Abstract
Here we describe an experimental murine model that allows for aerosolized antituberculosis drug efficacy testing. Intrapulmonary aerosol delivery of isoniazid, capreomycin, and amikacin to mice with pulmonary infection of Mycobacterium tuberculosis demonstrated efficacy in reducing pulmonary bacterial loads similar to that seen by standard drug delivery methods, even when lower concentrations of drugs and fewer doses were used in the aerosolized drug regimens. Interestingly, intrapulmonary delivery of isoniazid also reduced the bacterial load in the spleen.
TEXT
Tuberculosis (TB) is a chronic infectious disease with increasing incidence of drug resistance. Patients with drug-susceptible TB receive 6 to 9 months of combination drug treatment consisting of isoniazid (INH), rifampin (RIF), pyrazinamide, and ethambutol by the oral route for 2 months followed by INH and RIF for another 4 to 6 months. For patients with multidrug-resistant tuberculosis (MDR-TB) infection, current guidelines still recommend prolonged (up to 2 years) therapy using second-line TB drugs. Of the second-line drugs, parenterally administered antimicrobials of initial choice are amikacin, kanamycin, and capreomycin (1). The optimal duration for the treatment of MDR-TB with injectable agents is not known, and treatment with these agents is associated with substantial toxicity. New drugs that have the potential to shorten therapy, can be easily administered, and have low toxicity would be a great advance in TB drug development. An alternative approach is to use an aerosolized method to deliver high concentrations of anti-TB drugs locally to the site of the infection. Aerosolized drugs, unlike injectable agents, are easy to administer and provide higher concentrations at the local site of infection, thereby reducing systemic levels of exposure to the drug (4, 10–12, 14, 21). There have been several attempts to deliver aerosols of anti-TB drugs using microparticles, dry powder, and nebulized forms of drugs, but these approaches are also subject to inherent problems with formulation and delivery of the carrier vehicle (8, 9, 11, 13, 20, 22–25). An experimental murine model that utilizes intrapulmonary aerosol delivery of drugs and allows for local pulmonary administration of anti-TB drugs and efficacy testing will facilitate the development of new agents for anti-TB aerosol drug delivery. In addition, the murine model could be a tool for early experimental compounds with limited oral bioavailability to obtain an in vivo proof of concept, especially for natural product compounds (with high molecular weights) or those of peptide origin.
These studies compare the efficacies of commonly used anti-TB drugs when delivered by intrapulmonary aerosol delivery, oral administration, or the subcutaneous injection route using a low-dose aerosol infection of Mycobacterium tuberculosis in an immunocompetent murine model. INH, an orally administered drug, and the parenteral anti-TB drugs capreomycin and amikacin were chosen to validate the efficacy of intrapulmonary aerosol delivery versus that of standard routes of administration in mice experimentally infected with M. tuberculosis. All three drugs are currently used for human TB treatment and have been extensively studied in the murine model of TB infection used for drug testing (1, 5–7, 17–19). The purpose of this work is to obtain an in vivo proof of concept to demonstrate that when drugs are administered locally via intrapulmonary aerosol delivery to mice chronically infected with M. tuberculosis, they can have efficacy similar to that seen when drugs are administered by their standard route of administration (by injection or oral gavage), even when given at a lower dose and dosing frequency. The novel mouse model for efficacy testing of antituberculosis agents via intrapulmonary delivery described here has the potential to offer a new animal model for preclinical TB drug discovery and development.
Ten- to 12-week old BALB/c female mice with an average weight of 22 g were infected with a low-dose aerosol using the Glas-Col inhalation exposure system calibrated to deliver ∼50 to 100 Mycobacterium tuberculosis (Erdman strain, TMC107; ATCC 35801) bacilli into the lungs of each mouse. Three mice were sacrificed at day 1 postinfection to determine bacterial uptake. Whole lungs were homogenized in 1 ml of saline and plated on 7H11 agar plates. At the start of therapy, on day 24 postinfection, 6 mice were sacrificed to determine the bacterial load in the lungs. The bacterial load was determined using serial dilutions of homogenized organs that were plated on 7H11 agar plates as previously described (2). The bacterial load in each animal was expressed as the log10 numbers of CFU.
Drug therapy began on day 24 postaerosol infection. Mice received drug therapy (Table 1) via oral gavage or the subcutaneous injection route as reported earlier (3). The treatment regimens administered by intrapulmonary aerosol delivery of drugs and systemic delivery (oral gavage or subcutaneous injection) are shown in Table 1. Briefly, mice treated by oral gavage received INH (Sigma-Aldrich) five times a week at a dose of 25 mg/kg of body weight (550 μg/dose). Other groups of mice received capreomycin or amikacin (Sigma-Aldrich) by subcutaneous injection at a dose of 150 mg/kg (3,300 μg/dose). Groups of mice treated by intrapulmonary aerosol delivery with INH, capreomycin, or amikacin received 500 μg/dose three times per week. After 2 or 3 weeks of therapy, mice were euthanized; the lungs and spleens were homogenized, and the bacterial load was determined as indicated above. A statistical analysis was performed using a one-way analysis of variance followed by a pairwise comparison of treatment group means using the Tukey test.
Table 1.
Intrapulmonary and systemic drug delivery regimens
| Regimen | Dose amount (μg) and frequency (times per wk) for each group (n) |
||||
|---|---|---|---|---|---|
| Control (6) | Drug |
Drug diluenta (dH2O or PBS) (6) | |||
| INH (6) | Capreomycin (6) | Amikacin (6) | |||
| Intrapulmonary | 0/0 | 500/3 | 500/3 | 500/3 | 50/3 |
| Systemic | 0/0 | 550/5 | 3,300/5 | 3,300/5 | 50/5 |
Dose amounts for the drug diluents are in μl. dH2O, distilled water.
Mice were drug treated by intrapulmonary aerosol delivery using a microspray device (MicroSprayer, model IA-C; Penn-Century, Philadelphia, PA) attached to an FMJ-250 high-pressure syringe device (Penn-Century) as described earlier (15, 16). Briefly, mice were anesthetized using an isoflurane and oxygen mixture (5% isoflurane in oxygen at 4 liters/min; VIP 3000 isoflurane vaporizer) for about 10 min until animals were sedated. Each mouse was placed on its abdomen in a Perspex support adjusted to a 45° angle; the teeth were suspended up with an incisor loop located on top. During the intubation, the mouse was on continued isoflurane anesthesia. The mouth was opened and, with the help of a cotton tip, the tongue was extended. Then the MicroSprayer tip was aimed at the trachea and the formulation was sprayed. The mouse was taken off the support and laid in its cage until it awoke from the anesthesia (2 to 3 min). After administration of the anesthetic, the animals were monitored for regular breathing and behavior.
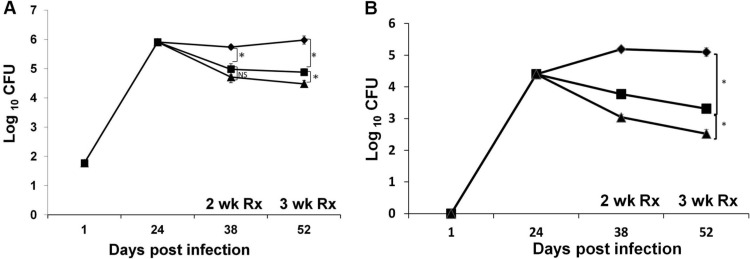
The results showed that the pulmonary bacterial load (determined as the average number of CFU for each group of mice treated with INH by oral gavage or intrapulmonary aerosol delivery) was significantly reduced after 2 and 3 weeks of either treatment (P < 0.05) (Fig. 1A). The bacterial load in lungs of the control mice treated with distilled water by intrapulmonary aerosol was not statistically different from that of untreated mice (P = 0.314) (data not shown). The reduction in the bacterial load after local pulmonary delivery of INH was similar to that of INH administered by oral gavage. Mice treated by the intrapulmonary aerosol received 6 (2 weeks of treatment) or 9 (3 weeks of treatment) doses, whereas the oral gavage groups received 10 or 15 doses during 2 or 3 weeks of treatment, respectively. Interestingly, when INH was delivered by intrapulmonary aerosol, there was also a reduction in the bacterial load of the spleen, albeit not to the same extent as when INH was administered by oral gavage (P < 0.05) (Fig. 1B).
Fig 1.
Bacterial loads in the lungs of M. tuberculosis-infected mice after treatment with INH delivered by intrapulmonary aerosol or oral gavage. Mice infected with M. tuberculosis (n = 6) were treated with INH by intrapulmonary aerosol delivery (squares) three times a week or oral gavage (triangles) five times a week. Rhomboids, control group of untreated mice. The graph shows the log10 numbers of CFU in the lung (A) and spleen (B). NS, not significant; Rx, treatment; *, P < 0.05.
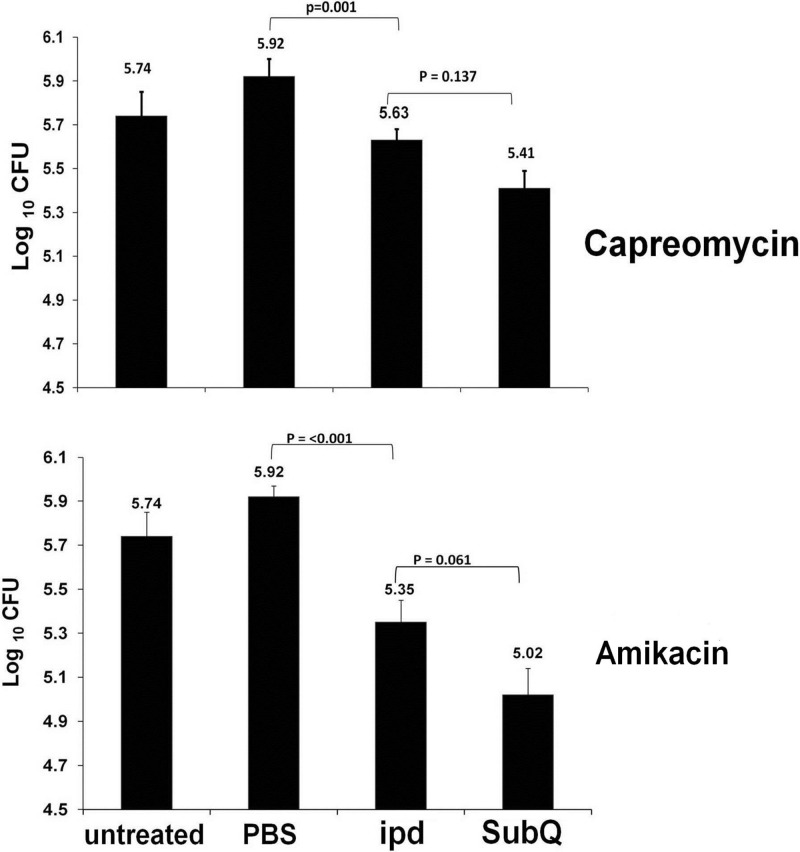
The efficacy of the intrapulmonary aerosol delivery for capreomycin and amikacin is shown in Fig. 2. Neither capreomycin nor amikacin is orally bioavailable. Mice treated by the intrapulmonary aerosol or by subcutaneous injection of capreomycin or amikacin demonstrated similar reductions of the pulmonary bacterial load after 3 weeks of treatment. During the 3 weeks of treatment, mice treated with capreomycin or amikacin received a total of 9 doses when delivered by intrapulmonary aerosol or a total of 15 doses by subcutaneous injection. Similarly, both drugs were administered at 500 μg/dose when delivered by the intrapulmonary aerosol and at 3,300 μg/dose when delivered by subcutaneous injection. The bacterial loads of controls treated with sterile phosphate-buffered saline (PBS) (diluents for the drugs) were statistically similar to those of untreated mice (P > 0.05). The bacterial load in the spleen of mice treated by either intrapulmonary aerosol or subcutaneous injection with capreomycin or amikacin did not differ significantly from that of the control mice treated with the drug diluents (P > 0.05) (data not shown).
Fig 2.
Bacterial loads in the lungs of M. tuberculosis-infected mice after capreomycin or amikacin intrapulmonary aerosol delivery or subcutaneous injection. Groups of mice (n = 6) were treated with amikacin or capreomycin by subcutaneous injection (subQ) at 3,300 μg/dose five times a week or aerosol intrapulmonary delivery (ipd) at 500 μg/dose three times a week. Other groups of M. tuberculosis-infected mice were used as controls (untreated) or treated by aerosol intrapulmonary delivery with 50 μl/dose three times a week of the PBS used as a drug diluent (PBS).
In conclusion, local pulmonary delivery of anti-TB drugs results in a reduction of the bacterial load similar to that of systemic administration via an oral or injection route. Less-frequent dosing of INH by the pulmonary aerosol route in these studies demonstrates a similar reduction of bacterial load, not only in the lungs but also in the spleen. Capreomycin and amikacin are drugs currently used as injectable drugs (1), but this study demonstrated that local pulmonary administration of capreomycin and amikacin has the potential to have improved efficacy in the lungs compared to that of systemic delivery. Less-frequent dosing and lower total doses of capreomycin and amikacin demonstrated reductions in the bacterial load in the lungs when delivered locally that were similar to those when delivered systemically by subcutaneous injection. Our results suggest that local aerosolized administration of INH, capreomycin, and amikacin in TB patients has the potential to improve TB treatment efficacy severalfold, easing drug administration, reducing the frequency of dosages and total drug dosages, diminishing patient discomfort caused by needle injections, and possibly reducing systemic toxicity. Further advanced studies of particle size to ensure intra-alveolar deposition and optimized pharmacokinetics are warranted.
ACKNOWLEDGMENTS
We acknowledge the staff of the Laboratory Animal Resources at Colorado State University (CSU) for their animal care. We kindly thank Elizabeth Donegan for her technical assistance. We thank Alan R. Schenkel and Marta Lishnevsky for their help and advice as well.
Support was provided by the National Institutes of Health (NIH) contract NO1 AI-95385 and task order HHSN272201000009I/01 at Colorado State University, as well as the Global Alliance for TB Drug Development.
Footnotes
Published ahead of print 30 April 2012
REFERENCES
- 1. Caminero JA, Sotgiu G, Zumla A, Migliori GB. 2010. Best drug treatment for multidrug-resistant and extensively drug-resistant tuberculosis. Lancet Infect. Dis. 10:621–629 [DOI] [PubMed] [Google Scholar]
- 2. De Groote MA, et al. 2011. Comparative studies evaluating mouse models used for efficacy testing of experimental drugs against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 55:1237–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. De Groote MA, et al. 2012. Importance of confirming data on the in vivo efficacy of novel antibacterial drug regimens against various strains of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 56:731–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hickey AJ, Misra A, Robertson BD. 2011. Optimisation of inhaled tuberculosis therapies and implications for host-pathogen interactions. Tuberculosis (Edinb.) 91:64. [DOI] [PubMed] [Google Scholar]
- 5. Klemens SP, DeStefano MS, Cynamon MH. 1993. Therapy of multidrug-resistant tuberculosis: lessons from studies with mice. Antimicrob. Agents Chemother. 37:2344–2347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Le Conte P, et al. 1994. Pharmacokinetics, toxicity, and efficacy of liposomal capreomycin in disseminated Mycobacterium avium beige mouse model. Antimicrob. Agents Chemother. 38:2695–2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lounis N, Ji B, Truffot-Pernot C, Grosset J. 1997. Comparative activities of amikacin against Mycobacterium avium complex in nude and beige mice. Antimicrob. Agents Chemother. 41:1168–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lu D, et al. 2010. Pulmonary immunization using antigen 85-B polymeric microparticles to boost tuberculosis immunity. AAPS J. 12:338–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lu D, et al. 2007. Poly (lactide-co-glycolide) microspheres in respirable sizes enhance an in vitro T cell response to recombinant Mycobacterium tuberculosis antigen 85B. Pharm. Res. 24:1834–1843 [DOI] [PubMed] [Google Scholar]
- 10. Misra A, et al. 2011. Inhaled drug therapy for treatment of tuberculosis. Tuberculosis (Edinb.) 91:71–81 [DOI] [PubMed] [Google Scholar]
- 11. Muttil P, et al. 2007. Inhalable microparticles containing large payload of anti-tuberculosis drugs. Eur. J. Pharm. Sci. 32:140–150 [DOI] [PubMed] [Google Scholar]
- 12. Muttil P, Wang C, Hickey AJ. 2009. Inhaled drug delivery for tuberculosis therapy. Pharm. Res. 26:2401–2416 [DOI] [PubMed] [Google Scholar]
- 13. O'Hara P, Hickey AJ. 2000. Respirable PLGA microspheres containing rifampicin for the treatment of tuberculosis: manufacture and characterization. Pharm. Res. 17:955–961 [DOI] [PubMed] [Google Scholar]
- 14. Paraf J, Zivy P, Fournier E. 1955. Aerosol therapy and cationic tensio-active drugs in pulmonary tuberculosis. Sem Hop. 31:4163–4164 (In French.) [PubMed] [Google Scholar]
- 15. Rosas-Taraco AG, et al. 2009. Intrapulmonary delivery of XCL1-targeting small interfering RNA in mice chronically infected with Mycobacterium tuberculosis. Am. J. Respir. Cell Mol. Biol. 41:136–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rosas-Taraco AG, et al. 2011. Local pulmonary immunotherapy with siRNA targeting TGFbeta1 enhances antimicrobial capacity in Mycobacterium tuberculosis infected mice. Tuberculosis (Edinb.) 91:98–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rosenthal IM, Zhang M, Almeida D, Grosset JH, Nuermberger EL. 2008. Isoniazid or moxifloxacin in rifapentine-based regimens for experimental tuberculosis? Am. J. Respir. Crit. Care Med. 178:989–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rosenthal IM, et al. 2007. Daily dosing of rifapentine cures tuberculosis in three months or less in the murine model. PLoS Med. 4:e344 doi:10.1371/journal.pmed.0040344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sanders WE, Jr, Hartwig C, Schneider N, Cacciatore R, Valdez H. 1982. Activity of amikacin against Mycobacteria in vitro and in murine tuberculosis. Tubercle 63:201–208 [DOI] [PubMed] [Google Scholar]
- 20. Sethuraman VV, Hickey AJ. 2002. Powder properties and their influence on dry powder inhaler delivery of an antitubercular drug. AAPS PharmSciTech. 3:E28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sharma A, Sharma S, Khuller GK. 2004. Lectin-functionalized poly (lactide-co-glycolide) nanoparticles as oral/aerosolized antitubercular drug carriers for treatment of tuberculosis. J. Antimicrob. Chemother. 54:761–766 [DOI] [PubMed] [Google Scholar]
- 22. Shi S, Hickey AJ. 2010. PLGA microparticles in respirable sizes enhance an in vitro T cell response to recombinant Mycobacterium tuberculosis antigen TB10.4-Ag85B. Pharm. Res. 27:350–360 [DOI] [PubMed] [Google Scholar]
- 23. Suarez S, et al. 2001. Airways delivery of rifampicin microparticles for the treatment of tuberculosis. J. Antimicrob. Chemother. 48:431–434 [DOI] [PubMed] [Google Scholar]
- 24. Suarez S, et al. 2001. Respirable PLGA microspheres containing rifampicin for the treatment of tuberculosis: screening in an infectious disease model. Pharm. Res. 18:1315–1319 [DOI] [PubMed] [Google Scholar]
- 25. Wang C, Hickey AJ. 2010. Isoxyl aerosols for tuberculosis treatment: preparation and characterization of particles. AAPS PharmSciTech. 11:538–549 [DOI] [PMC free article] [PubMed] [Google Scholar]