Abstract
The antigenicity of seasonal human influenza virus changes continuously; thus, a cross-protective influenza vaccine design needs to be established. Intranasal immunization with an influenza split-virion (SV) vaccine and a mucosal adjuvant induces cross-protection; however, no mucosal adjuvant has been assessed clinically. Formalin-inactivated intact human and avian viruses alone (without adjuvant) induce cross-protection against the highly pathogenic H5N1 avian influenza virus. However, it is unknown whether seasonal human influenza formalin-inactivated whole-virion (WV) vaccine alone induces cross-protection against strains within a subtype or in a different subtype of human influenza virus. Furthermore, there are few reports comparing the cross-protective efficacy of the WV vaccine and SV vaccine-mucosal adjuvant mixtures. Here, we found that the intranasal human influenza WV vaccine alone induced both the innate immune response and acquired immune response, resulting in cross-protection against drift variants within a subtype of human influenza virus. The cross-protective efficacy conferred by the WV vaccine in intranasally immunized mice was almost the same as that conferred by a mixture of SV vaccine and adjuvants. The level of cross-protective efficacy was correlated with the cross-reactive neutralizing antibody titer in the nasal wash and bronchoalveolar fluids. However, neither the SV vaccine with adjuvant nor the WV vaccine induced cross-reactive virus-specific cytotoxic T-lymphocyte activity. These results suggest that the intranasal human WV vaccine injection alone is effective against variants within a virus subtype, mainly through a humoral immune response, and that the cross-protection elicited by the WV vaccine and the SV vaccine plus mucosal adjuvants is similar.
INTRODUCTION
Influenza viruses belong to the Orthomyxoviridae family and are a major cause of respiratory disease in humans. Pandemics of influenza A virus are responsible for substantial mortality and morbidity, particularly in high-risk groups, which include the elderly and individuals with chronic underlying medical conditions (31). Natural infection can confer resistance to virus infection to a certain degree (5, 18) and provides more protection against antigenic drift variants within a given influenza virus subtype and against viruses from different subtypes (18, 20, 27, 28). However, the current human influenza split-virion (SV) vaccine, in which hemagglutinin is the major component, provides protection only against the homologous virus strain. The antigenicity of seasonal human influenza virus changes continuously because of the frequent mutation of viral genes, including the gene for hemagglutinin (31). Therefore, it is necessary to establish a cross-protective clinical influenza vaccine design.
In animal models, intranasal immunization with an influenza virus SV vaccine with adjuvant induces cross-protection and in vivo virus clearance against drift variants within a subtype and against different subtypes, as shown by Tamura et al. (11, 24, 26, 27) in mice that were immunized intranasally with SV vaccines and cholera toxin B subunit (CTB) as an adjuvant. They also found that intranasal immunization, but not subcutaneous or intraperitoneal immunization, with the vaccine and CTB induces cross-reactive IgA antibody production in the respiratory tract, leading the authors to suggest that the cross-protection and in vivo virus clearance are associated with a sufficient level of secreted IgA antibody. However, the toxin causes severe diarrhea and nasal discharge, so a safer and more effective adjuvant is needed for intranasal influenza virus vaccines in clinical use. Recent studies have demonstrated that synthetic double-stranded RNA polyriboinosinic acid-polyribocytidylic acid [poly(I·C)], modified pulmonary surfactant, and poly(γ-glutamic acid) nanoparticles (γ-PGA-NPs) are safe and potent candidates for use as an adjuvant in mucosal influenza virus vaccines (8, 13, 14). These materials induce dendritic cell (DC) activation, which plays an important role in mucosal adjuvant activity. However, these adjuvants, like most other mucosal adjuvants that have been development worldwide, have not been assessed clinically (3).
Takada et al. (21) demonstrated that the intranasal immunization of mice with formalin-inactivated forms of several HN types of intact human and avian influenza A viruses alone (without adjuvant) induces cross-protection against the highly pathogenic H5N1 avian influenza virus. The currently available and licensed influenza vaccines for humans are formalin-inactivated whole-virion (WV) and SV vaccines, although these vaccines are only injected intramuscularly. Takada et al. raised the possibility that the WV vaccine is a candidate for a cross-protective intranasal vaccine for clinical use. However, it is still unknown whether a seasonal human influenza WV vaccine alone would induce cross-protection against strains within a subtype or in a different subtype of human influenza virus. Furthermore, there are few reports comparing the in vivo cross-protection and cross-reactive virus clearance of the WV vaccine and SV vaccine-mucosal adjuvant mixtures. In addition, it is not clear whether the in vivo cross-protection or cross-reactive virus clearance elicited by intranasal immunization with these vaccines is associated with neutralizing antibody production or cell-mediated immune responses. In the present study, we sought to address these questions using WV vaccines and SV vaccines with mucosal adjuvants.
MATERIALS AND METHODS
Animals, virus strains, inactivated whole-virion vaccine, split-virion vaccine, and adjuvant.
Female BALB/c mice were obtained from Japan SLC Inc. (Hamamatsu, Japan) and Charles River Japan (Yokohama, Japan) and bred in our facility at the National Institute of Biomedical Innovation. All of the mice used in this study were 6 weeks old. The human influenza A virus strains A/New Caledonia/20/99 (H1N1), A/Solomon Islands/3/2006 (H1N1), A/Brisbane/59/2007 (H1N1), A/Hiroshima/52/2005 (H3N2), and B/Malaysia/2506/2004 were kindly provided by Takato Odagiri (National Institute of Infectious Diseases, Tokyo, Japan), and the mouse-adapted human influenza virus strain A/PR/8/34 (H1N1) was kindly provided by Hideki Hasegawa (National Institute of Infectious Diseases).
The viruses were grown in MDCK cells. Mouse-adapted strains of A/Solomon Islands/3/2006, A/Brisbane/59/2007, A/New Caledonia/20/99, A/Hiroshima/52/2005, and B/Malaysia/2506/2004 were generated as follows. Mice were inoculated with virus (108 PFU in 40 μl PBS), and their lungs were harvested 3 days later. The lung tissue was homogenized in PBS (one set of lungs per 1 ml PBS) and spun, and the supernatants were harvested. Naïve mice were inoculated with the supernatant (40 μl/mouse). These steps were repeated for a total of 10 inoculations. The mouse-adapted viruses were then grown once in MDCK cells, and the mouse 50% lethal dose (MLD50) for the viruses was assessed. The MLD50s of A/Solomon Islands/3/2006, A/Brisbane/59/2007, A/New Caledonia/20/99, A/Hiroshima/52/2005, and B/Malaysia/2506/2004 strains were 5 × 105, 5 × 105, 5 × 105, 5 × 105, and 5 × 104 PFU, respectively. The influenza WV vaccines, in which the influenza virus particles were fixed with PBS containing 0.02% formalin for 1 month, and SV vaccines, which contained all of the proteins from the virus particles, of which hemagglutinin was the major component (about 30% of the total protein), were prepared from influenza strains at Kanonji Institute, The Research Foundation for Microbial Diseases of Osaka University (Kanonji, Japan). We used synthetic double-stranded RNA polyriboinosinic acid-polyribocytidylic acid [poly(I·C)] (Sigma, St. Louis, MO) and γ-PGA-NPs as mucosal adjuvants.
Synthesis and preparation of γ-PGA-NPs.
γ-PGA (number-average molecular weight, 380,000) was kindly donated by Meiji Seika Co., Ltd. (Tokyo, Japan). Nanoparticles composed of γ-PGA hydrophobic derivatives were prepared as previously described (2, 14–16). Briefly, γ-PGA was hydrophobically modified with l-phenylalanine ethylester (l-Phe) (Sigma, St. Louis, MO) in the presence of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (WSC) (Wako Pure Chemical Industries, Osaka, Japan). The purified γ-PGA-graft-l-Phe was characterized by 1H nuclear magnetic resonance (NMR) to determine the degree of l-Phe grafting. In this study, γ-PGA-graft-l-Phe with a 53% degree of grafting was used. Nanoparticles composed of γ-PGA-graft-l-Phe were prepared by a precipitation and dialysis method. The γ-PGA-graft-l-Phe (10 mg) was dissolved in 1 ml of dimethyl sulfoxide (DMSO), and an equal volume of saline was added, yielding a translucent solution. The solution, in cellulose membrane tubing (50,000 molecular weight cutoff), was then dialyzed against distilled water to remove the organic solvents. The size of the NPs was measured by a dynamic light-scattering (DLS) method using a Zetasizer Nano ZS (Malvern Instruments, United Kingdom). The mean diameter of the NPs was about 200 nm.
Vaccination.
BALB/c mice (6 weeks old) were anesthetized and inoculated subcutaneously (inoculation volume, 200 μl) or intranasally (inoculation volume, 20 μl) with phosphate-buffered saline (PBS), PBS containing a mixture of SV vaccine (1 μg) with either γ-PGA-NPs (100 μg) or poly(I·C) (10 μg), or PBS containing WV vaccine (1 μg) on days 0 and 21.
Virus challenge.
To assess mouse mortality upon virus infection, 14 days after the final immunization the mice were inoculated intranasally with 40 μl of PBS containing one of the following mouse-adapted viral strains at the indicated concentration: 1,000 PFU (20× LD50) of A/PR/8/34, 106 PFU (20× LD50) of B/Malaysia/2506/2004, or 107 PFU (20× LD50) of A/Solomon Islands/3/2006, A/Brisbane/59/2007, A/New Caledonia/20/99, or A/Hiroshima/52/2005. The mortality and weight loss of the mice were assessed daily for up to 14 days thereafter, and the loss of 20% of total body weight was used as the endpoint. To measure virus clearance in the lungs of the vaccine-immunized mice, the mice were inoculated intranasally with 20 μl of PBS containing 104 or 105 PFU of influenza virus 14 days after the final immunization. Two days later, the bronchoalveolar lavage (BAL) fluid was collected for virus titration by a plaque assay (21).
RNA isolation, cDNA synthesis, and real-time PCR.
The expression of cytokines in the nasal-associated lymphoid tissues (NALTs) and cervical lymph nodes of vaccinated or influenza-virus-infected mice was examined as described previously (8), with some modifications. Mice were inoculated intranasally with influenza virus or immunized intranasally with the WV vaccine or the SV vaccine with γ-PGA-NPs. The NALTs, cervical lymph nodes, and spleen were collected at various time points up to 72 h after virus or vaccine administration. The mRNA levels of cytokines in the tissues were measured by real-time quantitative PCR after reverse transcription as follows. Total RNA was prepared from the mouse NALTs, cervical lymph nodes, and spleen (3 mice per tissue type) with TRIzol reagent (Invitrogen, Carlsbad, CA), and cDNA was synthesized using Superscript III reverse transcriptase (Invitrogen) according to the manufacturer's instructions. Real-time quantitative PCR was performed using the Applied Biosystems 7300 real-time PCR system (Applied Biosystems, Foster City, CA) with the SYBR Premix Ex Taq II kit (TaKaRa Bio Inc., Kyoto, Japan) and the primers (Sigma Genosys, Ishikari, Japan) listed in Table 1 (8, 22), which were designed with Primer Express (Applied Biosystems). The cytokine targets examined were gamma interferon (IFN-α), IFN-β, IFN-γ, interleukin-4 (IL-4), IL-6, and IL-12 p40. PCR was carried out in a volume of 25 μl with the following steps: initial denaturation at 95°C for 2 min, followed by 45 cycles of 94°C for 30 s, 60°C for 30 s, and 72°C for 34 s. For each sample, PCR was performed in triplicate. The relative mRNA level of each transcript was determined by the ΔΔ threshold cycle (ΔΔCT) method using β-actin as the reference gene.
Table 1.
Primers used for real-time quantitative PCR
| Gene | Sequence (5′–3′) |
Reference | |
|---|---|---|---|
| Forward | Reverse | ||
| IFN-α | ATGCCCAGCAGATCAAGAAG | GGTGGAGGTCATTGCAGAAT | 22 |
| IFN-β | GCTCCTGGAGCAGCTGAATG | TCCGTCATCTCCATAGGGATCT | 8 |
| IFN-γ | AGCCAGATTATCTCTTTCTACCTC | GCAATACTCATGAATGCATCCTTT | 8 |
| IL-4 | CGCCATGCACGGAGATG | CGAGCTCACTCTCTGTGGTGTT | 8 |
| IL-6 | CCAGAAACCGCTATGAAGTTCCT | CACCAGCATCAGTCCCAAGA | 8 |
| IL-12 p40 | AGCTCGCAGCAAAGCAAGGT | TGGAGACACCAGCAAAACGA | 8 |
| β-Actin | ATGAAGATCAAGATCATTGCTCCTC | ACATCTGCTGGAAGGTGGACAG | 22 |
Measurement of the anti-influenza virus neutralizing antibody titer.
The neutralizing antibody titer in the mouse nasal wash and BAL fluids was determined by a microcytopathic effect (CPE) neutralizing test. For this test, the nasal wash and BAL fluids from 5 mice were pooled and then treated with receptor-destroying enzyme (RDE) as described previously (19). The RDE-treated samples were concentrated 200 times (a 20-fold final concentration compared to the original [1 ml per mouse] nasal wash and BAL fluid samples) by microcon (50,000 molecular weight cutoff; Millipore, Billerica, MA), and the concentrated samples were then subjected to 2-fold serial dilutions. The diluted samples (100 μl) were mixed 1:1 (vol/vol) with a suspension containing 100 50% tissue culture doses (TCID50) of various influenza virus strains and incubated at 37°C for 1 h. The mixtures were then transferred onto MDCK cell monolayers in 96-well plates and incubated at 37°C for 1 h. The cells were washed with minimal essential medium (MEM) supplemented with 0.01 M HEPES, 0.2% bovine albumin, and 1 μg/ml trypsin (MEM maintenance medium), 100 μl of MEM maintenance medium was added to each well, and the cells were incubated at 37°C in a 5% CO2 incubator. The CPE status was observed 96 h after the infection. The culture medium was removed by absorption, and the adherent MDCK cells were fixed with 4% formaldehyde-PBS for more than 1 h. The formaldehyde-PBS was removed by absorption, and the fixed cells were stained with 0.1% naphthol blue black for 30 min. After the plates were washed, 50 μl of 0.1 M NaOH was added to each well, and the A595 was determined using a microplate reader. The neutralizing antibody titer was expressed as the reciprocal of the highest dilution of BAL or nasal wash fluid that yielded 50% neutralization of 100 TCID50 of virus.
CTL assay.
The cytotoxic T-lymphocyte (CTL) assay was performed as described previously (7), with some modifications. BALB/c mice were inoculated intranasally with PBS, SV vaccine with γ-PGA-NPs, or WV vaccine twice on days 0 and 21, or they were infected intranasally with a nonlethal dose of influenza virus once, on day 0, and then all the mice were challenged on day 35 with an influenza virus strain. The same strain was used to infect P815 target cells for the CTL assay. Lymphocytes were harvested from the lungs 5 days postchallenge, and red blood cell-depleted cell suspensions were prepared for use as effector cells. CD8 T cells were purified from lung lymphocytes using the Dynal mouse CD8 cell negative isolation kit (Life Technologies, Inc., Rockville, MD). To prepare the target cells, 5 × 106 P815 cells (JCRB Cell Bank, Ibaraki, Japan) were suspended in 1 ml of RPMI 1640 medium supplemented with 5 × 10−5 M β-mercaptoethanol in fetal bovine serum (FBS)-free RPMI 1640 medium and incubated with 5 × 107 PFU of influenza virus for 1 h at 37°C, 5% CO2. The cells were then washed three times with RPMI 1640 medium supplemented with 10% FBS and 5 × 10−5 M β-mercaptoethanol (complete RPMI 1640 medium). For the cytotoxicity assays, the infected P815 target cells were labeled with Na51CrO4 (GE Healthcare Bio-Science Corp., Piscataway, NJ) as described previously (6, 32). The labeled cells were suspended at 105 cells/ml in complete RPMI 1640 medium, and 100 μl of this suspension was added to each well of 96-well round-bottomed microtiter plates. Effector cells, also in 100 μl of complete RPMI 1640 medium, were then added at an effector cell-to-target cell ratio of 50:1. The plates were incubated for 5 h at 37°C, 5% CO2, 100 μl of the supernatant was removed, and the concentration of 51Cr released was determined using a TopCount NXT microplate scintillation luminescence counter (Perkin-Elmer). Specific lysis was determined as the mean percent lysis for triplicate wells, and values were calculated using the formula [(experimental counts per minute [cpm] − spontaneous cpm)/(maximal release cpm − spontaneous cpm)] × 100.
Statistical evaluations.
To evaluate the differences in mortality between groups, Fisher's exact test was performed using Statcel2 software (OMS, Tokyo, Japan). To analyze the data in the other experiments, nonparametric Student's t tests were used. A P value of <0.05 was considered significant.
RESULTS
Cross-protection against several influenza virus strains in mice intranasally immunized with WV vaccine or SV vaccine plus adjuvants.
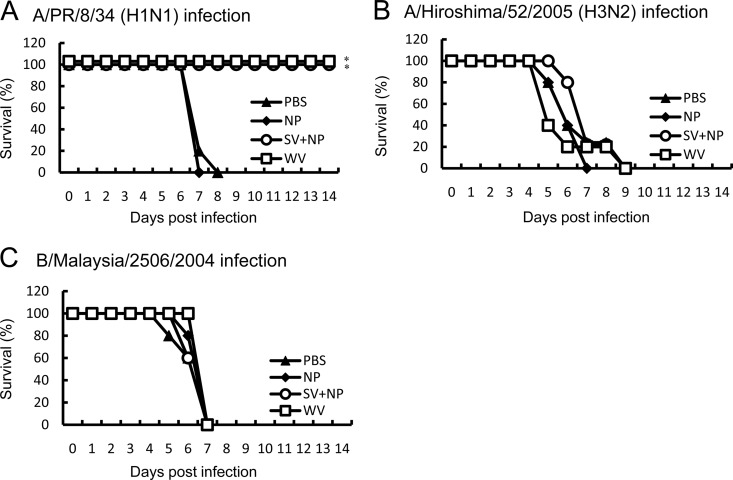
We determined the cross-protective activity in the intranasally immunized mice. Mice were intranasally inoculated with 20 μl PBS or 20 μl PBS containing 1 μg A/PR/8/34 WV vaccine or 1 μg A/PR/8/34 SV vaccine with a mucosal adjuvant. Since we previously demonstrated (14) that the mixture of 1 μg influenza A/PR/8/34 SV vaccine and 100 μg γ-PGA-NPs cross-protects 100% of mice against A/New Caledonia/20/99 infection, we mainly used 100 μg γ-PGA-NPs as the mucosal adjuvant in the present study. We examined the survival of the intranasally immunized mice after infection with A/PR/8/34, A/Hiroshima/52/2005, or B/Malaysia/2506/2004. All of the intranasally PBS- or γ-PGA-NP-injected mice lost more than 20% of their body weight and died. In contrast, all mice intranasally immunized with the WV vaccine or the SV vaccine with γ-PGA-NPs lost less than 10% of their body weight after A/PR/8/34 infection (data not shown) and survived (Fig. 1A). However, infection with the subtype virus strain A/Hiroshima/52/2005 or B/Malaysia/2506/2004 resulted in more than 20% body weight loss and death (Fig. 1B and C).
Fig 1.
Cross-protection of mice against the homotype or subtype influenza virus strains by intranasal immunization with A/PR/8/34 vaccines. Groups of mice (5 per group) were inoculated twice intranasally with PBS, 100 μg of γ-PGA-NPs (NP), a mixture of 1 μg of A/PR/8/34 SV vaccine and 100 μg of γ-PGA-NPs (SV+NP), or 1 μg of A/PR/8/34 WV vaccine (WV). Fourteen days after the final immunization, the mice were infected with A/PR/8/34 (H1N1; 103 PFU) (A), A/Hiroshima/53/2005 (H3N2; 107 PFU) (B), or B/Malaysia/2506/2004 (106 PFU) (C), and the mortality was assessed. *, P < 0.05 versus the group inoculated intranasally with PBS.
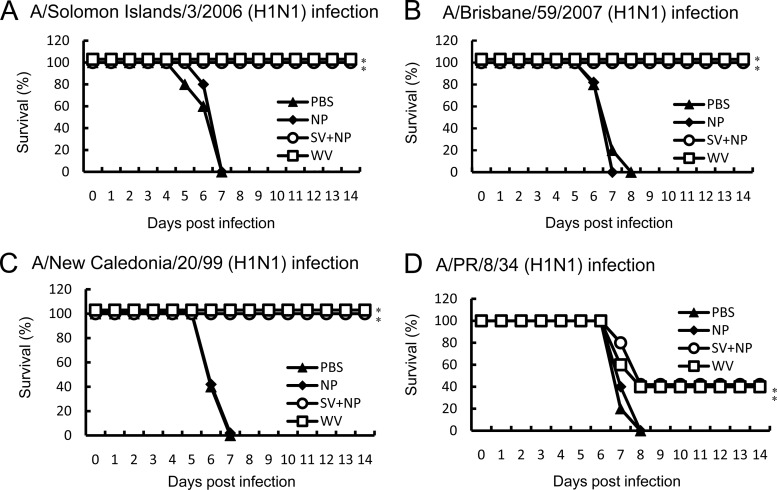
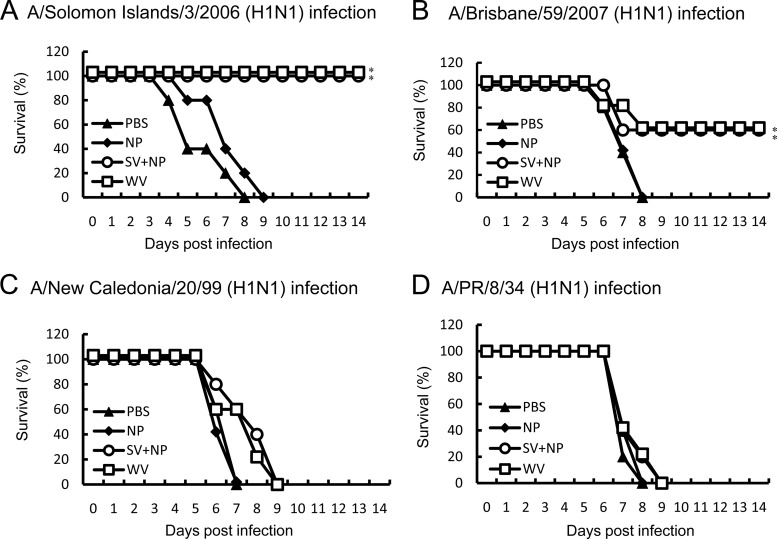
We next compared the mortality of mice infected with H1N1 variants of influenza virus after being intranasally immunized with A/Solomon Islands/3/2006 (H1N1) vaccines. All of the intranasally PBS- or γ-PGA-NP-injected mice lost more than 20% of their body weight after the virus infection and died (Fig. 2). In contrast, all of the mice intranasally immunized with the WV vaccine or the SV vaccine with γ-PGA-NPs lost less than 10% of their weight after infection with A/Solomon Islands/3/2006, A/Brisbane/59/2007, or A/New Caledonia/20/99 and survived (Fig. 2A, B, and C), and 40% of the immunized mice also survived after the infection with A/PR/8/34 (Fig. 2D).
Fig 2.
Cross-protection of mice against drift variants within a viral subtype by intranasal immunization with A/Solomon Islands/3/2006 vaccines. Groups of mice (5 per group) were inoculated twice intranasally with PBS, 100 μg of γ-PGA-NPs (NP), a mixture of 1 μg of A/Solomon Islands/3/2006 SV vaccine and 100 μg of γ-PGA-NPs (SV+NP), or 1 μg of A/Solomon Islands/3/2006 WV vaccine (WV). Fourteen days after the final immunization, the mice were infected with A/Solomon Islands/3/2006 (H1N1; 107 PFU) (A), A/Brisbane/59/2007 (H1N1; 107 PFU) (B), A/New Caledonia/20/99 (H1N1; 107 PFU) (C), or A/PR/8/34 (H1N1; 103 PFU) (D), and the mortality was assessed. *, P < 0.05 versus the group inoculated intranasally with PBS.
We also determined whether subcutaneous immunization with A/Solomon Islands/3/2006 vaccines induced cross-protection against the drift variants within a subtype strain. The subcutaneous vaccine immunizations induced 100% survival against A/Solomon Island/3/2006, whereas they induced 60, 0, and 0% survival against A/Brisbane/59/2007, A/New Caledonia/20/99, and A/PR/8/34, respectively (Fig. 3).
Fig 3.
Cross-protection of mice against drift variants within a viral subtype by subcutaneous immunization with A/Solomon Islands/3/2006 vaccines. Groups of mice (5 per group) were inoculated twice subcutaneously with PBS, 100 μg of γ-PGA-NPs (NP), a mixture of 1 μg of A/Solomon Islands/3/2006 SV vaccine and 100 μg of γ-PGA-NPs (SV+NP), or 1 μg of A/Solomon Islands/3/2006 WV vaccine (WV). Fourteen days after the final immunization, the mice were infected with A/Solomon Islands/3/2006 (H1N1; 107 PFU) (A), A/Brisbane/59/2007 (H1N1; 107 PFU) (B), A/New Caledonia/20/99 (H1N1; 107 PFU) (C), or A/PR/8/34 (H1N1; 103 PFU) (D), and the mortality was assessed. *, P < 0.05 versus the group inoculated intranasally with PBS.
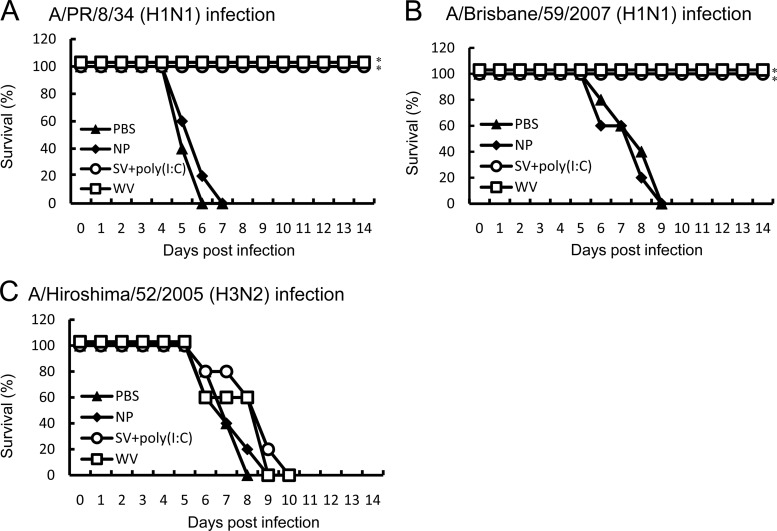
We also compared the protective efficacy against several influenza virus strains in mice intranasally immunized with A/PR/8/34 WV vaccine or A/PR/8/34 SV vaccine plus poly(I·C) as the adjuvant. We previously showed that the cross-protective immunity by intranasal immunization with SV vaccine plus 100 μg γ-PGA-NPs is equal to that with SV vaccine plus 10 μg poly(I·C) (14). As shown in Fig. 4, both vaccines provided cross-protection against A/PR/8/34 and A/Brisbane/59/2007 but not against A/Hiroshima/52/2005.
Fig 4.
Cross-protection of mice against the homotype, a drift variant within the subtype, or different subtypes of an influenza virus strain by intranasal immunization with A/PR/8/34 WV vaccine and its SV vaccine plus poly(I·C). Groups of mice (5 per group) were inoculated twice intranasally with PBS, 10 μg of poly(I·C), a mixture of 1 μg of A/PR/8/34 SV vaccine and 10 μg of poly(I·C) [SV+poly(I·C)], or 1 μg of A/PR/8/34 WV vaccine (WV). Fourteen days after the final immunization, the mice were infected with A/PR/8/34 (H1N1; 103 PFU) (A), A/Brisbane/59/2007 (H1N1; 107 PFU) (B), or A/Hiroshima/53/2005 (H3N2; 107 PFU) (C), and the mortality was assessed. *, P < 0.05 versus the group inoculated intranasally with PBS.
Cross-reactive in vivo virus clearance against several influenza virus strains in mice intranasally immunized with WV vaccine or SV vaccine plus γ-PGA-NPs.
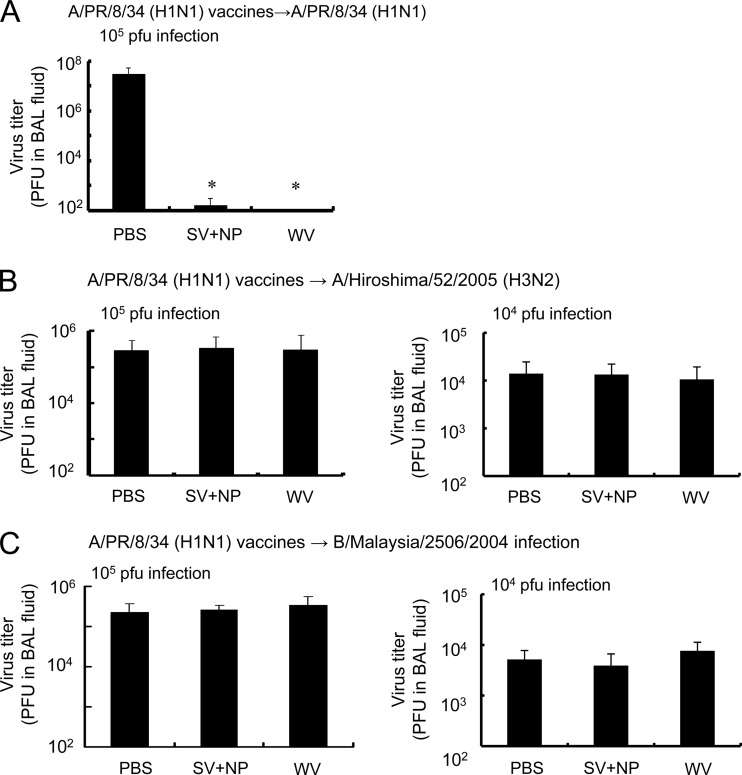
We determined virus clearance against the homotype influenza virus and subtyped influenza strains in mice intranasally immunized with WV vaccine or SV vaccine plus γ-PGA-NPs. Mice intranasally immunized with A/PR/8/34 were infected with 104 or 105 PFU of A/PR/8/34, A/Hiroshima/52/2005, or B/Malaysia/2506/2004 14 days after the final immunization. Two days after the infection, the BAL fluid was harvested and the viral titer assessed. Intranasal immunization with the WV vaccine significantly decreased the titer of A/PR/8/34 (Fig. 5A). The inhibitory effect on the virus titer in mice immunized with the WV vaccine was almost the same as that in mice immunized with the SV vaccine and γ-PGA-NPs (Fig. 5A). On the other hand, neither type of vaccine decreased the titer of A/Hiroshima/52/2005 or B/Malaysia/2506/2004 in the BAL fluid (Fig. 5B and C).
Fig 5.
In vivo clearance of homotype and subtype of influenza virus strains by intranasal immunization of mice with A/PR/8/34 vaccines. Groups of mice (5 per group) were inoculated intranasally with PBS, a mixture of 1 μg of A/PR/8/34 SV vaccine and 100 μg of γ-PGA-NPs (SV+NP), or 1 μg of A/PR/8/34 WV vaccine (WV) as described in Materials and Methods. Fourteen days after the final immunization, the mice were infected with 104 or 105 PFU of A/PR/8/34 (H1N1), A/Hiroshima/53/2005 (H3N2), or B/Malaysia/2506/2004. Two days after the infection, the BAL fluid was harvested and the number of PFU of A/PR/8/34 (A), A/Hiroshima/52/2005 (B), or B/Malaysia/2506/2004 (C) was assessed. Bars represent the means ± standard deviations from 5 mice. *, P < 0.05 versus the group of mice inoculated intranasally with PBS.
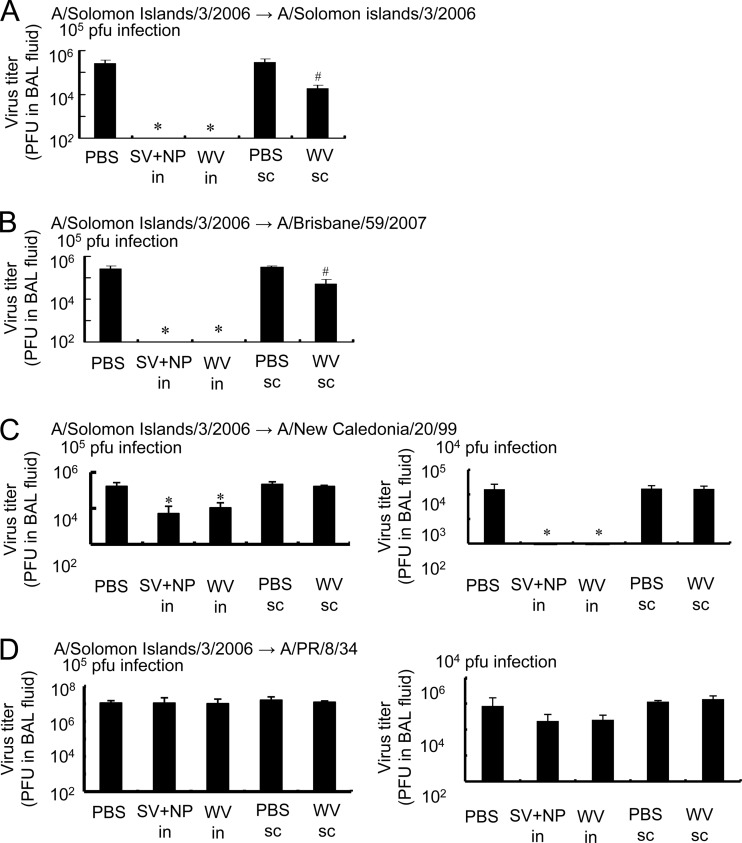
To determine the in vivo virus clearance against H1N1 variants of influenza virus, we examined the virus titer in the BAL fluid of mice immunized with the A/Solomon Islands/3/2006 WV vaccine or SV vaccine with γ-PGA-NPs 2 days after intranasal viral infection. As shown in Fig. 6A and B, intranasal immunization with either the WV vaccine or the SV vaccine with γ-PGA-NPs decreased the titer of A/Solomon Islands/3/2006 or A/Brisbane/59/2007, infected at 105 PFU, to nondetectable levels. Furthermore, the intranasal immunization decreased the titer of A/New Caledonia/20/99, infected at 104 PFU, to nondetectable levels, but the titer of A/PR/8/34 was decreased slightly, but not significantly, by the immunization (Fig. 6C and D). The in vivo virus clearance by immunization with the WV vaccine was almost the same as that with the mixture of SV vaccine and γ-PGA-NPs. In addition, subcutaneous immunization with the A/Solomon Islands/3/2006 WV vaccine decreased the titer of A/Solomon Islands/3/2006 and A/Brisbane/59/2007 to trace levels, but not that of A/New Caledonia/20/99 or A/PR/8/34 (Fig. 6).
Fig 6.
In vivo clearance of H1N1 variants of influenza virus by the intranasal immunization of mice with A/Solomon Islands/3/2006 vaccines. Groups of mice (5 per group) were inoculated intranasally with PBS (PBS i.n.), a mixture of 1 μg A/Solomon Islands/3/2006 SV vaccine and 100 μg of γ-PGA-NPs (SV+NP i.n.), or 1 μg of A/Solomon Islands/3/2006 WV vaccine (WV i.n.), as described in Materials and Methods. Other groups of mice (5 per group) were immunized subcutaneously with PBS (PBS s.c.) or 1 μg of A/Solomon Islands/3/2006 WV vaccine (WV s.c.) as described in Materials and Methods. Fourteen days after the final immunization, the mice were infected with 104 or 105 PFU of A/Solomon Islands/3/2006, A/Brisbane/59/2007, A/New Caledonia/20/99, or A/PR/8/34. Two days after the infection, the BAL fluid was harvested and the PFU value for A/Solomon islands (A), A/Brisbane/59/2007 (B), A/New Caledonia/20/99 (C), or APR/8/34 (D) was assessed. Bars represent the means ± standard deviations from 5 mice. *, P < 0.05 versus the group of mice inoculated intranasally with PBS. #, P < 0.05 versus the group of mice inoculated subcutaneously with PBS.
Induction of IFN and Th1- and Th2-related cytokines by the intranasal administration of WV vaccine or SV vaccine with γ-PGA-NPs.
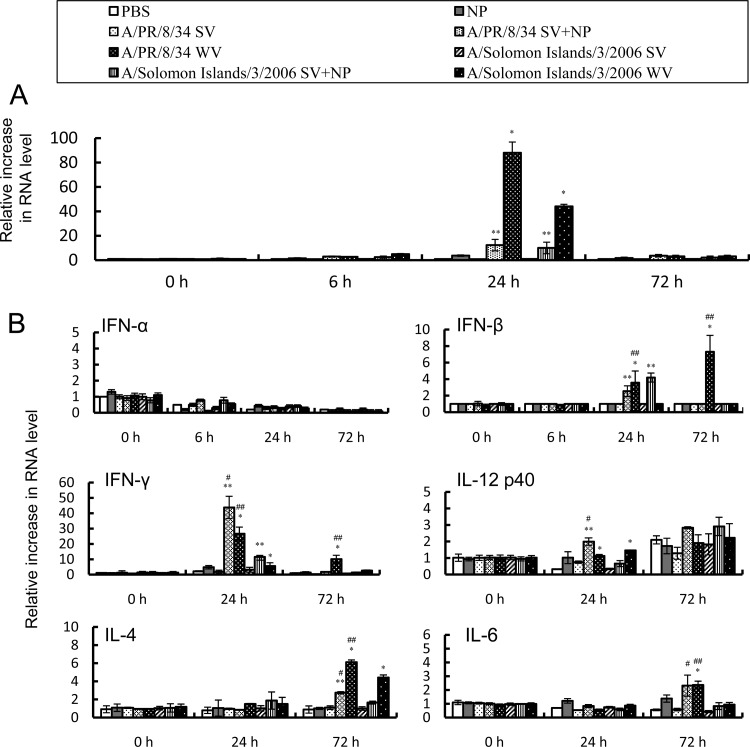
We previously demonstrated (14) that adding γ-PGA-NPs to an intranasal SV vaccine enhances the protective immune responses. Since γ-PGA-NPs activate dendritic cells (DCs) in the mucosa to enhance Th1 and Th2 responses (14), we determined whether intranasal immunization with WV vaccine alone activated DCs. To do this, we examined the mRNA expression of interferons and cytokines in the NALTs and cervical lymph nodes. IFN-α (Fig. 7A), but not IFN-β, IFN-γ, IL-4, IL-6, or IL-12 p40 (data not shown), was induced in the NALTs 24 h after vaccination with WV vaccine or with SV vaccine with γ-PGA-NPs, and it returned to its basal level by 72 h. However, the SV vaccine alone did not induce the expression of IFN-α (Fig. 3A). On the other hand, the mRNA expression levels of IFN-β, IFN-γ, IL-4, and IL-6, but not of IFN-α or IL-12 p40 in the cervical lymph nodes, were increased 24 and/or 72 h after vaccination (Fig. 7B). Taken together, these results indicate that intranasal immunization with WV vaccine alone or with a mixture of SV vaccine and γ-PGA-NPs activated DC cells in the NALTs to induce both Th1 and Th2 responses in the cervical lymph nodes. Although we also examined the expression level of cytokines in the spleen, the levels were not detectable (data not shown).
Fig 7.
Expression of cytokine mRNAs in the NALTs and cervical lymph nodes. Total RNA was extracted from the NALTs and cervical lymph nodes of mice intranasally treated with PBS, 1 μg of γ-PGA-NPs (NP), 1 μg of SV vaccine (SV), SV vaccine with γ-PGA-NPs (SV+NP), or WV vaccine (WV) from A/PR/8/34 and A/Solomon Islands/3/2006. The mRNA levels of IFN-α in the NALTs (A) and of IFN-β, IFN-γ, IL-4, IL-6, and IL-12 p40 in the cervical lymph nodes (B) were determined by real-time RT-PCR (n = 3). *, P < 0.05 for WV compared to PBS; **, P < 0.05 for SV+NP compared to NP; #, P < 0.05 for A/PR/8/34 SV+NP compared to A/Solomon Islands/3/2006 SV+NP; ##, P < 0.05 for A/PR/8/34 WV compared to A/Solomon Islands/3/2006 WV.
Cross-reactive anti-influenza neutralizing antibodies against several influenza viruses in mice intranasally immunized with WV vaccine or SV vaccine plus γ-PGA-NPs.
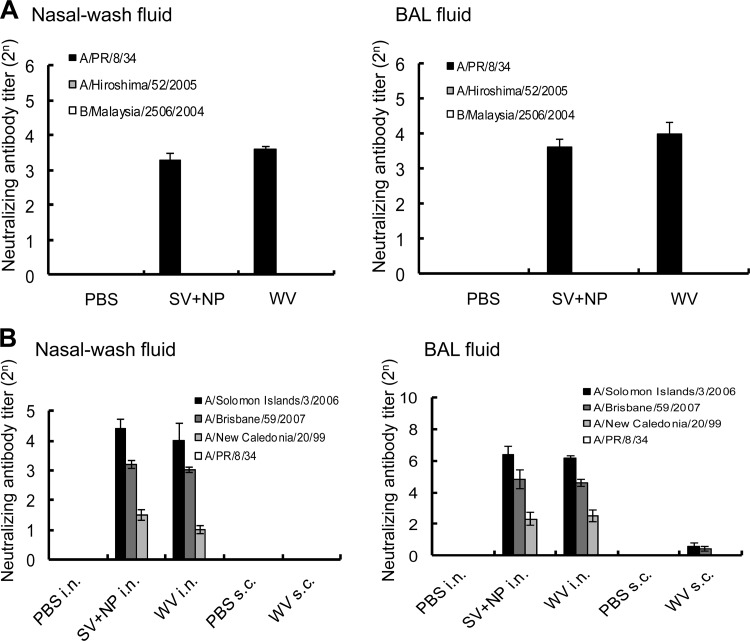
Although we tried to determine the neutralizing antibody titer in the nasal wash and BAL fluids of both groups of immunized mice, the antibody could not be detected (data not shown). Since the collection of the nasal wash and BAL fluids involved diluting them in 1 ml of PBS, we concentrated both types of fluid 20 times and found that the neutralizing antibody titer in the concentrated samples could be measured. As shown in Fig. 8A, neutralizing antibodies for A/PR/8/34 were detected in the concentrated samples of both fluids from mice immunized with the WV vaccine. The level of neutralizing antibody titer in the mice immunized with the WV vaccine was almost the same as that in mice immunized with the SV vaccine and γ-PGA-NPs. However, no immunization-induced secretion of neutralizing antibodies for A/Hiroshima/52/2005 or B/Malaysia/2506/2004 was observed in the nasal wash or BAL fluid of mice treated with either vaccine (Fig. 8A).
Fig 8.
Cross-reactive anti-influenza neutralizing antibody titer in the nasal wash and BAL fluids of mice intranasally immunized with A/PR/8/34 vaccines. (A) Groups of mice (50 per group) were inoculated twice intranasally with PBS, 100 μg of γ-PGA-NPs (NP), a mixture of 1 μg of A/PR/8/34 SV vaccine and 100 μg of γ-PGA-NPs (SV+NP), or 1 μg of A/PR/8/34 WV vaccine (WV). Fourteen days after the final immunization, the BAL and nasal wash fluids were harvested. Fluids from 5 mice were pooled, pretreated with RDE, and concentrated. The neutralizing antibody titers for A/PR/8/34 (H1N1), A/Hiroshima/52/2005 (H3N2), and B/Malaysia/2506/2005 in the concentrated samples were assessed. Bars represent the means ± standard deviations from 10 concentrated samples. (B) Anti-influenza neutralizing antibody titer for drift variants within a virus subtype in the nasal wash and BAL fluids of mice intranasally immunized with A/Solomon Islands/3/2006 vaccines. Groups of mice (50 per group) were inoculated twice intranasally with PBS, 100 μg of γ-PGA-NPs (NP), a mixture of 1 μg of A/Solomon islands/3/2006 SV vaccine and 100 μg of γ-PGA-NPs (SV+NP), or 1 μg of A/Solomon Islands/3/2006 WV vaccine (WV). Fourteen days after the final immunization, the BAL and nasal wash fluids were harvested. Fluids from 5 mice were pooled, pretreated with RDE, and concentrated. The neutralizing antibody titers for A/Solomon Islands/3/2006, A/Brisbane/59/2007, A/New Caledonia/20/99, and A/PR/8/34 were then assessed. Bars represent the means ± standard deviations from 10 concentrated samples.
We further determined the neutralizing antibody titer against H1N1 variants of influenza virus in mice intranasally immunized with A/Solomon Islands/3/2006 WV vaccine. Neutralizing antibodies for A/Solomon Islands/3/2006, A/Brisbane/59/2007, and A/New Caledonia/20/99, but not A/PR/8/34, were detected in the nasal wash and BAL fluids of the immunized mice (Fig. 8B). The neutralizing antibody titers in the mice immunized with the WV vaccine were not significantly greater than those in the mice immunized with the SV vaccine and γ-PGA-NPs. For both immunization groups, the highest neutralizing antibody titer was against A/Solomon Islands/3/2006, and the next highest was against A/Brisbane/59/2007 (Fig. 8B). In addition, we subcutaneously immunized mice with the A/Solomon Islands/3/2006 WV vaccine and detected no neutralizing antibodies for any of the strains tested in the nasal wash fluid (Fig. 8B). The BAL fluid of the subcutaneously immunized mice contained trace amounts of neutralizing antibodies for A/Solomon Islands/3/2006 and A/Brisbane/59/2007; however, the titers were much lower than those in mice immunized intranasally with the WV vaccine (Fig. 8B).
Cross-reactive CTL activity against several influenza viruses in mice intranasally immunized with WV vaccine or SV vaccine plus γ-PGA-NPs.
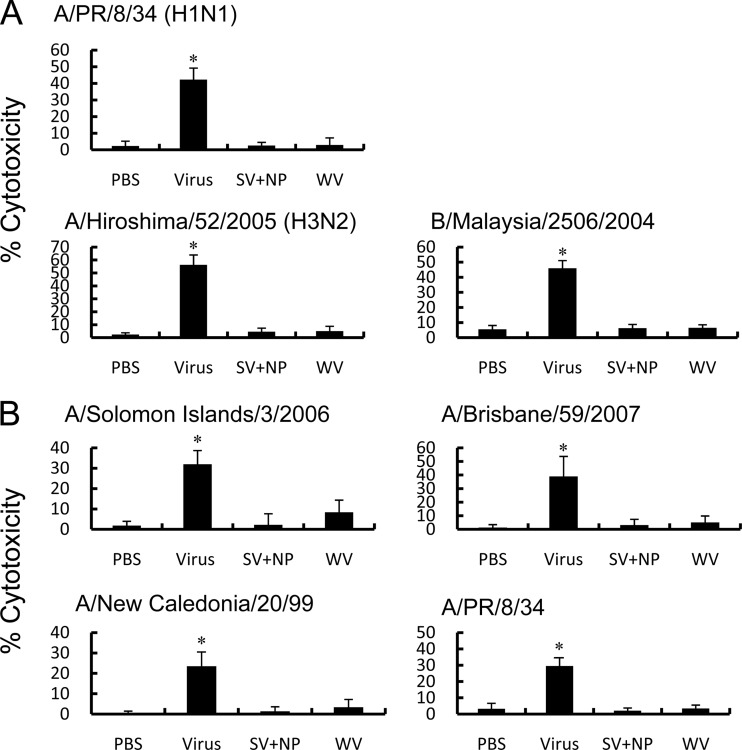
We next determined whether intranasal immunization with the WV vaccine or the mixture of SV vaccine and γ-PGA-NPs enhanced the influenza virus-specific CTL activity. Sublethal infection with one inoculation of A/PR/8/34 induced CTL activity for A/PR/8/34, A/Hiroshima/52/2005, and B/Malaysia/2506/2004. However, intranasal immunization with the A/PR/8/34 WV vaccine or its SV vaccine with γ-PGA-NPs did not significantly induce CTL activity for any virus tested (Fig. 9A). We also examined the CTL activity for the H1N1 variants, and we found that intranasal immunization with A/Solomon Islands/3/2006 WV vaccine or its SV vaccine with γ-PGA-NPs did not significantly induce CTL activity for any of the test strains (Fig. 9B). Although the percentage of effector CD8 T cells in the lungs of infected mice was 1.5 times greater than that of vaccine-immunized, infected mice (49 versus 32%), the enrichment of CD8 T cells in the lungs of the vaccine-immunized mice (the percentage of CD8 T cells in the CD8 T cell-enriched cells is 76%) did not significantly induce cytotoxicity (data not shown).
Fig 9.
Cross-reactive anti-influenza CTL activity in intranasally immunized mice. (A) Mice (5 mice per group) were injected intranasally twice with PBS, a mixture of 1 μg of A/PR/8/34 SV vaccine and 100 μg of γ-PGA-NPs (SV+NP), or 1 μg of A/PR/8/34 WV vaccine (WV) on days 0 and 21, or they were infected with A/PR/8/34 (10 PFU; Virus) once on day 0. The mice then were challenged with A/PR/8/34 (102 PFU), A/Hiroshima/52/2005 (105 PFU), or B/Malaysia/2506/2004 (105 PFU) on day 35. Lymphocytes in the lungs were harvested 5 days postchallenge, and the lymphocytes (effector cells) were mixed with 51Cr-labeled P815 cells infected with the challenged influenza virus (target cells) at an effector cell-to-target cell ratio of 50:1. Following a 5-h incubation at 37°C, 5% CO2, 100 μl of the supernatant was examined for the concentration of 51Cr released. (B) Mice were injected twice intranasally with PBS, a mixture of 1 μg of A/Solomon Islands/3/2006 SV vaccine and 100 μg of γ-PGA-NPs (SV+NP), or 1 μg of A/Solomon islands/3/2006 WV vaccine (WV) on days 0 and 21, or they were infected with A/Solomon Islands/3/2006 (105 PFU; Virus) once on day 0. The mice were challenged with A/Solomon Islands/3/2006 (103 PFU), A/Brisbane/59/2007 (105 PFU), A/New Caledonia/20/99 (105 PFU), or A/PR/8/34 (102 PFU) on day 42. Lymphocytes in the lungs were harvested 5 days postchallenge, pooled, and mixed with 51Cr-labeled P815 cells infected with the challenged influenza virus, and the CTL assay was performed as described for panel A. *, P < 0.05 versus the group of mice immunized with PBS.
DISCUSSION
In the present study, we found that intranasal immunization with a human seasonal influenza WV vaccine alone induced cross-protection against drift variants within a subtype of human influenza virus. The cross-protection conferred by the WV vaccine alone was almost as strong as that elicited by a mixture of SV vaccine and a mucosal adjuvant candidate, γ-PGA-NPs or poly(I·C).
Although other studies have demonstrated that mucosal immunization with several forms of influenza vaccine induce cross-reactive anti-influenza virus immune responses, the roles of influenza-specific humoral and cell-mediated immunity in the cross-protection and cross-reactive virus clearance achieved by intranasal immunization with formalin-inactivated WV vaccine or SV vaccine with mucosal adjuvant have been unclear. Furuya et al. (7) demonstrated that intranasal immunization with γ-irradiated inactivated influenza virus induces a cross-protection against subtyped influenza virus strains. They also found that the cross-protection is due to influenza-specific CTL activity (cell-mediated immunity) but not to humoral immunity, and that the cross-reactive CTL activity was not induced by a formalin-inactivated WV vaccine (7). In the present study, we found that intranasal immunization with a WV vaccine or an SV vaccine with γ-PGA-NPs protected mice from the homotype virus and some variants within the subtype but not from viruses of a different subtype (Fig. 1, 2, 4, 5, and 6). Accordingly, the immunization produced neutralizing antibodies against the homotype virus and the variants but not against viruses of a different subtype (Fig. 8). Furthermore, intranasal immunization with a WV vaccine or an SV vaccine with γ-PGA-NPs did not significantly induce cross-reactive CTL activity (Fig. 9). These results suggest that cross-reactive neutralizing antibodies contribute to the cross-reactive in vivo virus clearance caused by intranasal immunization with a WV vaccine or with a mixture of SV vaccine and adjuvant.
Several reports have suggested that the advantage of nasal vaccination against influenza virus infection is the induction of antibodies at the mucosal epithelium, which results in an efficient production of cross-protective immunity (23, 25). However, there are few reports examining the cross-reactive neutralizing antibodies in the mucosa of mice immunized intranasally with WV vaccine versus mixtures of SV vaccines and adjuvants in relation to cross-reactive in vivo virus clearance in the mucosa. In fact, several studies have reported that neutralizing antibody activity could not be detected in the mucosa (8, 14, 21). However, this finding may reflect the amount of dilution necessary to obtain the nasal wash and BAL fluids. Here, because the normal mucus volume in the nasal mucosa is 3.0 mm3 and that of the ciliated epithelia of the trachea and lung is 2.8 mm3 (9), the extracted nasal wash and BAL fluids were each diluted more than 300 times. Since we suspected that this dilution of the mucus was responsible for the inability to detect neutralizing antibodies, we concentrated the nasal wash and BAL fluids 20-fold and found that the neutralizing antibody titer could be determined.
Intranasal immunization with the WV vaccine induced a much higher titer of neutralizing antibody against the homotype virus in the nasal wash and BAL fluids than did subcutaneous immunization with the WV vaccine (Fig. 8B). Furthermore, the cross-protection and clearance of the homotype virus from the lungs of mice immunized with the intranasal WV vaccine was greater than that of mice immunized with a subcutaneous WV vaccine (Fig. 2, 3, and 6). These results suggested that intranasal immunization with a WV vaccine provides more effective protection from the homotype virus and from variants within a subtyped virus than does subcutaneous WV vaccine immunization.
Seasonal human influenza A virus continuously evolves by undergoing mutations, thus altering the antigenic sites of the surface antigens in hemagglutinin (HA) and neuraminidase (NA) (1, 4, 29, 31). Current subcutaneous human influenza vaccines induce neutralizing IgG antibody responses to the highly variable influenza HA protein; therefore, the immunization protects against homologous but not antigenically distinct viruses, including drift variants and different viral subtypes. On the other hand, several reports have demonstrated that intranasal immunization induces cross-protection against intrasubtype drift variants and different viral subtypes, which led us to hypothesize that the neutralizing antibodies induced by intranasal immunization recognize a highly conserved region of the antigen that most influenza viruses contain. In the present study, intranasal immunization with A/Solomon Islands/3/2006 vaccines induced protection and neutralizing antibody responses against A/Brisbane/59/2007 and A/New Caledonia/20/99 but less so against A/PR/8/34 (Fig. 2 and 6); all of these viruses are of the H1N1 subtype.
We investigated the similarity of the HA amino acid sequences from the GenBank database using DNASIS Pro v.3.0 software (Hitachi Solutions, Tokyo, Japan). The percent identity of the HA in A/Brisbane/59/2007 (GenBank accession number ADI99532.1), A/New Caledonia/20/99 (GenBank accession number ABQ10137.1), and A/PR/8/34 (GenBank accession number ABO21709.1) compared to the HA in A/Solomon Islands/3/2006 (GenBank accession number ABU99069.1) was 98.1, 96.9, and 83.2%, respectively. Furthermore, the intranasal immunization did not protect against different subtypes of viruses, for which the homology of the HA might be much lower than that of variants within a viral subtype (Fig. 1 and 4). These findings suggest that the greater the sequence variation of the viral protein, the lower the activity of the neutralizing antibodies will be. However, it remains to be discovered which region of the viral antigen is responsible for the cross-protective neutralizing antibody recognition of drift variants within a viral subtype and in different subtypes.
Adding a mucosal adjuvant, i.e., γ-PGA-NPs or poly(I·C), is necessary to induce cross-protection by intranasal immunization with an SV vaccine, whereas a WV vaccine can induce cross-protection when used alone. We demonstrated in the present study that intranasal immunization with a WV vaccine or with an SV vaccine plus γ-PGA-NPs, but not SV vaccine alone, induced IFN-α/β and Th1- and Th2-related cytokines (Fig. 7), suggesting that the WV vaccine alone induces both the innate immune response and acquired immune response. While the induced innate immune response, in which plasmacytoid dendritic cells (pDCs) produce type I interferon, might be essential for the immunogenicity of WV vaccines (12), other studies suggest that pDCs and interferon-controlled pathways do not significantly contribute to the generation of CTL activity or to the antibody response to influenza virus infection (17, 30). On the other hand, Jordan et al. (10) reported that the exposure of naïve B cells to cytokine IL-4 and/or antigen leads to a priming state in which the subsequent aggregation of major histocompatibility complex II molecules induces the mobilization of calcium ions and cell proliferation. Therefore, we speculate that an increased production of IL-4 in the cervical lymph nodes of mice immunized intranasally with WV vaccine alone or with SV vaccine plus mucosal adjuvant contributes to the priming of naïve B cells together with the antigen, resulting in cross-protection, including an enhanced anti-influenza neutralizing antibody response. However, it remains unknown why the CTL activity is rarely induced while Th1 cytokines are highly induced.
We found in the present study that intranasal WV immunization, as well as intranasal immunization with a mixture of SV vaccine and mucosal adjuvant, induced cross-protection against the homotype virus and drift variants within a human viral subtype. The cross-protection was correlated with the cross-reactive neutralizing antibody titer in the nasal cavity and lungs; therefore, humoral immunity in the mucosal area might play a role in effective cross-reactive virus clearance. Influenza virus vaccines for humans need to be able to induce effective cross-protection with minimal side effects. Although intranasal immunization with an SV vaccine plus mucosal adjuvant induces cross-protection, mucosal adjuvants have not been assessed clinically. A cold-adaptive live influenza vaccine for intranasal injection, FluMist, is available clinically in the United States and Europe but not in Japan. However, it can be used in individuals from age 2 to 49 years and can result in viral replication, leading to influenza-like syndromes. The WV vaccine is licensed clinically only for intramuscular injection; therefore, it needs to be determined whether intranasal immunization with the WV vaccine is safe and has rare side effects. However, since viral reactivation cannot occur with the WV vaccine, it may be a good candidate for an intranasal seasonal influenza vaccine for clinical use in the near future.
ACKNOWLEDGMENTS
We thank Takato Odagiri and Hideki Hasegawa (National Institute of Infectious Diseases) for providing the viruses.
This study was partly supported by grants from the Japanese Ministry of Health, Welfare, and Labor, the Japanese Ministry of Education, Culture, Sports, Science, and Technology, and CREST, Japan Science and Technology Agency.
Footnotes
Published ahead of print 2 May 2012
REFERENCES
- 1. Agrawal AS, et al. 2010. Genetic characterization of circulating seasonal influenza A viruses (2005–2009) revealed introduction of oseltamivir resistant H1N1 strains during 2009 in eastern India. Infect. Genet. Evol. 10:1188–1198 [DOI] [PubMed] [Google Scholar]
- 2. Akagi T, Kaneko T, Kida T, Akashi M. 2005. Preparation and characterization of biodegradable nanoparticles based on poly(gamma-glutamic acid) with l-Phenylalanine as a protein carrier. J. Control Rel. 108:226–236 [DOI] [PubMed] [Google Scholar]
- 3. Amorij JP, Hinrichs W, Frijlink HW, Wilschut JC, Huckriede A. 2010. Needle-free influenza vaccination. Lancet Infect. Dis. 10:699–711 [DOI] [PubMed] [Google Scholar]
- 4. Bean WJ, et al. 1992. Evolution of the H3 influenza virus hemagglutinin from human and nonhuman hosts. J. Virol. 66:1129–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Belshe RB. 2004. Current status of live attenuated influenza virus vaccine in the US. Virus Res. 103:177–185 [DOI] [PubMed] [Google Scholar]
- 6. Bennink JR, Yewdell JW, Smith GL, Moss B. 1986. Recognition of cloned influenza virus hemagglutinin gene products by cytotoxic T lymphocytes. J. Virol. 57:786–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Furuya Y, et al. 2010. Cytotoxic T cells are the predominant players providing cross-protective immunity induced by gamma-irradiated influenza A viruses. J. Virol. 84:4212–4221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ichinohe T, et al. 2005. Synthetic double-stranded RNA poly(I:C) combined with mucosal vaccine protects against influenza virus infection. J. Virol. 79:2910–2919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ito R, et al. 2003. Roles of anti-hemagglutinin IgA and IgG antibodies in different sites of the respiratory tract of vaccinated mice in preventing lethal influenza pneumonia. Vaccine 21:2362–2371 [DOI] [PubMed] [Google Scholar]
- 10. Jordan MB, Mills DM, Kappler J, Marrack P, Cambier JC. 2004. Promotion of B cell immune responses via an alum-induced myeloid cell population. Science 304:1808–1810 [DOI] [PubMed] [Google Scholar]
- 11. Kikuta K, et al. 1990. Cross-protection against influenza B type virus infection by intranasal inoculation of the HA vaccines combined with cholera toxin B subunit. Vaccine 8:595–599 [DOI] [PubMed] [Google Scholar]
- 12. Koyama S, et al. 2010. Plasmacytoid dendritic cells delineate immunogenicity of influenza vaccine subtypes. Sci. Transl. Med. 2:25ra24 doi:10.1126/scitranslmed.3000759 [DOI] [PubMed] [Google Scholar]
- 13. Mizuno D, Ide-Kurihara M, Ichinomiya T, Kubo I, Kido H. 2006. Modified pulmonary surfactant is a potent adjuvant that stimulates the mucosal IgA production in response to the influenza virus antigen. J. Immunol. 176:1122–1130 [DOI] [PubMed] [Google Scholar]
- 14. Okamoto S, et al. 2009. Poly(gamma-glutamic acid) nano-particles combined with mucosal influenza virus hemagglutinin vaccine protects against influenza virus infection in mice. Vaccine 27:5896–5905 [DOI] [PubMed] [Google Scholar]
- 15. Okamoto S, et al. 2007. Influenza hemagglutinin vaccine with poly(gamma-glutamic acid) nanoparticles enhances the protection against influenza virus infection through both humoral and cell-mediated immunity. Vaccine 25:8270–8278 [DOI] [PubMed] [Google Scholar]
- 16. Okamoto S, et al. 2008. Single dose of inactivated Japanese encephalitis vaccine with poly(gamma-glutamic acid) nanoparticles provides effective protection from Japanese encephalitis virus. Vaccine 26:589–594 [DOI] [PubMed] [Google Scholar]
- 17. Price GE, Gaszewska-Mastarlarz A, Moskophidis D. 2000. The role of alpha/beta and gamma interferons in development of immunity to influenza A virus in mice. J. Virol. 74:3996–4003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Quan FS, Compans RW, Nguyen HH, Kang SM. 2008. Induction of heterosubtypic immunity to influenza virus by intranasal immunization. J. Virol. 82:1350–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rowe T, et al. 1999. Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J. Clin. Microbiol. 37:937–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Smirnov YA, Gitelman AK, Govorkova EA, Lipatov AS, Kaverin NV. 2004. Influenza H5 virus escape mutants: immune protection and antibody production in mice. Virus Res. 99:205–208 [DOI] [PubMed] [Google Scholar]
- 21. Takada A, Matsushita S, Ninomiya A, Kawaoka Y, Kida H. 2003. Intranasal immunization with formalin-inactivated virus vaccine induces a broad spectrum of heterosubtypic immunity against influenza A virus infection in mice. Vaccine 21:3212–3218 [DOI] [PubMed] [Google Scholar]
- 22. Takenaka N, et al. 2011. Overexpression of phospholipase Cepsilon in keratinocytes upregulates cytokine expression and causes dermatitis with acanthosis and T-cell infiltration. Eur. J. Immunol. 41:202–213 [DOI] [PubMed] [Google Scholar]
- 23. Tamura S. 2010. Studies on the usefulness of intranasal inactivated influenza vaccines. Vaccine 28:6393–6397 [DOI] [PubMed] [Google Scholar]
- 24. Tamura S, et al. 1991. Cross-protection against influenza A virus infection by passively transferred respiratory tract IgA antibodies to different hemagglutinin molecules. Eur. J. Immunol. 21:1337–1344 [DOI] [PubMed] [Google Scholar]
- 25. Tamura S, Hasegawa H, Kurata T. 2010. Estimation of the effective doses of nasal-inactivated influenza vaccine in humans from mouse-model experiments. Jpn. J. Infect. Dis. 63:8–15 [PubMed] [Google Scholar]
- 26. Tamura S, et al. 1992. Cross-protection against influenza virus infection afforded by trivalent inactivated vaccines inoculated intranasally with cholera toxin B subunit. J. Immunol. 149:981–988 [PubMed] [Google Scholar]
- 27. Tamura SI, et al. 1992. Superior cross-protective effect of nasal vaccination to subcutaneous inoculation with influenza hemagglutinin vaccine. Eur. J. Immunol. 22:477–481 [DOI] [PubMed] [Google Scholar]
- 28. Tumpey TM, Renshaw M, Clements JD, Katz JM. 2001. Mucosal delivery of inactivated influenza vaccine induces B-cell-dependent heterosubtypic cross-protection against lethal influenza A H5N1 virus infection. J. Virol. 75:5141–5150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. 1992. Evolution and ecology of influenza A viruses. Microbiol. Rev. 56:152–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wolf AI, et al. 2009. Plasmacytoid dendritic cells are dispensable during primary influenza virus infection. J. Immunol. 182:871–879 [DOI] [PubMed] [Google Scholar]
- 31. Wright PF, Neuman G, Kawaoka Y. 2007. Orthomyxoviruses, p 1691–1740 In Knipe DM, et al. (ed), Fields virology, 5th ed Lippincott-Raven, Philadelphia, PA [Google Scholar]
- 32. Yewdell JW, Bennink JR, Smith GL, Moss B. 1985. Influenza A virus nucleoprotein is a major target antigen for cross-reactive anti-influenza A virus cytotoxic T lymphocytes. Proc. Natl. Acad. Sci. U. S. A. 82:1785–1789 [DOI] [PMC free article] [PubMed] [Google Scholar]