Abstract
ST-246, a novel compound that inhibits egress of orthopoxvirus from mammalian cells, is being tested as a treatment for pathogenic orthopoxvirus infections in humans. This phase I, double-blind, randomized, crossover, exploratory study was conducted to compare the pharmacokinetics (PK) of a single daily 400-mg oral dose of ST-246 polymorph form I versus polymorph form V administered to fed, healthy human volunteers. Both forms appeared to be well tolerated, with no serious adverse events. The order of administration of the two forms had no effect on the results of the PK analyses. Form I and form V both exhibited comparable plasma concentration versus time profiles, but complete bioequivalence between the two forms was not found. Maximum drug concentration (Cmax) met the bioequivalence criteria, as the 90% confidence interval (CI) was 80.6 to 96.9%. However, the area under the concentration-time curve from time zero to time t (AUC0-t) and AUC0-∞ did not meet the bioequivalence criteria (CIs of 67.8 to 91.0% and 73.9 to 104.7%, respectively). The extent of absorption of form I, as defined by AUC0-∞, was 11.7% lower than that of form V. Since ST-246 form I is more thermostable than form V, form I was selected for further development and use in all future studies.
INTRODUCTION
In the 1970s, a global eradication campaign using principally vaccination against variola virus, the etiologic agent of the highly communicable disease smallpox, successfully removed the virus from the environment. However, by the late 1990s, remaining virus stockpiles engendered concern over the potential use of variola virus as a biological weapon (3, 4, 9, 12). In an effort to prepare for this possibility, testing of existing licensed medications for antipoxviral activity and characterization of animal models of infection that could be used to predict efficacy in humans (8) were initiated. In 1999, an Institute of Medicine panel recommended development of new antiviral drugs against smallpox, especially for medications that can be taken orally (5). Vaccination, while effective at immunizing vulnerable populations, has a lag period for antibody formation, carries the risk of certain severe side effects, and may not be immediately or universally available for distribution in the event of a large-scale biological attack. Since there are currently no U.S. Food and Drug Administration-approved therapies that can alter the outcome of disease or potentially prevent disease in a population that has been exposed (5), there is clearly a need for a safe, small-molecule, oral medication that is highly effective against variola virus and possibly other zoonotic poxviruses, such as monkeypox and cowpox viruses (2).
ST-246 (tecovirimat), the low-molecular-weight synthetic compound benzamide, N-[(3aR,4R,4aR,5aS,6S,6aS)-3,3a,4,4a,5,5a,6,6a-octahydro-1,3-dioxo-4,6-ethenocycloprop[f]isoindol-2(1H)-yl]-4-(trifluoromethyl), was discovered through a deliberate effort to develop orally available antiviral drugs for use in biodefense (1, 13). High-throughput screening of more than 350,000 compounds identified a lead compound with low toxicity and good inhibitory activity against vaccinia virus. Approximately 200 chemical analogs were synthesized and tested against a panel of agents, including variola virus, and the most active analog was chosen for further evaluation. In a number of animal studies, oral administration of ST-246 not only protected nonhuman primates from variola and monkeypox viruses but also protected mice from lethal infection with vaccinia virus, cowpox virus, and ectromelia virus (10, 13) and squirrels from severe monkeypox disease (11), implying that ST-246 could also be used to control vaccination complications and to prevent or treat zoonotic poxvirus disease. The compound ST-246 is the first in its class and is chemically unrelated to any substance currently in use for human or veterinary therapy.
ST-246 is poorly soluble in the physiologically relevant pH range of 1.2 to 7.4. ST-246 can exist as different crystalline polymorphs/hydrates, with the major forms being form I (monohydrate), form III (monohydrate), and form V (hemihydrate). ST-246 form V (C19H15F3N2O3 · 0.5H2O) was used in investigational new drug (IND)- and new drug application (NDA)-enabling animal toxicological studies, early animal efficacy studies, and two previous clinical (phase I, single- and multiple-dose pharmacokinetic [PK] and safety studies) human studies. However, form I, a monohydrate (C19H15F3N2O3 · H2O), was later found to be the most thermodynamically stable form of ST-246 and therefore was proposed for further development, testing, and use in human clinical trials. An initial bridging comparative PK study of ST-246 form V versus form I was conducted in cynomolgus monkeys (Macaca fascicularis), and no consistent difference in systemic exposure was noted between the two forms (6).
This crossover clinical study was developed and conducted in order to compare the PK, safety, and tolerability of a single 400-mg daily oral dose of ST-246 form V (reference drug) versus form I (test drug) and to better understand the PK profile of the thermodynamically stable form (form I) for possible phase II development. The PK differences between the previously used form (form V) and form I will be used to model the dose for future animal efficacy and human safety trials.
MATERIALS AND METHODS
ST-246 products.
ST-246 form I was administered as an oral dose of 400 mg (two 200-mg capsules) (lot 8F076). ST-246 form I was produced by Albemarle, South Haven, MI. ST-246 form V was administered as an oral dose of 400 mg (two 200-mg capsules) (lot 5J054). ST-246 form V was produced by Pharmacore, Highpoint, NC. All inactive ingredients and excipients are generally recognized as safe (GRAS) and are U.S. Pharmacopeia (USP)/National Formulary (NF) grade.
Study design and population.
This study was a phase I, randomized, double-blind, crossover, exploratory study to compare the PK (area under the concentration-time curve [AUC] variables and maximum concentration of drug in serum [Cmax]) of a single 400-mg (two 200-mg capsules) oral dose of ST-246 form I (test drug) versus ST-246 form V (reference drug) and to evaluate the safety and tolerability of both forms in fed, healthy volunteers.
The study protocol and informed consent forms were reviewed and approved by an Institutional Review Board (IRB). At the screening visit, subjects or their legally acceptable representatives provided written consent to participate in the study after having been informed about the nature and purpose of the study, participation and termination conditions, and risks and benefits of treatment. Twelve of 63 screened individuals (males and nonpregnant females of 18 to 50 years of age [inclusive]) were accepted into the study (see “Statistical analysis”) and were randomized to one of the following sequences: form I and then form V or form V and then form I.
Subjects were screened for eligibility within a 28-day period prior to enrollment. Those chosen were in good physical health, had routine laboratory test results and electrocardiograms (ECGs) within the normal range, and remained nonmedicated from screening through 72 h after the last study dose. Laboratory assessments (hematology, chemistry, and urinalysis) were performed at screening and on days 1, 2, 10, 12, and 14 (or early termination visit). Pregnancy test screening was done on days 1 and 10 for women of childbearing potential, and drug/alcohol abuse screening was also performed on days 1 and 10. An ECG (12-lead; Mortara ELI 150/250) was performed on day 1 and then 2, 4, 8, and 23 h after the first treatment dose. After the washout period from days 2 to 10, a second treatment dose was given on day 11, and ECGs were taken as for the first treatment.
Venous blood sample collection.
To determine the PK of ST-246, a baseline (0 h) venous blood sample was obtained on day 1, followed by serial blood draws after medication administration. All subjects received a single, 400-mg dose (two 200-mg capsules) of either form I or form V of ST-246, administered orally within 30 min after a standard light meal (400 to 450 cal; approximately 25% fat), which was shown in a previous clinical study to enhance bioavailability (6). Postdose (treatment 1) blood samples for PK analyses were taken at 0.5, 1, 2, 3, 4, 8, 12, 24, 36, 48, and 72 h. A postdose urine sample was obtained on day 2. A washout period occurred during study days 2 to 10, so treatment 2 occurred on day 11. At this time, those subjects originally receiving form I of ST-246 received a single, 400-mg dose (two 200-mg capsules) of form V, and vice versa. Blood sampling for PK analyses following treatment 2 was performed as for that following treatment 1. Plasma samples were collected and stored at −70°C until analyzed for Cmax, time to maximum drug concentration (Tmax), terminal half-life (t1/2), AUC, and renal clearance (CLR).
Bioanalysis.
ST-246 in human plasma specimens was quantified by a validated liquid chromatography and tandem mass spectrometry method using an analog of ST-246 as an internal standard. The assay range for ST-246 was 50 to 4,000 ng/ml. All analytical runs met acceptance criteria consistent with regulatory and industry recommendations. Acceptance criteria for calibration standards were back-calculated concentrations of at least 75% of all standards within ±15.0% relative error of the nominal values (within ±20.0% relative error of the nominal value for the lower limit of quantification standard).
Human plasma (10 μl) was extracted with an ammonium hydroxide-methanol solution, and the supernatant was injected onto a Phenomenex Luna C18 column (30 by 2 mm; 5-um particle size). The mobile phase consisted of water containing 0.05% acetic acid and 0.05% NH4OH. ST-246 was eluted using a 20% to 90% gradient of methanol containing 0.05% acetic acid and 0.05% NH4OH from 0.2 to 0.5 min, with a flow rate of 300 μl/min. Fractions were analyzed on a Sciex API 4000 triple-quadrupole mass spectrometer, using turbo spray ionization in the negative ion mode. Analytes were detected by multiple reactions monitoring the m/z 375.1-to-282.9 transition for ST-246 and the m/z 340.9-to-248.9 transition for the internal standard.
Pharmacokinetic analysis.
The pharmacokinetic parameters Cmax, Tmax, AUC from time zero to time t (AUC0-t), AUC0-∞, and t1/2 were estimated for ST-246 by applying a noncompartmental analysis using WinNonlin, professional edition, version 5.0.1 (Pharsight Corporation).
Statistical analysis.
Because the study design incorporated a comparison of plasma exposures of polymorphs, a nonreplicated statistical design that provided average or population data for PK comparison was chosen. Time points for PK calculations were selected based on the results of phase I, single-dose and multidose studies. Form V was considered the reference group, and form I was considered the test group. The selected time points covered sampling in and around Cmax, and the last time point (72 h) accounted for sample collection beyond 3 half-lives of ST-246.
Based on a previous clinical study of healthy volunteers where the mean AUC0-t was ∼13,000 ng-h/ml (standard deviation, ∼3,500 ng-h/ml) following a single 400-mg dose of ST-246 form V (7), it was determined statistically (using nQuery, version 4.0) that 12 subjects would provide a statistical power of 80% to detect a 25% difference in reference mean between the test and reference doses at the 0.05 significance level (2-sided) for this crossover design. Data summaries were presented by sequence group or form, as appropriate. Continuous variables (e.g., age) were summarized by the number of subjects, mean, standard deviation, median, minimum, maximum, and number of missing values. Categorical variables (e.g., race) were summarized by frequencies and percentages of subjects in each category. For frequency tables by time point, subjects with missing data were not included in the denominator for percent calculations. Two analytical approaches to the continuous PK variables were used. First, a parametric (normal theory) general linear model was applied to AUC0-t, AUC0-∞, t1/2, Cmax, and Tmax. Analysis of variance for a 2-way crossover design was employed to examine the differences between the 2 forms. Second, AUC0-t, AUC0-∞, and Cmax were analyzed on a log scale to assess bioequivalence (BE) between form I (the test compound) and form V (the reference compound). The scheduled collection date and time were used to summarize data collected at multiple time points (e.g., vital signs). If repeated laboratory tests were performed, the values from the earlier tests were summarized. All analyses and summaries were produced using SAS, version 9.1.3.
Safety assessments.
The general safety of ST-246 was evaluated by use of reports of adverse events (AEs), vital signs, physical examinations (PEs), hematology, blood chemistry for electrolytes and liver function, urinalysis, and ECGs. Subjects were expected to participate in follow-up telephone evaluations 4 weeks after receipt of the final dose of ST-246 (form I or V).
RESULTS
Demographics.
A total of 12 subjects were enrolled and randomized to one of the following sequences: form I and then form V or form V and then form I. These subjects were all included in the safety analysis. The mean age of subjects in the study was approximately 34 years. Ten subjects were male, and two were female. Ten subjects were white, and two were black. Nine of 12 subjects reported their ethnicity as Hispanic or Latino; the remaining 3 subjects (2 white and 1 black) were not Hispanic or Latino. Subjects in all treatment groups were comparable for all other characteristics.
Pharmacokinetics.
This study compared the PK profiles of ST-246 form I and form V capsules following a single oral dose administration. This objective was achieved through the collection and analysis of plasma samples from 12 of 12 subjects for PK assessment of form I and from 11 of 12 subjects for assessment of form V. Pharmacokinetic parameter results are summarized in Table 1. Outlier values were excluded from the t1/2 analyses for three subject PK profiles. Both the 48- and 72-h values for two subjects, one for form I and one for form V, and the 72-h value for one form I subject were excluded because concentrations at these time points were slightly above those at previous time points (but within the normal observed values for the population) and the elimination rate constant could not be estimated. As illustrated in Table 1, the mean elimination half-lives for the two treatments were comparable (27.4 and 29.2 h for form I and form V, respectively). The extrapolated percent AUC values (comparing AUC0-t to AUC0-∞) were 17% and 15% for form I and form V, respectively. Thus, the study design was adequate to measure more than 80% of the AUC by using the 72-h sampling interval. There were significant treatment effects for AUC0-t (P = 0.0048) and Cmax (P = 0.0422) but not for AUC0-∞, Tmax, or t1/2. There did not appear to be complete BE between the 2 forms of ST-246. Neither time period nor the form I-form V treatment sequence showed statistically significant effects for any of the variables tested, with the exception of a significant period effect for t1/2. As shown in Table 2, Cmax met the BE criteria because the 90% confidence interval (CI) was 80.6 to 96.9%, which lies within the FDA-recommended BE criterion limits of 80 to 125%. However, AUC0-t and AUC0-∞ did not meet the BE criteria, as their 90% CIs were 67.8 to 91.0% and 73.9 to 104.7%, respectively. The extent of absorption (as defined by AUC0-∞) of form I was 11.7% lower than that of form V.
Table 1.
Summary of ST-246 plasma PK parameter estimates (PK population)a
| Form | Statistic | AUC0-t (h-ng/ml) | AUC0-∞ (h-ng/ml) | AUC(extrap) (%) | t1/2 (h) | Cmax (ng/ml) | Tmax (h) |
|---|---|---|---|---|---|---|---|
| Form I | n | 12 | 11 | 11 | 11 | 12 | 12 |
| Mean | 15,625 | 19,922 | 17.4 | 27.4 | 1,068.9 | 3.8 | |
| SD | 5,449 | 6,544 | 7.8 | 13.1 | 294.3 | 1.5 | |
| CV% | 34.9 | 32.8 | 44.9 | 47.8 | 27.5 | 39.6 | |
| Geometric mean | 14,816 | 19,050 | 15.7 | 24.7 | 1,026.9 | 3.5 | |
| Median | 14,151 | 17,202 | 13.2 | 25.1 | 1,170 | 3.5 | |
| Minimum | 8,054 | 13,959 | 5.7 | 10.9 | 525 | 2 | |
| Maximum | 26,597 | 31,059 | 30.4 | 56.5 | 1,590 | 8 | |
| No. of missing values | 0 | 1 | 1 | 1 | 0 | 0 | |
| Form V | n | 11 | 8 | 8 | 8 | 11 | 11 |
| Mean | 20,065 | 21,983 | 15.3 | 29.2 | 1,230.2 | 3.8 | |
| SD | 6,745 | 9,331 | 10.8 | 22 | 348.6 | 1.6 | |
| CV% | 33.6 | 42.4 | 70.8 | 75.4 | 28.3 | 41.9 | |
| Geometric mean | 19,021 | 20,409 | 12.4 | 23.1 | 1,185 | 3.6 | |
| Median | 19,399 | 19,465 | 12.5 | 16.6 | 1,180 | 4 | |
| Minimum | 10,399 | 11,947 | 4.5 | 11.5 | 732 | 2 | |
| Maximum | 30,974 | 39,058 | 37.5 | 69.5 | 1,940 | 8 | |
| No. of missing values | 0 | 3 | 3 | 3 | 0 | 0 |
For a given variable and drug form, the geometric mean was not calculated if any of the values were 0. AUC0-∞, area under the drug concentration-time curve from time zero to infinity; AUC0-t, area under the drug concentration-time curve from time zero to time t, where t is the last time point with a drug concentration at the lowest obtainable quantification or above; AUC(extrap), extrapolated area under the curve; t1/2, terminal half-life; Cmax, maximum plasma concentration; CV%, coefficient of variance; SD, standard deviation; Tmax, time to maximum plasma concentration
Table 2.
Bioequivalence analysis of ST-246 plasma PK parameter estimates (PK population)
| Variable | Geometric meana |
Ratio (%)b | 90% CIc | Power | ANOVA | |
|---|---|---|---|---|---|---|
| Test (n = 12) | Reference (n = 11) | |||||
| ln(Cmax) (ng/ml) | 1,026.9 | 1,161.8 | 88.4 | 80.6–96.9 | 0.99 | 11.82 |
| ln(AUC0–t) (ng–h/ml) | 14,816.3 | 18,865.4 | 78.5 | 67.8–91.0 | 0.63 | 19.10 |
| ln(AUC0–∞) (ng–h/ml) | 19,305.4 | 21,945.3 | 88 | 73.9–104.7 | 0.40 | 16.59 |
Geometric mean for test formulation (form I) or reference formulation (form V), based on least-squares mean of log-transformed parameter values.
Ratio of test geometric mean to reference geometric mean.
Two products are considered bioequivalent if 90% CIs for the relative mean Cmax, AUClast, and AUC∞ of the test and reference are within 80% to 125%.
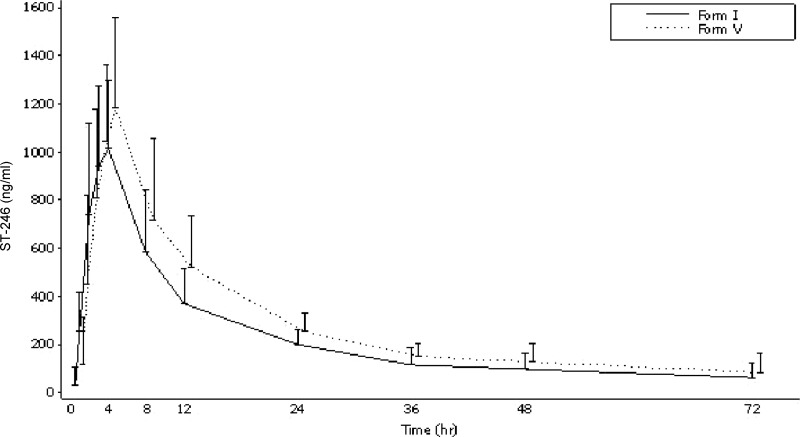
Both forms I and V exhibited comparable plasma concentration-time profiles, as shown in Fig. 1. ST-246 concentrations were generally measurable during 48 to 72 h of the 72-hour blood sampling period.
Fig 1.
Mean (standard deviation) ST-246 plasma concentrations over time (PK population). The lower SD bars are not included, as in some cases the mean minus SD resulted in a negative value.
Safety.
No clinically significant safety concerns were found during the study. All subjects completed treatment period 1. One subject in the form I-form V group withdrew consent due to a death in his family during the washout period. This subject completed all treatment period 1 assessments, missed treatment period 2, and returned 3 to 4 weeks later to complete a discontinuation visit. Three subjects, two in the form I-form V group (33.3%) and one in the form V-form I group (16.7%), reported a total of 4 treatment-emergent adverse events (TEAEs), none of which were deemed related to the study drug. One subject reported neck pain during both inpatient stays, with the second occurrence ongoing at the time of study completion; the subject could not be reached for the telephone follow-up. Other AEs included headache and underarm tenderness. No safety issues noted during the study met IRB reporting criteria. No deaths, severe AEs (SAEs), or other significant AEs were reported.
There also were no clinically significant changes in weight or vital signs, PEs, serum chemistry, hematology, or urinalysis variables. A review of the QTcF (QT correction by Fridericia's formula) intervals and the pharmacokinetic-pharmacodynamic relationships for form I and form V revealed that there were no significant effects of ST-246 on cardiac repolarization.
DISCUSSION
Based on the literature, it is well known that a drug substance has several polymorphic forms and that control of the polymorphic form is critical for quality of a drug product. Different polymorphic forms may differ in physicochemical properties and may affect oral absorption for drugs that are delivered as solid dosing forms. Based on FDA classification, ST-246 is a Biopharmaceutics Classification System (BCS) class II drug, so solubility could be the major factor limiting oral absorption. ST-246 polymorphic forms were found to have different hydration and crystallinity properties but to exhibit similar solubilities in the physiologically relevant pH range and hence are expected to provide comparable plasma profiles. For commercial product development, it is important to select a polymorphic form that is stable and can be made consistently.
The primary objective of this phase I, randomized, double-blind, crossover study of fed, healthy subjects was to compare the pharmacokinetics of a single oral dose (400 mg) of ST-246 polymorph form I (test drug) versus ST-246 polymorph form V (reference drug) through comparison of plasma exposure (AUC) and peak plasma concentration (Cmax) parameters, with the resulting data used to modify the dose for future clinical development if there were a significant difference between different forms. No clinically significant safety concerns were found with either form during this study. The order of administration of the two forms of ST-246 had no effect on the results of PK analyses. Both forms exhibited comparable plasma concentration versus time profiles. Complete bioequivalence between the two forms was not found, but both forms of drug provided adequate plasma exposure in humans at a 400-mg dose, which is much higher than that needed for efficacy in animals. The extent of absorption (as defined by AUC0-∞) of form I was 11.7% lower than that of form V.
ST-246 form I is safe and more stable than form V, and a 400-mg dose of form I provides plasma exposure well above that demonstrated for efficacy in a nonhuman primate model. Based on these data, the dose (400 mg) and form (form I) of ST-246 were confirmed as appropriate for future clinical investigations.
ACKNOWLEDGMENTS
We acknowledge our colleagues at the National Institutes of Health, National Institute of Allergy and Infectious Diseases, and the Office of Biodefense Research Affairs for their continued support of this program.
This project was funded in whole or in part with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under contract HHSN266200600014C.
Robert Jordan, Tove' C. Bolken, Kevin F. Jones, Shanthakumar R. Tyavanagimatt, and Dennis E. Hruby are shareholders of SIGA Technologies, Inc.
Footnotes
Published ahead of print 23 April 2012
REFERENCES
- 1. Bailey TR, et al. 2007. N-(3,3a,4,4a,5,5a,6,6a-octahydro-1,3-dioxo-4,6-ethenocycloprop[f]isoindol-2-(1H)-yl)carboxamides: identification of novel orthopoxvirus egress inhibitors. J. Med. Chem. 50:1442–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baker RO, Bray M, Huggins JW. 2003. Potential antiviral therapeutics for smallpox, monkeypox and other orthopoxvirus infections. Antiviral Res. 57:13–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Henderson DA. 1999. The looming threat of bioterrorism. Science 283:1279–1282 [DOI] [PubMed] [Google Scholar]
- 4. Henderson DA, et al. 1999. Smallpox as a biological weapon: medical and public health management. JAMA 281:2127–2137 [DOI] [PubMed] [Google Scholar]
- 5. Institute of Medicine of the National Academies 1999. Assessment of future scientific needs for live variola virus. National Academy Press, Washington, DC: [PubMed] [Google Scholar]
- 6. Jordan R, et al. 2008. Single-dose safety and pharmacokinetics of ST-246, a novel orthopoxvirus egress inhibitor. Antimicrob. Agents Chemother. 52:1721–1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jordan R, et al. 2010. Safety and pharmacokinetics of the antiorthopoxvirus compound ST-246 following repeat oral dosing in healthy adult subjects. Antimicrob. Agents Chemother. 54:2560–2566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. LeDuc JW, Damon I, Relman DA, Huggins J, Jahrling PB. 2002. Smallpox research activities: U.S. interagency collaboration, 2001. Emerg. Infect. Dis. 8:743–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. National Institute of Allergy and Infectious Diseases 2002. NIAID biodefense research agenda for CDC category A agents. National Institute of Allergy and Infectious Diseases, Bethesda, MD [Google Scholar]
- 10. Quenelle DC, et al. 2007. Efficacy of delayed treatment with ST-246 given orally against systemic orthopoxvirus infections in mice. Antimicrob. Agents Chemother. 51:689–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sbrana E, et al. 2007. Efficacy of the antipoxvirus compound ST-246 for treatment of severe orthopoxvirus infection. Am. J. Trop. Med. Hyg. 76:768–773 [PubMed] [Google Scholar]
- 12. Whitley RJ. 2003. Smallpox: a potential agent of bioterrorism. Antiviral Res. 57:7–12 [DOI] [PubMed] [Google Scholar]
- 13. Yang G, et al. 2005. An orally bioavailable antipoxvirus compound (ST-246) inhibits extracellular virus formation and protects mice from lethal orthopoxvirus challenge. J. Virol. 79:13139–13149 [DOI] [PMC free article] [PubMed] [Google Scholar]