Abstract
ASP2151 (amenamevir) is a helicase-primase inhibitor against herpes simplex virus type 1 (HSV-1), HSV-2, and varicella-zoster virus. To evaluate the anti-HSV activity of ASP2151, susceptibility testing was performed on viruses isolated from patients participating in a placebo- and valacyclovir-controlled proof-of-concept phase II study for recurrent genital herpes. A total of 156 HSV strains were isolated prior to the dosing of patients, and no preexisting variants with less susceptibility to ASP2151 or acyclovir (ACV) were detected. ASP2151 inhibited HSV-1 and HSV-2 replication with mean 50% effective concentrations (EC50s) of 0.043 and 0.069 μM, whereas ACV exhibited mean EC50s of 2.1 and 3.2 μM, respectively. Notably, the susceptibilities of HSV isolates to ASP2151 and ACV were not altered after dosing with the antiviral agents. Taken together, these results demonstrate that ASP2151 inhibits the replication of HSV clinical isolates more potently than ACV, and HSV resistant to this novel helicase-primase inhibitor as well as ACV may not easily emerge in short-term treatment for recurrent genital herpes patients.
INTRODUCTION
Genital herpes infections are caused by herpes simplex virus type 1 (HSV-1) or HSV-2, which are widely prevalent pathogens belonging to the human herpesvirus family, and are characterized by the formation of painful vesicles or small, grouped ulcers in the genital region. After primary infection, HSV establishes latency in sensory ganglia, which is followed by recurrent episodes of reactivation (20). HSV subtypes differ with respect to epidemiology, natural history, and propensity for recurrence (3, 15); for instance, HSV-1 genital infections are typically milder and less prone to recurrence than those of HSV-2 (1, 16, 24, 26, 27), whereas the latter virus is the more frequent cause of genital herpes (11, 17). Although no effective treatment exists for the eradication of genital herpes, several antiviral drugs are available to treat outbreak episodes and minimize disease symptoms.
Since the late 1970s, several synthetic nucleoside analogues, including acyclovir (ACV), penciclovir, valacyclovir (VCV), and famciclovir, have been developed for treating HSV infections (9, 10). These antiviral agents, as well as ganciclovir and valganciclovir, which also display anti-HSV activity, function as viral DNA polymerase inhibitors and, to some extent, DNA chain terminators during viral DNA replication but require prior phosphorylation by viral thymidine kinase (TK) and cellular kinases to form active triphosphates. Hence, HSV can develop cross-resistance to nucleoside analogues through mutation of viral TK and/or DNA polymerase genes (18). Because viral TK is not essential for viral replication, TK-deficient HSV mutants are readily detectable among pools of wild-type HSV, at frequencies ranging from 10−4 to 10−3 (2, 8, 19, 21). Since the most commonly isolated ACV-resistant variants of HSV have a TK-deficient phenotype, exposure to ACV might promote the selection and enrichment of ACV-resistant HSV in the clinical setting. Currently, foscarnet and cidofovir are the only antiviral agents approved for the treatment of severe HSV infections that do not require TK-mediated phosphorylation. However, both drugs are available only for parenteral use, can be difficult to tolerate, and have potentially serious side effects, including renal failure. Thus, the development of a novel class of anti-HSV agents with a mechanism of action that targets a viral protein essential for replication is desirable.
ASP2151 is a nonnucleoside, oxadiazol-phenyl-containing drug that targets the helicase-primase complex of HSV-1, HSV-2, and varicella-zoster virus (VZV) (6). Since this protein complex is essential for HSV DNA synthesis, helicase-primase inhibitors such as ASP2151 represent a potentially effective treatment option for genital herpes. Notably, ASP2151 has demonstrated more-potent in vitro antiviral activity than ACV against a limited number of laboratory stocks and clinically isolated strains of HSV-1, HSV-2, and VZV (6). In addition, ASP2151 has excellent oral bioavailability and potent anti-HSV activities in animal models (6, 14, 23).
The aim of the present study was to evaluate the antiviral activities of ASP2151 against a broad range of clinical HSV isolates obtained from patients participating in a placebo- and VCV-controlled proof-of-concept study of ASP2151 (3-day treatment course) for recurrent genital herpes (23). In addition, we also monitored changes in the susceptibility of HSV isolates to ASP2151 before and after the short-term dosing of patients with ASP2151.
MATERIALS AND METHODS
Antiviral compounds.
ASP2151 (international nonproprietary name, amenamevir) was synthesized at Astellas Pharma, Inc. (Tokyo, Japan). ACV was purchased from a commercial supplier (Sigma-Aldrich, St. Louis, MO).
Clinical specimens.
HSVs were isolated from genital herpes patients enrolled in a phase II, double-blinded, multicenter, randomized, active- and placebo-controlled proof-of-concept study at 26 sites throughout the United States between June 2007 and August 2008 (23). A signed informed consent form was obtained from each patient before the initiation of any study-specific procedure. A total of 156 HSV isolates (5 HSV-1 and 151 HSV-2 isolates) were recovered from the positive viral cultures of genital swab samples collected before the dosing of patients with placebo, VCV (500 mg twice daily for 3 days), or ASP2151 (100, 200, or 400 mg once daily for 3 days or 1,200 mg one-shot). Of these patients, 106 HSV isolates (3 HSV-1 and 103 HSV-2 isolates) were also collected from the same patients within or after the 3-day treatment period.
PRA.
HSV isolates successfully obtained from the first (average of 0.6 h before treatment) and last (average of 46.8 h after the initiation of treatment) positive culture for each patient were tested for sensitivity to ASP2151 and ACV using a plaque reduction assay (PRA), as previously described (25). Briefly, human embryonic lung fibroblast (HELF) cells were seeded into 12-well plates (2 × 105 cells/well) and incubated at 37°C until the cells formed a monolayer. After removal of the growth medium, the cells were infected with HSV-1 or HSV-2 (50 to 100 PFU/well), and the plates were then incubated for 1 h at 37°C. After the viral inocula were aspirated, cells were treated with the test compound until clear plaques appeared. The cells were then fixed with 10% formalin in phosphate-buffered saline and stained with a 0.8% crystal violet solution. The number of plaques present in each well was determined by counting under a microscope. The 50% effective concentration (EC50), which is the concentration to reduce the plaque number by 50%, was calculated using linear regression analysis. The antiviral activity of ASP2151 was fitted to the sampling time of the respective isolates using the linear regression program of GraphPad Prism (GraphPad Software, San Diego, CA). ACV- and ASP2151-sensitive and resistant HSV controls were repeatedly tested for validation. A sensitive clinical isolate (sensitive control) was included in each run. ACV resistance was defined by an EC50 of ≥11.1 μM (2.5 μg/ml) and a ≥4-fold increase over the EC50 for the sensitive control included in the run. Since there are no recognized in vitro breakpoints for defining resistance to ASP2151, we set provisional guidelines for this study. Preexisting resistance to ASP2151 was defined by an EC50 ≥4-fold increased over that for the sensitive control and greater than the mean + 3 standard deviations (SD) based on aggregate data for the sensitive control. Resistance associated with ASP2151 treatment was defined by satisfying the criteria described above and by a ≥4-fold increase in the EC50 over the EC50 against the pretherapy isolate obtained from any given subject.
Statistical analyses.
Statistical analyses were performed using the SAS software program (SAS Institute, Carey, NC), and a P value of <0.05 was considered statistically significant. In the PRA, results were analyzed using the paired Student t test for comparisons of samples obtained before and after dosing patients with antiviral agents.
RESULTS AND DISCUSSION
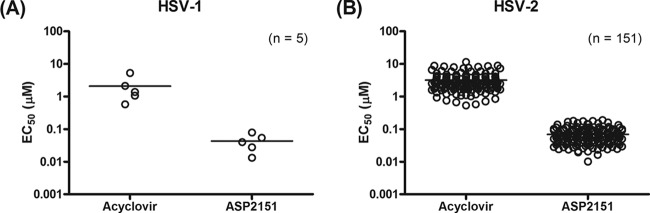
PRA analysis was performed to test the antiviral activities of ACV and ASP2151 against a total of 156 clinical HSV isolates (HSV-1, n = 5; HSV-2, n = 151). Figure 1 shows the susceptibilities to ACV and ASP2151 of HSV isolates obtained from predose subjects. The EC50s (means ± standard errors [SE]) of ACV and ASP2151 against the HSV isolates were 2.1 ± 0.8 and 0.043 ± 0.011 μM, respectively, for HSV-1 and 3.2 ± 0.2 and 0.069 ± 0.003 μM, respectively, for HSV-2. The PRA results indicate that ASP2151 inhibits the replication of a broad range of clinical HSV isolates at concentrations lower than those of ACV. Importantly, no ASP2151- or ACV-resistant HSV clinical isolates were detected prior to the dosing of patients. The ratios of EC50s for ASP2151 and ACV to data for the sensitive control ranged from 0.1 to 3.9 and 0.2 to 3.6, respectively.
Fig 1.
Susceptibilities of clinical HSV-1 and HSV-2 isolates to acyclovir and ASP2151. Antiviral activities of acyclovir and ASP2151 against 5 HSV-1 and 151 HSV-2 isolates obtained from subjects before the first dosing with antiviral agents were measured using the plaque reduction assay with HELF cells. Each symbol represents the value for one experiment performed in triplicate.
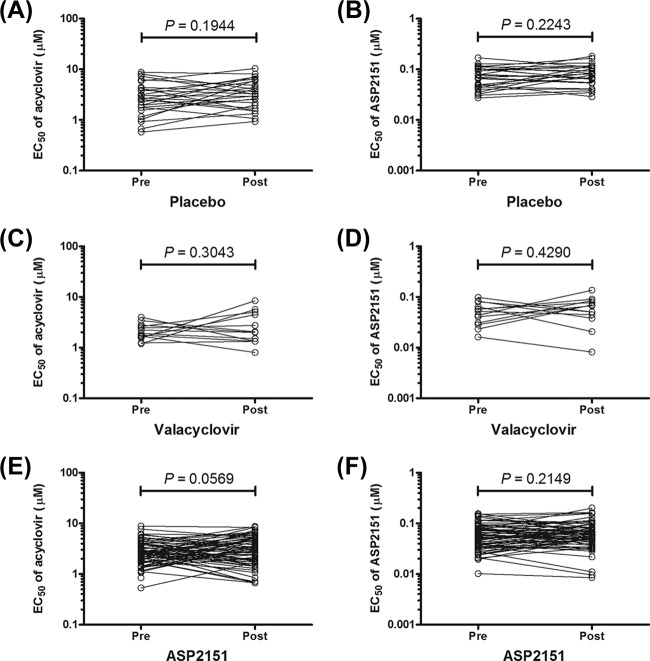
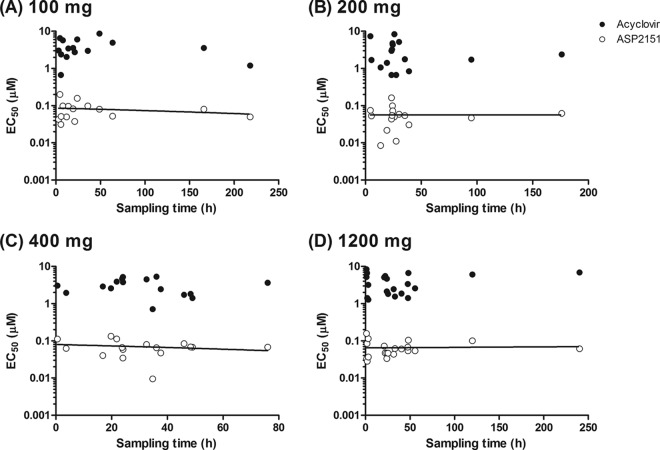
We next investigated whether the susceptibilities of HSV isolates to the two antiviral agents were altered within or following the 3-day dosing period of patients with placebo (n = 26), VCV (500 mg; n = 13), or ASP2151 (100, 200, 400, and 1,200 mg; n = 15, 16, 16, and 20, respectively). The overall susceptibility of the HSV strains to ASP2151 and ACV was not altered after the dosing (P > 0.05) (Fig. 2). The mean antiviral activities of ASP2151 against the HSV isolates obtained from patients pre- and post-dosing with ASP2151 (100, 200, 400, and 1,200 mg) did not significantly differ (P = 0.2243, 0.4402, 0.8034, and 0.2770, respectively) (Table 1). In addition, the HSV isolates from patients treated with VCV did not show significantly decreased susceptibility to ACV (Fig. 2 and Table 1). Although ASP2151 dosing did not result in significant alteration of susceptibility of HSV isolates to ACV (Fig. 2), a slight difference in the mean EC50s of ACV was obtained between pre- and post-dosing with 100 mg ASP2151 (P = 0.013) (Table 1). However, since no dose dependency of ASP2151 was evident with regard to alteration on susceptibility to ACV, ASP2151 does not likely have the ability to select ACV resistance. Importantly, a trend toward resistance development with increasing duration of treatment also was not detected (Fig. 3). When the relationship between the antiviral activity of ASP2151 and sampling time for the HSV isolates was analyzed, the 95% confidence intervals for the slope of the plotted line determined using a linear regression model ranged from −0.0006 to 0.0003, −0.0005 to 0.0005, −0.001 to 0.0006, and −0.0003 to 0.0003 for isolates obtained from patients given 100, 200, 400, and 1,200 mg ASP2151, respectively. This analysis indicates that no correlation exists between the antiviral activity of ASP2151 and sampling time for the isolates. Together, these findings suggest that no ASP2151- or ACV-resistant HSV would be detected after the short-term treatment of recurrent genital herpes patients with the respective antiviral agents.
Fig 2.
Susceptibilities of HSV isolated from subjects before and after the first dosing with antiviral agents. Antiviral activities of acyclovir and ASP2151 against clinical isolates obtained from patients pre- or post-dosing with placebo (A and B) (n = 26), valacyclovir (C and D) (n = 13), or ASP2151 (E and F) (n = 67) were measured using the plaque reduction assay with HELF cells. Each symbol represents the value of one experiment performed in triplicate. Statistical analyses were performed using the paired Student t test for comparisons between results obtained pre- and post-dosing of patients with the antiviral agents.
Table 1.
Susceptibilities to acyclovir and ASP2151 of clinical isolates obtained from patients treated with antiviral agents
| Antiviral agent administered and dose (mg) | No. of isolatesa | Result forb: |
|||||
|---|---|---|---|---|---|---|---|
| Acyclovir |
ASP2151 |
||||||
| EC50 (μM) |
P valuec | EC50 (μM) |
P valuec | ||||
| Pre | Post | Pre | Post | ||||
| Placebo | 26 | 3.3 ± 0.4 | 3.9 ± 0.5 | 0.1944 | 0.076 ± 0.007 | 0.084 ± 0.008 | 0.2655 |
| Valacyclovir, 500 | 13 | 2.2 ± 0.2 | 3.0 ± 0.6 | 0.3043 | 0.052 ± 0.007 | 0.062 ± 0.009 | 0.4290 |
| ASP2151 | |||||||
| 100 | 15 | 2.6 ± 0.3 | 3.8 ± 0.6 | 0.0132 | 0.065 ± 0.011 | 0.081 ± 0.012 | 0.2243 |
| 200 | 16 | 2.8 ± 0.3 | 3.0 ± 0.6 | 0.7497 | 0.060 ± 0.001 | 0.056 ± 0.009 | 0.4402 |
| 400 | 16 | 3.2 ± 0.6 | 3.1 ± 0.4 | 0.8904 | 0.067 ± 0.009 | 0.069 ± 0.008 | 0.8034 |
| 1,200 | 20 | 3.1 ± 0.3 | 3.9 ± 0.5 | 0.1324 | 0.059 ± 0.006 | 0.065 ± 0.007 | 0.2770 |
Number of isolates that were collected both before and after the first dosing of patients.
Data represent the mean EC50s and standard errors of triplicate determinations for viruses isolated from patients before (Pre) and after (Post) dosing.
Comparisons between pre- and postdosing EC50s were performed using the paired Student t test.
Fig 3.
Relationship between antiviral activity and sampling time. Antiviral activities of acyclovir (filled circles) and ASP2151 (open circles) against clinical isolates obtained from the last positive culture for each patient after treatment with 100 mg (A), 200 mg (B), 400 mg (C), or 1,200 mg (D) of ASP2151 were measured using the plaque reduction assay with HELF cells. The antiviral activities were plotted versus the sampling time of each isolate. Correlations were analyzed using a linear regression model.
The emergence of highly virulent mutant HSV with resistance to antiviral drugs is of concern for the effective treatment of genital herpes. Although ACV-resistant mutants were previously detected at relatively high frequencies (10−4 to 10−3) in tissue culture (2, 18, 19, 21), no resistant HSV was found among the clinical isolates in the present study. This finding is consistent with the fact that the reported incidence of ACV-resistant HSV has not significantly increased among immunocompetent patients despite the widespread use of ACV (5, 18), although ACV resistance is problematic in immunocompromised patients, in which <5% of isolates are reported to be ACV resistant (7, 12).
In in vitro studies, mutants resistant to the HSV helicase-primase inhibitor AIC316 (also called BAY 57-1293) were detected at 10 to 100 times the expected background frequency (<10−6) for 16% to 20% of clinical isolates following incubation in the presence of 0.8 to 3 μM AIC316 (4, 22). However, no viral resistance to AIC316 was observed after 28 days of treatment in a phase II trial involving genital herpes patients (13). In the present study, we also found that all clinical HSV isolates were susceptible to ASP2151, with no preexisting resistant variants detected, and that the overall susceptibility of the HSV strains to ASP2151 was not markedly altered after dosing (Fig. 1 and 2). We have shown that the frequency of ASP2151-resistant variants in in vitro cell cultures is lower than that of ACV-resistant variants, and that the ASP2151-resistant strains exhibit lower growth ability than the parent strain (data not shown). Therefore, the ASP2151-resistant virus might not be isolated or may be difficult to select for after short-term treatment in the clinic. Based on our present findings and the literature cited above (5, 13, 18), preexisting variants resistant to ASP2151 are unlikely to be detected, and ASP2151 might not select for resistant HSV in immunocompetent patients with recurrent genital herpes. Nevertheless, further investigations are necessary to determine if resistant strains of HSV would be likely to emerge in the clinical setting, notably during long-term suppressive treatment for genital herpes.
In conclusion, our results demonstrate that the helicase-primase inhibitor ASP2151 prevented the replication of HSV clinical isolates more potently than ACV. Moreover, HSV resistant to this novel helicase-primase inhibitor as well as ACV may not easily emerge in short-term treatment for recurrent genital herpes patients.
ACKNOWLEDGMENT
This work was supported by and conducted at Astellas Pharma Inc.
Footnotes
Published ahead of print 23 April 2012
REFERENCES
- 1. Ashley RL, Wald A. 1999. Genital herpes: review of the epidemic and potential use of type-specific serology. Clin. Microbiol. Rev. 12:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bacon TH, Levin MJ, Leary JJ, Sarisky RT, Sutton D. 2003. Herpes simplex virus resistance to acyclovir and penciclovir after two decades of antiviral therapy. Clin. Microbiol. Rev. 16:114–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Benedetti J, Corey L, Ashley R. 1994. Recurrence rates in genital herpes after symptomatic first-episode infection. Ann. Intern. Med. 121:847–854 [DOI] [PubMed] [Google Scholar]
- 4. Biswas S, Smith C, Field HJ. 2007. Detection of HSV-1 variants highly resistant to the helicase-primase inhibitor BAY 57-1293 at high frequency in 2 of 10 recent clinical isolates of HSV-1. J. Antimicrob. Chemother. 60:274–279 [DOI] [PubMed] [Google Scholar]
- 5. Chibo D, Druce J, Sasadeusz J, Birch C. 2004. Molecular analysis of clinical isolates of acyclovir resistant herpes simplex virus. Antiviral Res. 61:83–91 [DOI] [PubMed] [Google Scholar]
- 6. Chono K, et al. 2010. ASP2151, a novel helicase-primase inhibitor, possesses antiviral activity against varicella-zoster virus and herpes simplex virus types 1 and 2. J. Antimicrob. Chemother. 65:1733–1741 [DOI] [PubMed] [Google Scholar]
- 7. Christophers J, et al. 1998. Survey of resistance of herpes simplex virus to acyclovir in northwest England. Antimicrob. Agents Chemother. 42:868–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Collins P, Darby G. 1991. Laboratory studies of herpes simplex virus strains resistant to acyclovir. Rev. Med. Virol. 1:19–28 [Google Scholar]
- 9. De Clercq E. 1993. Antivirals for the treatment of herpesvirus infections. J. Antimicrob. Chemother. 32(Suppl. A):121–132 [DOI] [PubMed] [Google Scholar]
- 10. De Clercq E, Field HJ. 2006. Antiviral prodrugs—the development of successful prodrug strategies for antiviral chemotherapy. Br. J. Pharmacol. 147:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Engelberg R, Carrell D, Krantz E, Corey L, Wald A. 2003. Natural history of genital herpes simplex virus type 1 infection. Sex. Transm. Dis. 30:174–177 [DOI] [PubMed] [Google Scholar]
- 12. Field HJ. 2001. Herpes simplex virus antiviral drug resistance—current trends and future prospects. J. Clin. Virol. 21:261–269 [DOI] [PubMed] [Google Scholar]
- 13. Huang M, et al. 2011. No emergence of resistance under treatment with the novel HSV inhibitor AIC316 in persons with genital herpes, abstr. V-2941b. Abstr. 51st Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC [Google Scholar]
- 14. Katsumata K, et al. 2011. Effect of ASP2151, a herpesvirus helicase-primase inhibitor, in a guinea pig model of genital herpes. Molecules 16:7210–7223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kinghorn GR. 1994. Epidemiology of genital herpes. J. Int. Med. Res. 22(Suppl. 1):14A–23A [PubMed] [Google Scholar]
- 16. Lafferty WE. 2002. The changing epidemiology of HSV-1 and HSV-2 and implications for serological testing. Herpes 9:51–55 [PubMed] [Google Scholar]
- 17. Lafferty WE, Coombs RW, Benedetti J, Critchlow C, Corey L. 1987. Recurrences after oral and genital herpes simplex virus infection. Influence of site of infection and viral type. N. Engl. J. Med. 316:1444–1449 [DOI] [PubMed] [Google Scholar]
- 18. Morfin F, Thouvenot D. 2003. Herpes simplex virus resistance to antiviral drugs. J. Clin. Virol. 26:29–37 [DOI] [PubMed] [Google Scholar]
- 19. Parris DS, Harrington JE. 1982. Herpes simplex virus variants restraint to high concentrations of acyclovir exist in clinical isolates. Antimicrob. Agents Chemother. 22:71–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pellet PE, Roizman B. 2007. The family Herpesviridae: a brief introduction, p 2479–2499 In Knipe DM, et al. (ed), Fields virology, 5th ed, vol 2 Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 21. Shin YK, Cai GY, Weinberg A, Leary JJ, Levin MJ. 2001. Frequency of acyclovir-resistant herpes simplex virus in clinical specimens and laboratory isolates. J. Clin. Microbiol. 39:913–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sukla S, et al. 2010. Mismatch primer-based PCR reveals that helicase-primase inhibitor resistance mutations pre-exist in herpes simplex virus type 1 clinical isolates and are not induced during incubation with the inhibitor. J. Antimicrob. Chemother. 65:1347–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tyling S, et al. 20 February 2012. ASP2151 for the treatment of genital herpes: a randomized, double-blind, placebo- and valacyclovir-controlled, dose-finding study. J. Infect. Dis. doi:10.1093/infdis/jis019 [DOI] [PubMed] [Google Scholar]
- 24. Wald A, Zeh J, Selke S, Ashley RL, Corey L. 1995. Virologic characteristics of subclinical and symptomatic genital herpes infections. N. Engl. J. Med. 333:770–775 [DOI] [PubMed] [Google Scholar]
- 25. Weinberg A, Leary JJ, Sarisky RT, Levin MJ. 2007. Factors that affect in vitro measurement of the susceptibility of herpes simplex virus to nucleoside analogues. J. Clin. Virol. 38:139–145 [DOI] [PubMed] [Google Scholar]
- 26. Whitley RJ, Kimberlin DW, Roizman B. 1998. Herpes simplex viruses. Clin. Infect. Dis. 26:541–553 [DOI] [PubMed] [Google Scholar]
- 27. Whitley RJ, Roizman B. 2001. Herpes simplex virus infections. Lancet 357:1513–1518 [DOI] [PubMed] [Google Scholar]