Abstract
UVC light has long been known to be highly germicidal but has not been much developed as a therapy for infections. This study investigated the potential of UVC light for the prophylaxis of infections developing in highly contaminated superficial cutaneous wounds. In vitro studies demonstrated that the pathogenic bacteria Pseudomonas aeruginosa and Staphylococcus aureus were inactivated at UVC light exposures much lower than those needed for a similar effect on mammalian keratinocytes. Mouse models of partial-thickness skin abrasions infected with bioluminescent P. aeruginosa and S. aureus were developed. Approximately 107 bacterial cells were inoculated onto wounds measuring 1.2 by1.2 cm on the dorsal surfaces of mice. UVC light was delivered at 30 min after bacterial inoculation. It was found that for both bacterial infections, UVC light at a single radiant exposure of 2.59 J/cm2 reduced the bacterial burden in the infected mouse wounds by approximately 10-fold in comparison to those in untreated mouse wounds (P < 0.00001). Furthermore, UVC light increased the survival rate of mice infected with P. aeruginosa by 58.3% (P = 0.0023) and increased the wound healing rate in mice infected with S. aureus by 31.2% (P < 0.00001). DNA lesions were observed in the UVC light-treated mouse wounds; however, the lesions were extensively repaired by 48 h after UVC light exposure. These results suggested that UVC light may be used for the prophylaxis of cutaneous wound infections.
INTRODUCTION
Cutaneous infections are ubiquitous and the most common infections, ranging in severity from minor superficial infections to, under rare circumstances, life-threatening diseases, particularly in immunocompromised patients (14, 15). The major etiological agents implicated in cutaneous infections include Gram-negative Pseudomonas aeruginosa and Gram-positive Staphylococcus aureus (29). Of more concern is the fact that these infections are becoming worrisome due to bacterial resistance to conventional antibiotics (26, 33). As a result, novel topical bactericides are required.
UVC light is electromagnetic irradiation at wavelengths of 200 to 280 nm. Although it has been known for the last 100 years that UVC light (especially at 240 to 280 nm) is highly bactericidal, the use of UVC light to treat actual infections in wounds or other anatomical situations remains at an early stage of development (13). The mechanism of UVC light inactivation of bacteria is to damage the genetic material in bacterial cells (5). UVC light-induced damage to the DNA of a bacterium often results from the dimerization of pyrimidine residues. In particular, thymine produces cyclobutane pyrimidine dimers (CPD). When DNA is damaged, it becomes very difficult for the nucleic acids to replicate, and if replication does occur, it often produces a defect that prevents the bacterium from being viable. We recently reported that the topical application of UVC radiation could prevent and treat superficial infections in mice caused by the fungal pathogen Candida albicans (8). Furthermore, we have also used UVC light to treat onychomycosis in an ex vivo toenail model (10) and studied the potential of UVC light to destroy microorganisms on catheters (11). In the present study, using novel mouse models of cutaneous wound infections with bioluminescent bacteria combined with noninvasive imaging, we investigated the potential of UVC light for the prophylaxis of bacterial infections and possible UVC-induced damage to host tissue.
The mouse is an ideal organism for the exploration of human infectious diseases. Despite some differences, the immune systems of mice and humans are similar and they can often be challenged with the same or similar pathogens (3, 7). In addition, mouse models have the advantage of using animals that are inexpensive to purchase and maintain, thereby allowing the performance of studies with enough samples to yield statistically significant conclusions. The wounding procedure is quick, is simple to perform under anesthesia, requires no specialized equipment, and yields a highly reproducible result (7).
MATERIALS AND METHODS
UVC light source.
UVC light was delivered using a low-pressure mercury vapor lamp (American UV Co., Lebanon, IN). Emission spectrum measurement of this lamp using a spectroradiometer (SPR-01; Luzchem Research, Inc., Ottawa, Ontario, Canada) showed a peak emission at 254 ± 2 nm. The irradiance was adjusted by manipulating the distance between the UVC lamp and the irradiated target and measured using a model IL-1700 research radiometer-photometer (International Light, Inc., Newburyport, MA).
Bacterial strains and growth conditions.
Gram-negative P. aeruginosa and Gram-positive S. aureus were investigated, and stable bioluminescent strains were a kind gift from Xenogen Corp. (Alameda, CA). The bioluminescent strain of P. aeruginosa (Xen05) was derived from ATCC 19660 (strain 180), which is noted for its invasive properties in mouse wound models (31). The bioluminescent strain of S. aureus (Xen8.1) was derived from pathogenic strain ATCC 8325-4, which has been shown to cause localized infections after intramuscular injection into mice (17). Bacterial cells were grown in brain heart infusion (BHI) medium in an orbital shaking incubator (37°C, 100 rpm) to an optical density at 600 nm of 0.6 to 0.8, which corresponds to 108 CFU/ml (mid-log phase). This suspension was then centrifuged, washed with phosphate-buffered saline (PBS), and resuspended in PBS at the appropriate cell density prior to experimentation.
Confluent monolayer keratinocyte cultures.
A mouse immortalized keratinocyte cell line, PAM 212, was used. To obtain monolayer keratinocyte cultures, keratinocytes were inoculated into 35-mm petri dishes (∼200 cells/dish) in RPMI 1640 medium (Sigma-Aldrich Co., St. Louis, MO) supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin. The dishes were then incubated at 37°C in a humidified atmosphere of 5% CO2 for 18 to 24 h prior to experimentation.
Mouse model of skin abrasion infections.
Adult female BALB/c mice (Charles River Laboratories, Wilmington, MA) 7 to 8 weeks old and weighing 16 to 18 g were used. Mice were housed one per cage (to prevent attacks on wounds) and had access to food and water ad libitum. The mice were maintained on a 12-h light-dark cycle at a room temperature of 21°C.
For S. aureus infection, mice were given two intraperitoneal (i.p.) injections of cyclophosphamide (Sigma-Aldrich) before bacterial inoculation on day 0. The first dose, 150 mg cyclophosphamide per kg mouse body weight (150 mg/kg) on day −4, was followed by a second dose of 100 mg/kg on day −1. This treatment reduced peripheral blood neutrophils to <100/μl blood, fostering an environment in the mice that made them more vulnerable to infection. For P. aeruginosa infection, no cyclophosphamide was necessary.
Before the creation of skin abrasions, mice were anesthetized by i.p. injection of a ketamine-xylazine cocktail and then shaved on the dorsal surfaces using an electric fur clipper. Mouse skin was then scraped with no. 15 scalpel blades until a reddened area appeared (just short of drawing blood) (7, 23). This procedure resulted in first-degree skin abrasions with most of the epidermis removed (Fig. 1B). Each wound measured approximately 1.2 by 1.2 cm. One drop (60 μl) of a prepared bacterial suspension containing 107 CFU was then inoculated onto the mouse wound by using a micropipette (0 to 200 μl) and smeared uniformly with the micropipette tip. The minimum bacterial inoculum required to develop infections in mice was determined by preliminary experiments. In routine experiments, the bacterial densities (CFU/ml) of the bacterial suspensions were further confirmed by in vitro colony-forming assay. This dual measurement of viability gave additional confirmation of the reliability of the results obtained.
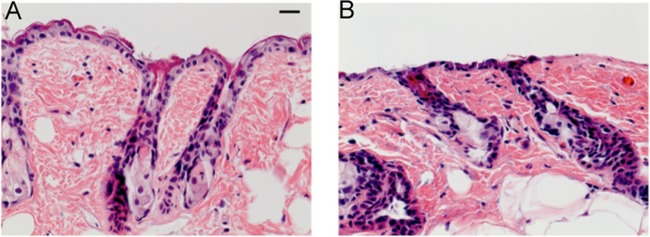
Fig 1.
Hematoxylin-and-eosin-stained histology of representative BALB/c mouse skin samples. (A) Intact skin. (B) Skin abrasion. Scale bar, 20 μm.
Bioluminescence imaging.
The bioluminescence imaging setup consisted of an intensified charge-coupled device camera (model C2400-30H; Hamamatsu Photonics, Bridgewater, NJ), a camera controller, a light-tight imaging chamber, an image processor, and a color monitor. Using the photon-counting mode, an image can be obtained by detecting and integrating individual photons emitted by the bacterial cells. Prior to imaging, mice were anesthetized and placed on an adjustable stage in the imaging chamber with the infected wounds positioned directly under the camera. A gray-scale background image of each wound was made, and this was followed by a bioluminescence image of the same region displayed in a false color scale ranging from pink (most intense) to blue (least intense) and superimposed on the gray-scale image. Using ARGUS software (Hamamatsu), the signal from the bioluminescence image was quantified as relative luminescence units (RLU), which are proportional to corresponding bacterial CFU counts (19).
UVC light irradiation of monolayer keratinocyte cultures.
Prior to UVC light exposure, the PAM212 culture medium was replaced with PBS and the lids were removed. For each trial, five culture dishes were exposed to UVC light for 0, 3, 6, 9, and 12 s, respectively, at an irradiance of 1.6 mW/cm2. After UVC light exposure, the PBS was removed from the 35-mm petri dishes. The cells were then detached using 2 ml trypsin–0.25% EDTA solution (Sigma-Aldrich) per dish and recultured in 150-mm petri dishes inoculated with 50 ml fresh culture medium. The 150-mm dishes were then incubated at 37°C for 10 days to grow keratinocyte colonies to visible sizes before the assay of cell viability. Culture medium in the 150-mm dishes was refreshed every 3 days. After 10 days of incubation, the culture medium was removed from the 150-mm dishes and keratinocyte viability was assessed by staining of the keratinocyte colonies in the dishes with crystal violet solution. The stained keratinocyte colonies were then counted.
UVC light inactivation of bacteria in vitro.
Experiments were carried out using two different types of bacterial culture: monolayer culture on agar plates and suspension in PBS. Bacterial monolayers were directly compared with cellular monolayers, while, since bacterial cells are smaller than mammalian cells, the susceptibilities of bacteria and keratinocytes to UVC light were compared in a fairer manner (bacterial suspension versus keratinocyte monolayer).
For the experiments using bacterial monolayers, prior to UVC light exposure, bacterial suspensions (P. aeruginosa or S. aureus) with a density of approximately 108 CFU/ml were subjected to five 10-fold serial dilutions in PBS. Aliquots (10 μl) from the serial dilutions were streaked onto square BHI plates in the order of most (1:105) to least (1:1) diluted (22). This procedure resulted in bacterial cell monolayer cultures with five 10-fold serial dilutions on BHI plates. The BHI plates were then exposed to UVC light at an irradiance of 0.072 mW/cm2 for 0, 10, 20, 30, 40, and 50 s, respectively. After UVC light exposure, the BHI plates were incubated at 37°C for 18 to 24 h before the surviving cells were counted.
For the experiments using bacterial suspensions, a 3-ml bacterial suspension containing 108 CFU/ml in PBS was placed into a 35-mm petri dish at room temperature to give a liquid depth of 3 mm. The suspension then was exposed to UVC light at an irradiance of 1.6 mW/cm2 with the dish lid removed. During UVC light exposure, the bacterial suspension was stirred with a miniature magnetic bar. Aliquots (40 μl) of the bacterial suspension were withdrawn at 0, 3, 6, 9, and 12 s. Post-UVC light exposure CFU counts were then determined by serial dilution on BHI agar plates (22). Colonies were allowed to grow for 18 to 24 h at 37°C before counting. All in vitro experiments were performed in triplicate.
UVC light prophylaxis of infections in mouse skin abrasions.
UVC light was delivered to mouse skin abrasions at 30 min after bacterial inoculation with an irradiance of 2.7 mW/cm2. Groups of mice (n = 11 to 12) were given a total UVC light exposure of 2.59 J/cm2 (a total illumination time of 16 min) in aliquots with bioluminescence imaging taking place after each aliquot of light. In addition, the same number of mice without UVC light prophylaxis were used as the controls. During UVC light exposure, the areas surrounding the mouse wounds were masked using aluminum foil. Bacterial luminescence from mouse wounds was recorded daily after UVC light prophylaxis until the infections were cured (characterized by the disappearance of bacterial luminescence).
Immunohistochemical analyses of UVC light-induced DNA lesions in mouse skin abrasions.
UVC light was delivered to the skin abrasions at an antimicrobial exposure level of 2.59 J/cm2. Skin biopsy specimens were taken from the irradiated wounds before and immediately, 24 h, and 48 h after UVC light exposure. They were fixed in 10% phosphate-buffered formalin at 4°C for 18 to 24 h, processed, and then embedded in paraffin. Serial skin tissue sections (4 μm) were then prepared, deparaffinized, and rehydrated. Skin sections were incubated for 30 min at room temperature with Anti-Thymine Dimer monoclonal antibody (Kaniya Biomedical Company, Seattle, WA), which reacts specifically with UV-induced CPD. The antibody dilution was 1:50. Skin sections were then incubated with mouse-on-mouse biotinylated anti-mouse IgG reagent (secondary antibody; Vector Labs, Burlingame, CA) for 10 min at room temperature. The skin sections were then incubated in the dark for 5 min at room temperature with Fluorescein Avidin DCS (Vector Labs). Between incubations, the skin sections were extensively washed in PBS. The skin sections were then mounted with coverslips using mounting medium with 4′,6-diamidino-2-phenylindole (DAPI; Vector Labs) used as a nuclear counterstain and observed using an Olympus Fluoview FV1000 MPE multiphoton microscope (Olympus Co., Tokyo, Japan).
Statistical analyses.
Data are given as means ± standard deviations. The statistical significance of differences between the rates of in vitro bacterial cell and keratinocyte inactivation by UVC light were determined using Student's t test. The area-under-the-curve (AUC) data, which represent the time courses of bacterial luminescence of skin abrasions (i.e., the overall bacterial burdens of mouse wounds) or the time courses of wound areas (i.e., the overall healing rate of mouse wounds), were calculated using numerical integration. Differences in the AUC between untreated control and treated mouse groups were compared also using Student's t test. Kaplan-Meier survival or wound-healing curves were compared by the use of a log-rank test. In all statistical analyses, P values of <0.05 were considered statistically significant.
RESULTS
In vitro susceptibilities of bacteria and keratinocytes to UVC light inactivation.
Figure 2 compares the in vitro susceptibilities of bacterial cells and keratinocytes to UVC light inactivation. The log10-transformed inactivation curves approximately followed the first-order kinetic model (39), i.e., log10 (N/N0) = −kHH, where N is the CFU count at radiant exposure H, N0 is the initial CFU count, kH is the cell inactivation rate coefficient (34), and kH can be obtained from the slope of the inactivation curve, i.e., kH = Δlog10N/ΔH.
Fig 2.
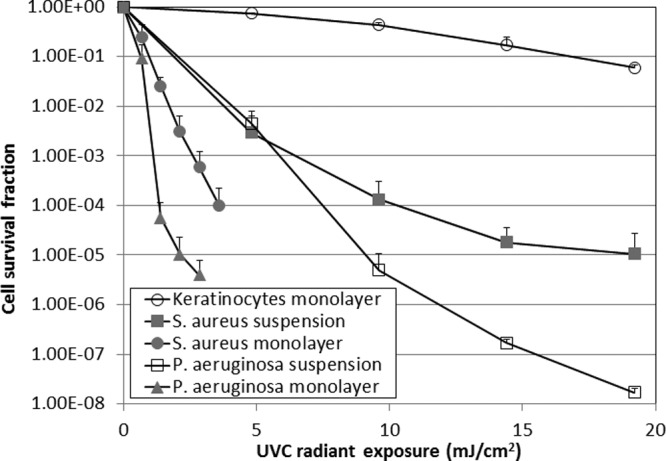
Dose-dependent in vitro cell survival fractions of bacteria and keratinocytes in response to UVC light irradiation. Bars show standard deviations.
The results show that both P. aeruginosa and S. aureus were inactivated at UVC light exposures much lower than that needed for a similar effect on keratinocytes. To achieve a 1-log10-cycle (90%) reduction of cell viability, the required UVC light exposures for keratinocyte monolayer, P. aeruginosa monolayer, S. aureus monolayer, P. aeruginosa suspension, and S. aureus suspension cultures were, respectively, 16.8, 0.64, 0.94, 2.04, and 1.90 mJ/cm2. The mean kH values for keratinocyte monolayer, P. aeruginosa monolayer, S. aureus monolayer, P. aeruginosa suspension, and S. aureus suspension cultures were 0.06, 1.90, 1.11, 0.40, and 0.26 cm2/mJ, respectively (keratinocyte monolayer versus P. aeruginosa monolayer, P = 0.00026; keratinocyte monolayer versus S. aureus monolayer, P = 0.0025; keratinocyte monolayer versus P. aeruginosa suspension, P = 1.6e-06; keratinocyte monolayer versus S. aureus suspension, P = 0.043).
UVC light prophylaxis of mouse skin abrasions infected with P. aeruginosa.
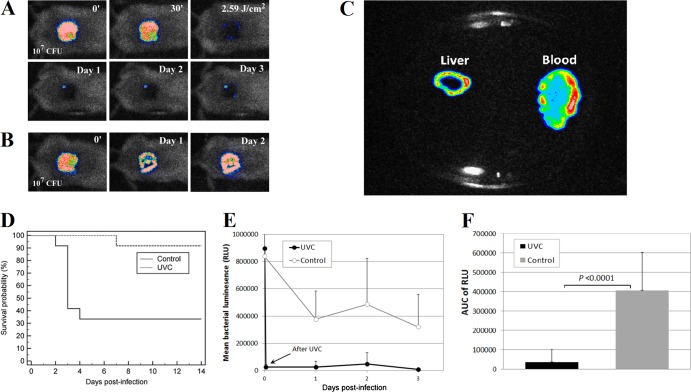
Figure 3A and B are successive bacterial luminescence images of representative P. aeruginosa-infected mouse skin abrasions with and without UVC light prophylaxis, respectively. After an exposure of 2.59 J/cm2 UVC light, a bacterial luminescence reduction of >2 log10 cycles was observed in the UVC light-treated wound (Fig. 3A). Modest bacterial luminescence remained after UVC light exposure (Fig. 3A). In contrast, infection was fully established in the mouse without UVC light prophylaxis (Fig. 3B). The untreated mouse died on day 3 after bacterial inoculation, and luminescent P. aeruginosa was detected in the blood and liver cultures of the mouse (Fig. 2C), indicating that the mouse died of sepsis. Overall, 91.6% (11 out of 12) of the mice survived after UVC light prophylaxis while only 33.3% (4 out of 12) of the mice survived without UVC light prophylaxis (P = 0.0023, Fig. 3D). Most of the deaths (6 out of 8) in the nontreated group occurred on day 3 after bacterial inoculation. Figure 3E shows the time courses of the mean bacterial luminescence of the UVC light-treated (n = 12) and untreated (n = 12) mice from day 1 to day 3. The AUC of the bioluminescence time courses (from day 1 to day 2, i.e., before most of the deaths occurred) were (3.72 ± 6.27) ×104 and (4.07 ± 1.95) ×105 for UVC light-treated and untreated mice, respectively (P < 0.00001, Fig. 3F), indicating that a >10-fold reduction of the bacterial burden resulted from UVC light prophylaxis.
Fig 3.
(A and B) Successive bacterial luminescence images of representative mouse skin abrasions infected with 107 CFU of P. aeruginosa with and without UVC light prophylaxis, respectively. The 0′ bacterial luminescence image was taken immediately after bacterial inoculation; the 30′ image was taken 30 min after bacterial inoculation and just prior to UVC light irradiation; the 2.59 J/cm2 image was taken immediately after 2.59 J/cm2 UVC light had been delivered; and the day 1, 2, and 3 images were taken 24, 48, and 72 h, respectively, after bacterial inoculation, respectively. (C) Bioluminescence image of blood and liver cultures (on an agar plate) from the untreated mouse shown in panel B. (D) Kaplan-Meier survival curves of UVC light-treated mouse skin abrasions (n = 12) and untreated mouse skin abrasions (n = 12). (E) Time courses of mean bacterial luminescence of infected skin abrasions with and without UVC light prophylaxis, respectively. Bars show standard deviations. (F) Mean areas under the bioluminescence-versus-time curves (from days 1 and 2 in the two-dimensional coordinate system in panel E), representing the overall bacterial burdens of mouse wounds. Bars show standard deviations.
UVC light prophylaxis for mouse skin abrasions infected with S. aureus.
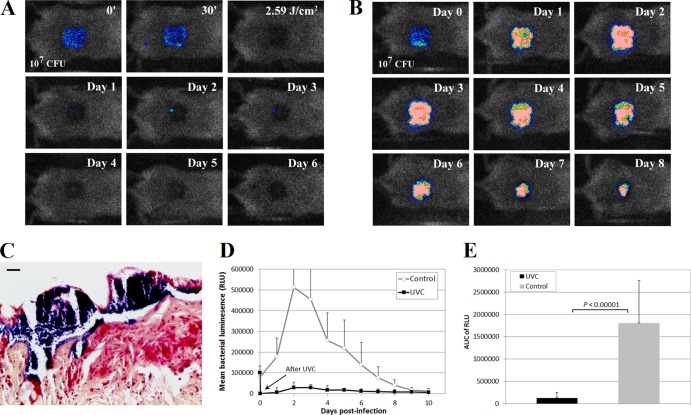
Figure 4A and B show successive bacterial luminescence images of representative S. aureus-infected mouse skin abrasions with and without UVC light prophylaxis. After exposure to 2.59 J/cm2 UVC light, bacterial luminescence was completely eradicated in the UVC light-treated wound. Only modest reoccurrence of bacterial luminescence was observed after UVC light exposure (Fig. 4A), while in the untreated wound strong bacterial luminescence remained until day 7 (Fig. 4B). Gram staining evaluation of the skin abrasions infected with S. aureus and without UVC light prophylaxis revealed evidence of biofilm formation in the wounds within 24 h after bacterial inoculation (Fig. 4C).
Fig 4.
(A and B) Successive bacterial luminescence images of representative mouse skin abrasions infected with 107 CFU of S. aureus with and without UVC light prophylaxis, respectively. (C) S. aureus skin abrasion infection subjected to Gram staining. A biopsy sample was taken at 24 h after bacterial inoculation. Evidence of biofilm growth (blue) was observed in the wound. (D) Time courses of mean bacterial luminescence of infected skin abrasions with (n = 11) and without (n = 12) UVC light prophylaxis. (E) Mean areas under the bioluminescence-versus-time curves (in the two-dimensional coordinate system in panel D), representing the overall bacterial burdens of mouse wounds. Bars show standard deviations.
Figure 4D displays the time courses from day 1 to day 10 of the mean bacterial luminescence of the mouse wounds treated by exposure to 2.59 J/cm2 UVC light at 30 min after bacterial inoculation (n = 11) or left untreated (n = 12). Statistical comparison of the AUC of the bioluminescence time courses in Fig. 4D demonstrated that UVC light significantly reduced the bacterial burdens of the infected wounds (Fig. 4E). The average AUC of the UVC light-treated and untreated wounds were (1.26 ± 0.13) ×105 and (1.81 ± 0.95) ×106, respectively, a more-than-10-fold difference (P < 0.00001). No deaths occurred in the S. aureus-infected mice.
Figure 5A shows the time courses of the mean normalized wound area of the S. aureus-infected skin abrasions with and without UVC light prophylaxis. It can be seen that UVC light promoted the wound healing rate in UVC light-treated mouse wounds in comparison to that in untreated ones. The AUC of the time courses of the mean normalized wound area of the skin abrasions with and without UVC light prophylaxis were 6.89 ± 1.18 and 10.01 ± 1.72 (Fig. 5B, P < 0.00001), respectively, indicating a 31.2% increase in the wound healing rate in the UVC light-treated mouse wounds. Wound healing represented as a Kaplan-Meier plot is shown in Fig. 5C. The mean time to complete healing was 11.9 ± 2.6 days for the UVC light-treated mouse wounds and 16.0 ± 2.0 days for the nontreated mouse wounds (P = 0.0006).
Fig 5.
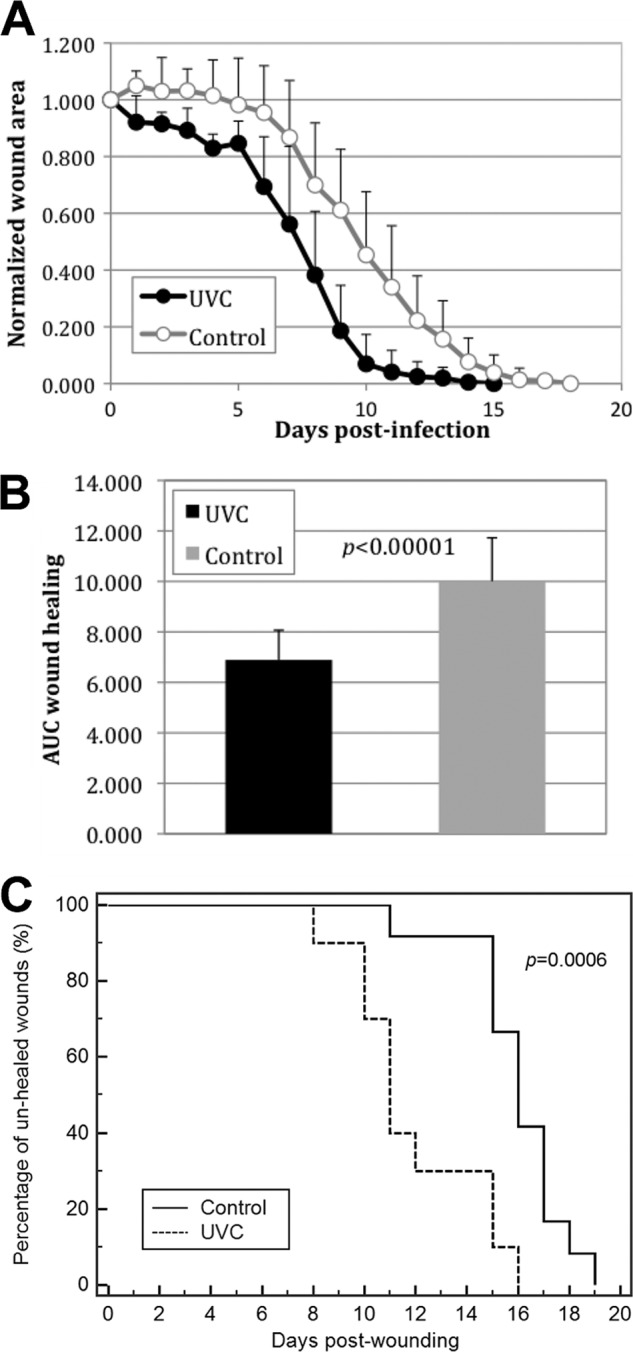
(A) Time courses of mean normalized wound areas of S. aureus-infected skin abrasions with and without UVC light prophylaxis. Bars show standard deviations. (B) Mean areas under the normalized wound area-versus-time curves (in the two-dimensional coordinate system in panel A). Bars show standard deviations. (C) Kaplan-Meier wound-healing curves of S. aureus-infected mouse abrasions with and without UVC light prophylaxis.
Effect of UVC light exposure on mouse skin abrasions.
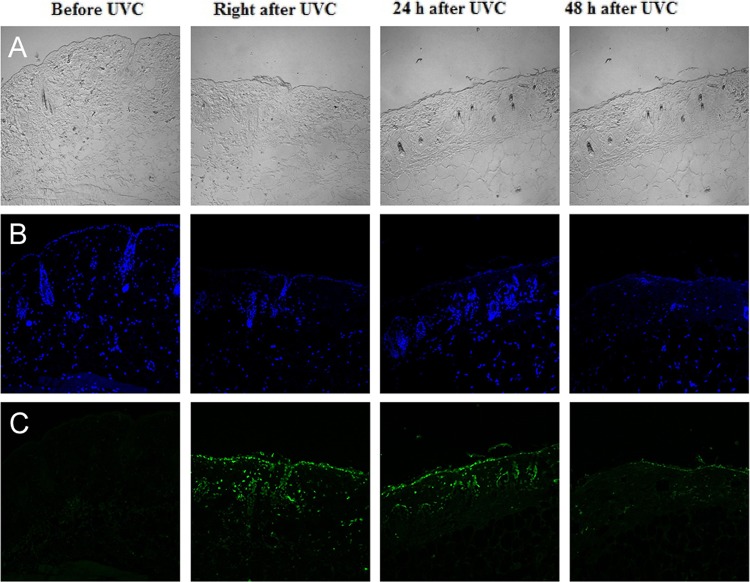
Figure 6 depicts the effect of UVC light at an antimicrobial exposure level (2.59 J/cm2) on mouse skin abrasions. CPD-positive nuclei were observed in the immunofluorescence micrograph of a mouse skin biopsy specimen taken immediately after UVC light exposure. However, the damage was extensively repaired within 48 h, as shown in the skin biopsy specimen taken at 48 h after UVC light exposure, where only traces of fluorescence remained.
Fig 6.
Immunohistochemical analyses of DNA lesions in representative mouse skin abrasions after UVC light exposure. Biopsy specimens were taken before and immediately, 24 h, and 48 h after UVC light irradiation. (A) Micrographs of the morphologies of skin abrasions. (B) Micrographs of DAPI counterstaining of cell nuclei. (C) Immunofluorescence micrographs of CPD in skin cell nuclei.
DISCUSSION
For a long time, wound infections were always successfully treated with topical and systemic antibiotics; however, the rapid emergence of multiantibiotic-resistant strains of bacteria has become a considerable concern. Traumatic wounds may contain significant amounts of nonperfused tissue due to compromise of the capillary circulation. These factors seriously limit the use of systemic antibiotics. One the other hand, the use of topical antibiotics is even more controversial since it has been suggested that such an approach induces antibiotic resistance faster than the use of systemic antibiotics (32, 38).
In this study, we investigated the use of UVC light for the prophylaxis of both lethal P. aeruginosa and nonlethal S. aureus skin wound infections in mice. The results indicated that by rapidly eradicating the pathogenic bacteria in mouse wounds, UVC light can significantly reduce the mortality rate in mice that developed otherwise lethal P. aeruginosa infections and can also accelerate wound healing in mice with otherwise S. aureus infections. Although there is some evidence of UVC light damage to host tissue, it is quickly repaired by DNA repair enzymes.
In vitro tests in the present study showed that the inactivation rate coefficients of monolayer P. aeruginosa and S. aureus cells were approximately 31.7- and 18.5-fold higher than that of monolayer keratinocytes. Taking into account the fact that keratinocytes are bigger than bacterial cells, we also compared the susceptibilities of keratinocytes and bacteria to UVC light inactivation by comparing bacterial suspensions and keratinocyte monolayers. The inactivation coefficients of P. aeruginosa and S. aureus suspensions were still 6.7- and 4.3-fold higher than that of keratinocyte monolayers. These results suggested that selective inactivation of pathogenic microorganisms can be achieved under appropriate UVC light exposures.
It is considered that prolonged UV (especially UVB) irradiation is a major inducer of DNA damage in human skin and an important environmental mutagen and carcinogen (24). In the present study, we did observe UVC light-induced DNA damage in mouse wounds after exposure to antimicrobial UVC light fluences. However, we also observed that these lesions were rapidly repaired by the DNA-repairing enzymes of the host within 48 h. It has been reported in an animal study that UVC light is less carcinogenic than UVB light because of its more superficial penetration depth: “Abnormal differentiation of a layer of cells that is committed to being sloughed off anyway (UVC light) is not harmful, whereas mutation of the basal cells (UVA or UVB) may result in skin cancer” (35). On the other hand, it has been reported that UVB exposure is an effective treatment option for a large number of cutaneous disorders in humans with excellent safety profiles. An analysis of 3,867 patients receiving UVB over an 18-year period, with a median number of 29 treatments and 352 patients receiving 100 or more treatments with more than 6 months of follow-up for each patient, showed no increase in skin cancers of any kind (21). For wound infections, we expect that only a limited number of repetitions of UVC light would be required, while the UV-induced carcinogenic mutation is a long-term effect.
In the case of P. aeruginosa infection, UVC light was able to save mice from death due to sepsis. This is due to the highly virulent and invasive nature of P. aeruginosa as a pathogen. Rapid killing of bacteria in the wound by a topical antimicrobial agent is required because otherwise the bacteria invade the tissue and soon gain access to the bloodstream (20). We also observed the benefit of accelerated wound healing due to the eradication of S. aureus from contaminated wounds. Previous studies reported that UVC light per se could stimulate wound healing. It was found that UVC light-induced fibronectin release led to increased healing via wound contraction (27). UVC light (254 nm) at radiant exposures of 15 to 60 mJ/cm2 promoted the expressions of transforming growth factor beta and basic fibroblast growth factor in rat wounds and might be beneficial in accelerating wound healing (36, 37).
To establish S. aureus infection in mouse wounds, we used cyclophosphamide for temporary immunosuppression of mice. The reduction in peripheral blood neutrophils begins to reverse approximately 4 to 5 days after the second challenge with cyclophosphamide, and this contributes to the clearing of bacteria and wound healing in the untreated group. Future studies may be worth doing with chronically immunosuppressed mice. With immunosuppressed mice, we expect to see a better therapeutic outcome of UVC light prophylaxis than observed in the present study, because the clearance of bacteria and subsequently wound healing would be slower in the nontreated mice because of their permanent immunosuppression.
P. aeruginosa and S. aureus fall within the ESKAPE group of pathogens (i.e., Enterococcus faecium, S. aureus, Klebsiella pneumoniae, Acinetobacter baumannii, P. aeruginosa, and Enterobacter species) for which novel therapies are needed, as discussed by the Infectious Diseases Society of America (2, 30). Since the onset of U.S. military operations in Iraq and Afghanistan, wound infections caused by the ESKAPE pathogens are the leading cause of death and morbidity among wounded service members (4, 28). Overuse of broad-spectrum antibiotics is the most important short-term concern facing military caregivers (4, 16). As a result, a promising antimicrobial application of UVC light might be the prevention of combat-related wound infections.
UVC light is a nonpharmacological approach that is likely to be noninjurious to wounds. Furthermore, it is unlikely that complete resistance to UVC light can be induced in pathogenic bacteria, although it is possible that variants with enhanced DNA repair systems may emerge. Even though Deinococcus radiodurans is one of the most UV-resistant bacterial species known, a fluence of UVC light four times higher than that needed for Escherichia coli will produce equivalent killing (1). A UVC lamp could be easily miniaturized to make it portable, light, and battery powered. It is also easy to operate and can even be issued to each service member as standard equipment. This approach might be particularly useful in combat situations, where injured service members have to be medevaced to receive appropriate care and transportation can often be delayed due to adverse combat conditions.
In our previous studies, we have demonstrated the efficacies of another light-based therapy, antimicrobial photodynamic therapy (aPDT), for various skin and soft tissue infections (6, 9, 12, 18, 25). Unlike aPDT, UVC light inactivates microorganisms without using exogenous photosensitizers and therefore is easier to operate and inexpensive. In addition, the use of UVC light for disinfection does not generate chemical residues. Another fact of aPDT is that currently there is no highly effective photosensitizer that has been approved by the FDA. Due to the limited penetration of skin and tissue by UVC light, UVC light is usually more efficient in the prophylaxis of infections or treatment of superficial infections.
Taken together, the data show that UVC light prophylaxis can significantly reduce the bacterial burden in both lethal P. aeruginosa wound infections and nonlethal S. aureus wound infections in mice and subsequently reduce the mortality rate of mice and promote faster wound healing. UVC light-induced DNA lesions can be rapidly repaired within 48 h after UVC light exposure. In conclusion, UVC light may be a potential prophylactic for cutaneous wound infections.
ACKNOWLEDGMENTS
We thank Jie Zhao from the Photopathology and Microscopy Core at the Wellman Center for her generous assistance in immunohistochemical staining.
This study was supported in part by an Airlift Research Foundation Extremity Trauma research grant (109421 to T.D.), by a COTA/Smith & Nephew grant (2012-16 to T.D.), and by the NIH (grant RO1AI050875 to M.R.H.). We have no conflict of interest.
Footnotes
Published ahead of print 7 May 2012
REFERENCES
- 1. Battista JR. 1997. Against all odds: the survival strategies of Deinococcus radiodurans. Annu. Rev. Microbiol. 51:203–224 [DOI] [PubMed] [Google Scholar]
- 2. Boucher HW, et al. 2009. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 48:1–12 [DOI] [PubMed] [Google Scholar]
- 3. Buer J, Balling R. 2003. Mice, microbes and models of infection. Nat. Rev. Genet. 4:195–205 [DOI] [PubMed] [Google Scholar]
- 4. Calhoun JH, Murray CK, Manring MM. 2008. Multidrug-resistant organisms in military wounds from Iraq and Afghanistan. Clin. Orthop. Relat. Res. 466:1356–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chang JC, et al. 1985. UV inactivation of pathogenic and indicator microorganisms. Appl. Environ. Microbiol. 49:1361–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dai T, Bil de Arce VJ, Tegos GP, Hamblin MR. 2011. Blue dye and red light, a dynamic combination for prophylaxis and treatment of cutaneous Candida albicans infections in mice. Antimicrob. Agents Chemother. 55:5710–5717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dai T, et al. 2011. Animal models of external traumatic wound infections. Virulence 2:296–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dai T, et al. 2011. Ultraviolet-C light for treatment of Candida albicans burn infection in mice. Photochem. Photobiol. 87:342–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dai T, et al. 2009. Photodynamic therapy for Acinetobacter baumannii burn infections in mice. Antimicrob. Agents Chemother. 53:3929–3934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dai T, Tegos GP, Rolz-Cruz G, Cumbie WE, Hamblin MR. 2008. Ultraviolet C inactivation of dermatophytes: implications for treatment of onychomycosis. Br. J. Dermatol. 158:1239–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dai T, et al. 2011. Ultraviolet-C irradiation for prevention of central venous catheter-related infections: an in vitro study. Photochem. Photobiol. 87:250–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dai T, Tegos GP, Zhiyentayev T, Mylonakis E, Hamblin MR. 2010. Photodynamic therapy for methicillin-resistant Staphylococcus aureus infection in a mouse skin abrasion model. Lasers Surg. Med. 42:38–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dai T, Vrahas MS, Murray CK, Hamblin MR. 2012. Ultraviolet C irradiation: an alternative antimicrobial approach to localized infections? Expert Rev. Anti Infect. Ther. 10:185–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dryden MS. 2010. Complicated skin and soft tissue infection. J. Antimicrob. Chemother. 65(Suppl 3):iii35–44 [DOI] [PubMed] [Google Scholar]
- 15. Dryden MS. 2009. Skin and soft tissue infection: microbiology and epidemiology. Int. J. Antimicrob. Agents 34(Suppl 1):S2–S7 [DOI] [PubMed] [Google Scholar]
- 16. Eardley WG, Brown KV, Bonner TJ, Green AD, Clasper JC. 2011. Infection in conflict wounded. Philos. Trans. R. Soc. Lond. B Biol. Sci. 366:204–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Francis KP, et al. 2000. Monitoring bioluminescent Staphylococcus aureus infections in living mice using a novel luxABCDE construct. Infect. Immun. 68:3594–3600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gad F, Zahra T, Francis KP, Hasan T, Hamblin MR. 2004. Targeted photodynamic therapy of established soft-tissue infections in mice. Photochem. Photobiol. Sci. 3:451–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hamblin MR, O'Donnell DA, Murthy N, Contag CH, Hasan T. 2002. Rapid control of wound infections by targeted photodynamic therapy monitored by in vivo bioluminescence imaging. Photochem. Photobiol. 75:51–57 [DOI] [PubMed] [Google Scholar]
- 20. Hamblin MR, Zahra T, Contag CH, McManus AT, Hasan T. 2003. Optical monitoring and treatment of potentially lethal wound infections in vivo. J. Infect. Dis. 187:1717–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hearn RM, Kerr AC, Rahim KF, Ferguson J, Dawe RS. 2008. Incidence of skin cancers in 3867 patients treated with narrow-band ultraviolet B phototherapy. Br. J. Dermatol. 159:931–935 [DOI] [PubMed] [Google Scholar]
- 22. Jett BD, Hatter KL, Huycke MM, Gilmore MS. 1997. Simplified agar plate method for quantifying viable bacteria. Biotechniques 23:648–650 [DOI] [PubMed] [Google Scholar]
- 23. Kraft WG, Johnson PT, David BC, Morgan DR. 1986. Cutaneous infection in normal and immunocompromised mice. Infect. Immun. 52:707–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lisby S, Gniadecki R, Wulf HC. 2005. UV-induced DNA damage in human keratinocytes: quantitation and correlation with long-term survival. Exp. Dermatol. 14:349–355 [DOI] [PubMed] [Google Scholar]
- 25. Lu Z, et al. 2010. Photodynamic therapy with a cationic functionalized fullerene rescues mice from fatal wound infections. Nanomedicine (Lond.) 5:1525–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. May L, et al. 2011. Self-reported incidence of skin and soft tissue infections among deployed US military. Travel Med. Infect. Dis. 9:213–220 [DOI] [PubMed] [Google Scholar]
- 27. Morykwas MJ, Mark MW. 1998. Effects of ultraviolet light on fibroblast fibronectin production and lattice contraction. Wounds 10:111–117 [Google Scholar]
- 28. Murray CK, et al. 2009. Recovery of multidrug-resistant bacteria from combat personnel evacuated from Iraq and Afghanistan at a single military treatment facility. Mil. Med. 174:598–604 [DOI] [PubMed] [Google Scholar]
- 29. Rennie RP, Jones RN, Mutnick AH. 2003. Occurrence and antimicrobial susceptibility patterns of pathogens isolated from skin and soft tissue infections: report from the SENTRY Antimicrobial Surveillance Program (United States and Canada, 2000). Diagn. Microbiol. Infect. Dis. 45:287–293 [DOI] [PubMed] [Google Scholar]
- 30. Rice LB. 2008. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. J. Infect. Dis. 197:1079–1081 [DOI] [PubMed] [Google Scholar]
- 31. Rosenthal SM. 1967. Local and systemic therapy of pseudomonas septicemia in burned mice. Ann. Surg. 165:97–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ross JI, et al. 2003. Antibiotic-resistant acne: lessons from Europe. Br. J. Dermatol. 148:467–478 [DOI] [PubMed] [Google Scholar]
- 33. Sebeny PJ, Riddle MS, Petersen K. 2008. Acinetobacter baumannii skin and soft-tissue infection associated with war trauma. Clin. Infect. Dis. 47:444–449 [DOI] [PubMed] [Google Scholar]
- 34. Sinton LW, Hall CH, Lynch PA, Davies-Colley RJ. 2002. Sunlight inactivation of fecal indicator bacteria and bacteriophages from waste stabilization pond effluent in fresh and saline waters. Appl. Environ. Microbiol. 68:1122–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sterenborg HJ, van der Putte SC, van der Leun JC. 1988. The dose-response relationship of tumorigenesis by ultraviolet radiation of 254 nm. Photochem. Photobiol. 47:245–253 [DOI] [PubMed] [Google Scholar]
- 36. Suo W, Guo H, Wang X, Wang D. 2003. Effect of ultraviolet C light on the expression of basic fibroblast growth factor in rat wounds. Chin. J. Phys. Med. Rehabil. 25:651–654 [Google Scholar]
- 37. Suo W, Wang X, Wang D. 2002. Effect of ultraviolet C irradiation on expression of transforming growth factor-β in wound. Chin. J. Rehabil. Theory Pract. 8:5–7 [Google Scholar]
- 38. Wyatt TD, Ferguson WP, Wilson TS, McCormick E. 1977. Gentamicin resistant Staphylococcus aureus associated with the use of topical gentamicin. J. Antimicrob. Chemother. 3:213–217 [DOI] [PubMed] [Google Scholar]
- 39. Xiong R, Xie G, Edmondson AE, Sheard MA. 1999. A mathematical model for bacterial inactivation. Int. J. Food Microbiol. 46:45–55 [DOI] [PubMed] [Google Scholar]