatibility complex molecules. J. Infect. Dis. 191: 1842–1853 [DOI] [PubMed] [Google Scholar]
- 2. Arreygue-Garcia NA, et al. 2008. Augmented serum level of major histocompatibility complex class I-related chain A (MICA) protein and reduced NKG2D expression on NK and T cells in patients with cervical cancer and precursor lesions. BMC Cancer. 8: 16 doi: 10.1186/1471-2407-8-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carrington M, et al. 2005. Hierarchy of resistance to cervical neoplasia mediated by combinations of killer immunoglobulin-like receptor and human leukocyte antigen loci. J. Exp. Med. 201: 1069–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cutts FT, et al. 2007. Human papillomavirus and HPV vaccines: a review. Bull. World Health Org. 85: 719–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Doníz-Padilla L, et al. 2011. Analysis of expression and function of the inhibitory receptor ILT2 in lymphocytes from patients with autoimmune thyroid disease. Eur. J. Endocrinol. 165: 129–136 [DOI] [PubMed] [Google Scholar]
- 6. Frazer IH. 2009. Interaction of human papillomaviruses with the host immune system: a well evolved relationship. Virology 384: 410–414 [DOI] [PubMed] [Google Scholar]
- 7. Garcia-Iglesias T, et al. 2009. Low NKp30, NKp46 and NKG2D expression and reduced cytotoxic activity on NK cells in cervical cancer and precursor lesions. BMC Cancer. 9: 186 doi: 10.1186/1471-2407-9-186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gumá M, et al. 2004. Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood 104: 3664–3671 [DOI] [PubMed] [Google Scholar]
- 9. Hasan UA, et al. 2007. TLR9 expression and function is abolished by the cervical cancer-associated human papillomavirus type 16. J. Immunol. 178: 3186–3197 [DOI] [PubMed] [Google Scholar]
- 10. Ince MN, et al. 2004. Increased expression of the natural killer cell inhibitory receptor CD85j/ILT2 on antigen-specific effector CD8 T cells and its impact on CD8 T-cell function. Immunology 112: 531–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kanodia S, Fahey L, Kast W. 2007. Mechanisms used by human papillomaviruses to escape the host immune response. Curr. Cancer Drug Targets 7: 79–89 [DOI] [PubMed] [Google Scholar]
- 12. Lee SJ, et al. 2001. Both E6 and E7 oncoproteins of human papillomavirus 16 inhibit IL-18-induced IFN-gamma production in human peripheral blood mononuclear and NK cells. J. Immunol. 167: 497–504 [DOI] [PubMed] [Google Scholar]
- 13. Merlo A, et al. 2001. CD85/LIR-1/ILT2 and CD152 (cytotoxic T lymphocyte antigen 4) inhibitory molecules down-regulate the cytolytic activity of human CD4+ T-cell clones specific for Mycobacterium tuberculosis. Infect. Immun. 69: 6022–6029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Monsiváis-Urenda A, et al. 2007. Analysis of expression and function of the inhibitory receptor ILT2 (CD85j/LILRB1/LIR-1) in peripheral blood mononuclear cells from patients with systemic lupus erythematosus (SLE). J. Autoimmun. 29: 97–107 [DOI] [PubMed] [Google Scholar]
- 15. Monsiváis-Urenda A, et al. 2010. Influence of human cytomegalovirus infection on the NK cell receptor repertoire in children. Eur. J. Immunol. 40: 1418–1427 [DOI] [PubMed] [Google Scholar]
- 16. Nakajima H, et al. 2003. Transcriptional regulation of ILT family receptors. J. Immunol. 171: 6611–6620 [DOI] [PubMed] [Google Scholar]
- 17. Paust S, von Andrian UH. 2011. Natural killer cell memory. Nat. Immunol. 12: 500–508 [DOI] [PubMed] [Google Scholar]
- 18. Prod'homme V, et al. 2007. The human cytomegalovirus MHC class I homolog UL18 inhibits LIR-1+ but activates LIR-1− NK cells. J. Immunol. 178: 4473–4481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Renoux VM, et al. 2011. Human papillomavirus entry into NK cells requires CD16 expression and triggers cytotoxic activity and cytokine secretion. Eur. J. Immunol. 41: 3240–3252 [DOI] [PubMed] [Google Scholar]
- 20. Sayós J, Martínez-Bariocanal A, Kitzig F, Bellón T, López-Botet M. 2004. Recruitment of C-terminal Src kinase by the leukocyte inhibitory receptor CD85j. Biochem. Biophys. Res. Commun. 324: 640–647 [DOI] [PubMed] [Google Scholar]
- 21. Scott M, Nakagawa M, Moscicki AB. 2001. Cell-mediated immune response to human papillomavirus infection. Clin. Diagn. Lab. Immunol. 8: 209–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Seliger B, Abken H, Ferrone S. 2003. HLA-G and MIC expression in tumors and their role in anti-tumor immunity. Trends Immunol. 24: 82–87 [DOI] [PubMed] [Google Scholar]
- 23. Shah W, et al. 2011. A reversed CD4/CD8 ratio of tumor-infiltrating lymphocytes and a high percentage of CD4+ FOXP3+ regulatory T cells are significantly associated with clinical outcome in squamous cell carcinoma of the cervix. Cell. Mol. Immunol. 8: 59–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stanley M. 2008. Immunobiology of HPV and HPV vaccines. Gynecol. Oncol. 109(Suppl. 2): S15–S21 [DOI] [PubMed] [Google Scholar]
- 25. Suzich JA, et al. 1995. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proc. Natl. Acad. Sci. U. S. A. 92: 11553–11557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Textor S, et al. 2008. Activating NK cell receptor ligands are differentially expressed during progression to cervical cancer. Int. J. Cancer 123: 2343–2353 [DOI] [PubMed] [Google Scholar]
- 27. Wang SS, et al. 2002. Comprehensive analysis of human leukocyte antigen class I alleles and cervical neoplasia in 3 epidemiologic studies. J. Infect. Dis. 186: 598–605 [DOI] [PubMed] [Google Scholar]
- 28. Wang SS, et al. 2001. Human leukocyte antigen class I and II alleles and risk of cervical neoplasia: results from a population-based study in Costa Rica. J. Infect. Dis. 184: 1310–1314 [DOI] [PubMed] [Google Scholar]
- 29. Yang R, et al. 2005. Papillomavirus capsid mutation to escape dendritic cell-dependent innate immunity in cervical cancer. J. Virol. 79: 6741–6750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zheng N, et al. 2011. Up-regulation of HLA-G expression in cervical premalignant and malignant lesions. Tissue Antigens 77: 218–224 [DOI] [PubMed] [Google Scholar]