Abstract
Possible smallpox reemergence drives research for third-generation vaccines that effectively neutralize variola virus. A comparison of neutralization assays using different substrates, variola and vaccinia (Dryvax and modified vaccinia Ankara [MVA]), showed significantly different 90% neutralization titers; Dryvax underestimated while MVA overestimated variola neutralization. Third-generation vaccines may rely upon neutralization as a correlate of protection.
TEXT
Previous studies (6) suggested that the quantitative titers of human serum plaque reduction neutralization tests (PRNTs) differed when variola or vaccinia virus was used as the neutralization target. Similar observations were made during Acambis2000 vaccine studies in postimmunization nonhuman primate sera (10). This study was designed to evaluate the correlation between PRNTs when different virus species or strains were used as the neutralization target. This study is the first to look extensively at these differences using the same set of well-defined human postvaccination sera. If the ability to neutralize variola is the desired outcome of smallpox vaccination, then understanding the relative significances of variola and vaccinia neutralization titers is a critical surrogate measure, especially if previous measures of vaccine efficacy (i.e., the Jennerian pustule or “take”) are not available for third-generation vaccines and if variola stocks are destroyed. The comparable efficacy of a vaccination regimen using vaccinia (Dryvax or modified vaccinia Ankara [MVA]) or variola virus as the neutralizing target is presented elsewhere (3, 4).
Sera (from 46 participants) from a National Institutes of Health-funded smallpox vaccine trial (DMID 02-017) were evaluated at Saint Louis University (SLU) and at the Centers for Disease Control and Prevention (CDC). Twenty participants received MVA (IMVAMUNE) subcutaneously (SC) (1 × 108 50% tissue culture infective dose units [TCID50]; 2 doses, 1 month apart), 15 received MVA intramuscularly (IM) (1 × 108 TCID50; 2 doses, 1 month apart), and 11 received Dryvax vaccination by scarification (1 dose) (4). MVA is a replication-deficient, less-reactogenic third-generation smallpox vaccine (1, 7, 9). Sera at peak response times postvaccination were evaluated using variola, Dryvax, and MVA PRNTs. Individuals were evaluated 28 to 30 days post-Dryvax vaccination or 14 days after the second MVA dose.
At SLU, serum samples were tested in a qualified PRNT assay using an American Type Culture Collection (ATCC) strain of MVA (catalog number VR-1508) or Dryvax (2, 8) as the neutralization reference virus. The Dryvax PRNT was modified by substituting MVA for Dryvax as the neutralizing target and by identifying plaques through immunostaining in place of crystal violet staining. Sonicated MVA virus was diluted to ∼30 to 50 PFU/well. An equal volume of diluted MVA was mixed with each serial 2-fold dilution of heat-inactivated serum or medium and incubated overnight at 37°C. Each serum-virus mixture and virus-medium mixture (virus-only control) was inoculated onto BSC-40 cell monolayers, an adsorption time of 1 h was utilized, and then the plates were incubated for 2 days at 37°C to allow for plaque formation. Plates were fixed with cold acetone/methanol (50/50) for 1 h at 2 to 8°C. Plaques were then elucidated by immunostaining using anti-vaccinia antibody (rabbit anti-vaccinia; ViroStat, Portland, ME) as the primary antibody followed by goat anti-rabbit IgG conjugated to horseradish peroxidase (Kirkegaard and Perry, Gaithersburg, MD). The substrate used was the enhanced orange system (Kirkegaard and Perry, Gaithersburg, MD). Immunostained plaques were counted using a dissecting microscope.
Variola PRNT assays were performed at the CDC using a method adapted from that previously described (3, 4). Duplicate 2-fold dilutions of sera were prepared in RPMI 1640 supplemented with 2% fetal bovine serum, mixed with variola virus strain Solaimen (final serum dilutions of 1:10 to 1:40 for prebleeds and 1:40 to 1:1,280 for postvaccination sera), and incubated at 35°C overnight. Medium alone was used to quantitate the virus-only control. Positive (sera from previously vaccinated persons) and negative serum controls were used to confirm that the assay was performed within predetermined parameters (5). The positive control, vaccinia immune globulin intravenous (human) (Cangene Corporation, Winnipeg, Canada), was used at dilutions of 1:1,000 to 1:32,000 based on prior knowledge of vaccinia-neutralizing capacity. After overnight incubation, one milliliter of the serum-virus or control-virus mixture was added to BSC-40 cell monolayers and adsorbed for 1 h, and an additional milliliter of medium was applied. Plaques developed over 72 h and were counted following crystal violet staining of cell monolayers.
Linear regression analysis was applied to a log transformation of each individual's serum dilutions to facilitate linear interpolation of actual 90% PRNT titers at peak postvaccination response. The medians and interquartile ranges at 90% neutralization were calculated for each neutralization target overall and by vaccine treatment group; the geometric mean titers (GMTs) were also calculated (Table 1). The overall 90% PRNT titers for the different orthopoxvirus neutralization targets (Dryvax, MVA, and variola) were compared using the Wilcoxon signed-rank test; nonparametric statistics were used, as the data are not normally distributed. A P value of <0.05 was considered statistically significant. The 90% PRNT titers for the different orthopoxvirus neutralization targets were displayed graphically (Fig. 1).
Table 1.
90% PRNT medians and geometric mean titers for different orthopoxvirus neutralization targets by vaccination regimen
| Orthopoxvirus neutralization target | Each vaccination regimen (n) |
All vaccines (n = 46) | ||
|---|---|---|---|---|
| MVA SC (20) | MVA IM (15) | Dryvax (11) | ||
| Dryvax | ||||
| 90% PRNT median (interquartile range) | 35.0 (13.0–95.5) | 14.0 (11.0–33.0) | 71.0 (29.0–167.0) | 32.5 (12.0–93.0) |
| GMT | 30.9 | 18.0 | 47.5 | 28.7 |
| MVA | ||||
| 90% PRNT median (interquartile range) | 245.0 (61.5–673.0) | 117.0 (92.0–247.0) | 10.0 (4.0–59.0) | 110.5 (18.0–345.0) |
| GMT | 152.9 | 156.0 | 15.5 | 89.3 |
| Variola | ||||
| 90% PRNT median (interquartile range) | 63.4 (49.9–130.2) | 78.1 (42.6–104.8) | 41.7 (14.1–64.1) | 63.4 (41.7–108.0) |
| GMT | 83.9 | 73.7 | 35.9 | 65.6 |
Fig 1.
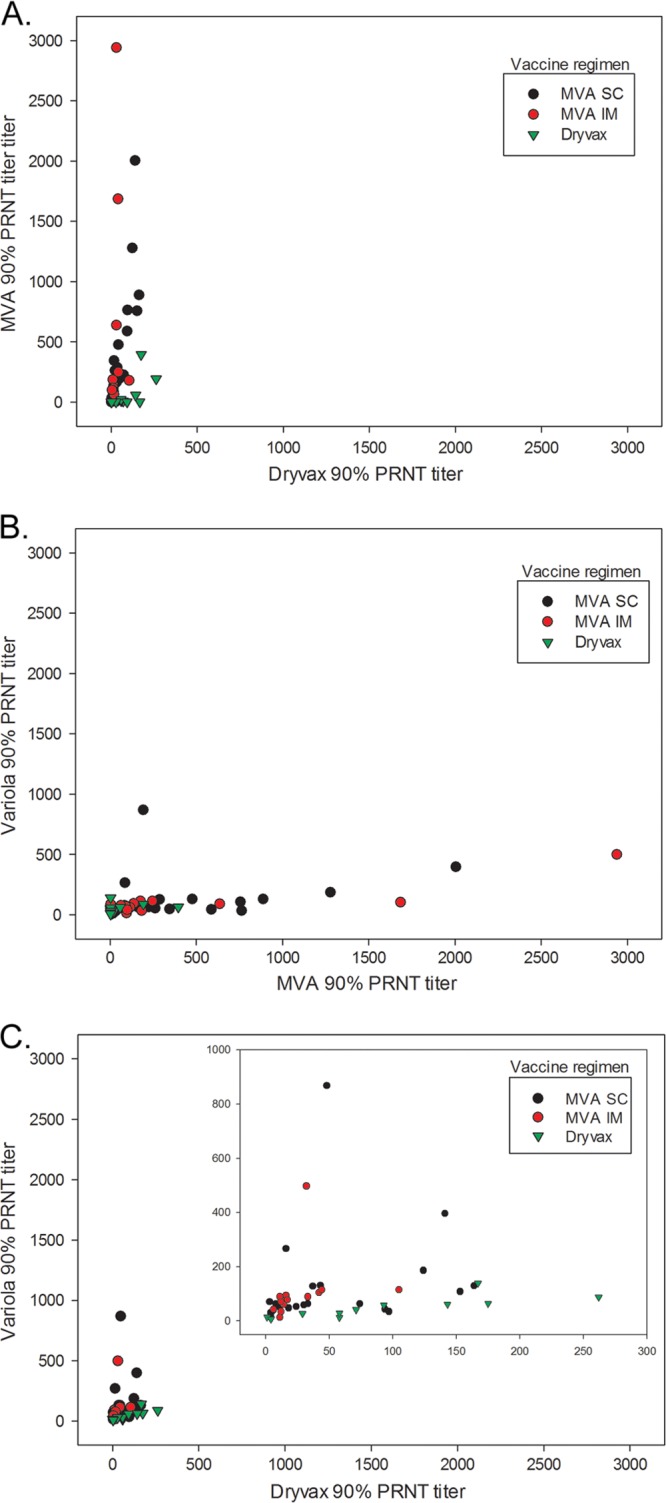
90% PRNT titers by vaccination regimen for Dryvax versus MVA (A), MVA versus variola (B), and Dryvax versus variola (inset graph, reduced x and y axis scales) (C).
Median 90% PRNT titers for each neutralization target were compared, and GMTs were presented (Table 2). Statistically significant differences were noted between the overall 90% PRNT titers for Dryvax and variola, MVA and variola, and Dryvax and MVA (Table 2). Graphical analyses did not reveal visual relationships between the 90% PRNT titers for the comparison of the three different neutralization targets. Graphs of the MVA 90% PRNT titers with the Dryvax and variola 90% PRNT titers show a clustering of titers at <500, with the MVA PRNT titer higher than the corresponding Dryvax or variola PRNT titer for some individuals (Fig. 1A and B). The graph of the Dryvax 90% PRNT with the variola 90% PRNT showed a clustering of titers at <200, with the variola 90% PRNT higher than the corresponding Dryvax 90% PRNT for some individuals (Fig. 1C).
Table 2.
Comparison of 90% PRNT titers for orthopoxvirus neutralization targets
| Target comparison | Respective medians | P value |
|---|---|---|
| Dryvax vs variola | 32.5 and 63.4 | 0.008 |
| MVA vs variola | 110.5 and 63.4 | 0.0007 |
| Dryvax vs MVA | 32.5 and 110.5 | <.0001 |
Previous studies (6) suggesting that PRNTs differed when variola or vaccinia virus was used as the neutralization target are largely anecdotal, with evaluation of human convalescent and postvaccination sera as well as some hyperimmune sera raised in other species. In addition, the results were not truly comparable, as different sets of sera were used for the variola and vaccinia neutralization tests. This article is the first to look extensively at the differences in PRNT results for the same set of well-defined postvaccination sera using different viruses (variola and vaccinia) and different strains (MVA and Dryvax) as the substrate. Significant differences in PRNT titers were observed using different viruses as the substrate for neutralization when all subjects, regardless of vaccination regimen, are combined. Using Dryvax as the neutralization antigen results in significantly lower 90% PRNT titers than using variola as the neutralization antigen. Using MVA as the neutralization antigen results in significantly higher 90% PRNT titers than using variola as the neutralization antigen. The mechanisms for this observation are uncertain and likely include subtle antigenic differences between viruses and between the vaccine regimens. PRNT titer differences were not due to variances in viral preparation quality, since the amount of virus used is limited and calculations of genomes/PFU for variola and Dryvax were similar (variola, ∼147 genomes/PFU; vaccinia, ∼100 genomes/PFU). Although the Dryvax PRNT protocol had to be modified to include immunohistochemical (IHC) staining in order to elucidate plaques formed by MVA, staining techniques were the same for the Dryvax and variola virus PRNTs. Therefore, it is unlikely that differences in 90% PRNTs observed between the neutralization viral antigens are due to differences in PRNT techniques. This analysis, in combination with data from other vaccine trials, may assist in determining if neutralization titers with vaccinia virus as the target can be bridged to variola virus-neutralizing titers. Furthermore, a better understanding of neutralizing capacity is invaluable for third-generation vaccines which do not produce the historic correlate of protection (the “take”).
ACKNOWLEDGMENTS
We acknowledge the assistance of Mark Challberg and Robert Johnson of DMID/NIAID for their assistance in facilitating this study.
This study was funded by grant number N01-AI-25464 from the National Institutes of Health, Bethesda, MD.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Footnotes
Published ahead of print 16 May 2012
REFERENCES
- 1. Antoine G, Scheiflinger F, Dorner F, Falkner FG. 1998. The complete genomic sequence of the modified vaccinia Ankara strain: comparison with other orthopoxviruses. Virology 244: 365–396 [DOI] [PubMed] [Google Scholar]
- 2. Belshe RB, et al. 2004. Dose-dependent neutralizing-antibody responses to vaccinia. J. Infect. Dis. 189: 493–497 [DOI] [PubMed] [Google Scholar]
- 3. Damon IK, et al. 2009. Evaluation of smallpox vaccines using variola neutralization. J. Gen. Virol. 90: 1962–1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Frey SE, et al. 2007. Clinical and immunologic responses to multiple doses of IMVAMUNE (modified vaccinia Ankara) followed by Dryvax challenge. Vaccine 25: 8562–8573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Karem KL, et al. 2005. Characterization of acute-phase humoral immunity to monkeypox: use of immunoglobulin M enzyme-linked immunosorbent assay for detection of monkeypox infection during the 2003 North American outbreak. Clin. Diagn. Lab. Immunol. 12: 867–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McCarthy K, Downie W. 1948. An Investigation of immunological relationships between the viruses of variola, vaccinia, cowpox and ectromelia by neutralization tests on the chorio-allantois of chick embryos. Br. J. Exp. Pathol. 29: 501–510 [Google Scholar]
- 7. Meyer H, Sutter G, Mayr A. 1991. Mapping of deletions in the genome of the highly attenuated vaccinia virus MVA and their influence on virulence. J. Gen. Virol. 72(Part 5): 1031–1038 [DOI] [PubMed] [Google Scholar]
- 8. Newman FK, et al. 2003. Improved assay to detect neutralizing antibody following vaccination with diluted or undiluted vaccinia (Dryvax) vaccine. J. Clin. Microbiol. 41: 3154–3157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vollmar J, et al. 2006. Safety and immunogenicity of IMVAMUNE, a promising candidate as a third generation smallpox vaccine. Vaccine 24: 2065–2070 [DOI] [PubMed] [Google Scholar]
- 10. Weltzin R, et al. 2003. Clonal vaccinia virus grown in cell culture as a new smallpox vaccine. Nat. Med. 9: 1125–1130 [DOI] [PubMed] [Google Scholar]


