Abstract
Infections caused by Mycobacterium abscessus and Mycobacterium massiliense are on the rise among humans. Although macrolides, including clarithromycin (CLR) and azithromycin (AZM), are key antibiotics for the treatment of M. abscessus and M. massiliense infections, treatment regimens for these infections are still largely undefined. In this study, we evaluated the in vitro, ex vivo, and in vivo activities of moxifloxacin (MXF) in combination with macrolides against clinically isolated M. abscessus and M. massiliense strains. Overall, CLR, AZM, and MXF alone showed activity against both species in vitro, ex vivo, and in vivo. When MXF was combined with a macrolide against M. abscessus isolates, antagonism was observed in 65.4% (17/26) of the strains with CLR and 46.2% (12/26) of the strains with AZM in vitro as well as in 66.7% (10/15) of the strains with CLR and 40.0% (6/15) of the strains with AZM in macrophages as determined by the fractional inhibitory concentration index. In contrast, either indifferent or synergistic effects of the MXF-macrolide combinations were observed against only M. massiliense strains. Moreover, a murine infection model showed similar results. Antagonism between the MXF and macrolide combinations was observed in five out of seven M. abscessus strains, while indifferent and synergistic effects for these combinations were observed for three of the six M. massiliense strains tested, respectively. In conclusion, the activity of MXF in combination with a macrolide differed for M. abscessus and M. massiliense infections and the addition of MXF to macrolide therapy had no benefit for the treatment of M. abscessus infections.
INTRODUCTION
Mycobacterium abscessus is the most common etiologic agent of lung diseases that are caused by the rapidly growing mycobacteria (RGM) (12, 13, 18), and it has emerged as an important pathogen in patients with cystic fibrosis (25, 33, 36). M. abscessus is resistant to most antibiotics currently available and thus is very difficult to treat (23, 24, 34). Isolates are usually susceptible only to some parenteral agents (amikacin, cefoxitin, and imipenem) and to oral macrolides (clarithromycin [CLR] and azithromycin [AZM]) (12, 13, 18). Combination therapy of intravenous amikacin with cefoxitin or imipenem and an oral macrolide for 2 to 4 months has been recommended by the American Thoracic Society (ATS)/Infectious Diseases Society of America (IDSA) and many other experts (12, 13, 18). After initial therapy, macrolide administration plus at least one other antibiotic agent to which the organism is susceptible should be used for treatment. However, this option is limited because of high in vitro resistance rates to the various other oral agents used against M. abscessus isolates (12, 13, 18).
Oral fluoroquinolones have also been used in the treatment of RGM infection. Although fluoroquinolones cannot be used as a single-drug therapy due to the risk of developing resistance mutations (45), some experts have suggested that “holding” regimens of a macrolide plus a fluoroquinolone may be helpful during periods between pulsed intravenous antibiotic therapies (14). Fluoroquinolones have been used in many patients during combination treatment of M. abscessus lung disease in clinical practices (23, 24), but the combined activities of a fluoroquinolone with a macrolide against M. abscessus have not been evaluated systemically in experimental studies or clinical trials.
Recently, M. abscessus was divided into three separate subspecies: Mycobacterium abscessus sensu stricto, Mycobacterium massiliense, and Mycobacterium bolletii (1, 3). M. massiliense is now recognized as a separate species from M. abscessus, and treatment response rates to CLR-based antibiotic therapy are much higher in patients with M. massiliense than in those with M. abscessus lung disease (28, 31). To gain greater insight into the optimal therapeutic strategy for M. abscessus and M. massiliense infections, we evaluated the in vitro, ex vivo, and in vivo activities of moxifloxacin (MXF) in combination with macrolides against clinical isolates of M. abscessus and M. massiliense.
MATERIALS AND METHODS
Clinical isolate sources, growth conditions, and inoculum preparation.
A total of 62 clinical isolates consisting of 31 M. abscessus and 31 M. massiliense isolates were recovered from patients who were diagnosed at the Samsung Medical Center (Seoul, South Korea). All patients met the diagnostic criteria for nontuberculous mycobacterium (NTM) lung disease published by the American Thoracic Society (18). The data are part of an ongoing prospective observational cohort study investigating NTM lung disease (ClinicalTrials.gov identifier NCT00970801). The study protocol was approved by the institutional review board of the Samsung Medical Center (IRB approval 2008-09-016), and written informed consent was obtained from all participants. Two reference strains, M. abscessus ATCC 19977T (ATCC, Manassas, VA) and M. massiliense CIP108297T (Institut Pasteur, Paris, France), were always included as controls for each set of experiments.
Precise species identification of M. abscessus and M. massiliense was performed using sequence analysis targeting the rpoB and hsp65 genes as well as an analysis of the variable-number tandem repeat (VNTR) profile as previously described (2, 8). All strains were initially cultured in 7H9 broth (Becton, Dickinson, Franklin Lakes, NJ) supplemented with oleic acid-albumin-dextrose-catalase (OADC; Becton, Dickinson, Sparks, MD) for 10 days at 37°C. Single-cell suspensions of each strain were prepared as previously described with slight modifications (41). Seed lots of each strain were kept in small aliquots at −80°C until use. Tenfold serial dilutions from seed lots of each strain were plated on Middlebrook 7H10 agar (Becton, Dickinson) to quantify the number of organisms per milliliter.
Antimicrobials.
MXF was kindly provided by Bayer Schering Pharma AG (Berlin, Germany). Two macrolides, CLR and AZM, were purchased from Tokyo Chemical Industry Co. (TCI, Tokyo, Japan) and LKT Laboratories, Inc. (St. Paul, MN), respectively. A common diluent (0.03% acetic acid solution) for all of the antibiotics was included as a control in all of the experiments. All of the stock drug solutions were freshly prepared for each experiment and filter sterilized using a 0.22-μm polycarbonate syringe filter (Millipore Corp., Bedford, MA).
In vitro susceptibility testing.
MICs of all tested drugs were determined by broth microdilution assays according to the protocol set by the Clinical and Laboratory Standards Institute (CLSI) (11). Briefly, the drugs at final concentrations ranging from 0.125 to 128 μg/ml in 7H9-OADC broth were added to 96-well plates in 2-fold serial dilutions. The final inoculum size was adjusted to 104 CFU/ml. Controls included the inoculum without drug added (no-drug control) and the 1:100-diluted inoculum (99% control). The MIC of each drug was defined as the lowest drug concentration that inhibited more than 99% of the bacterial growth observed in the drug-free medium after incubation at 37°C for 72 h. The MIC50 and MIC90 levels were interpreted as the lowest concentration of antibiotics that inhibited 50% and 90% of the isolates, respectively. All assays were independently performed three times in triplicate.
Mice and preparation of bone marrow-derived macrophages (BMDMs).
Specific-pathogen-free female C57BL/6 mice at 5 to 6 weeks of age were purchased from Japan SLC, Inc. (Shijuoka, Japan), and maintained under barrier conditions in a biosafety level 2 (BL-2) biohazard animal facility at the Medical Research Center of Chungnam National University. The animals were fed a sterile commercial mouse diet and provided with water ad libitum. The animal experiments complied with the ethical and experiment regulations for animal care at Chungnam National University (approval no. 2008-04).
Murine bone marrow-derived macrophages (BMDMs) were differentiated for 6 days in macrophage colony-stimulating factor (M-CSF)-containing media, as described previously (27). Briefly, bone marrow cells from the femur and tibia were cultured in Dulbecco's modified Eagle's medium (DMEM; HyClone, Logan, UT) containing 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 10% fetal bovine serum, and 20 ng/ml of recombinant M-CSF (R&D Systems, Minneapolis, MN) at 37°C in the presence of 5% CO2. After 6 days, nonadherent cells were removed, and the differentiated macrophages were incubated in antibiotic-free DMEM until use.
Antibiotic activities against intracellular bacteria within macrophages.
The activities of drug combinations between macrolides and MXF were evaluated for a reference strain and 14 clinical isolates of each species, M. abscessus and M. massiliense, using a murine bone marrow-derived macrophage system. These clinical isolates of each species were selected based on the similarity of their MICs to that of MXF (ranging from 0.5 to 2 μg/ml).
Differentiated BMDMs were adjusted to a density of 5.0 × 105/well in a 24-well tissue culture plate (Corning Life Sciences, Acton, MA) and were infected with each strain of M. abscessus and M. massiliense for 4 h at the ratio of 1 bacterium to 1 macrophage. This infectious dose of mycobacteria did not affect the macrophage viability during the experiments, and thus, the drug activity could be measured only against intracellular bacteria. The infected BMDMs were washed twice with DMEM to remove extracellular bacteria and were cultivated for 24 h at 37°C in 5% CO2 to allow the intracellular mycobacteria to adapt. To compare the antibiotic activities of each individual drug and the MXF-macrolide combinations against the intracellular bacteria, the mycobacterium-infected macrophages were treated with 10 μg/ml of each drug or MXF-macrolide combinations at the same concentrations followed by incubation for an additional 48 h. At the indicated times (before and after treatment), the macrophages were lysed with distilled water, and the number of CFU within the cell lysates was determined using the plate counting method for cultures grown on 7H10-OADC.
Evaluation of treatment efficacy in mice.
Six clinical isolates of M. abscessus and 5 clinical isolates of M. massiliense as well as the two reference strains were used for the therapeutic evaluation of each drug and the MXF-macrolide combinations in mycobacterium-infected mice. Before conducting the experiments, the minimum lethal dose (MLD) of each strain was determined using six mice per strain, and 4-fold dilutions of the MLD (4.0 × 106 to 1.0 × 107 CFU) were subsequently used in the in vivo experiments. Six-week-old female C57BL/6J mice (36 mice per strain, 6 mice per drug) were infected with the selected dose of each strain by intravenous injection via the tail vein. At 4 days postinfection, treatment was initiated with the antimicrobial agents (100 mg/kg of body weight/day of each drug alone or in combination with 100 mg/kg/day of MXF). The dose of each antibiotic was selected based on the results of previous studies that determined the effective dose of each drug in murine infection models of other mycobacteria (5, 6, 32). Antimicrobial agents were administered daily by oral gavage for 12 days. Control mice for each strain received the same volume of 0.03% acetic acid in distilled water over the same period. At 16 days postinfection, six mice per group were euthanized, and their livers, spleens, and lungs were aseptically collected for bacterial counts. The numbers of viable bacteria in the organs were determined by plating serial dilutions of the organ homogenates onto 7H10-OADC agar plates. Colonies were counted after 5 days of incubation at 37°C. The differences detected when comparing the drug-treated groups and control groups as well as among the drug-treated groups (drug alone versus combinations) are represented as the means of the log10 CFU ± the standard deviation (SD) for each group of mice. This experiment was performed twice.
Evaluation of combined drug action.
Evaluation of the antimycobacterial activities of each drug alone and of the MXF-macrolide combinations, the fractional inhibitory concentration (FIC) based on the MICs in vitro, and the FICs based on CFU counts in macrophages and mice are described below.
(i) Evaluation of combination activity in vitro.
The effects of the antibiotic combinations were determined by the fractional inhibitory concentration (FIC) based on the MICs of each drug alone and of each drug combination as previously described with slight modifications (19, 20, 26, 39). The concentration range of each antibiotic in combination ranged from 1/16 the MIC to 4 times the MIC. The FIC index was calculated as follows: (MIC of drug A in combination)/(MIC of drug A alone). The following definitions were used: synergism, FIC index of ≤0.5; indifference, FIC index of >0.5 and <2; and antagonism, FIC index of ≥2. All tests were performed in triplicate, and the results were averaged. For the evaluations of the combined effects of MXF and macrolides, drug A was defined as CLR or AZM. Synergy has been defined as a 2-fold reduction in the MIC of the combination of antibiotics compared with each antibiotic alone. Antagonism has been defined as a 2-fold increase in the MIC when a combination of antibiotics is used. The in vitro interactions of the individual macrolides in combination with MXF are summarized in Table 2.
Table 2.
In vitro antimicrobial activities of the combinations of a macrolide and moxifloxacin against M. abscessus and M. massiliense
| Species and agents | No. (%) of isolates with combination activitya |
||
|---|---|---|---|
| Synergism | Indifference | Antagonism | |
| M. abscessus (n = 26) | |||
| CLR-MXF | 1 (3.8) | 8 (30.8) | 17 (65.4) |
| AZM-MXF | 1 (3.8) | 13 (50.0) | 12 (46.2) |
| M. massiliense (n = 28) | |||
| CLR-MXF | 11 (39.3) | 16 (57.1) | 1 (3.6) |
| AZM-MXF | 10 (35.7) | 16 (57.1) | 2 (7.1) |
The effects of antibiotic combinations were determined by the fractional inhibitory concentration (FIC): synergism, FIC index of ≤0.5; indifference, FIC index of >0.5 and <2.0; antagonism, FIC index of ≥2.0.
(ii) Determination of combination activity in macrophages and in mice.
To determine the effect of antibiotic interactions in macrophages and in mice, the antibiotic activity of the combination treatment was defined as the log10 CFU reduction as previously reported with slight modifications (32, 40). Briefly, the antimycobacterial activity was assessed by counting the CFU from the macrophage lysates or the homogenates of inoculated organs on 7H10 agar supplemented with 10% OADC and determining the log10 CFU per ml after 5 days.
In these calculations, x refers to the Δlog10 CFU from the control obtained with the drug combination and y refers to the lowest Δlog10 CFU from the control obtained with each drug used alone. The interaction between the drugs was assessed quantitatively by adopting the x/y quotient method described by Hoffner et al. (20). An x/y value of 1 indicated that there was no interaction between the two drugs and was interpreted as indifference, an x/y value of <0.5 indicated synergy, and an x/y value of >2.0 indicated antagonism.
Statistical analysis.
The results in the text and tables are reported as the mean ± standard deviation or as the number (percentage) of strains. Comparisons between single drugs and drug combinations in the same strain were analyzed using Wilcoxon's matched pairs test. The differences between the treatment groups in the same species were determined using the Mann-Whitney nonparametric test. A comparison of treatment efficacies for M. abscessus and M. massiliense with each drug combination was performed with a chi-squared test. The differences were considered significant at a P value of <0.05. All statistical analyses were performed using GraphPad Prism software (version 4.03; GraphPad Software, San Diego, CA).
RESULTS
In vitro susceptibilities to macrolides and MXF.
The antimicrobial activities of the macrolides were tested against 31 M. abscessus and 31 M. massiliense isolates in addition to the two reference strains. The MIC data for CLR, AZM, and MXF against M. abscessus and M. massiliense are shown in Table 1. Overall, the MIC range of CLR was lower than that of AZM for both M. abscessus and M. massiliense. According to the CLSI, ≤2 μg/ml is regarded as susceptible and ≥8 μg/ml is regarded as resistant to CLR and ≤1 μg/ml is regarded as susceptible and ≥4 μg/ml is regarded as resistant to MXF (11). However, the breakpoint for AZM against M. abscessus and M. massiliense has not been established. In this study, M. abscessus and M. massiliense isolates were considered susceptible if the MIC of AZM was ≤16 μg/ml and were considered resistant at ≥32 μg/ml. These values were derived from both the distribution of MICs and the MIC90 of CLR.
Table 1.
In vitro antimicrobial activities of macrolides and moxifloxacin against 62 clinical isolates of M. abscessus and M. massiliense and each reference strain
| Species (no. of isolates) | Agent | No. of strains distributed at each MIC (μg/ml) |
Resistance rate, no. of strains (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | ≥64 | |||
| M. abscessus (32) | AZM | 4 | 5 | 15 | 2 | 3 | 3 | 6a (18.8) | |||
| CLR | 3 | 15 | 5 | 2 | 1 | 1 | 1 | 1 | 3 | 6a (18.8) | |
| MXF | 6 | 7 | 10 | 9 | |||||||
| M. massiliense (32) | AZM | 1 | 2 | 6 | 23 | ||||||
| CLR | 20 | 12 | |||||||||
| MXF | 12 | 10 | 3 | 3 | 3b | 1 | 4b (12.5) | ||||
Six isolates of M. abscessus that were resistant to both macrolides due to a point mutation in the 23S rRNA were excluded from the subsequent experiments.
Four isolates of M. massiliense that were resistant to moxifloxacin were excluded from the subsequent experiments.
Among the M. abscessus isolates, six strains showed initial drug resistance to both macrolides due to a point mutation in the adenine at position 2058 (A2058) in the 23S rRNA gene (data not shown). In contrast, none of the M. massiliense strains were resistant to either macrolide. Interestingly, 4 of the 31 M. massiliense isolates and the reference strain displayed MXF resistance, while the MICs of MXF for all of the M. abscessus strains did not exceed 2 μg/ml. The strains that showed initial resistance to any drug were ruled out for subsequent experiments to prevent interference in the determination of the combination activities of each drug. Thus, M. abscessus and M. massiliense clinical isolates with no considerable variation in MIC values upon each antimicrobial agent were used to investigate the effects of the combined drugs. MXF exhibited the lowest range of MIC values against the clinical isolates tested in the study (≤0.25 to 2 μg/ml for M. abscessus and ≤0.25 to 8 μg/ml for M. massiliense), while AZM had a wider range of MIC values than did MXF or CLR.
In vitro activities of macrolide-MXF combinations.
The activities of the drug combinations CLR-MXF and AZM-MXF were compared with those of single drugs for each of the strains that were found to be susceptible to each of the three drugs (Table 2). When antagonism was defined as an FIC index of >2.0, 17 (65.4%) and 12 (46.2%) of 26 M. abscessus isolates showed antagonism for the CLR-MXF and AZM-MXF combinations, respectively. Notably, synergism resulting from the combination of MXF with either CLR or AZM was observed for one strain only.
However, when the macrolide and MXF combinations were tested against the 28 M. massiliense strains, both CLR-MXF and AZM-MXF combinations generally resulted in an indifferent interaction for the same 16 isolates (57.1%). The synergistic effects of CLR-MXF and AZM-MXF were observed for 11 isolates (39.3%) and 10 isolates (35.7%), respectively. However, antagonism was observed for one isolate (3.6%) when combining MXF with CLR and for two isolates (7.1%) when MXF was combined with AZM. A statistical analysis using a chi-squared test showed a significant difference in the responses of the M. abscessus and M. massiliense species to the MXF-macrolide combinations (P < 0.005).
Activities of macrolide-MXF combinations against intracellular bacteria.
Next, we investigated the effect of the drug combinations on intracellular bacteria in comparison with the effects of the single drugs using the x/y quotient method (Table 3). Among the 15 M. abscessus strains susceptible to macrolides and MXF, 10 (66.7%) of the strains showed antagonism for the CLR-MXF combination, and 6 (40.0%) of the strains showed antagonism for the AZM-MXF combination when the antagonistic effect was defined as an x/y value of >2.0. Synergism for both drug combinations was also observed in one strain, which was the same strain that showed synergism in vitro. In contrast, a large proportion of the M. massiliense strains revealed indifference for CLR-MXF (60.0%) and AZM-MXF (66.7%). A synergistic effect was observed in five M. massiliense (33.3%) strains when treated with CLR-MXF and three strains (20%) when treated with AZM-MXF. A statistical analysis using a chi-squared test showed a significant difference between the M. abscessus and M. massiliense groups for the effects of the MXF-macrolide combinations (P < 0.005).
Table 3.
Evaluation of the antimicrobial activities of drug combinations against intracellular bacteria in bone marrow-derived macrophages
| Species and agents | No. (%) of isolates with combination activitya |
||
|---|---|---|---|
| Synergism | Indifference | Antagonism | |
| M. abscessus (n = 15) | |||
| CLR-MXF | 1 (6.6) | 4 (26.7) | 10 (66.7) |
| AZM-MXF | 1 (6.6) | 8 (53.3) | 6 (40.0) |
| M. massiliense (n = 15) | |||
| CLR-MXF | 5 (33.3) | 9 (60.0) | 1 (6.7) |
| AZM-MXF | 3 (20.0) | 10 (66.7) | 2 (13.3) |
The effects of antibiotic combinations were determined by adopting the x/y quotient method. Synergism, an x/y value of ≤0.5; indifference, a value of >0.5 and <2.0; antagonism, a value of ≥2.0.
In vivo activities of macrolide-MXF combinations.
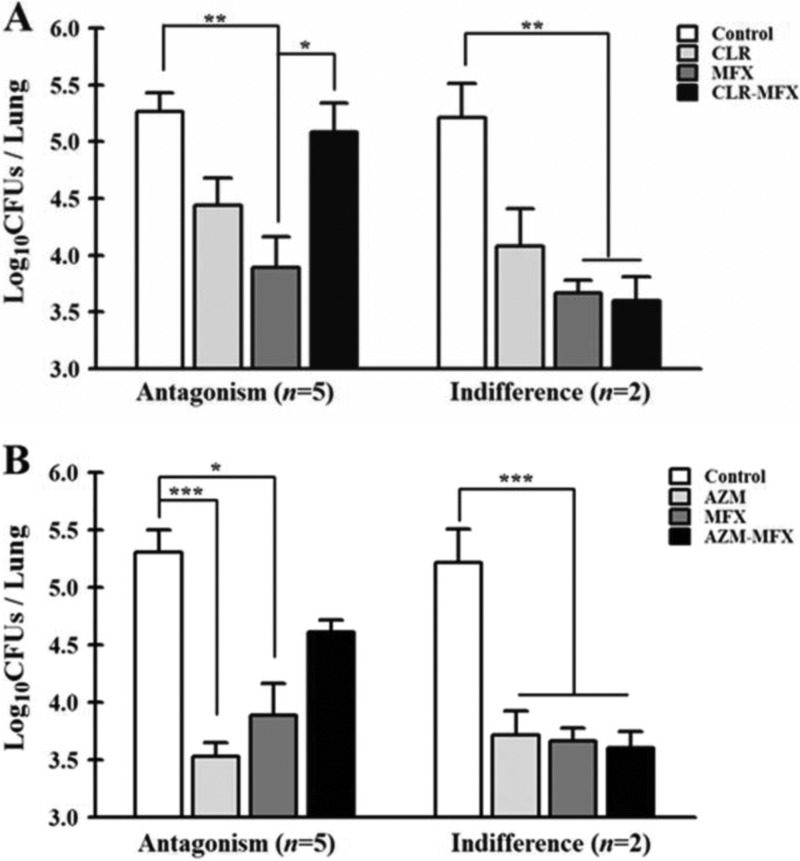
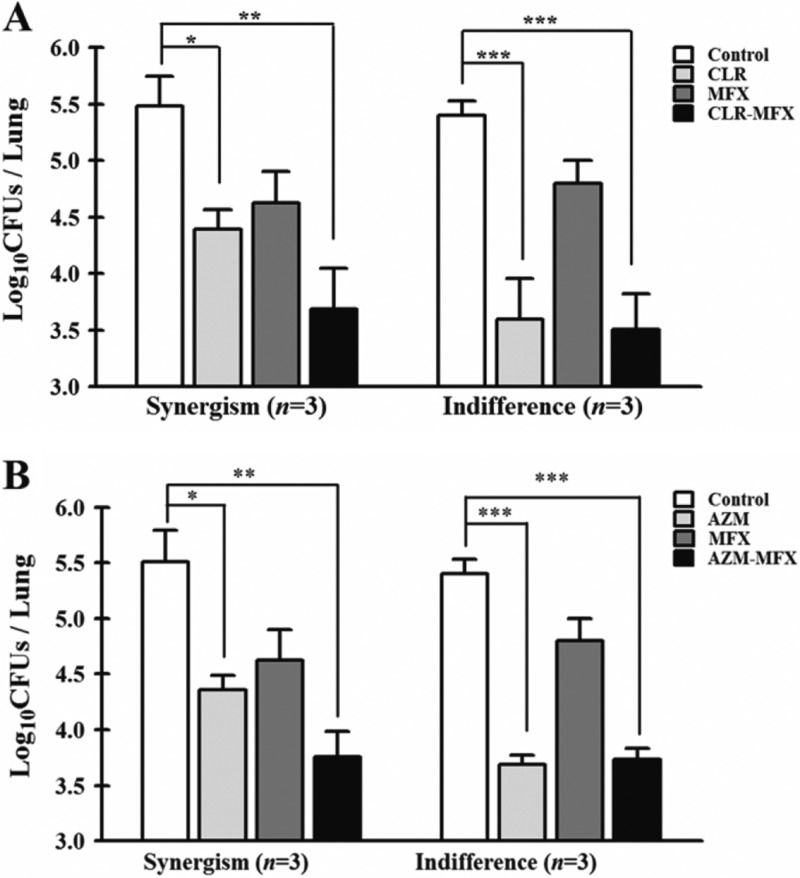
The therapeutic efficacies of single drugs and drug combinations were evaluated in a murine model of M. abscessus and M. massiliense infection. Treatment with each drug alone, CLR-MXF, and AZM-MXF was initiated 3 days postinfection and administered daily for 12 days. All experiments were performed twice, and similar results were obtained for both experiments. Although the activity of each drug differed depending on the tested strains, most mice that received macrolides or MXF alone revealed a significant decrease in the bacterial load in all of the organs examined (P < 0.001) compared to the control mice. The therapeutic effects of each drug alone and the drug combination treatments against each M. abscessus and M. massiliense-infected mice are shown in Tables S1 and S2 in the supplemental material. Similar to the results obtained from the in vitro and ex vivo investigations, the combination of a macrolide and MXF was not superior to either drug alone in the treatment of M. abscessus infection. Among the 7 strains tested, 5 and 2 strains showed antagonism and an indifferent effect for both macrolide-MXF combinations, respectively (Fig. 1). In contrast, in vivo synergism between CLR-MXF and AZM-MXF was observed against 3 of the M. massiliense strains (Fig. 2). Indifferent effects in the M. massiliense-infected mice were observed for 3 strains for both of the macrolide-MXF combinations (Fig. 2). A statistical analysis using a chi-squared test showed a significant difference between the M. abscessus and M. massiliense groups for treatment with the MXF-macrolide combinations (P < 0.005).
Fig 1.
Activities of clarithromycin, azithromycin, and moxifloxacin alone and macrolide-moxifloxacin combinations against 7 M. abscessus isolates in mice. (A) Significant differences are shown in the in the log10 CFU/lung between treatments with clarithromycin alone and with the clarithromycin-moxifloxacin combination. (B) Significant differences in the log10 CFU/lung between treatments with azithromycin alone and with the azithromycin-moxifloxacin combination. The P value versus control mice was evaluated using Wilcoxon's matched pairs test. *, P > 0.05; **, P > 0.005; and ***, P > 0.001.
Fig 2.
Activities of clarithromycin, azithromycin, and moxifloxacin alone and macrolide-moxifloxacin combinations against 6 M. massiliense isolates in mice. (A) Significant differences in the log10 CFU/lung between treatments with clarithromycin alone and with the clarithromycin-moxifloxacin combination. (B) Significant differences in the log10 CFU/lung between treatments with azithromycin alone and with the azithromycin-moxifloxacin combination. The P value versus control mice was evaluated using Wilcoxon's matched pairs test.
DISCUSSION
Treatment of the most common NTM pathogens improved with the introduction of macrolide-containing regimens, but the treatment outcome for M. abscessus lung disease remains disappointing (22–24, 34), because of the relatively few therapeutic alternatives for the treatment compared to other mycobacteria. Thus, novel therapeutic approaches are needed because clinical failures are frequently encountered.
Fluoroquinolones such as MXF showed good in vitro activity against M. abscessus clinical isolates in some studies (21, 38). In fact, MXF is an attractive treatment option because it can be administered orally for a long duration. However, there is only limited evidence regarding the clinical efficacy of MXF against M. abscessus and M. massiliense.
Our results clearly demonstrated two findings: (i) MXF hinders the activity of macrolides and (ii) the antagonism between MXF and the macrolide was relatively common against the M. abscessus isolates compared to the M. massiliense isolates. Thus, our study demonstrated that combining macrolides with MXF provided no advantage for the treatment of M. abscessus lung disease.
MXF has been reported to have clinical efficacy for M. abscessus infection. A recent prospective observational study demonstrated that CLR-MXF combination therapy for postacupuncture cutaneous M. abscessus infections resulted in a more rapid resolution of the cutaneous lesions than did the use of a CLR-amikacin combination (9). Moreover, MXF has been shown to be effective against experimentally induced M. abscessus keratitis and in human cases of that disorder (7, 10, 37). However, MXF obviously interferes with CLR activity in over 65% of the tested strains of M. abscessus in our models, indicating that many factors should be considered in explaining such a different result. For example, the pharmacokinetic and pharmacodynamic properties of MXF may differ according to the type of disease (cutaneous infection versus pulmonary infection), and differences in the drug dose and therapeutic regimens could also influence the results.
In contrast to the efficacy of medication regimens for nonpulmonary diseases such as skin infection, no antibiotic regimen based on in vitro susceptibilities has been shown to produce effective treatment for patients with M. abscessus lung disease (18). A lack of correlation between the in vitro results and the in vivo effects regarding drug activity was also observed in this study. For example, MXF showed activity in vitro superior to that of CLR and AZM against both M. abscessus and M. massiliense. However, monotherapy with MXF was less effective than that with AZM in the murine infection model. Thus, our results indicate that it may be difficult to predict the in vivo therapeutic effects of MXF based on its in vitro MIC test result. These results are in agreement with those of a similar study conducted on the Mycobacterium avium complex (32). Factors that could account for this may be differences in the ability of the strain to grow in the medium versus in vivo or differences in the penetrating power of the antibiotics. Similarly, many studies have demonstrated that MXF and macrolides are more effective during bacterial replication than when the bacterial numbers approach the maximal values in stationary phase (15, 16, 42).
Another factor that could affect the response to antibiotics is the anatomic location of infection or between strains isolated from hosts in different geographical regions. Notably, the in vitro susceptibilities for MXF against M. abscessus varied with the isolation source as well as between countries and patients infected with the same strain. A previous study conducted using 21 strains of M. abscessus in Japan found that the MIC of MXF ranged from 2 to 32 μg/ml with an MIC90 of 32 μg/ml (35). Similarly, a study conducted in Taiwan with 98 M. abscessus clinical isolates reported an MIC range of 0.064 to 32 μg/ml, also with an MIC90 of 32 μg/ml (22). However, a study performed in South Korea using 74 M. abscessus isolates showed that the MIC90 of MXF was 2 μg/ml and that only five strains exceeded an MIC of 4 μg/ml of MXF (38). Interestingly, the MICs of MXF of M. abscessus isolates from 12 patients varied significantly more than the MICs of other antibiotics, such as CLR and amikacin, in an outbreak of postacupuncture cutaneous infections caused by M. abscessus species belonging to the same genotype (9). Thus, there are significant geographic differences and patient variations in the MICs of MXF against M. abscessus, and comparisons of the activity of MXF are complicated by differences in the drug regimens and/or strains used in the studies. Additional studies on different M. abscessus and M. massiliense strains are needed to substantiate these observations.
In our study, the MICs of different antibiotics are reported according to the species. To date, differential identification of M. abscessus and M. massiliense has not been intensively studied because they cause the same spectrum of diseases and because it is difficult to discriminate between the two species using traditional molecular methods (e.g., PCR-restriction fragment length polymorphism or sequence analysis of rpoB and hsp65) due to a lack of polymorphisms in the region being tested (8, 17, 29). However, recent studies suggest that the antibiotic susceptibilities and treatment outcomes differ significantly between the two species (31). In this study, M. massiliense showed marked susceptibility to CLR, while six isolates of M. abscessus were resistant to macrolides, results which are similar to previously reported findings (30). In contrast, MXF showed a wider range of MICs against M. massiliense than did M. abscessus according to CLSI criteria for RGM. In the same context, treatment efficacy for M. massiliense with MXF alone was less than that for M. abscessus in our murine infection model (Fig. 1 and 2). Thus, it is important to discriminate between these two species prior to treatment because an empirical addition of antibiotics to treat M. abscessus and M. massiliense may be potentially counterproductive.
The exact reasons for the antagonistic effect of MXF on the actions of the macrolide are not known, but one possible explanation has been suggested in previous studies. Tomioka et al. showed that CLR decreases the activities of gatifloxacin and levofloxacin against M. avium complex strains in vitro (44). Kohno et al. also reported that the activity of CLR against M. avium complex strains was attenuated when combined with fluoroquinolones, including MXF, using both in vitro and in vivo models (32). Another study by Bermudez et al. revealed that the MXF-AZM combination was significantly less active than AZM alone for the treatment of mice infected with M. avium (4). In addition, protein synthesis inhibitors, such as CLR, interfere with the lethal antibacterial activities of fluoroquinolones (43).
A limitation of this study is that we did not investigate whether the combinations at various concentrations of each drug provided better antimycobacterial activity. An additional shortcoming of the present study is that we employed a systemic infection model via intravenous injection rather than an aerosol infection model.
Conclusively, the present study provides evidence that MXF negatively influences the treatment outcome in experimental models of M. abscessus infection when combined with macrolides. Our study demonstrates, for the first time, that MXF has significantly different effects on M. abscessus and M. massiliense.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a grant of the Korea Health Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A100027), and the Mid-career Researcher Program through an NRF grant funded by the MEST (2011-0015546).
Footnotes
Published ahead of print 7 May 2012
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1. Adekambi T, Berger P, Raoult D, Drancourt M. 2006. rpoB gene sequence-based characterization of emerging non-tuberculous mycobacteria with descriptions of Mycobacterium bolletii sp. nov., Mycobacterium phocaicum sp. nov. and Mycobacterium aubagnense sp. nov. Int. J. Syst. Evol. Microbiol. 56:133–143 [DOI] [PubMed] [Google Scholar]
- 2. Adekambi T, Drancourt M. 2004. Dissection of phylogenetic relationships among 19 rapidly growing Mycobacterium species by 16S rRNA, hsp65, sodA, recA and rpoB gene sequencing. Int. J. Syst. Evol. Microbiol. 54:2095–2105 [DOI] [PubMed] [Google Scholar]
- 3. Adekambi T, et al. 2004. Amoebal coculture of “Mycobacterium massiliense” sp. nov. from the sputum of a patient with hemoptoic pneumonia. J. Clin. Microbiol. 42:5493–5501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bermudez LE, et al. 2001. Activity of moxifloxacin by itself and in combination with ethambutol, rifabutin, and azithromycin in vitro and in vivo against Mycobacterium avium. Antimicrob. Agents Chemother. 45:217–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bermudez LE, et al. 2007. EDP-420, a bicyclolide (bridged bicyclic macrolide), is active against Mycobacterium avium. Antimicrob. Agents Chemother. 51:1666–1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bermudez LE, Petrofsky M, Kolonoski P, Young LS. 1998. Emergence of Mycobacterium avium populations resistant to macrolides during experimental chemotherapy. Antimicrob. Agents Chemother. 42:180–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Caballero AR, et al. 2006. Effectiveness of fluoroquinolones against Mycobacterium abscessus in vivo. Curr. Eye Res. 31:23–29 [DOI] [PubMed] [Google Scholar]
- 8. Choi GE, et al. 2011. Efficient differentiation of Mycobacterium abscessus complex isolates to the species level by a novel PCR-based variable-number tandem-repeat assay. J. Clin. Microbiol. 49:1107–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Choi WS, et al. 2011. Clarithromycin and amikacin vs. clarithromycin and moxifloxacin for the treatment of post-acupuncture cutaneous infections due to Mycobacterium abscessus: a prospective observational study. Clin. Microbiol. Infect. 17:1084–1090 [DOI] [PubMed] [Google Scholar]
- 10. Chung SH, et al. 2006. Mycobacterium abscessus keratitis after LASIK with IntraLase femtosecond laser. Ophthalmologica 220:277–280 [DOI] [PubMed] [Google Scholar]
- 11. Clinical and Laboratory Standards Institute 2011. Susceptibility testing of mycobacteria, nocardiae, and other aerobic actinomycetes; approved standard, 2nd ed, document no. M24-A2. Clinical and Laboratory Standards Institute, Wayne, PA: [PubMed] [Google Scholar]
- 12. Colombo RE, Olivier KN. 2008. Diagnosis and treatment of infections caused by rapidly growing mycobacteria. Semin. Respir. Crit. Care Med. 29:577–588 [DOI] [PubMed] [Google Scholar]
- 13. Daley CL, Griffith DE. 2010. Pulmonary non-tuberculous mycobacterial infections. Int. J. Tuberc. Lung Dis. 14:665–671 [PubMed] [Google Scholar]
- 14. De Groote MA, Huitt G. 2006. Infections due to rapidly growing mycobacteria. Clin. Infect. Dis. 42:1756–1763 [DOI] [PubMed] [Google Scholar]
- 15. Eick S, Pfister W. 2004. Efficacy of antibiotics against periodontopathogenic bacteria within epithelial cells: an in vitro study. J. Periodontol. 75:1327–1334 [DOI] [PubMed] [Google Scholar]
- 16. Greendyke R, Byrd TF. 2008. Differential antibiotic susceptibility of Mycobacterium abscessus variants in biofilms and macrophages compared to that of planktonic bacteria. Antimicrob. Agents Chemother. 52:2019–2026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Griffith DE. 2011. The talking Mycobacterium abscessus blues. Clin. Infect. Dis. 52:572–574 [DOI] [PubMed] [Google Scholar]
- 18. Griffith DE, et al. 2007. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 175:367–416 [DOI] [PubMed] [Google Scholar]
- 19. Hewlett PS. 1969. Measurement of the potencies of drug mixtures. Biometrics 25:477–487 [PubMed] [Google Scholar]
- 20. Hoffner SE, Svenson SB, Kallenius G. 1987. Synergistic effects of antimycobacterial drug combinations on Mycobacterium avium complex determined radiometrically in liquid medium. Eur. J. Clin. Microbiol. 6:530–535 [DOI] [PubMed] [Google Scholar]
- 21. Huang TS, et al. 2008. Antimicrobial resistance of rapidly growing mycobacteria in western Taiwan: SMART program 2002. J. Formos. Med. Assoc. 107:281–287 [DOI] [PubMed] [Google Scholar]
- 22. Huang YC, et al. 2010. Clinical outcome of Mycobacterium abscessus infection and antimicrobial susceptibility testing. J. Microbiol. Immunol. Infect. 43:401–406 [DOI] [PubMed] [Google Scholar]
- 23. Jarand J, et al. 2011. Clinical and microbiologic outcomes in patients receiving treatment for Mycobacterium abscessus pulmonary disease. Clin. Infect. Dis. 52:565–571 [DOI] [PubMed] [Google Scholar]
- 24. Jeon K, et al. 2009. Antibiotic treatment of Mycobacterium abscessus lung disease: a retrospective analysis of 65 patients. Am. J. Respir. Crit. Care Med. 180:896–902 [DOI] [PubMed] [Google Scholar]
- 25. Jonsson BE, et al. 2007. Molecular epidemiology of Mycobacterium abscessus, with focus on cystic fibrosis. J. Clin. Microbiol. 45:1497–1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Katou K, et al. 2005. Combined effects of panipenem and aminoglycosides on methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa in vitro. Chemotherapy 51:387–391 [DOI] [PubMed] [Google Scholar]
- 27. Kaushik RS, Uzonna JE, Zhang Y, Gordon JR, Tabel H. 2000. Innate resistance to experimental African trypanosomiasis: differences in cytokine (TNF-alpha, IL-6, IL-10 and IL-12) production by bone marrow-derived macrophages from resistant and susceptible mice. Cytokine 12:1024–1034 [DOI] [PubMed] [Google Scholar]
- 28. Kim HS, et al. 2012. Serial CT findings of Mycobacterium massiliense pulmonary disease compared with Mycobacterium abscessus disease after treatment with antibiotic therapy. Radiology 263:260–270 [DOI] [PubMed] [Google Scholar]
- 29. Kim HY, et al. 2010. Mycobacterium massiliense is differentiated from Mycobacterium abscessus and Mycobacterium bolletii by erythromycin ribosome methyltransferase gene (erm) and clarithromycin susceptibility patterns. Microbiol. Immunol. 54:347–353 [DOI] [PubMed] [Google Scholar]
- 30. Kim HY, et al. 2008. Proportions of Mycobacterium massiliense and Mycobacterium bolletii strains among Korean Mycobacterium chelonae-Mycobacterium abscessus group isolates. J. Clin. Microbiol. 46:3384–3390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Koh WJ, et al. 2011. Clinical significance of differentiation of Mycobacterium massiliense from Mycobacterium abscessus. Am. J. Respir. Crit. Care Med. 183:405–410 [DOI] [PubMed] [Google Scholar]
- 32. Kohno Y, et al. 2007. In vitro and in vivo activities of novel fluoroquinolones alone and in combination with clarithromycin against clinically isolated Mycobacterium avium complex strains in Japan. Antimicrob. Agents Chemother. 51:4071–4076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Levy I, et al. 2008. Multicenter cross-sectional study of nontuberculous mycobacterial infections among cystic fibrosis patients, Israel. Emerg. Infect. Dis. 14:378–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lyu J, et al. 2011. Outcomes in patients with Mycobacterium abscessus pulmonary disease treated with long-term injectable drugs. Respir. Med. 105:781–787 [DOI] [PubMed] [Google Scholar]
- 35. Miyasaka T, et al. 2007. In vitro efficacy of imipenem in combination with six antimicrobial agents against Mycobacterium abscessus. Int. J. Antimicrob. Agents 30:255–258 [DOI] [PubMed] [Google Scholar]
- 36. Olivier KN, et al. 2003. Nontuberculous mycobacteria. I: multicenter prevalence study in cystic fibrosis. Am. J. Respir. Crit. Care Med. 167:828–834 [DOI] [PubMed] [Google Scholar]
- 37. Pacheco PA, Tam PM. 2010. Oral moxifloxacin and topical amikacin for Mycobacterium abscessus keratitis after laser in situ keratomileusis. J. Cataract Refract. Surg. 36:843–846 [DOI] [PubMed] [Google Scholar]
- 38. Park S, et al. 2008. In vitro antimicrobial susceptibility of Mycobacterium abscessus in Korea. J. Korean Med. Sci. 23:49–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Perichon B, Courvalin P. 2006. Synergism between beta-lactams and glycopeptides against VanA-type methicillin-resistant Staphylococcus aureus and heterologous expression of the vanA operon. Antimicrob. Agents Chemother. 50:3622–3630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rouan MC, et al. 2012. Pharmacokinetics and pharmacodynamics of TMC207 and its N-desmethyl metabolite in a murine model of tuberculosis. Antimicrob. Agents Chemother. 56:1444–1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shin SJ, Han JH, Manning EJ, Collins MT. 2007. Rapid and reliable method for quantification of Mycobacterium paratuberculosis by use of the BACTEC MGIT 960 system. J. Clin. Microbiol. 45:1941–1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Spoering AL, Lewis K. 2001. Biofilms and planktonic cells of Pseudomonas aeruginosa have similar resistance to killing by antimicrobials. J. Bacteriol. 183:6746–6751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tomioka H, Sano C, Sato K, Shimizu T. 2002. Antimicrobial activities of clarithromycin, gatifloxacin and sitafloxacin, in combination with various antimycobacterial drugs against extracellular and intramacrophage Mycobacterium avium complex. Int. J. Antimicrob. Agents 19:139–145 [DOI] [PubMed] [Google Scholar]
- 44. Tomioka H, et al. 1999. Comparative in vitro antimicrobial activities of the newly synthesized quinolone HSR-903, sitafloxacin (DU-6859a), gatifloxacin (AM-1155), and levofloxacin against Mycobacterium tuberculosis and Mycobacterium avium complex. Antimicrob. Agents Chemother. 43:3001–3004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wallace RJ, Jr, et al. 1990. Activities of ciprofloxacin and ofloxacin against rapidly growing mycobacteria with demonstration of acquired resistance following single-drug therapy. Antimicrob. Agents Chemother. 34:65–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.