Abstract
Only anecdotal data are available on the pharmacokinetics (PK) of miltefosine in children suffering from visceral leishmaniasis (VL). While failure rates were higher in children with VL, steady-state concentrations appeared lower than those seen with adults. We hypothesized that the current linear dosage (in milligrams per kilogram of body weight) is too low for children and that a new dosing algorithm based on an appropriate body size model would result in an optimal exposure. A population PK analysis was performed on three historic pooled data sets, including Indian children, Indian adults, and European adults. Linear and allometric scaling of PK parameters by either body weight or fat-free mass (FFM) was evaluated for body size models. Based on the developed PK model, a dosing algorithm for miltefosine in children and adults was proposed and evaluated in silico. The population PK model employing allometric scaling fitted best to the pooled miltefosine data. Allometric scaling by FFM reduced between-subject variability, e.g., for drug clearance, from 49.6% to 32.1%. A new allometric miltefosine dosing algorithm was proposed. Exposure to miltefosine was lower in children than adults receiving 2.5 mg/kg/day: a Cmax of 18.8 μg/ml was reached by 90% of adults and 66.7% of children. The allometric daily dose resulted in similar levels of exposure to miltefosine for adults and children. The use of a new allometric dosing algorithm for miltefosine in VL patients results in optimal exposure to miltefosine in both adults and children and might improve clinical outcome in children.
INTRODUCTION
Visceral leishmaniasis (VL) or kala-azar (Hindi for “black fever”) has been classified as one of the world's “most neglected diseases” (62), and of all the parasitic diseases, it ranks third in terms of morbidity and mortality after only malaria and lymphatic filariasis (38). The World Health Organization (WHO) reported in 2004 that leishmaniasis is responsible for around 50,000 deaths and almost 2 million disability-adjusted life-years per year, approximately half of which can be attributed to children from low-income countries (38, 60), but in reality the burden of leishmaniasis is probably much larger due to massive underreporting of both cases and deaths in the often remote areas where leishmaniasis is prevalent (47).
Miltefosine (hexadecylphosphocholine; marketed by Paladin Laboratories Inc. as Impavido) is the newest addition to the small repertory of antileishmanial drugs and to date is the only drug that can be administered orally. It has achieved relatively high cure rates in the treatment of visceral (8, 26, 41, 52, 54), New World cutaneous (11, 44, 48, 50, 57), Old World cutaneous (30, 56) and even difficult-to-treat mucocutaneous (49, 51) leishmaniasis.
During the development of miltefosine, two clinical trials were designed to investigate the efficacy and safety of miltefosine in children (<12 years of age) in which a total of 119 pediatric patients were enrolled, employing dosages linearly extrapolated from the daily adult dosage (in milligrams per kilogram of body weight) (7, 53). The per-protocol cure rates obtained in these trials, however, tended to be lower than those previously observed in adult patients. In a more recent large phase IV trial conducted in India and Nepal, 358 children were treated with the same daily dose of 2.5 mg/kg as the adults in that trial (8). Most notably, a significant difference in levels of efficacy was also observed in that trial between children and adults: almost twice as many children as adults showed therapy failure on miltefosine in that trial (6.4% children versus 3.4% adults) while receiving the same milligram-per-kilogram dose (8).
A reasonable explanation for the observed lower efficacy in children might be relative underdosing in children. However, little is known about the clinical pharmacokinetics (PK) of miltefosine and even less is known about the pharmacokinetics in a pediatric population. The most extensive pharmacokinetic data, from adults, were previously published by our group in a report in which we observed that miltefosine kept accumulating in patients until the end of treatment and had an extremely long terminal elimination half-life of about 31 days (17). The sparsely published pharmacokinetic data that are available from the clinical trials on miltefosine performed in India indicated that the pharmacokinetics differ remarkably between adults and children. The reported miltefosine plasma concentrations in the last week of treatment were considerably higher in adults (administered 100 mg/day for 28 days) than in children (administered 2.5 mg/kg/day for 28 days): 70 μg/ml in adults (median value, day 23) versus 24 μg/ml in children (mean value, days 26 to 28) (20).
Dosing of miltefosine in children is, at the moment, neither rationally nor thoroughly experimentally derived. To reduce the paucity of information from pediatric population pharmacokinetic studies on miltefosine, we conducted a population pharmacokinetic modeling study on three existing pharmacokinetic data sets for children and adults that used data from both the Indian subcontinent and Europe. Our objective was to identify and evaluate a new dosing algorithm which produces in children a profile of drug exposure similar to that observed in adults suffering from leishmaniasis as a first approach to the establishment of a rational treatment design for miltefosine.
MATERIALS AND METHODS
Patient populations and pharmacokinetic data.
Pharmacokinetic data from three different studies and data sets were used: one pediatric study (“Pediatric Indian”) (53) and one adult study (“Adult Indian”) (26) with patients with relatively low body weights, both performed in India, and one adult study (“Adult European”) (17, 56) with patients with relatively high body weights, performed in Europe.
In the Pediatric Indian study, miltefosine was orally administered at a dosage of either 1.5 or 2.5 mg/kg of body weight/day for a total of 28 days. Plasma samples were collected on day 2 and days 26, 27, and 28 of treatment (53).
The Adult Indian study contained 4 dosing groups, who received miltefosine orally in a dosage regimen of 50 mg/day for 6 weeks, 50 mg/day for 1 week plus 3 weeks of 100 mg/day, 100 mg/day for 4 weeks, or 100 mg/day for 1 week plus 3 weeks of 150 mg/day, as reported previously. Plasma samples were taken predose at various time points during and after treatment. On day 22 of treatment, samples were taken at 0, 2, 4, 6, 8, 12, and 24 h (26).
In the Adult European study, miltefosine was orally administered at a dosage of 150 mg/day for a total of 28 days. Plasma samples were taken predose at various time points during and after end of treatment, with a very long follow-up (17).
Miltefosine concentrations were determined using validated bioanalytical methods employing liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS). For the Pediatric Indian and Adult Indian studies, a validated quantitative method was used with a lower limit of quantitation (LLOQ) of 5 ng/ml, based on a previously reported method for the structural analog perifosine (31). For the Adult European study, a previously reported validated quantitative method was used with an LLOQ of 4 ng/ml (16).
Population pharmacokinetic analysis.
All calculations, simulations, and estimations were performed on a dual-core desktop computer running NONMEM VI (level 2.0) (6), the R statistical software package (version 2.14; http://cran.r-project.org) (43), and Perl-speaks-NONMEM (PsN, version 2.3.1; http://psn.sourceforge.net) (32, 33). Pirana (version 2.4; an interface to NONMEM, PsN, and our cluster; http://www.pirana-software.com) was used for run deployment and analysis (29). Xpose (version 4.0; http://xpose.sourceforge.net) (27), an R-based model building aid, was used for graphical model evaluation.
The first-order conditional estimation procedure with interaction between between-subject variability and residual error components was used throughout. The minimal value of the objective function (equal to minus twice the log likelihood) provided by NONMEM was used as a goodness-of-fit characteristic, in addition to comparisons of, e.g., parameter values and standard errors of parameter estimates. Furthermore, performance of the models was assessed via goodness-of-fit plots using Xpose and Pirana.
(i) Structural pharmacokinetic model.
An open two-compartment model with first-order absorption and linear elimination from the central compartment had previously been developed on the Adult European data set (17). Absorption rate (ka), clearance (elimination clearance [CL] and intercompartmental clearance [Q]), and volume of distribution (central volume of distribution [V2] and peripheral volume of distribution [V3]) were the primary pharmacokinetic parameters estimated. Secondary parameters, such as elimination half-life, were calculated from these primary parameters. Bioavailability (F) was unknown, and therefore, parameters were estimated relative to the bioavailability (CL/F, V/F, etc.). Between-subject variability in the pharmacokinetic parameters was estimated with an exponential model. Residual variability was modeled with a proportional error model with separate estimates for each of the three different data sets (Pediatric Indian, Adult Indian, Adult European), since these data sets were obtained from three distinct clinical trials, with different populations and different analytical methods.
(ii) Body size models and descriptors.
Several body size descriptors and body size models were considered to account for the effect of body size on the pharmacokinetics of miltefosine.
Fat-free mass (FFM) in kilograms was estimated from total body weight (WT) in kilograms, height (H) in meters, and weight for height standard (WHS) in kilograms per square meter as follows (25):
| (1) |
where WHSmax is 42.92 or 37.99 kg/m2 and WHS50 is 30.93 or 35.98 kg/m2 for males or females, respectively (25).
To investigate the effect of size, different models were evaluated where parameters were scaled linearly by either WT (equation 2) or FFM (equation 3) or scaled allometrically by WT (equation 4) or FFM (equation 5). For example, for drug clearance, the following equations were used:
| (2) |
| (3) |
| (4) |
| (5) |
where CL/Fi represents the clearance of the i-th individual, θ1 represents the typical value of clearance, ηi represents the between-subject random effect with a mean of 0 and a variance of ω2, WTi represents the body weight of the i-th individual, WTstd is a standard body weight (set at 60 kg), FFMi represents the calculated fat-free body mass (see Eq. 1) of the i-th individual, FFMstd represents a standard fat-free body mass (set at 53 kg), and PWR represents the allometric power exponent. For clearance, the allometric PWR value was fixed at 0.75, and for volume of distribution, the value was fixed at 1.0, based on the biological principles that support these values (2, 3, 22, 58).
The ability of the body size models to reduce the unexplained between-subject variability and to improve the goodness-of-fit of the model (ΔOFV, difference in objective function value) was assessed. A visual predictive check (VPC) was used to assess the predictive performance of the models (28).
Development and evaluation of a new dosing algorithm.
Based on the body size model which best described and fitted the pharmacokinetic data, a maintenance dose algorithm was developed incorporating the most appropriate body size model and descriptor.
This new dosing algorithm was evaluated by simulating pharmacokinetic curves for pediatric patients (n = 1,000 individuals) and adult patients (n = 1,000 individuals) with the same anthropometric properties as the subjects in the original Pediatric Indian trial and the Adult Indian trial, respectively. Final typical pharmacokinetic parameter and covariance estimates from the previous population pharmacokinetic analysis were used in the Monte Carlo simulations. Systemic drug exposures were compared between children and adults receiving the currently used 2.5-mg/kg/day dosage or a dose according to the dosing algorithm proposed here, both for a total of 28 days. All body sizes were assigned randomly from a log-normal distribution taken from the original studies, and dosages were calculated from the simulated body sizes and were rounded to the nearest 10 mg (the smallest commercially available capsule of miltefosine). The simulation, thus, was consistent with the process of dosing as it should occur at the bedside. Systemic exposure to miltefosine was assessed through prediction plots, while the values for miltefosine plasma concentration at the end of treatment (CEOT) and area under the plasma concentration-time curve from zero to end of treatment (AUC0-EOT) were compared between children and adults, and between the two dose regimens.
RESULTS
Demographics.
In Table 1, the characteristics of the patients in the three studies are summarized. See “Patient populations and pharmacokinetic data” in Materials and Methods for references of the studies and an overview of the exact designations used here to refer to each study. Table 1 shows that both the adult and pediatric Indian patients were smaller and had a much lower relative fat mass than the adult European patients. The estimated ratio of fat-free mass to total body weight was around 95% for the Indian children and around 90% for Indian adults, while for the European adults this value was around 75%. The Indian adult patients were also younger (minimum age of 12 years) than the European adult patients (Table 1).
Table 1.
Baseline characteristics of patients in three distinct clinical trials included in the population pharmacokinetic analysis
| Parameter | Value for study groupa |
||
|---|---|---|---|
| Pediatric Indian (n = 53) | Adult Indian (n = 26) | Adult European (n = 17) | |
| Indication | VL | VL | CL |
| Ethnicity | Indian | Indian | Caucasian |
| No. of patients [no. male/no. female] | 39 [23/16] | 40 [30/10] | 31 [30/1] |
| Age (yr) | 7 (3–11) | 18.5 (12–50) | 24 (19–49) |
| Height (cm) | 108 (80–135) | 152.5 (108–180) | 184 (175–200) |
| Body wt (kg) | 15 (9–23) | 35.5 (16–58) | 85 (70–113) |
| Fat-free mass (kg)b | 13.9 (8.58–22.6) | 31.6 (15.4–49.3) | 64.6 (52.9–81.2) |
| Body mass index (kg m−2) | 12.8 (9.57–15.7) | 15.2 (11.0–23.2) | 25.1 (20.0–28.8) |
| No. of PK measurementsc | 4 (4–4) | 18 (16–19) | 11 (8–19) |
All values are median values (range) unless stated otherwise. CL, cutaneous leishmaniasis; VL, visceral leishmaniasis; PK, pharmacokinetic.
Fat-free mass was calculated by the formula of Janmahasatian et al. (25).
Data represent measurements after start of treatment, per patient.
Population pharmacokinetic analysis.
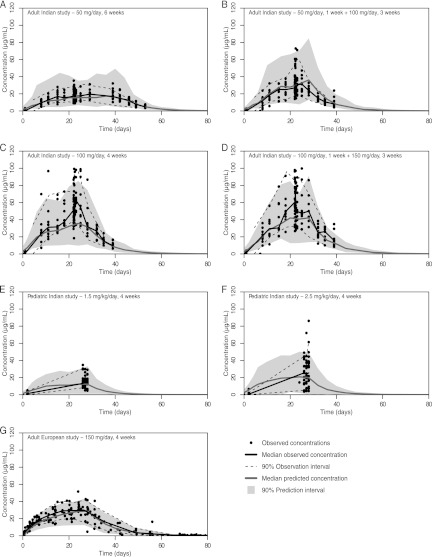
The observed miltefosine plasma concentration-versus-time data that were used in the population analysis are shown in Fig. 1, stratified by the different dosing regimens in the three distinct clinical studies. Of a total of 1,196 observations, only one sample was below the LLOQ, and that sample was ignored in the analysis.
Fig 1.
Visual predictive checks for the population pharmacokinetic model employing allometric scaling based on fat-free mass. The dots represent the observed concentrations, the black line indicates the median observed concentration, and the dotted lines show the 5th and 95th percentiles of the observations (indicating the 90% observation interval). The dark gray line indicates the median predicted concentration from 1,000 simulated individuals, and the gray area shows the 90% prediction interval of the model predicted values. The plots are stratified for each different dosing regimen that was used in the respective clinical trials (Adult Indian study, 4 regimens, plots A to D; Pediatric Indian study, 2 regimens, plots E and F; Adult European study, 1 regimen, plot G).
The base model (equation 2) with linear scaling by WT was successfully fitted to the pooled miltefosine pharmacokinetic data. The model with pharmacokinetic parameters scaled linearly by FFM (equation 3) performed better in terms of relative change in objective function value (ΔOFV) and reduction of between-subject variability than the linear model scaled by WT (equation 2) (see Table 2). However, both allometrically scaled models (equations 4 and 5) did perform much better than both the linear models (equations 2 and 3): the allometric scaling reduced the between-subject variability of both clearance (CL) and central volume of distribution (V2) compared to the linear models and had a better goodness-of-fit to the data (the OFV decreased by 31.4). Allometric scaling by FFM reduced the between-subject variability by 35.3% for CL (from 49.6% to 32.1%; Table 2) and by 20.2% for V2 (from 42.7% to 34.1%; Table 2), thereby adding explanatory power to the model. The estimation of the allometric power exponent for CL yielded a value of 0.667, with the value for V2 fixed to 1. However, in comparison to using a fixed value for CL of 0.75, there was only a very slight decrease in both OFV (−1.2 ΔOFV) and between-subject variabilities of CL (29.4% versus 32.1%) and V2 (33.2% versus 34.1%) and thus little improvement of model fit. Given the body of knowledge on the biological principles behind the allometric power exponents, the fixed values of 0.75 for CL and 1 for V2 were therefore preferred in the final model. Conventional goodness-of-fit plots (observed versus individual and model-predicted concentrations, conditional weighted residuals versus time and model-predicted concentrations) did not show any obvious trends, indicating that the model fit was adequate (plots not shown).
Table 2.
Comparison of the performances of miltefosine population pharmacokinetic models: differences in objective function values and relative between-subject variabilities
| Model | Corresponding equation | ΔOFVa | % BSV [relative % change]b |
|
|---|---|---|---|---|
| CL/F | V2/F | |||
| 1 (linear scaling by WT) | 2 | 0 | 49.6 [0] | 42.7 [0] |
| 2 (linear scaling by FFM) | 3 | −22.7 | 42.8 [-13.8] | 37.4 [-12.3] |
| 3 (allometric scaling by WT) | 4 | −42.0 | 35.1 [-29.3] | 37.7 [-11.7] |
| 4 (allometric scaling by FFM) | 5 | −63.9 | 32.1 [-35.3] | 34.1 [-20.2] |
ΔOFV (difference in objective function value) was calculated as ([OFV model value] − [OFV model 1 value]), where model 1 was used as the base model. A negative ΔOFV indicates a better fit of the model.
Between-subject variability (BSV) was calculated using the between-subject variance (ω2). BSV values from the base model (model 1) were used as reference values to calculate percent change between the models of the respective parameters.
Figure 1 shows the VPC of the final model with allometric scaling by FFM plotted over the observed values. The VPC indicated a sufficiently predictive performance of the two-compartment model for the two dosing regimens in the Pediatric Indian study, the four regimens in the Adult Indian study, and the single regimen in the Adult European study. Table 3 shows the final parameter estimates of the model with allometric scaling by FFM.
Table 3.
Final parameter estimates from the population pharmacokinetic model with allometric scaling by fat-free mass
| Parameter | Estimate (relative SE [%]) | % between-subject variability (relative SE [%]) |
|---|---|---|
| Absorption rate (ka) (h−1) | 0.416 (11.5) | 18.2 (115.5) |
| Clearance (CL/F) (liters/day/53 kg FFMa) | 3.99 (3.5)c | 32.1 (18.4) |
| Volume of central compartment (V2/F) (liters/53 kg FFM) | 40.1 (4.5)c | 34.1 (27.3) |
| Intercompartmental clearance (Q/F) (liters/day) | 0.0347 (18.3) | NEb |
| Volume of peripheral compartment (V3/F) (liters) | 1.75 (8.2) | NE |
| Residual variability, Pediatric Indian study (%) | 54.5 (5.5) | NE |
| Residual variability, Adult Indian study (%) | 34.3 (3.7) | NE |
| Residual variability, Adult European study (%) | 34.8 (6.9) | NE |
FFM, fat-free mass.
NE, not estimated.
Estimate is given for a standardized fat-free mass of 53 kg. Between-subject variabilities in CL/F and V2/F correlated with a correlation coefficient of 0.92.
Residual variability was estimated separately for the three different studies and appeared to be higher in the Pediatric Indian study (54.5%) than in both adult studies (34.3% and 34.8% for the Adult Indian and European study, respectively), which is also illustrated by the variability of observed values depicted in Fig. 1. The individual parameter estimates were used to calculate the elimination half-lives for the pediatric Indian population, the adult Indian population, and the adult European population. The typical initial elimination half-lives were estimated to be 4.99, 5.86, and 7.18 days for the Pediatric Indian, Adult Indian, and Adult European studies, respectively, while the values determined for the typical terminal elimination half-life were similar for all three study populations and were estimated at 35.5 days.
Development and evaluation of a new dose algorithm.
The final population pharmacokinetic model with allometric scaling by FFM which best fitted the data was used to perform Monte Carlo simulations. Following from this, an allometric maintenance dose was adapted from the adult “standard” dose, resulting in the following allometric dose algorithm:
| (6) |
where standard FFM is set at 53 kg and the standard dose is 150 mg, which is the maximal tolerable daily dose in adults. This allometric dose algorithm was transformed to a dosing table (Table 4) according to body weight and height and the resulting FFM according to equation 1. The dose was rounded to the nearest 10 mg, based on the smallest available miltefosine capsules.
Table 4.
Daily allometric miltefosine dose for males and females based on fat-free mass
| Weight (kg) | Total daily allometric miltefosine dose (mg) for patient of indicated height (cm)a |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 60 | 70 | 80 | 90 | 100 | 110 | 120 | 130 | 140 | 150 | 175 | 200 | |
| Males | ||||||||||||
| 9 | 30 | 40 | 40 | 40 | 40 | |||||||
| 12 | 40 | 40 | 40 | 50 | 50 | 50 | ||||||
| 15 | 40 | 50 | 50 | 60 | 60 | 60 | ||||||
| 20 | 50 | 60 | 60 | 70 | 70 | 70 | ||||||
| 25 | 60 | 70 | 70 | 80 | 80 | 80 | ||||||
| 30 | 80 | 80 | 90 | 90 | 90 | 100 | ||||||
| 35 | 80 | 90 | 90 | 100 | 100 | 100 | 110 | |||||
| 40 | 80 | 90 | 100 | 100 | 110 | 110 | 120 | 130 | ||||
| 45 | 90 | 90 | 100 | 110 | 110 | 120 | 130 | 130 | ||||
| 50 | 90 | 100 | 100 | 110 | 120 | 120 | 130 | 140 | ||||
| 55 | 90 | 100 | 110 | 120 | 120 | 130 | 140 | 150 | ||||
| 60 | 90 | 100 | 110 | 120 | 130 | 130 | 150 | 150b | ||||
| 65 | 100 | 110 | 110 | 120 | 130 | 140 | 150 | 150b | ||||
| 75 | 100 | 110 | 120 | 130 | 140 | 140 | 150b | 150b | ||||
| 85 | 100 | 110 | 120 | 130 | 140 | 150 | 150b | 150b | ||||
| Females | ||||||||||||
| 9 | 30 | 30 | 30 | 30 | 30 | |||||||
| 12 | 30 | 30 | 40 | 40 | 40 | 40 | ||||||
| 15 | 40 | 40 | 40 | 50 | 50 | 50 | ||||||
| 20 | 50 | 50 | 50 | 60 | 60 | 60 | ||||||
| 25 | 60 | 60 | 60 | 70 | 70 | 70 | ||||||
| 30 | 60 | 60 | 70 | 70 | 80 | 80 | 80 | |||||
| 35 | 60 | 70 | 70 | 80 | 80 | 80 | 90 | 90 | ||||
| 40 | 70 | 70 | 80 | 80 | 90 | 90 | 90 | 100 | 110 | |||
| 45 | 70 | 80 | 80 | 90 | 90 | 100 | 100 | 110 | 110 | |||
| 50 | 70 | 80 | 80 | 90 | 100 | 100 | 100 | 110 | 120 | |||
| 55 | 70 | 80 | 90 | 90 | 100 | 100 | 110 | 120 | 130 | |||
| 60 | 70 | 80 | 90 | 100 | 100 | 110 | 110 | 120 | 130 | |||
| 65 | 80 | 80 | 90 | 100 | 110 | 110 | 120 | 130 | 140 | |||
| 75 | 80 | 90 | 100 | 100 | 110 | 120 | 120 | 140 | 150 | |||
| 85 | 80 | 90 | 100 | 110 | 120 | 120 | 130 | 150 | 150b | |||
The total daily dose was calculated with equation 6 and rounded to the nearest 10 mg (smallest available capsule). To reduce the risk of gastrointestinal side effects upon intake, daily doses are best divided into three and given with 8-h intervals between doses.
A dose of 150 mg is currently considered to be the maximal tolerable dose that can be administered on a daily basis to a patient.
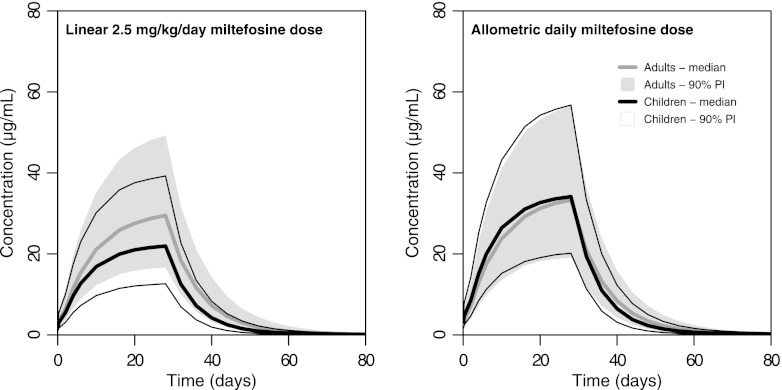
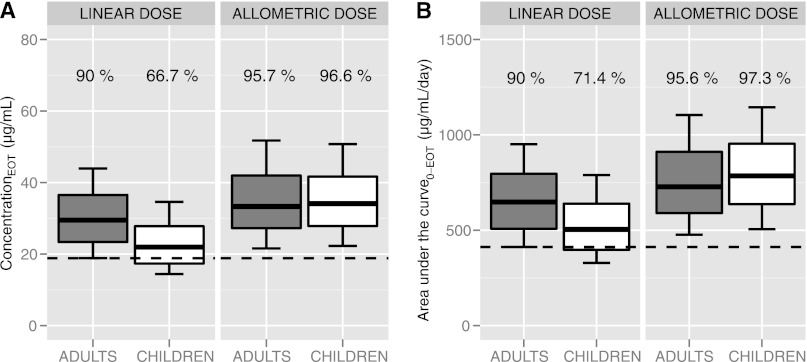
Pharmacokinetic curves were simulated for (i) 1,000 Indian adults receiving 2.5 mg/kg/day of miltefosine, (ii) 1,000 Indian children receiving 2.5 mg/kg/day, (iii) 1,000 Indian adults receiving a new allometric dose (Table 4), and (iv) 1,000 Indian children receiving the new allometric dose (Table 4), all with the same means and variances of body weights as the subjects in the respective original trials included in the population pharmacokinetic analysis but with their own estimates of between-subject variability. The prediction intervals and predicted median concentrations resulting from these simulations (Fig. 2) clearly demonstrate the discrepancy in exposure between children and adults administered the same linear dose (2.5 mg/kg/day). Consistent with the results from the population pharmacokinetic analysis, that dose led to underexposure to miltefosine in children compared to adults, as both median values and 90% prediction interval boundaries were lower in children. Conversely, the proposed allometric dose led to comparable miltefosine exposures in children and adults (Fig. 2). This is corroborated by the relative probability of reaching a minimal miltefosine exposure. An AUC0-EOT value of 412 μg/ml/day or higher was achieved by 90% of adults receiving 2.5 mg/kg/day, while only 71.4% of children on this dose reached this level of exposure (Fig. 3). In contrast, 95.6% and 97.3% of the adults and children, respectively, receiving the allometric dose proposed here reached this minimal target value of exposure (Fig. 3). Comparison of the miltefosine CEOT data shows similar results (Fig. 3). When administered the linear (milligrams per kilogram) dose, only 66.7% of children reached the CEOT that was reached by 90% of adults (18.8 μg/ml), while the allometric dose led to comparable proportions of both children and adults reaching this concentration (95.7% and 96.6%, respectively, above the target concentration).
Fig 2.
Comparison of miltefosine exposure levels in children and adults: predicted miltefosine concentrations following different dosage regimens. (A) Predicted miltefosine concentration-time curves and intervals for the currently recommended linear 2.5-mg/kg/day miltefosine dose for 28 days. (B) Allometric daily miltefosine dose for 28 days proposed here. The areas of data show the 90% prediction intervals (90% PI; 5th and 95th percentiles) for adults (in gray) and children (between thin black lines); the thicker gray and black lines indicate the median predicted concentrations for adults and children, respectively.
Fig 3.
Comparison of miltefosine exposure levels in children and adults: predicted concentration at the end of treatment and the area under the plasma concentration-time curve. These box plots represent distributions of the central miltefosine exposure following from Monte Carlo simulations of 1,000 adults (in gray) and 1,000 children (in white) receiving either the linear miltefosine dose (2.5 mg/kg/day) or the allometric daily miltefosine dose proposed here (Table 4 and equation 6). (A) Concentration at the end of treatment (CEOT). (B) Area under the concentration-time curve from start to end of treatment (AUC0-EOT). The pharmacokinetic target to be attained was the minimal adult exposure, set at the value that was attained by 90% of the adults receiving the linear dose (indicated by the dashed line); the percentages above the box plots show the proportions of individuals reaching this target.
DISCUSSION
The presented population pharmacokinetic model for miltefosine adequately predicts miltefosine exposure in Indian children, Indian adults, and European adults. The differences in body dimensions between these highly heterogeneous populations were high, with respective median body weights of 15, 35.5, and 85 kg. The differences in pharmacokinetics could best be explained with allometric scaling by FFM. This body size descriptor was found to be the best related to drug clearance and volume of distribution. Following from this, a new dose algorithm was developed, resulting in similar levels of systemic exposure to miltefosine between Indian children and Indian adults.
The treatment of VL patients has improved over the past years, as the international scientific attention has increased and several not-for-profit organizations have made it a priority to develop new chemical entities, drugs, and combination treatments for this fatal neglected disease (10, 13, 24, 61). Unfortunately, the currently available drugs for treatment of VL featured several lacunas during their development, which may have been in part due to the difficulty of performing clinical trials in the resource-limited settings where VL is present. For miltefosine, for example, the pharmacokinetic studies during the clinical development were inadequate and have remained largely unpublished. Although significant deviations in drug accumulation were detected in comparisons between children and adults, no further research was done on dosage requirements and pharmacokinetic-pharmacodynamic relationships in children (20, 53). The first pediatric studies with miltefosine, however, already indicated differences in efficacy between children and adults. These pediatric trials employed dosages linearly extrapolated from the “milligram per kilogram” adult dose. In the phase I-phase II dose-finding study, 21 patients were treated with 1.5 mg/kg/day and 18 patients with 2.5 mg/kg/day of miltefosine for a total of 28 days. The per-protocol cure rates in both treatment groups were lower than was previously observed in adult patients receiving 2.5 mg/kg/day (90% and 88%, respectively, versus 97%) (7, 26, 53, 54). This difference in efficacy was confirmed in a large phase IV trial, where twice as many children as adults failed cure while receiving the same 2.5-mg/kg dose (8). The milligram-per-kilogram dosing of miltefosine can be regarded as biologically inappropriate for scaling over a wider range of body weights, as it apparently does not result in similar levels of efficacy and systemic exposure to miltefosine (21).
It is scientifically widely accepted that the relationship between size and metabolic functions (such as drug clearance) in organisms can appropriately be scaled by an allometric power model (45, 58, 59). Such allometric models have been widely used to investigate and explain the effect of size on the pharmacokinetics of a variety of compounds, including analgesics and antimicrobial agents (2–4, 9, 23, 40), and the results imply that the metabolism of these drugs is not linearly related to changes in size. Notwithstanding the fact that the value of the allometric power exponent remains a point of discussion (36), in this study, fixed values of 0.75 for clearance and 1 for volume of distribution were chosen based on the biological principles that support these values (2, 45, 58). Moreover, estimating the allometric power exponent for clearance of miltefosine improved only marginally the performance of the model. In this study, we showed that allometric scaling of clearance and central volume of distribution for miltefosine also resulted in adequate fit of a population pharmacokinetic model to data from pediatric and adult patients with very diverse body weights.
During model development, we also considered the application of allometric scaling of the peripheral volume of distribution and intercompartmental clearance; however, we have chosen not to incorporate this due to the peculiar distribution pattern of miltefosine. Miltefosine is amphipathic and structurally similar to membrane lipids. Incorporation of miltefosine in cell membranes has been demonstrated in vitro (39, 42, 55) and would explain the extremely slow uptake and release from the (small) peripheral compartment. Therefore, we expect that a relationship between these peripheral distribution parameters and measures of body size is unlikely and not well supported. When applied in our pharmacokinetic model, allometric scaling resulted in a small and probably not very relevant decrease in goodness of fit. More importantly, the results of the simulation study were not altered (data not shown). Ultimately, we have chosen not to incorporate allometric scaling for these distribution parameters.
Not only total body weights but also the relative contributions of fat to WT were very different between the Indian children, Indian adults, and European adults included in our analysis. Allometric scaling by FFM reduced the interindividual differences in clearance more than allometric scaling by WT (Table 2). Fat contributes very little to the metabolic capacity of the body; thus, FFM might be the best descriptor for size in allometric models, certainly when there is a high variability in leanness between patients (2). On the other hand, miltefosine is a relatively lipophilic compound and, at least in rats, there is distribution of miltefosine in fat tissue to a small degree (37). Nevertheless, allometric scaling by FFM also reduced between-subject variability of the central volume of distribution more than any other scaling method (Table 2), including when, e.g., clearance was scaled by FFM and volume of distribution by WT (data not shown). Another alternative approach to assess the influence of the relative contribution of fat to body size on the pharmacokinetic parameters would be the estimation of normal fat mass (NFM) per individual parameter as a body size descriptor, using a parameter-specific fat factor (FfatP) which accounts for different contributions of fat mass, as described previously by Anderson and Holford (5):
| (7) |
In the present study, Ffat was estimated to be 0 for each parameter (data not shown); thus, FFM alone was the most appropriate body size descriptor. However, in other cases the use of NFM would allow for a continuous parameter to distinguish between body size models based on FFM and WT.
The currently recommended milligram-per-kilogram dose resulted in a substantially lower miltefosine exposure in children than in adults, while, on the other hand, the allometric dose led to similar levels of minimal miltefosine exposure in both patient groups. The probability of attaining minimal exposure in children similar to the level in adults with the allometric dose proposed here was evaluated, making use of Monte Carlo pharmacokinetic simulations. Monte Carlo pharmacokinetic simulations are a useful approach for the identification of pharmacokinetic-pharmacodynamic breakpoints of, e.g., antibiotics (1, 12, 15, 19, 34, 35, 63); however, the MIC values used for antibiotics are difficult to establish for Leishmania parasites because of their intracellular nature and the difficulty of drug sensitivity testing (14, 46). Intracellular concentrations, within the macrophages, to which the Leishmania parasites are exposed in these in vitro experiments have never been reported and deserve more attention in future experiments. In this study, only miltefosine regimens for monotherapy were evaluated and compared. Nevertheless, relative underexposure to miltefosine of children compared to adults can also be expected when miltefosine is similarly dosed on a milligram-per-kilogram basis for combination therapies.
The allometric dosage algorithm that we advise results in a higher absolute daily dose in children or adults with very low body weights than the currently advised 2.5-mg/kg dosage. An easy-to-use table to be used in clinical practice following from the proposed allometric miltefosine dosing algorithm is presented in Table 4. For the lowest weight category (9 to 12 kg), this would result in a daily absolute amount of miltefosine 1.7 to 1.5 times higher than the current 2.5-mg/kg dose. The main side effects of miltefosine are mild to moderate vomiting and diarrhea, which are related to a direct effect of miltefosine on the gastrointestinal tract upon administration of the miltefosine dose, instead of a systemic effect of the drug. However, to minimize the risk of gastrointestinal side effects, we suggest dividing the dose as much as possible throughout the day, while the current 2.5-mg/kg dosage is often administered as a single daily dose. Intake of (fatty) food concurrently with the administration of the miltefosine dose also minimizes the gastrointestinal side effects during miltefosine administration and is therefore recommended (18). Systemic toxic effects of miltefosine are most notably reversible hepatotoxicity and, to a lesser degree, nephrotoxicity (8). These drug effects are related to the systemic drug exposure and thus are not thought to differ between lower- and higher-weight categories using the allometric dosing algorithm, since levels of drug exposure in both categories are similar, as shown in this study. In contrast, hepato- and nephrotoxicity seem to be lower in pediatric patients: e.g., in a large phase IV trial, reversible elevation of creatinine was seen in ∼20% of the adults (including severe cases with Common Toxicity Criteria-3 increases), while this was observed in only ∼10% of the children in the trial (no severe cases) (8). This observation is in line with the hypothesis and outcome of our study in that the systemic exposure to miltefosine is lower in children than in adults given the same 2.5-mg/kg daily dosage. It is therefore important to investigate whether this presented optimal allometric dosage of miltefosine improves clinical outcome in children with VL at levels similar to those seen with adult patients. This trial would also reveal whether the higher absolute dose from our allometric dosing algorithm leads to toxicities in the lower body weight categories and whether these toxicities limit the applicability of this dosing algorithm.
In conclusion, the currently applied dose of 2.5 mg/kg/day results in a substantially lower exposure to miltefosine in children than in adults. We recommend employment of an allometric dosing table for miltefosine in the treatment of VL patients, the use of which results in similar levels of exposure to miltefosine for adults and children and might improve clinical outcome in children. An easy-to-use table is available for implementation of this dose in the clinic. More data are urgently needed on the pharmacokinetics of miltefosine in VL, specifically in children, to better define the role and dosing of miltefosine in (combination) therapy regimens and further improve the treatment of this fatal neglected disease.
ACKNOWLEDGMENTS
We thank Paladin Labs Inc. (Montreal, Canada) for making the pharmacokinetic data from the Adult Indian and Pediatric Indian studies available to us for the specific purpose of this study.
We report no conflicts of interest.
We declare that we did not receive any specific funding for this study. Thomas Dorlo was supported by a personal scholarship from the Graduate School of Medical Sciences of the Academic Medical Center, University of Amsterdam.
Footnotes
Published ahead of print 14 May 2012
REFERENCES
- 1. Ambrose PG, et al. 2009. Use of a clinically derived exposure-response relationship to evaluate potential tigecycline-Enterobacteriaceae susceptibility breakpoints. Diagn. Microbiol. Infect. Dis. 63:38–42 [DOI] [PubMed] [Google Scholar]
- 2. Anderson BJ, Holford NHG. 2008. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu. Rev. Pharmacol. Toxicol. 48:303–332 [DOI] [PubMed] [Google Scholar]
- 3. Anderson BJ, McKee AD, Holford NH. 1997. Size, myths and the clinical pharmacokinetics of analgesia in paediatric patients. Clin. Pharmacokinet. 33:313–327 [DOI] [PubMed] [Google Scholar]
- 4. Anderson BJ, Allegaert K, Van den Anker JN, Cossey V, Holford NHG. 2007. Vancomycin pharmacokinetics in preterm neonates and the prediction of adult clearance. Br. J. Clin. Pharmacol. 63:75–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Anderson BJ, Holford NHG. 2009. Mechanistic basis of using body size and maturation to predict clearance in humans. Drug Metab. Pharmacokinet. 24:25–36 [DOI] [PubMed] [Google Scholar]
- 6. Beal SL, Boeckmann AJ, Sheiner LB. 2006. NONMEM users guides. Icon Development Solutions, Ellicott City, MD [Google Scholar]
- 7. Bhattacharya SK, et al. 2004. Efficacy and tolerability of miltefosine for childhood visceral leishmaniasis in India. Clin. Infect. Dis. 38:217–221 [DOI] [PubMed] [Google Scholar]
- 8. Bhattacharya SK, et al. 2007. Phase 4 trial of miltefosine for the treatment of Indian visceral leishmaniasis. J. Infect. Dis. 196:591–598 [DOI] [PubMed] [Google Scholar]
- 9. Bulitta JB, et al. 2011. Comparable population pharmacokinetics and pharmacodynamic breakpoints of cefpirome in cystic fibrosis patients and healthy volunteers. Antimicrob. Agents Chemother. 55:2927–2936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chatelain E, Ioset J-R. 2011. Drug discovery and development for neglected diseases: the DNDi model. Drug Des. Devel. Ther. 5:175–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chrusciak-Talhari A, et al. 2011. Randomized controlled clinical trial to access efficacy and safety of miltefosine in the treatment of cutaneous leishmaniasis caused by Leishmania (Viannia) guyanensis in Manaus, Brazil. Am. J. Trop. Med. Hyg. 84:255–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Courter JD, Kuti JL, Girotto JE, Nicolau DP. 2009. Optimizing bactericidal exposure for beta-lactams using prolonged and continuous infusions in the pediatric population. Pediatr. Blood Cancer 53:379–385 [DOI] [PubMed] [Google Scholar]
- 13. Croft SL, Olliaro P. 2011. Leishmaniasis chemotherapy—challenges and opportunities. Clin. Microbiol. Infect. 17:1478–1483 [DOI] [PubMed] [Google Scholar]
- 14. da Luz RI, Vermeersch M, Dujardin JC, Cos P, Maes L. 2009. In vitro sensitivity testing of Leishmania clinical field isolates: preconditioning of promastigotes enhances infectivity for macrophage host cells. Antimicrob. Agents Chemother. 53:5197–5203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Deshpande D, et al. 2010. Moxifloxacin pharmacokinetics/pharmacodynamics and optimal dose and susceptibility breakpoint identification for treatment of disseminated Mycobacterium avium infection. Antimicrob. Agents Chemother. 54:2534–2539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dorlo TPC, et al. 2008. Development and validation of a quantitative assay for the measurement of miltefosine in human plasma by liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 865:55–62 [DOI] [PubMed] [Google Scholar]
- 17. Dorlo TPC, et al. 2008. Pharmacokinetics of miltefosine in Old World cutaneous leishmaniasis patients. Antimicrob. Agents Chemother. 52:2855–2860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dorlo TPC, et al. 2011. Dynamics of parasite clearance in cutaneous leishmaniasis patients treated with miltefosine. PLoS Negl. Trop. Dis. 5:e1436 doi:10.1371/journal.pntd.0001436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Drusano GL, et al. 2011. Meropenem penetration into epithelial lining fluid in mice and humans and delineation of exposure targets. Antimicrob. Agents Chemother. 55:3406–3412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. German Drug Registration Authorities 2008. Impavido 10/50 mg Kapseln—Fachinformation. German Federal Institute for Drugs and Medical Devices, Bonn, Germany [Google Scholar]
- 21. Holford N. 2010. Dosing in children. Clin. Pharmacol. Ther. 87:367–370 [DOI] [PubMed] [Google Scholar]
- 22. Holford NH. 1996. A size standard for pharmacokinetics. Clin. Pharmacokinet. 30:329–332 [DOI] [PubMed] [Google Scholar]
- 23. Holford NHG, Ma SC, Anderson BJ. 2012. Prediction of morphine dose in humans. Paediatr. Anaesth. 22:209–222 [DOI] [PubMed] [Google Scholar]
- 24. Ioset J-R, Chang S. 2011. Drugs for Neglected Diseases initiative model of drug development for neglected diseases: current status and future challenges. Future Med. Chem. 3:1361–1371 [DOI] [PubMed] [Google Scholar]
- 25. Janmahasatian S, et al. 2005. Quantification of lean bodyweight. Clin. Pharmacokinet. 44:1051–1065 [DOI] [PubMed] [Google Scholar]
- 26. Jha TK, et al. 1999. Miltefosine, an oral agent, for the treatment of Indian visceral leishmaniasis. N. Engl. J. Med. 341:1795–1800 [DOI] [PubMed] [Google Scholar]
- 27. Jonsson EN, Karlsson MO. 1999. Xpose—an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput. Methods Programs Biomed. 58:51–64 [DOI] [PubMed] [Google Scholar]
- 28. Karlsson MO, Holford NHG. 2008. A tutorial on visual predictive checks, p 17, abstr 1434. Abstr. Annu. Meet. Popul. Approach Group Eur [Google Scholar]
- 29. Keizer RJ, van Benten M, Beijnen JH, Schellens JHM, Huitema ADR. 2011. Piraña and PCluster: a modeling environment and cluster infrastructure for NONMEM. Comput. Methods Programs Biomed. 101:72–79 [DOI] [PubMed] [Google Scholar]
- 30. Keynan Y, et al. 2008. Use of oral miltefosine for cutaneous leishmaniasis in Canadian soldiers returning from Afghanistan. Can. J. Infect. Dis. Med. Microbiol. 19:394–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Knebel NG, et al. 1999. Quantification of perifosine, an alkylphosphocholine anti-tumour agent, in plasma by pneumatically assisted electrospray tandem mass spectrometry coupled with high-performance liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 721:257–269 [DOI] [PubMed] [Google Scholar]
- 32. Lindbom L, Pihlgren P, Jonsson EN, Jonsson N. 2005. PsN-Toolkit—a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput. Methods Programs Biomed. 79:241–257 [DOI] [PubMed] [Google Scholar]
- 33. Lindbom L, Ribbing J, Jonsson EN. 2004. Perl-speaks-NONMEM (PsN)—a Perl module for NONMEM related programming. Comput. Methods Programs Biomed. 75:85–94 [DOI] [PubMed] [Google Scholar]
- 34. Lodise TP, et al. 2007. Pharmacodynamics of ceftazidime and meropenem in cerebrospinal fluid: results of population pharmacokinetic modelling and Monte Carlo simulation. J. Antimicrob. Chemother. 60:1038–1044 [DOI] [PubMed] [Google Scholar]
- 35. Lodise TP, et al. 2011. Penetration of vancomycin into epithelial lining fluid in healthy volunteers. Antimicrob. Agents Chemother. 55:5507–5511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mahmood I. 2010. Theoretical versus empirical allometry: facts behind theories and application to pharmacokinetics. J. Pharm. Sci. 99:2927–2933 [DOI] [PubMed] [Google Scholar]
- 37. Marschner N, Kötting J, Eibl H, Unger C. 1992. Distribution of hexadecylphosphocholine and octadecyl-methyl-glycero-3-phosphocholine in rat tissues during steady-state treatment. Cancer Chemother. Pharmacol. 31:18–22 [DOI] [PubMed] [Google Scholar]
- 38. Mathers CD, Ezzati M, Lopez AD. 2007. Measuring the burden of neglected tropical diseases: the global burden of disease framework. PLoS Negl. Trop. Dis. 1:e114 doi:10.1371/journal.pntd.0000114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ménez C, Buyse M, Farinotti R, Barratt G. 2007. Inward translocation of the phospholipid analogue miltefosine across Caco-2 cell membranes exhibits characteristics of a carrier-mediated process. Lipids 42:229–240 [DOI] [PubMed] [Google Scholar]
- 40. Nielsen EI, Sandström M, Honoré PH, Ewald U, Friberg LE. 2009. Developmental pharmacokinetics of gentamicin in preterm and term neonates: population modelling of a prospective study. Clin. Pharmacokinet. 48:253–263 [DOI] [PubMed] [Google Scholar]
- 41. Rahman M, Ahmed, et al. 2011. Phase IV trial of miltefosine in adults and children for treatment of visceral leishmaniasis (kala-azar) in Bangladesh. Am. J. Trop. Med. Hyg. 85:66–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rakotomanga M, Loiseau PM, Saint-Pierre-Chazalet M. 2004. Hexadecylphosphocholine interaction with lipid monolayers. Biochim. Biophys. Acta 1661:212–218 [DOI] [PubMed] [Google Scholar]
- 43. R Development Core Team 2011. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria [Google Scholar]
- 44. Rubiano LC, et al. 2012. Noninferiority of miltefosine versus meglumine antimoniate for cutaneous leishmaniasis in children. J. Infect. Dis. 205:684–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Savage VM, Deeds EJ, Fontana W. 2008. Sizing up allometric scaling theory. PLoS Comput. Biol. 4:e1000171 doi:10.1371/journal.pcbi.1000171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Seifert K, Escobar P, Croft SL. 2010. In vitro activity of anti-leishmanial drugs against Leishmania donovani is host cell dependent. J. Antimicrob. Chemother. 65:508–511 [DOI] [PubMed] [Google Scholar]
- 47. Singh SP, Reddy DCS, Rai M, Sundar S. 2006. Serious underreporting of visceral leishmaniasis through passive case reporting in Bihar, India. Trop. Med. Int. Health 11:899–905 [DOI] [PubMed] [Google Scholar]
- 48. Soto J, et al. 2004. Miltefosine for new world cutaneous leishmaniasis. Clin. Infect. Dis. 38:1266–1272 [DOI] [PubMed] [Google Scholar]
- 49. Soto J, et al. 2007. Treatment of Bolivian mucosal leishmaniasis with miltefosine. Clin. Infect. Dis. 44:350–356 [DOI] [PubMed] [Google Scholar]
- 50. Soto J, et al. 2008. Efficacy of miltefosine for Bolivian cutaneous leishmaniasis. Am. J. Trop. Med. Hyg. 78:210–211 [PubMed] [Google Scholar]
- 51. Soto J, et al. 2009. Efficacy of extended (six weeks) treatment with miltefosine for mucosal leishmaniasis in Bolivia. Am. J. Trop. Med. Hyg. 81:387–389 [PubMed] [Google Scholar]
- 52. Sundar S, et al. 1998. Trial of oral miltefosine for visceral leishmaniasis. Lancet 352:1821–1823 [DOI] [PubMed] [Google Scholar]
- 53. Sundar S, et al. 2003. Oral miltefosine treatment in children with mild to moderate Indian visceral leishmaniasis. Pediatr. Infect. Dis. J. 22:434–438 [DOI] [PubMed] [Google Scholar]
- 54. Sundar S, et al. 2002. Oral miltefosine for Indian visceral leishmaniasis. N. Engl. J. Med. 347:1739–1746 [DOI] [PubMed] [Google Scholar]
- 55. van Blitterswijk WJ, Hilkmann H, Storme GA. 1987. Accumulation of an alkyl lysophospholipid in tumor cell membranes affects membrane fluidity and tumor cell invasion. Lipids 22:820–823 [DOI] [PubMed] [Google Scholar]
- 56. van Thiel PPAM, et al. 2010. Miltefosine treatment of Leishmania major infection: an observational study involving Dutch military personnel returning from northern Afghanistan. Clin. Infect. Dis. 50:80–83 [DOI] [PubMed] [Google Scholar]
- 57. Vélez I, et al. 2010. Efficacy of miltefosine for the treatment of American cutaneous leishmaniasis. Am. J. Trop. Med. Hyg. 83:351–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. West GB, Brown JH, Enquist BJ. 1997. A general model for the origin of allometric scaling laws in biology. Science 276:122–126 [DOI] [PubMed] [Google Scholar]
- 59. West GB, Woodruff WH, Brown JH. 2002. Allometric scaling of metabolic rate from molecules and mitochondria to cells and mammals. Proc. Natl. Acad. Sci. U. S. A. 99(Suppl. 1):2473–2478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. World Health Organization 2004. The global burden of disease: update. World Health Organization, Geneva, Switzerland [Google Scholar]
- 61. Yamey G, Hotez P. 2007. Neglected tropical diseases. BMJ 335:269–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yamey G, Torreele E. 2002. The world's most neglected diseases. BMJ 325:176–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zobell JT, et al. 2011. Population pharmacokinetic and pharmacodynamic modeling of high-dose intermittent ticarcillin-clavulanate administration in pediatric cystic fibrosis patients. Clin. Ther. 33:1844–1850 [DOI] [PubMed] [Google Scholar]