Abstract
In bone marrow transplantation, the efficacy of ganciclovir in cytomegalovirus (CMV) disease treatment or prophylaxis remains partial. Because its hematological toxicity is dose limiting, optimization of the dosing schedule is required to increase its therapeutic index. The goal of our study was to describe the influence of the ganciclovir concentration and duration of exposure on cell survival and antiviral efficacy. The study was carried out in vitro on cultures of lymphoblastoid cells infected or not with the CMV AD169 reference strain and exposed to ganciclovir at different concentrations for 1, 2, 7, or 14 days. The data were analyzed by a mathematical model that allowed a quantitative characterization of ganciclovir pharmacodynamics and its variability. Simulations of the model were undertaken to determine the optimal concentration profile for maximizing the ganciclovir therapeutic index. Ganciclovir had very little toxic and antiviral effect, even at 20 mg liter−1, when the duration of exposure was ≤7 days. A biologically significant effect was observed only with a 14-day exposure. Complete inhibition of viral replication was obtained at 20 mg liter−1. The utility function, assuming equal weights for antiviral effect and toxicity, showed that maximal utility was reached around 10 mg liter−1. The optimal ganciclovir concentration profile consisted of maintaining the concentration at 20 mg liter−1 at the intervals 0 to 2 days and 7.58 to 9.58 days and a null concentration at other times. This optimal profile could be obtained by intravenous (i.v.) ganciclovir at 10 mg/kg of body weight twice daily (b.i.d.) at days 1, 2, 8.5, and 9.5 in stem cell transplant patients with normal renal function.
INTRODUCTION
Cytomegalovirus (CMV) infection remains a major complication of bone marrow transplantation (7, 11, 13, 17, 27). The major clinical signs of CMV infection in immunocompromised patients are pneumonitis, hepatitis, and intestinal disease.
Ganciclovir (GCV) or its prodrug, valganciclovir, remains the first-line treatment of CMV infection (1, 16). Ganciclovir is used as a prophylactic, preemptive, or curative treatment (16). Prophylactic and preemptive treatments have demonstrated similar reductions of mortality and morbidity in bone marrow transplantation (3, 16, 26). In allogeneic bone marrow recipients receiving prophylactic ganciclovir, the incidence of CMV disease varies between 30 and 60%, while the incidence of death related to CMV is 25 to 30% (26). A major risk factor for virological failure is a peak viral load of >20,000 copies/ml at the onset of treatment (odds ratio [OR], 5.88). The main risk factor for a peak viral load of >20,000 copies/ml is the presence of grade II to IV acute graft-versus-host disease (OR, 16) (28).
Ganciclovir is known to have hematological toxicity, neurotoxicity, and possibly hepatotoxicity (21). Hematological toxicity has been characterized in vitro on normal human hematopoietic progenitor cells. Ganciclovir inhibition was concentration dependent on both granulocyte-macrophage progenitors and erythroid progenitors (25). The hematological toxicity of ganciclovir may be enhanced by CMV itself. CMV has a particular tropism for bone marrow cells, and it is toxic for these cells by direct and indirect mechanisms (13). Ganciclovir-induced neutropenia is associated with a greater risk of mortality after bone marrow transplantation (23) and of nonviral opportunistic infections when the treatment duration is longer than 4 weeks (7).
Hence, the efficacy of ganciclovir in CMV disease treatment or prophylaxis remains partial. Because the hematological toxicity is dose limiting, optimization of the dosing schedule is required in order to increase the therapeutic index of ganciclovir. No firm correlation has been established between ganciclovir exposure and antiviral efficacy or toxicity (21). The interplay between the ganciclovir dosing rate and treatment duration with respect to anti-CMV efficacy has been partially characterized in immunodeficient mice (6), but the hematological toxicity has not been assessed in this study.
The main goal of our study was to describe the influence of the ganciclovir concentration and duration of exposure on cell survival and antiviral efficacy. The study was carried out in vitro on cultures of lymphoblastoid cells. The data were analyzed by a mathematical model that allowed quantitative characterization of ganciclovir pharmacodynamics and its variability. Simulations of the model were undertaken to determine the optimal concentration profile for maximizing the ganciclovir therapeutic index.
MATERIALS AND METHODS
Chemicals.
GCV [9-(1,3-dihydroxy-2-propoxymethyl) guanine] was provided by Roche Laboratories (Neuilly sur Seine, France). RPMI 1640 medium was purchased from Eurobio (Courtaboeuf, France), and minimum essential medium (MEM) was from Lonza Laboratories (Verviers, Belgium). Fetal calf serum was from Perbio Science (Bezons, France). Phosphate-buffered saline medium was from Jacques Boy (Reims, France). Penicillin/streptomyin (10,000 U/ml) and amphotericin B were purchased from Bio Whittaker Europe (Verviers, Belgium) and Bristol-Myers Squibb (Rueil Malmaison, France), respectively. Cyclosporine was from Sigma (Steinheim, Germany). The High-Pure viral nucleic acid kit was provided by Roche Diagnostic Laboratories (Mannheim, Germany).
The human cytomegalovirus (HCMV) strain AD 169 (ATCC VR-358) was propagated in human embryonic fibroblasts (MRC5 cell line; RD Biotech, Besançon, France) and stored at −80°C. This virus stock was thawed 10 days before lymphoblastoid cell infection. After 2 passages at low virus-to-cell ratios, HCMV was titrated by real-time quantitative PCR (18). Experiments in this study used a final stock of virus with a titer of 109 PFU/ml. After the preparation and titration of virus, it was immediately used to infect lymphoblastoid cells.
Infection of lymphoblastoid cell culture and exposure to ganciclovir.
Peripheral blood mononuclear cells (PBMCs) were isolated from the whole blood (10 cm3) of healthy pediatric donors by Ficoll density gradient centrifugation. B lymphoblastoid cell lines (BLCLs) were established from PBMCs by ex vivo infection with a laboratory strain of Epstein Barr virus (EBV 95-8), an effective procedure for inducing the long-term growth of certain human B lymphocytes (19). The BLCLs were then grown as suspension cultures in RPMI 1640 medium supplemented with 12% fetal calf serum, penicillin, streptomycin, and amphotericin B. The BLCL culture was performed at 37°C in a humidified atmosphere of 5% CO2. Cells were kept in the exponential phase over the course of the culture (14 days).
BLCLs (107 cells/ml) were infected with HCMV at 0.01 PFU/cell. The cell culture was incubated for 2 h at 37°C. After infection, the cells were washed in phosphate-buffered saline medium (PBS) three times to remove unattached and passively adsorbed virus. Cells were counted and resuspended in RPMI (106 cells/ml). To control the efficiency of the washing procedure, virus detection was performed by PCR after the last washing. Uninfected cells were treated in the same way as infected cells. GCV was added just after the last washing to infected and noninfected cells.
GCV stock solution was prepared at 1 mg/ml and diluted in physiological serum before the assay to obtain a range of GCV concentrations in BLCL culture medium from 1 to 20 mg/liter. The durations of GCV exposure were 1, 2, 7, and 14 days for each concentration (1, 5, 10, and 20 mg/liter), with three consecutive washings done to completely remove extracellular GCV. The total culture duration, independent of the GCV exposure periods, was 14 days to determine the long-term effects of the antiviral drug after removal from cell culture.
All experiments were done in triplicate.
Evaluation of ganciclovir toxicity and antiviral activity.
For evaluation of toxicity, the total cell number was determined using a Coulter particle counter and size analyzer (Z2; Beckman, Fullerton, CA) after 1, 2, 7, and 14 days of cell culture, independent of the GCV exposure duration.
Viable-cell numbers were determined using an Adam Counter (Labtech, Palaiseau, France). Samples were stained with fluorescent dye (propium iodide), which intercalated DNA to stain the nuclei of target cells, and fluorescent images were taken automatically and processed by image analysis software. Measurements were performed at day 7 (for the 1-, 2-, and 7-day GCV exposures) and at day 14 (for the 1-, 2-, 7-, and 14-day GCV exposures).
For evaluation of ganciclovir antiviral activity, the method of virus quantification was real-time PCR, as previously described (18). A High-Pure viral nucleic acid kit was used to extract HCMV DNA, and an Apparatus 7500 real-time PCR system (Applied Biosystems) was used to quantify HCMV DNA. Viral quantification was performed on virus stocks obtained on MRC5 cells (before BLCL infection) and on infected cells on day 0, day 1, day 2, day 7, and day 14 postinfection independent of GCV exposure duration.
Pharmacodynamic model for cell cultures without virus.
The typical profile of the cell number-versus-time curve showed initial decay until a nadir was reached, followed by an S-shaped growth phase and a final plateau phase. To describe the full profile, the variation of the viable-cell number with time, N(t), was modeled by the following equation:
| (1) |
In this equation, t is time and Kmax is the maximal growth rate constant that can be achieved during the 14-day experiment (day−1). GCV(t) is the ganciclovir concentration (mg liter−1). The parameter α1 (liters mg−1) is the growth inhibition constant of ganciclovir. A value of zero means no inhibition of cell growth by ganciclovir. Tr is the dimensionless reduced time. Tlag is the delay before growth begins (days). Tmax is the time at which the maximal growth rate is observed (days).
Pharmacodynamic model for cell cultures with virus.
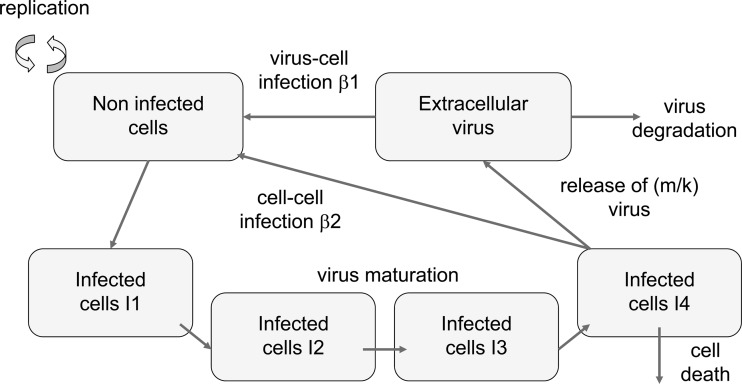
The structure of the infection model is illustrated in Fig. 1. The model assumes that the noninfected cells (N) are infected by extracellular virion particles and by transmission of virus from mature infected cells. The intensity of this process is characterized by two infectivity rate constants, β1 and β2, respectively. The infected cells go through a number of states (I1 to I4), accounting for the maturation of virus, i.e., the delay between cell infection and cell death. The rate constant for each step is k1 (days−1). Hence, the mean maturation time is 4/k1. In the state I4, the virus is released with a rate constant, m (days−1). The number of virion particles released by infected cells (the so-called reproductive number) is as follows: R = m/k1. The extracellular virion particles (V) are eliminated with a rate constant of Kv. Finally, an exponential decrease of the infectivity had to be introduced into the model to account for the late decay of the viral load in spite of a constant cell concentration in the control experiment with no ganciclovir. This exponential decrease was characterized by its half-life, Tinf (days).
Fig 1.
The pharmacodynamic model and its parameters.
The set of differential equations was as follows:
| (2) |
where
| (3) |
| (4) |
| (5) |
for j values of 2 to 4 (Fig. 1).
| (6) |
The observed cell number, C, is the sum of noninfected and infected cell concentrations. The observed viral load, VL, is approximated as the sum of the extracellular virus, the virus in I1 cells, and R times I4.
The intensity of the antiviral effect of ganciclovir was described as a linear function of its concentration. The effect of ganciclovir on cells and virus was assumed to be instantaneous, because the steady state of the ganciclovir triphosphate intracellular concentration is reached in less than 6 h (S. Cohen and J. Guitton, unpublished data). Several submodels were tested to determine the site of action of ganciclovir (with respect to our model of infection): inhibition of infectivity [β1 or β2 was multiplied by 1 − α2 · GCV(t)], inhibition of maturation [m was multiplied by 1 − α2 · GCV(t)], stimulation of virus elimination [Kv was multiplied by 1 + α3 · GCV(t)].
Parameter estimation and model building.
To account for interassay variability, the model was written as a mixed-effects model. Each parameter of the model was assumed to follow a log normal distribution. The parameters to be estimated were the median of all parameters and the variance of Kmax, Tmax, β1, β2, k1, m, Tinf, and Kv. All the parameters were estimated by nonlinear regression with NONMEM VII by the so-called FOCE method (2).
The strategy for model building was as follows. In the first step, the model was fitted to the cell number-versus-time data from cell cultures with no virus. In the second step, the model was fitted to the cell number- and viral load-versus-time data from cell cultures with virus by fixing α1 to the value estimated in the former step and α2 and α3 to zero. In this way, the antiviral effect of ganciclovir was not accounted for in the model. The post hoc estimates of Kmax, Tmax, β1, β2, k1, m, Tinf, and Kv were plotted against the concentration and the area under the concentration-time curve (AUC) of ganciclovir in order to detect any influence of ganciclovir exposure on these parameters. In the last step, the relationships between the parameters and the ganciclovir concentration were included in the model, i.e., α2 and α3 were estimated.
Hypothesis testing for, e.g., fixing a parameter to zero, was based on the likelihood ratio test. A P value of less than 0.05 was considered significant.
Simulation-based diagnostic tools were used for final model evaluation. A thousand individual simulated profiles were obtained by sampling in the distribution of the random effects of the mixed-effects model. A visual predictive check allowed us to compare the 90% prediction interval to the experimental data (30). Normalized prediction distribution errors (a criterion with high power to detect departures from the model) were calculated and plotted against time and ganciclovir concentration (4). These approaches do not rely on any approximation.
Simulations.
Using the final mixed-effects model, 400 cell number and viral-load profiles of the complete design (ganciclovir concentrations equal to 0, 1, 2, 5, 10, and 20 mg liter−1 with exposure to ganciclovir for 0, 1, 2, 7, or 14 days) were simulated by sampling in the distribution of the random effects. For each simulation, several metrics were calculated. To characterize the variation of cells over the 14 days of the experiment, the normalized cell AUC, AUCCN, was defined as:
| (7) |
The numerator is the AUC of noninfected cells, while the denominator is the AUC that would be observed if the cell number remained at the initial value for 14 days. A value of AUCCN greater than 1 indicates cell reproduction.
A similar index, the normalized AUC of viral load (AUCVN), was calculated to characterize the replication of CMV:
| (8) |
In this equation, AUCmin is the AUC of the viral load that would be observed if there was no replication of the virus. In this case, VL(t) would decrease exponentially from VL(0) to zero, and the corresponding AUCmin is equal to VL(0)/Kv. A value of AUCVN equal to zero means complete inhibition of viral replication by ganciclovir. A value greater than 1 indicates net replication of the virus.
The median and percentiles of AUCCN and AUCVN were calculated from the distribution of the 400 values for each set of conditions (ganciclovir concentration and duration of exposure). The median was taken as the point estimate of the effect of a given concentration of ganciclovir on cells and the viral load for a given duration of exposure. These point estimates were used to calculate the fraction of maximal effect as a function of the ganciclovir concentration (GCV):
| (9) |
| (10) |
All the simulations were carried out with NONMEM VII.
Optimization of the drug concentration profile.
Because ganciclovir toxic and antiviral effects are both concentration dependent, an optimal ganciclovir concentration profile that maximizes the antiviral effect while minimizing toxicity was calculated. To avoid confusion, the concentration profile of ganciclovir is referred to as the dosing schedule, D. Our approach was based on the concept of the utility function, U (20), which is a weighted sum of antiviral activity and nontoxicity evaluated over 14 days. In order to account for the variability of the cell and viral-load profiles under a given dosing schedule, the mean utility is maximized with respect to D. The mean utility is computed as the arithmetic mean of 400 values, obtained by generating 400 profiles of C(t) and VL(t) for a given D, using the mixed-effects model. In mathematical terms, U is defined as follows for the jth simulated profile of C(t) and VL(t):
| (11) |
A and T are the antiviral effect and the toxic effect associated with a given dosing schedule:
| (12) |
max(AUCVN) and max(AUCCN) are the maximal values that can be observed among the 400 profiles when no ganciclovir is added. (1 − T) may be regarded as the safety. In this way, A and (1 − T) are constrained to be in the range 0 to 1. w is the weight attributed to the antiviral effect on a scale from 0 to 1, while (1− w) is the weight attributed to safety. By construction, Uj is also in the range 0 to 1. In our study, w was fixed at 0.5.
The strategy to find the optimal dosing schedule comprised two steps. In the first step, the 14-day period was split into 7 consecutive periods of 2 days each. The mean utility was maximized with respect to the ganciclovir concentration in each period. In the second step, the 14-day AUC of ganciclovir was constrained to the value obtained in step 1, but the mean utility was maximized with respect to the time of onset of each period. All these calculations were done with NONMEM VII, using the LIKELIHOOD option.
Finally, the performances of two ganciclovir concentration profiles, AUCCN and AUCVN, were compared with respect to their utility. The first profile was the optimal profile; the other was a constant-concentration profile with the same ganciclovir 14-day AUC.
RESULTS
Parameter estimation and model building.
The model was first fitted to the cell number-versus-time data from cell cultures with no virus. The growth model fit the data well (data not shown), and the value of α1 was estimated at 0.026 with a relative standard error (SE) of 6%. The model was then fitted to the cell number- and viral load-versus-time data from virus-infected cell cultures by fixing α1 at 0.026, and α2 and α3 at zero. The post hoc estimates of Kmax, Tmax, β1, β2, k1, m, Tinf, and Kv were plotted against the concentration and the AUC of ganciclovir in order to detect any influence of ganciclovir on these parameters. It was observed that β1, β2, and m were approximately linearly related to the ganciclovir concentration. The other parameters showed no trend with respect to ganciclovir exposure. In particular, there was no visible relationship between the rate constant of viral elimination (Kv) and the exposure. Therefore, in the last step, α3 remained fixed at zero, while the relationships between β1, β2, and m and the ganciclovir concentration were included in the model. Because exposure to ganciclovir at 20 mg/liter for 14 days obviously resulted in complete inhibition of viral replication, α2 was fixed at 0.05 liter mg−1. Finally, because the uncertainty about the k1 and m estimates was large, these parameters were fixed at 4 and 60, respectively. In this way, the mean maturation time, 4/k1, was equal to 1 day (9), and the reproductive number, m/k1, was equal to 15 (10). This final model fit the data well (Table 1), as shown by the visual predictive check (Fig. 2) and the other criteria (not shown).
Table 1.
Parameters estimated in the final model
| Parameter | Value (RSEa) (%) |
|
|---|---|---|
| Median | CVb | |
| Kmax (day−1) | 0.234 (9) | |
| Tmax (day) | 2.39 (8) | 15 (19) |
| Tlag (day) | 0.74 (12) | |
| β1 (day−1 cell−1) | 0.276 × 10−6 (34 × 10−6) | 4 (75) |
| β2 (day−1 cell−1) | 10 × 10−6 (47 × 10−6) | 59 (89) |
| Tinf (day) | 2.5 (5) | |
| k1 (day−1) | 4 | |
| m (day−1) | 60 | |
| Kv (day−1) | 1 (7) | |
| α1 (liter mg−1) | 0.026 | |
| α2 (liter mg−1) | 0.050 | |
| α3 (liter mg−1) | 0 | |
RSE, relative standard error. Fixed parameters have no RSE.
CV, coefficient of variation.
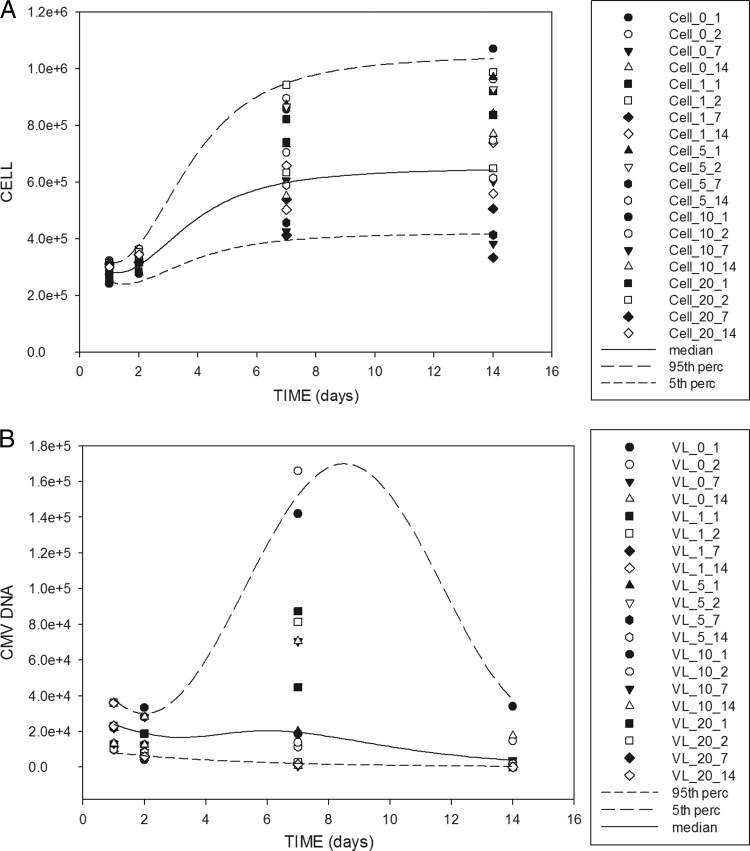
Fig 2.
Visual predictive check of the model. (A) Cell number (CL) versus time. (B) Viral load versus time. The points are the observations. The line is the median profile of 400 individual simulations. The dashed lines are the 5th and 95th percentiles (perc) of the 400 simulations. The symbols are coded CL_X_Y or VL_X_Y, where X is the ganciclovir concentration in mg/liter and Y is the exposure duration in days.
Simulations.
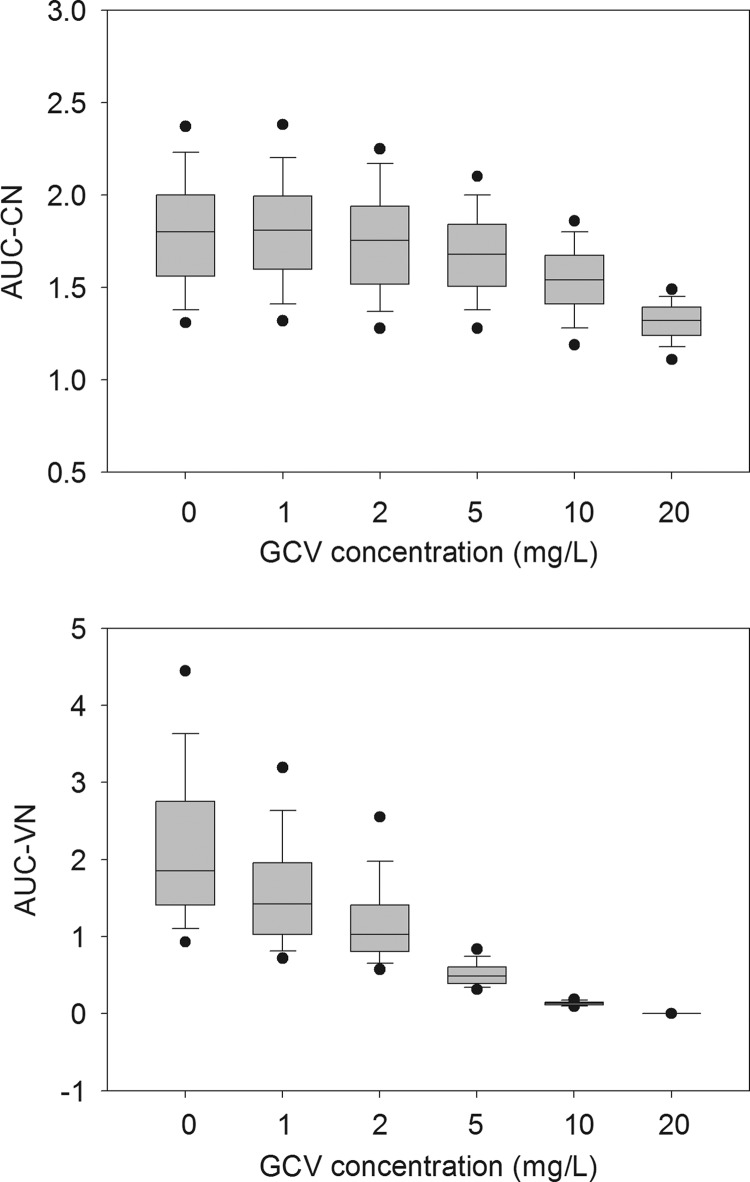
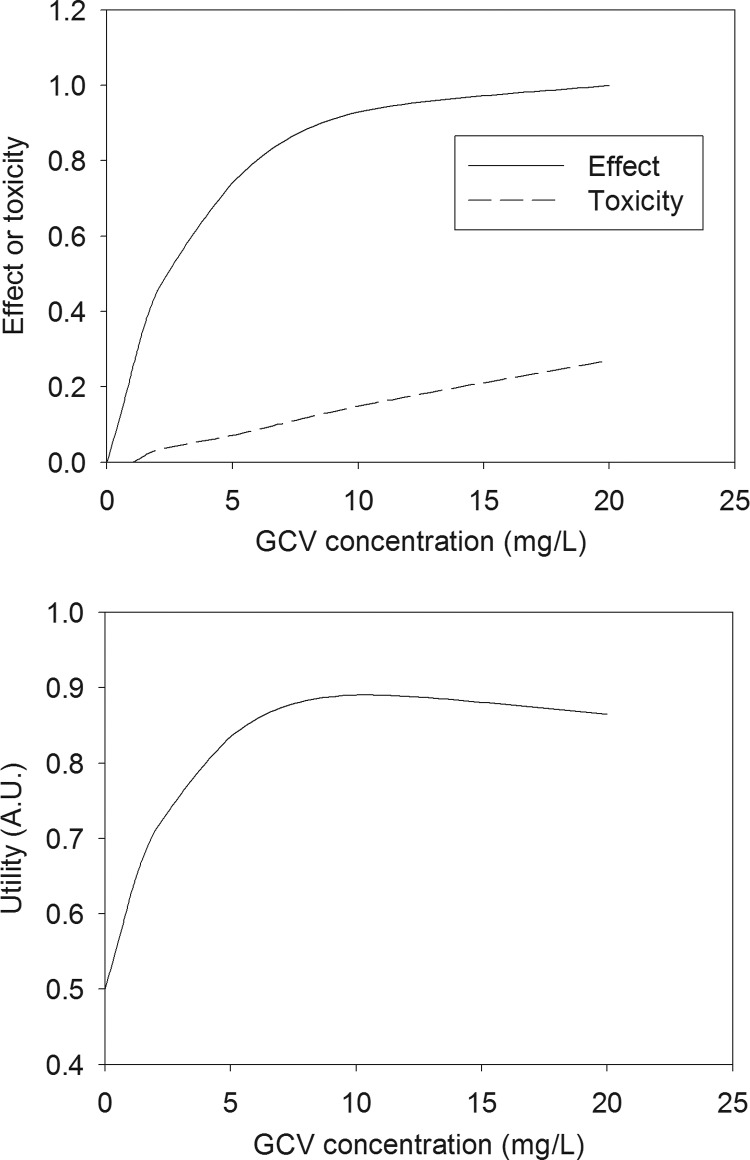
The median and percentiles of AUCCN and AUCVN, calculated from the distribution of 400 replicates under several sets of conditions, showed that ganciclovir had very little toxic and antiviral effect, even at 20 mg liter−1, when the duration of exposure was ≤7 days. Biologically, a significant effect was observed only with a 14-day exposure. The effect of exposure to different concentrations of ganciclovir for 14 days on the normalized AUC of viable cells and the normalized viral load is shown in Fig. 3. Although the interassay variability was quite high, complete inhibition of viral replication was obtained at 20 mg liter−1. The fraction of maximal effect as a function of exposure to a constant ganciclovir concentration for 14 days is shown in Fig. 4A. The corresponding utility function, assuming equal weights for antiviral effect and toxicity, is plotted in Fig. 4B. The plot shows that a maximal utility of ca. 0.9 is reached around 10 mg liter−1. Lower concentrations lead to low antiviral effect, while higher concentrations lead to unacceptable toxicity. Therefore, it makes sense to search for an optimal concentration profile aimed at maximizing the utility function.
Fig 3.
Effects of exposure to different concentrations of ganciclovir for 14 days on the normalized AUC of viable cells (top) and the normalized viral load (bottom). The line in each box is the median, the bounds of each box are the 25th and 75th percentiles, the lower and upper lines are the 5th and 95th percentiles, and the dots are the extreme values.
Fig 4.
(Top) Antiviral effect and cell toxicity versus concentration of ganciclovir after exposure for 14 days. (Bottom) Utility function of the ganciclovir concentration. A.U., arbitrary units.
Optimization of the drug concentration profile.
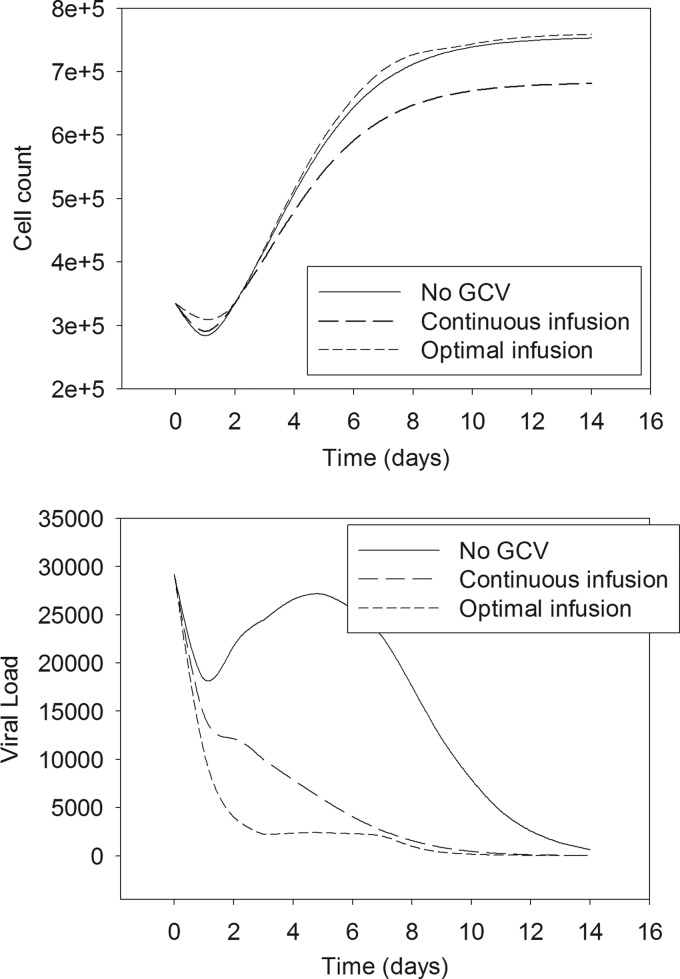
In the first step, the optimization procedure (i.e., maximizing the mean utility function with respect to the ganciclovir concentration in each 2-day period) showed that the optimal profile consisted of maintaining the ganciclovir concentration at 20 mg liter−1 in the first and the fifth 2-day periods, i.e., in the intervals 0 to 2 days and 8 to 10 days, and a null concentration at other times. In the second step, the optimal time for onset of each of these two periods was found to be 0 and 7.58 days, respectively. The optimal profile was finally determined to be ganciclovir concentrations of 20 mg liter−1 in the intervals 0 to 2 days and 7.58 to 9.58 days and a null concentration at other times. The AUC of the optimal profile is equivalent to that of a constant-concentration profile of 5.71 mg liter−1 over 14 days. The performances of the optimal profile and the constant profile, evaluated by simulation, are compared in Table 2. The optimal profile was significantly better on all criteria: higher mean utility, lower cell toxicity, and greater viral reduction. The kinetic profiles of cell and viral load under both dosing schedules are shown in Fig. 5 and compared to the profiles without treatment by ganciclovir. The optimal schedule results in almost no toxicity but in a marked antiviral effect.
Table 2.
Comparison of the optimal concentration profile with the constant concentration profile
| Parameter | Concn profilea |
|
|---|---|---|
| Constant | Optimal | |
| Utility | 0.79 (0.75–0.83) | 0.87 (0.83–0.91) |
| Normalized AUC of viable cells | 1.66 (1.49–1.84) | 1.83 (1.64–2.03) |
| Normalized AUC of viral load | 0.40 (0.33–0.55) | 0.10 (0.08–0.14) |
The numbers are medians (25th–75th percentiles) based on 400 individual simulations.
Fig 5.
Comparison of the influence of three concentration profiles of ganciclovir on the median cell concentration profile (top) and the median viral-load profile (bottom).
DISCUSSION
In this study, a pharmacodynamic model was derived and fitted to the cell number and viral load under different concentrations of ganciclovir and durations of exposure. The model is similar to models developed previously for HIV infection (12) and hepatitis C virus (HCV) infection (5), but the cell growth function of our model is more elaborate. Under our conditions, the cells were asynchronous at the beginning of the experiment, the cell count reached a plateau due to consumption of nutriments (the medium was not renewed during the experiment), and the cells were quiescent at the end of the experiment. These characteristics mimic the clinical situation of a primary infection in a bone marrow recipient. The antiviral drug concentration range was 0 to 20 mg/liter. This range must be compared with the mean (standard deviation [SD]) maximal concentration of 10 (2) mg/liter observed in stem cell transplant (SCT) patients receiving intravenous (i.v.) ganciclovir at 5 mg/kg of body weight twice daily (b.i.d.) (29). Higher ganciclovir concentrations might have been studied in our in vitro experiments to observe the entire toxicity-versus-concentration profile. This approach could have resulted in a different equation for the toxicity-versus-concentration model. Hence, a limitation of our current model is that it is not suitable for extrapolation beyond 20 mg/liter.
Ganciclovir toxicity for lymphoblastoid cells in the absence of virus could be characterized in spite of the complex shape of the cell growth curve. At the highest concentration (20 mg/liter), the growth rate constant was reduced by 52%. Hence, the ganciclovir 50% inhibitory concentration (IC50) is about 20 mg/liter for these cells. This value is higher than that observed with granulocyte-macrophage progenitors (0.7 to 4.8 mg/liter) and erythroid progenitors (0.4 to 7.4 mg/liter) (25).
Ganciclovir appeared to exert its antiviral effect by decreasing the infectivity and the release rate constant of CMV. This is consistent with the molecular mechanism of action of ganciclovir regarding inhibition of viral replication (15). Complete inhibition of viral replication by ganciclovir was observed at 20 mg/liter. These results were obtained with the CMV AD169 strain, whose IC50 is typically 0.9 mg/liter. The IC50 of clinical strains is typically 0.7 mg/liter (range, 0.2 to 1.9 mg/liter, depending on the assay) (21). Hence, similar patterns of activity should be observed with clinical strains, although the extra genes present in the clinical strains may alter their cell entry and possibly replication efficiency. Clinically, in the case of secondary infection, the immune reaction is susceptible to increased viral degradation (parameter Kv) and decreased infectivity (parameters β1 and β2) and the reproductive number (m/k1). If the IC50 is unchanged, greater efficacy of the treatment is expected, as observed by Emery et al. (10).
Because our model handles drug action by an empirical model, our approach is not restricted to a specific drug action mechanism and may possibly be applied to other drugs. For example, maribavir (24) and AIC246 (letermivir) (14) are new antiviral drugs with anti-CMV activity that are in phase III and phase II, respectively, of their clinical development. Their mechanism of action is different from that of ganciclovir. Their toxicity seems very low, and the target organs are different from those of ganciclovir. Our model may be applied to maribavir and letermivir by setting α1 equal to 0 (no toxicity for lymphoblastoid cells) and adjusting α2 to a suitable value to account for the fact that the IC50s of these drugs are lower than that of ganciclovir.
The kinetics of the viral load consisted of an initial decline, due to penetration of virus into cells, followed by a rebound, due to production and release of new virus. According to the model simulation, the rebound reached its maximum about 8 days after the beginning of the infection. The rate of increase in the CMV load in our in vitro model was consistent with the doubling time of the CMV load in the blood of bone marrow transplant recipients after allogeneic transplantation (9). The optimal ganciclovir concentration profile consisted of maintaining the concentration at 20 mg liter−1 in the intervals 0 to 2 days and 7.58 to 9.58 days and a null concentration at other times. Hence, the model-based optimization suggests that a high concentration of ganciclovir should be applied at the time of the onset of infection and at the time of viral rebound in order to maximize efficacy and minimize toxicity. This optimal profile could be obtained by i.v. ganciclovir at 10 mg/kg at 0, 12, 24, 36, 180, 192, 204, and 216 h in SCT patients with normal renal function.
The feasibility of a short treatment at a high dose is partly supported by the clinical study reported by Saleh et al. (22). In the study, valganciclovir was given at 900 mg b.i.d. for 1 or 2 weeks to CMV-positive HSCT patients. The complete response rate was over 92%, while severe neutropenia requiring granulocyte colony-stimulating factor occurred in 8 of 61 episodes (13%). In another study with the same dosing regimen, the mean (SD) peak concentration of ganciclovir was 8.8 (2.4) mg/liter (8). This value is comparable to that of the constant-concentration profile at 5.7 mg liter−1 for 14 days, equivalent to the AUC of the optimal profile determined in our study.
To conclude, the model-based analysis of our in vitro pharmacodynamic data suggests that the ganciclovir therapeutic index could be increased by infusions of 10 mg/kg b.i.d. at days 1, 2, 8.5, and 9.5 in SCT patients. This prediction should be evaluated in a clinical trial.
Footnotes
Published ahead of print 23 April 2012
REFERENCES
- 1. Atkinson K, et al. 1995. Prophylactic ganciclovir is more effective in HLA-identical family member marrow transplant recipients than in more heavily immune-suppressed HLA-identical unrelated donor marrow transplant recipients. Australasian Bone Marrow Transplant Study Group. Bone Marrow Transplant. 16:401–405 [PubMed] [Google Scholar]
- 2. Beal S, Sheiner LB, Boeckmann A, Bauer RJ. 2009. NONMEM user's guides (1989–2009). Icon Development Solutions, Ellicott City, MD [Google Scholar]
- 3. Boeckh M, et al. 1996. Cytomegalovirus pp65 antigenemia-guided early treatment with ganciclovir versus ganciclovir at engraftment after allogeneic marrow transplantation: a randomized double-blind study. Blood 88:4063–4071 [PubMed] [Google Scholar]
- 4. Brendel K, Comets E, Laffont C, Laveille C, Mentré F. 2006. Metrics for external model evaluation with an application to the population pharmacokinetics of gliclazide. Pharm. Res. 23:2036–2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dahari H, Lo A, Ribeiro RM, Perelson AS. 2007. Modeling hepatitis C virus dynamics: liver regeneration and critical drug efficacy. J. Theor. Biol. 247:371–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Duan J, et al. 1998. Dose and duration-dependence of ganciclovir treatment against murine cytomegalovirus infection in severe combined immunodeficient mice. Antiviral Res. 39:189–197 [DOI] [PubMed] [Google Scholar]
- 7. Einsele H, et al. 2000. Risk factors for treatment failures in patients receiving PCR-based preemptive therapy for CMV infection. Bone Marrow Transplant. 25:757–763 [DOI] [PubMed] [Google Scholar]
- 8. Einsele H, et al. 2006. Oral valganciclovir leads to higher exposure to ganciclovir than intravenous ganciclovir in patients following allogeneic stem cell transplantation. Blood 107:3002–3008 [DOI] [PubMed] [Google Scholar]
- 9. Emery VC, Cope AV, Bowen EF, Gor D, Griffiths PD. 1999. The dynamics of human cytomegalovirus replication in vivo. J. Exp. Med. 190:177–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Emery VC, Hassan-Walker AF, Burroughs AK, Griffiths PD. 2002. Human cytomegalovirus (HCMV) replication dynamics in HCMV-naive and -experienced immunocompromised hosts. J. Infect. Dis. 185:1723–1728 [DOI] [PubMed] [Google Scholar]
- 11. Forman SJ, Zaia JA. 1994. Treatment and prevention of cytomegalovirus pneumonia after bone marrow transplantation: where do we stand? Blood 83:2392–2398 [PubMed] [Google Scholar]
- 12. Herz AV, Bonhoeffer S, Anderson RM, May RM, Nowak MA. 1996. Viral dynamics in vivo: limitations on estimates of intracellular delay and virus decay. Proc. Natl. Acad. Sci. U. S. A. 93:7247–7251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Holberg-Petersen M, et al. 1996. Direct growth suppression of myeloid bone marrow progenitor cells but not cord blood progenitors by human cytomegalovirus in vitro. Blood 88:2510–2516 [PubMed] [Google Scholar]
- 14. Lischka P, et al. 2010. In vitro and in vivo activities of the novel anticytomegalovirus compound AIC246. Antimicrob. Agents Chemother. 54:1290–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Matthews T, Boehme R. 1988. Antiviral activity and mechanism of action of ganciclovir. Rev. Infect. Dis. 10(Suppl. 3):S490–S494 [DOI] [PubMed] [Google Scholar]
- 16. McGavin JK, Goa KL. 2001. Ganciclovir: an update of its use in the prevention of cytomegalovirus infection and disease in transplant recipients. Drugs 61:1153–1183 [DOI] [PubMed] [Google Scholar]
- 17. Mori T, et al. 2002. Dose-adjusted preemptive therapy for cytomegalovirus disease based on real-time polymerase chain reaction after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 29:777–782 [DOI] [PubMed] [Google Scholar]
- 18. Najioullah F, Thouvenod D, Lina B. 2001. Development of a real time PCR procedure including an internal control for the measurement of HCMV viral load. J. Virol. Methods 92:55–64 [DOI] [PubMed] [Google Scholar]
- 19. Neitzel H. 1986. A routine method for the establishment of permanent growing lymphoblastoid cell lines. Hum. Genet. 73:320–326 [DOI] [PubMed] [Google Scholar]
- 20. Ouellet D, et al. 2009. The use of a clinical utility index to compare insomnia compounds: a quantitative basis for benefit-risk assessment. Clin. Pharmacol. Ther. 85:277–282 [DOI] [PubMed] [Google Scholar]
- 21. Perrottet N, et al. 2009. Valganciclovir in adult solid organ transplant recipients: pharmacokinetic and pharmacodynamic characteristics and clinical interpretation of plasma concentration measurements. Clin. Pharmacokinet. 48:399–418 [DOI] [PubMed] [Google Scholar]
- 22. Saleh AJ, et al. 2010. High efficacy and low toxicity of short-course oral valganciclovir as pre-emptive therapy for hematopoietic stem cell transplant cytomegalovirus infection. Hematol. Oncol. Stem Cell Ther. 3:116–120 [DOI] [PubMed] [Google Scholar]
- 23. Salzberger B, Bowden RA, Hackman RC, Davis C, Boeckh M. 1997. Neutropenia in allogeneic marrow transplant recipients receiving ganciclovir for prevention of cytomegalovirus disease: risk factors and outcome. Blood 90:2502–2508 [PubMed] [Google Scholar]
- 24. Shannon-Lowe CD, Emery VC. 2010. The effects of maribavir on the autophosphorylation of ganciclovir resistant mutants of the cytomegalovirus UL97 protein. Herpesviridae. 1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sommadossi JP, Carlisle R. 1987. Toxicity of 3′-azido-3′-deoxythymidine and 9-(1,3-dihydroxy-2-propoxymethyl)guanine for normal human hematopoietic progenitor cells in vitro. Antimicrob. Agents Chemother. 31:452–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stocchi R, et al. 1999. A comparison of prophylactic vs pre-emptive ganciclovir to prevent cytomegalovirus disease after T-depleted volunteer unrelated donor bone marrow transplantation. Bone Marrow Transplant. 23:705–709 [DOI] [PubMed] [Google Scholar]
- 27. Tomonari A, et al. 2004. Ganciclovir-related neutropenia after preemptive therapy for cytomegalovirus infection: comparison between cord blood and bone marrow transplantation. Ann. Hematol. 83:573–577 [DOI] [PubMed] [Google Scholar]
- 28. Torre-Cisneros J, et al. 2010. Impact of initial cytomegalovirus viral load on efficacy of preemptive therapy with ganciclovir in allogeneic stem cell transplant recipients. Enferm. Infecc. Microbiol. Clin. 28:6–12 [DOI] [PubMed] [Google Scholar]
- 29. Wolfe EJ, et al. 1997. Pharmacokinetics of mycophenolate mofetil and intravenous ganciclovir alone and in combination in renal transplant recipients. Pharmacotherapy 17:591–598 [PubMed] [Google Scholar]
- 30. Yano Y, Beal SL, Sheiner LB. 2001. Evaluating pharmacokinetic/pharmacodynamic models using the posterior predictive check. J. Pharmacokinet. Pharmacodyn. 28:171–192 [DOI] [PubMed] [Google Scholar]