Abstract
Staphylococcus aureus small-colony variants (SCVs) persist intracellularly, which may contribute to persistence/recurrence of infections and antibiotic failure. We have studied the intracellular fate of menD and hemB mutants (corresponding to menadione- and hemin-dependent SCVs, respectively) of the COL methicillin-resistant S. aureus (MRSA) strain and the antibiotic pharmacodynamic profile against extracellular (broth) and intracellular (human THP-1 monocytes) bacteria. Compared to the parental strain, SCVs showed slower extracellular growth (restored upon medium supplementation with menadione or hemin), reduced phagocytosis, and, for the menD SCV, lower intracellular counts at 24 h postinfection. Against extracellular bacteria, daptomycin, gentamicin, rifampin, moxifloxacin, and oritavancin showed similar profiles of activity against all strains, with a static effect obtained at concentrations close to their MICs and complete eradication as maximal effect. In contrast, vancomycin was not bactericidal against SCVs. Against intracellular bacteria, concentration-effect curves fitted sigmoidal regressions for vancomycin, daptomycin, gentamicin, and rifampin (with maximal effects lower than a 2-log decrease in CFU) but biphasic regressions (with a maximal effect greater than a 3-log decrease in CFU) for moxifloxacin and oritavancin, suggesting a dual mode of action against intracellular bacteria. For all antibiotics, these curves were indistinguishable between the strains investigated, except for the menD mutant, which systematically showed a lower amplitude of the concentration-effect response, with markedly reduced minimal efficacy (due to slower growth) but no change in maximal efficacy. The data therefore show that the maximal efficacies of antibiotics are similar against normal-phenotype and menadione- and hemin-dependent strains despite their different intracellular fates, with oritavancin, and to some extent moxifloxacin, being the most effective.
INTRODUCTION
Small-colony variants (SCVs) are a naturally occurring subpopulation of bacteria with distinctive phenotypic features, among which the most characteristic is the formation of colonies having about 1/10 of the normal size. They have been described in many bacterial species and recovered in clinical samples from patients presenting persistent or recurrent infections (42). In Staphylococcus aureus, SCVs are mostly nonpigmented and nonhemolytic; their slow growth is due to auxotrophism for distinct growth factors such as menadione, hemin, and/or thymidine. Menadione- and hemin-dependent strains are defective in electron transport (35). Mutations in the menadione biosynthetic enzymes cause depletion in menaquinone, which normally forms a complex with cytochromes involved in electron transport, while failure to produce hemin blocks the synthesis of these cytochromes. Thymidine-dependent strains are deficient for dTMP synthesis and therefore unable to synthesize DNA (42). These SCVs often escape detection in routine laboratory testing because their auxotrophic character requires specific nutritional supplementation or prolonged culture (64). However, in epidemiological surveys looking specifically for their presence, SCVs of S. aureus are easily detected in a number of situations of chronic and relapsing infections. For instance, SCVs are observed in the sputum of 70% of patients suffering from cystic fibrosis (22), with about two-thirds of these strains harboring a thymidine-dependent phenotype (related to the chronic administration of trimethoprim-sulfamethoxazole in these patients) and one-third showing dependence on menadione or hemin or even double auxotrophism (12, 18, 21). Hemin- or menadione-dependent mutants are most frequent in osteomyelitis or device-associated infections, especially in patients treated with aminoglycosides (41, 42).
Small-colony variants show an enhanced ability to invade and persist in both phagocytic and nonphagocytic cells (2, 50, 56, 63, 65), which protects them from the immune system, makes them less accessible to antibiotics, and contributes to their survival. Therefore, the treatment of these intracellular forms requires the use of antibiotics not only displaying activity against SCVs but also presenting cellular pharmacokinetic and pharmacodynamic properties that enable them to act intracellularly (59).
In a previous study, we compared the intracellular activities of a series of antibiotics against a thymidine-dependent SCV isolated from a cystic fibrosis patient and its isogenic normal-phenotype counterpart (38). The present study focuses on hemin- and menadione-dependent SCVs, for which data are still lacking. We used in parallel genetically stable hemin- and menadione-dependent mutants of the well-characterized COL methicillin-resistant S. aureus (MRSA) strain (9, 66). This allowed us to compare the intracellular fates of these organisms, their extracellular and intracellular susceptibilities to antibiotics, and the influences of supplementation on these parameters. The studies were performed in a model of THP-1-infected monocytes that had been specifically developed for a quantitative assessment of the intracellular pharmacodynamics of antibiotics (7). These cells are indeed considered permissive to many intracellular bacteria (55), such as S. aureus (7), Listeria monocytogenes (48), Legionella pneumophila (53), Chlamydia spp. (68), Coxiella burnetii (17), Brucella spp. (26), Francisella tularensis (69), Yersinia pestis (67), and Mycobacterium tuberculosis (52). This cellular model therefore allows analysis of the true effect of antibiotics (in a pharmacological context) with minimal interference from cell defense mechanisms.
MATERIALS AND METHODS
Antibiotics and main reagents.
The following antibiotics were obtained as microbiological standards from their respective manufacturers: daptomycin from Novartis Pharma AG (Basel, Switzerland), moxifloxacin from Bayer HealthCare (Leverkusen, Germany), and oritavancin from The Medicines Company (Parsippany, NJ). The other antibiotics were obtained as the branded products commercialized in Belgium for human use (gentamicin and vancomycin as Geomycin and Vancocin [distributed in Belgium by GlaxoSmithKline s.a./n.v., Genval, Belgium] and rifampin as Rifadine [Merrell Dow Pharmaceuticals Inc., Strasbourg, France]). Human serum was obtained from healthy volunteers (Lonza, Basel, Switzerland) and stored at −80°C as pooled samples until use. Cell culture media and sera were from Invitrogen Corp. (Carlsbad, CA), and other reagents were from Sigma-Aldrich (St. Louis, MO) or Merck KGaA (Darmstadt, Germany).
Bacterial strains, susceptibility testing, and 24-h dose-response studies in broth.
We used four isogenic strains throughout this study, namely, S. aureus strain COL (wild-type [WT] hospital-acquired [HA] MRSA strain [14, 44]), its menD and hemB SCV mutants, and the hemB genetically complemented strain. The hemB and menD mutants were constructed by allelic replacement with an ermB cassette-inactivated hemB gene and an ermC cassette-inactivated menD gene, respectively (9, 66). MICs were determined by microdilution according to CLSI recommendations (13), and 24-h concentration-response studies in the acellular medium (broth) were performed in Mueller-Hinton broth as described previously (7). For experiments with oritavancin, 0.002% polysorbate (Tween) 80 was added to the medium according to CLSI recommendations to prevent drug binding to plastic surfaces (1). For studies with daptomycin, media were supplemented with 50 mg CaCl2/liter (16). Readings or colony counts were made after 24 or 48 h. The maintenance of the SCV character of the strains was checked at the end of all experiments by observation of the size of the colonies growing on agar plates. No reversion was observed.
Cells, cell infection, and morphological studies.
All experiments were conducted with human THP-1 cells (ATCC TIB-202 [American Tissue Culture Collection, Manassas, VA]), a myelomonocytic cell line that behaves like monocytes, showing moderate phagocytic activity and low cell defense mechanisms (47, 55). These cells were maintained in our laboratory as described previously (7). Cell infection, assessment of antibiotic activities, and morphological studies were performed exactly as described previously (7, 38), except that the concentration of gentamicin added to the culture medium of the controls (to prevent extracellular growth and the subsequent acidification of the medium) was reduced to 0.25× MIC to minimize its influence on S. aureus intracellular growth while still effectively preventing extracellular contamination (verified to be <5% of the total number of bacteria in samples under these conditions). Antibiotic activity was assessed by CFU counting (the large dilution of samples before spreading on agar plates for CFU counting ensured the absence of carryover effect [39]). The SCV character of the strains was maintained based on the observation of the size of the colonies. Electron microscopy was performed on samples prepared as previously described, with infection carried out at a bacterium/macrophage ratio of approximately 20 to allow visualization of a sufficiently large number of intracellular bacteria (7).
Curve fitting and statistical analyses.
Concentration-response studies were performed against both extracellular and intracellular bacteria, as described previously (7). Curve-fitting analyses were made using GraphPad Prism version 4.03 (GraphPad Software, San Diego, CA). Data were used to fit monophasic or biphasic sigmoidal functions (Hill equations with slope factors set to 1). The monophasic equation is
| (1) |
and the biphasic equation is
| (2) |
where (i) Y is the log10 of the change in CFU and X is the log10 of the antibiotic concentration (in multiples of the MIC in broth) in both functions, (ii) EC50 is the concentration at which the change in log10 CFU is halfway between Emin and Emax function in the monophasic function, and (iii) EC50_1 and EC50_2 are the concentrations at which the change in log10 CFU is halfway between Emin and the first plateau and between this first plateau and Emax, respectively, and fract is the fraction of the total response associated with the first phase in the biphasic function. This allowed us to obtain, for each condition, numeric values of (i) relative minimal efficacy (Emin; CFU increase in log10 units at 24 h compared to the original inoculum, as extrapolated for an infinitely low concentration of antibiotic), (ii) relative maximal efficacy (Emax; CFU decrease in log10 units at 24 h compared to the original inoculum, as extrapolated for an infinitely large antibiotic concentrations), and (iii) drug static concentration (Cs, concentration of antibiotic resulting in no apparent bacterial growth compared to the original inoculum as determined by graphical interpolation and, in the case of a biphasic response, the proportion of the total response that could be ascribed to the first and the second wave of CFU decrease). Statistical analyses were performed with GraphPad Instat version 3.06 (GraphPad Software).
RESULTS
Extracellular growth and effect of menadione or hemin supplementation.
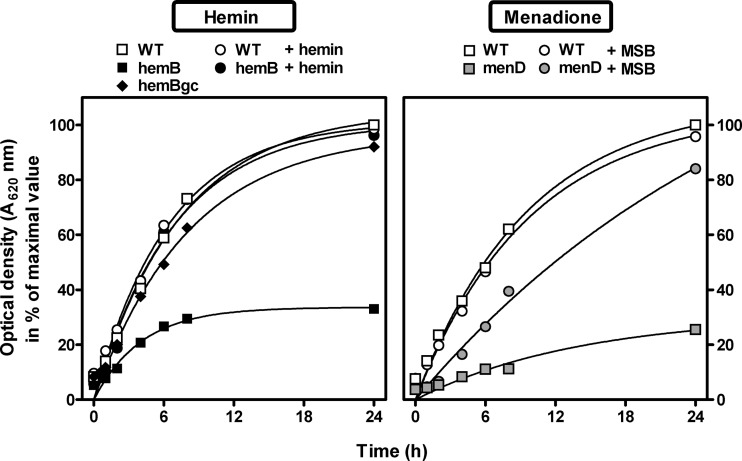
In the first series of experiments, we compared the growth rate of SCVs with that of the parental isogenic strain in broth, by following over time the optical density at 620 nm (OD620) of the bacterial suspension (Fig. 1). Compared to the wild-type isogenic strain, both menD and hemB mutants were characterized by a reduced apparent extracellular growth, reaching only 20 to 30% of the optical density of the parental strain at 24 h. Bacterial counts were recorded in parallel and confirmed this slower growth (CFUs for SCVs were ∼60% of the value measured for the wild-type strain). The genetically complemented hemB strain showed a growth similar to that of the parental strain.
Fig 1.
Extracellular growth (evaluated by optical density at 620 nm) of the strains under study under control conditions or in cation-adjusted Mueller-Hinton broth supplemented with hemin (2 mg/liter; left panels) or menadione sodium bisulfite (MSB; 2 mg/liter; right panels). WT, wild-type, normal-phenotype parental COL strain; hemB, hemB mutant; menD, menD mutant; hemBgc, genetically complemented hemB mutant; menDs, menD mutant in the presence of MSB.
Addition of hemin (0.5 to 4 mg/liter; data are shown for 2 mg/liter) fully restored the growth of the hemB mutant without affecting that of the parental strain. In contrast, the addition of 2 mg/liter menadione sodium bisulfite (MSB) (or menadione; data not shown) only partially increased the growth rate of the menD mutant without effect on the parental strain. Higher concentrations of MSB were tested but appeared toxic, as they caused a concentration-dependent decrease in the culture OD620 (data not shown).
Phagocytosis and intracellular growth.
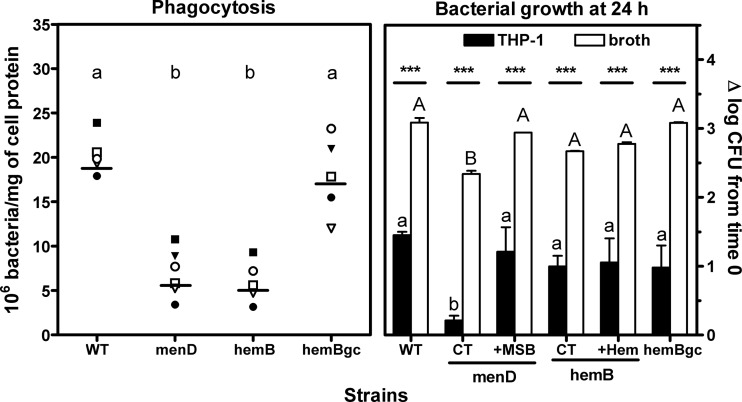
In a subsequent step, we compared the capacities of the parental strain, its two SCV derivatives, and the genetically complemented hemB strain to infect THP-1 monocytes (Fig. 2). Considering phagocytosis first (left panel), both SCV strains were less efficiently internalized by monocytes than were the normal-phenotype or hemB-complemented strains, which showed similar rates of uptake. To assess the intracellular growth (right panel), viable counts were measured after 24 h of incubation (with data obtained in broth presented in parallel for comparison). The growth rates of all strains were much lower intracellularly than in broth. Thus, the parental strain, the hemB mutant, and the genetically complemented strain gained 1 to 1.5 log CFU versus 2.3 to 3.1 log CFU in broth. Interestingly, the menD mutant did not show significant intracellular growth, with only a 0.2-log increase in CFU over the 24-h period. Yet, MSB supplementation brought the intracellular counts at 24 h of the menD mutant back to the value observed for the parental strain, while hemin supplementation had no effect on the hemB mutant.
Fig 2.
Comparative phagocytosis and intracellular versus extracellular survival of SCVs (menD and hemB) in THP-1 monocytes, compared to the isogenic S. aureus normal-phenotype strain (WT) and the hemB genetically complemented strain (hemBgc). (Left) Enumeration of cell-associated CFU after 1 h of phagocytosis by human monocytes. Each data set corresponds to the actual counts from 6 independent experiments performed in triplicate. The horizontal line indicates the corresponding mean value. (Right) Comparative extracellular (Mueller-Hinton broth, pH 7.4) and intraphagocytic (THP-1 monocytes) growth of strains after 24 h of incubation. For SCVs, media were supplemented with 2 mg/liter of MSB or hemin for comparison with control conditions (CT). Values are expressed as the change in CFU (in log scale) per ml of medium (broth) or per mg of cell protein compared to the initial inoculum. Results are means ± standard deviations of three independent determinations. For statistical analysis (by analysis of variance, with Tukey post hoc test), data with different letters indicate significant differences between strains; asterisks indicate significant differences between extracellular and intracellular values (P < 0.05).
Intracellular localization of bacteria.
Electron microscopy showed that all strains were present in membrane-delineated vacuoles, some of which contained several bacteria (Fig. 3).
Fig 3.
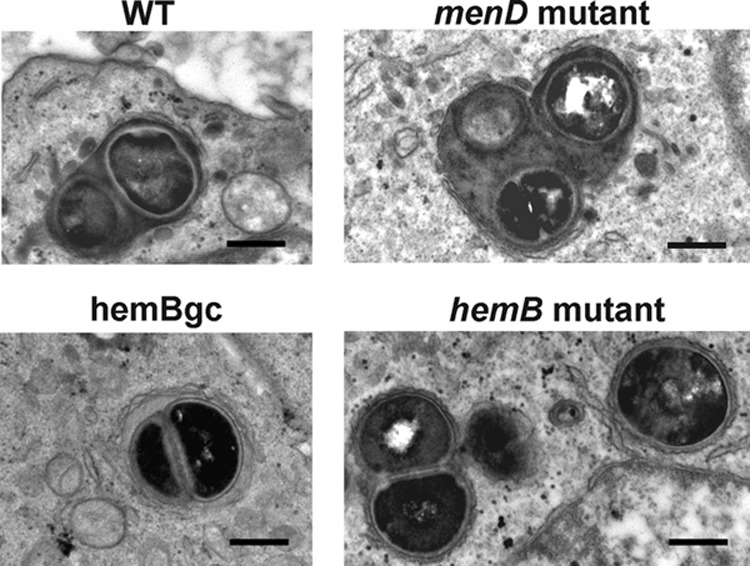
Morphological appearance of infected cells after 24 h of incubation. Both normal and SCV phenotypes were seen in membrane-bound structures with, in some cases, the appearance of several bacteria in a single vacuole, suggestive of intracellular multiplication. Bar, 500 nm.
Susceptibility testing.
The MICs of a large series of antibiotics were tested against the COL strain and its derivatives. It appeared susceptible to most of them except β-lactams, tetracycline, and trimethoprim-sulfamethoxazole (see Table S1 in the supplemental material). Six antibiotics were selected for this study based on their known bactericidal character in broth against S. aureus (β-lactams were not included because of the MRSA phenotype of the COL parental strain). MICs were measured in broth at pH 7.4 and 5.5, to mimic the pHs of the extracellular milieu and of the lysosomal environment, respectively (Table 1). Among these antibiotics, only gentamicin and moxifloxacin showed a significant (2-log2-dilution) increase in MIC against SCVs, which was reversed by hemin supplementation for the hemB strain but not by MSB for the menD strain. Oritavancin was slightly more active against the menD mutant than against the parental strain. When measured at acidic pH, MICs were globally similar, except for gentamicin, which showed higher MICs against the two SCVs. Again, susceptibility was recovered upon addition of hemin in tests involving the hemB strain but not upon addition of MSB in tests involving the menD strain.
Table 1.
MICs of antibiotics against bacterial strains and influence of medium supplementation in MSB or in hemin (24/48 h)a
| Antibiotic | pH | MIC (mg/liter) |
|||||
|---|---|---|---|---|---|---|---|
| Wild type |
menD mutant |
hemB mutant |
hemB genetically complemented strain | ||||
| Control | With MSB at 2 mg/liter | Control | With hemin at 2 mg/liter | ||||
| Vancomycin | 7.4 | 1/2 | 1 | 1 | 1/2 | 1 | 1 |
| 5.5 | 1/2 | 1 | 1 | 1 | 1 | 1 | |
| Daptomycin | 7.4 | 0.5/2 | 0.25/0.5 | 0.25/0.5 | 0.5 | 0.5 | 1 |
| 5.5 | 0.5 | 0.5 | 0.5 | 0.5 | 1 | 2 | |
| Gentamicin | 7.4 | 0.25 | 1 | 1 | 0.5 | 0.125/1 | 0.25/0.5 |
| 5.5 | 1 | 32/64 | 32/64 | 32 | 0.5 | 1 | |
| Rifampin | 7.4 | 0.016 | 0.016/0.03 | 0.016/0.06 | 0.016/0.03 | 0.016/0.06 | 0.016 |
| 5.5 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | |
| Moxifloxacin | 7.4 | 0.03/0.06 | 0.125 | 0.125 | 0.125/0.25 | 0.06/0.125 | 0.03/0.06 |
| 5.5 | 0.125/0.25 | 0.25/0.5 | 0.5 | 0.25/0.5 | 0.125 | 0.125/0.5 | |
| Oritavancin | 7.4 | 0.25/1 | 0.03/0.06 | 0.03/0.06 | 0.125 | 2/4 | 0.25 |
| 5.5 | 0.25/1 | 0.125 | 0.06/0.125 | 0.125/0.25 | 0.25/0.5 | 0.25/1 | |
Only values that were different at 48 h and at 24 h are indicated.
Activities of antibiotics against extracellular and intracellular S. aureus.
The activities of antibiotics were then examined in parallel against extracellular (in broth) and intracellular (infected THP-1 cells) bacteria after 24 h of incubation. We compared the parental wild-type strain with its menD and hemB mutants, the menD mutant in medium supplemented with 2 mg/liter MSB, and the genetically complemented hemB strain. Antibiotics were added to the medium over a wide range of concentrations, which allowed us to obtain the pertinent pharmacological descriptors of activity (relative minimal efficacy [Emin], relative maximal efficacy [Emax], and apparent static concentration [Cs]; see reference 7 and Materials and Methods for a description of the models used and of the corresponding parameters). A graphical representation of the data is presented in Fig. 4 and 5, with the numerical values for pharmacological descriptors shown in Table 2. All data are presented as a function of the extracellular concentration (expressed in multiples of the MIC measured at pH 7.4 for extracellular bacteria and at pH 5.5 for intracellular bacteria).
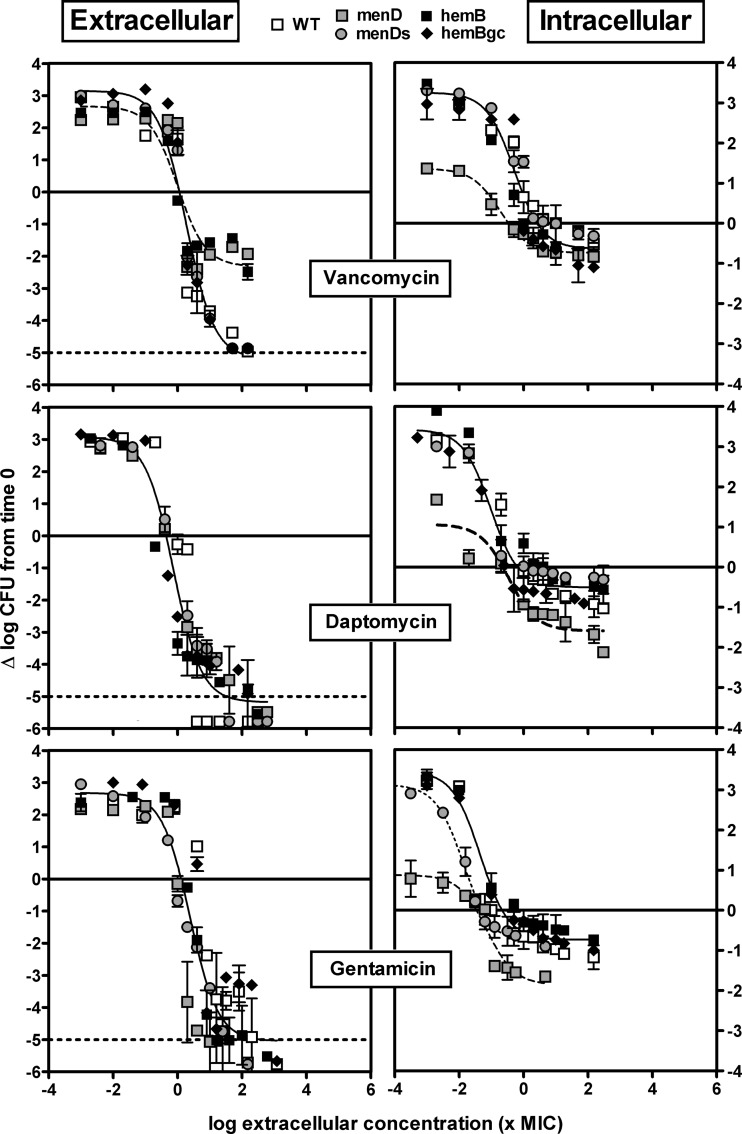
Fig 4.
Concentration-response curves of vancomycin, daptomycin, and gentamicin against the extracellular (broth, left panels) and intracellular (THP-1, right panels) forms of the S. aureus parental strain with the wild-type phenotype (WT), its menD mutant under control conditions (menD) or in medium supplemented with 2 mg/liter MSB (menDs), its hemB mutant (hemB), and the hemB genetically complemented mutant (hemBgc). Bacteria and infected cells were incubated in the presence of increasing concentrations of antibiotics (total drug) for 24 h. The ordinate shows the change in the number of CFU (log scale) per ml of broth (extracellular bacteria) or per mg of cell protein (intracellular bacteria). The solid horizontal line corresponds to an apparent static effect, and the dashed line corresponds to the limit of detection. The abscissa shows the drug concentration in broth (extracellular) or in the culture medium (intracellular) expressed in multiples of the MIC measured at pH 7.4 (extracellular), pH 5.5 (intracellular, except hemB strain), or pH 5.5 in the presence of hemin (hemB strain, based on data in Fig. 2 suggesting the availability of hemin-like compounds in the cellular medium). All values are means ± standard deviations (SD) of three independent determinations (when not visible, the SD bars are smaller than the size of the symbols). Experiments have been reproduced 3 times with similar results.
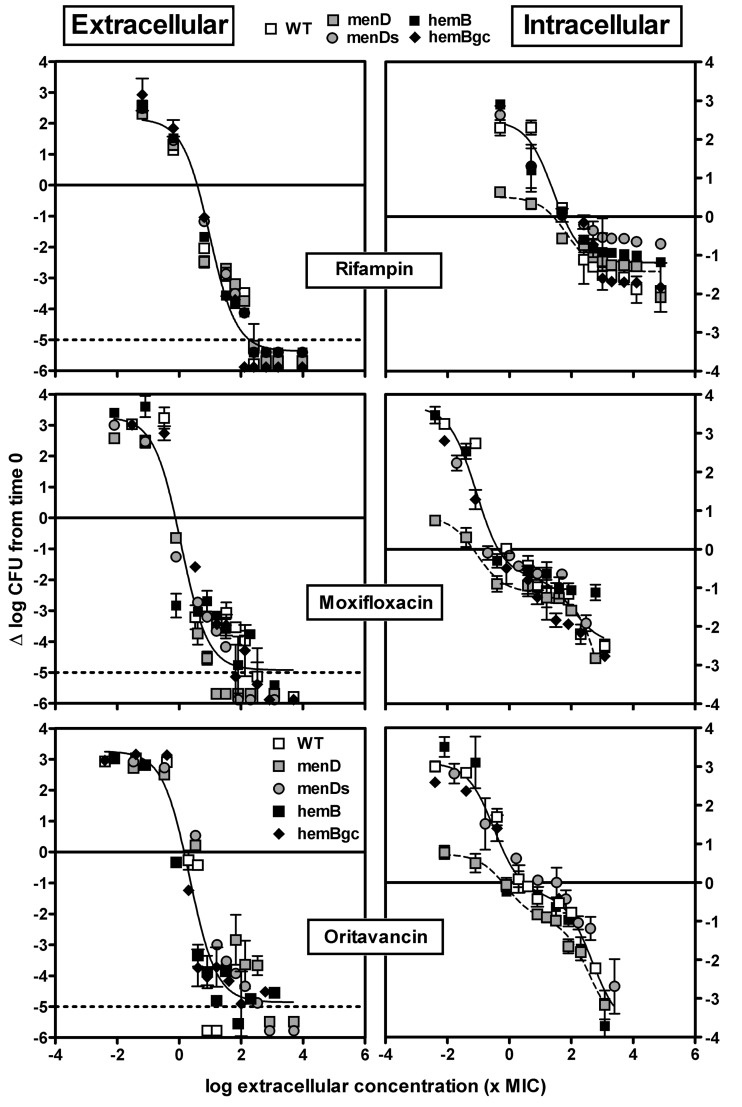
Fig 5.
Concentration-response curves of rifampin, moxifloxacin, and oritavancin against the extracellular (broth, left panels) and intracellular (THP-1, right panels) forms of the S. aureus parental strain with wild-type phenotype (WT), its menD mutant under control conditions (menD) or in medium supplemented with 2 mg/liter MSB (menDs), its hemB mutant (hemB), and the hemB genetically complemented mutant (hemBgc). Bacteria and infected cells were incubated in the presence of increasing concentrations of antibiotics (total drug) for 24 h. The ordinate shows the change in the number of CFU (log scale) per ml of broth (extracellular bacteria) or per mg of cell protein (intracellular bacteria). The plain horizontal line corresponds to an apparent static effect, and the dotted line corresponds to the limit of detection. The abscissa shows the drug concentration in broth (extracellular) or in the culture medium (intracellular) expressed in multiples of the MIC measured at pH 7.4 (extracellular), pH 5.5 (intracellular, except the hemB strain), or pH 5.5 in the presence of hemin (hemB strain, based on data in Fig. 2 suggesting the availability of hemin-like compounds in the cellular medium). All values are means ± standard deviations (SD) of three independent determinations (when not visible, the SD bars are smaller than the size of the symbols). Experiments have been reproduced 3 times with similar results.
Table 2.
Pertinent regression parametersa (with confidence intervals) and statistical analysisf of the dose-response curves illustrated in Fig. 4 and 5
| Antibiotic | Extracellular activity |
Intracellular activity |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Strain(s) | Eminb | Emaxc | Csd | R2 | Strain(s) | Eminb | Emaxc | Csd | Fracte | R2 | |
| Vancomycin | WT, menDs strain, hemBgc strain | 3.15 (2.61 to 3.68); a, A | <−5; a, A | 1.16 | 0.948 | WT, menDs strain, hemB strain, hemBgc strain | 3.26 (2.94 to 3.57); a, A | −0.64 (−0.91 to −0.37); a, B | 2.68 | NA | 0.916 |
| menD strain, hemB strain | 2.67 (1.84 to 3.50); a, A | −2.32 (−3.14 to −1.49); b, A | 1.16 | 0.847 | menD | 1.36 (1.14 to 1.58); b, B | −0.74 (−0.88 to −0.60); a, B | 0.34 | NA | 0.984 | |
| Daptomycin | All strains | 3.08 (2.52 to 3.63); a, A | <−5; a, A | 0.44 | 0.934 | WT, menDs strain, hemB strain, hemBgc strain | 3.43 (3.09 to 3.76); a, A | −0.51 (−0.67 to −0.34); a, B | 0.65 | NA | 0.940 |
| menD strain | 1.07 (0.27 to 1.86); b, B | −1.59 (−2.09 to −1.09); b, B | 0.23 | NA | 0.881 | ||||||
| Gentamicin | All strains | 2.68 (2.02 to 3.34); a, A | <−5; a, A | 1.29 | 0.879 | WT, hemB strain, hemBgc strain | 3.46 (3.15 to 3.77); a, A | −0.74 (−0.87 to −0.60); a, B | 0.22 | NA | 0.969 |
| menD strain | 0.88 (0.50 to 1.26); b, B | −1.86 (−2.38 to −1.33); b, B | 0.03 | NA | 0.948 | ||||||
| menDs strain | 3.13 (2.89 to 3.37); a, A | −0.82 (−1.02 to −0.62); a, B | 0.05 | NA | 0.993 | ||||||
| Rifampin | All strains | 2.16 (1.68 to 2.64); b, A | <−5; a, A | 3.90 | 0.948 | WT, menDs strain, hemB strain, hemBgc strain | 2.49 (2.04 to 2.93); a, A | −1.19 (−1.39 to −0.99); b, B | 56.38 | NA | 0.891 |
| menD strain | 0.51 (−0.07 to 1.10); b, B | −1.42 (−1.78 to −1.07); b, B | 25.32 | NA | 0.882 | ||||||
| Moxifloxacin | All strains | 3.27 (2.51 to 4.03); a, A | <−5; a, A | 0.76 | 0.908 | WT, menDs strain, hemB strain, hemBgc strain | 3.69 (3.13 to 4.25); a, A | <−2; c, B | 0.45 | 0.80 | 0.937 |
| menD strain | 0.85 (0.52 to 1.18); b, B | <−2; c, B | 0.07 | 0.72 | 0.990 | ||||||
| Oritavancin | All strains | 3.27 (2.56 to 3.97); a, A | <−5; a, A | 1.42 | 0.907 | WT, menDs strain, hemB strain, hemBgc strain | 3.10 (2.72 to 3.49); a, A | <−2; c, B | 3.0 | 0.50 | 0.951 |
| menD strain | 0.77 (0.41 to 1.06); b, B | <−2; c, B | 0.63 | 0.36 | 0.991 | ||||||
Calculated based on sigmoidal regressions with a Hill coefficient of 1 for extracellular data and for intracellular data with vancomycin, daptomycin, gentamicin, and rifampin and based on biphasic sigmoidal regressions with Hill coefficients of 1 for intracellular data with moxifloxacin and oritavancin.
Increase in CFU (in log10 units) from the corresponding original inoculum as extrapolated for an infinitely low concentration of antibiotic (mean with 95% confidence interval).
Decrease in CFU (in log10 units) from the corresponding original inoculum as extrapolated for an infinitely large concentration of antibiotic (mean with 95% confidence interval). For moxifloxacin and oritavancin, the plateau value was not reached intracellularly, preventing us from calculating accurate Emax values. It was therefore estimated as being greater than a 5-log (extracellular) or a 2-log (intracellular) decrease (<-5 or <-2 in the table).
Concentration (multiple of the MIC) resulting in no apparent bacterial growth as determined by graphical interpolation. The MICs used were values at pH 7.4 for extracellular activity, values at pH 5.5 for intracellular activity for all strains from the hemB mutant, and the value at pH 5.5 in the presence of hemin for the hemB mutant based on the data from Fig. 2 suggesting the availability of hemin-like compounds in the cellular medium.
Proportion of the total response that could be ascribed to the first wave of CFU decrease in the biphasic curve. NA, not applicable.
For statistical analysis per column (one-way ANOVA with Tukey test for multiple comparisons between each parameter for all drugs), values with different lowercase letters are significantly different from each other (P < 0.05); for statistical analysis per row (unpaired, two-tailed t test between corresponding parameters of extracellular and intracellular activities), values with different uppercase letters are significantly different from each other (P < 0.05).
Figure 4 shows the concentration-response profiles of the 3 antibiotics with moderate activity against SCVs (MICs, ≥0.25 mg/liter), and Fig. 5 shows the concentration-response profiles for those displaying lower MICs and which previously proved the most active against a thymidine-dependent SCV of S. aureus (38). Numerical data are presented overall in Table 2, and data for individual strains and antibiotics are presented in Tables S2 and S3 in the supplemental material.
Considering extracellular bacteria (left panels, Fig. 4 and 5), a monophasic sigmoidal regression was found to fit the data for each antibiotic and for each strain. For all strains, Emin (bacterial growth in the absence of antibiotic) values were similar (all comprised between approximately a 2- and a 3-log10-CFU increase over the initial inoculum), and the static concentrations (Cs; in multiples of the MIC in broth) were similar for all antibiotics (all at a value close to the corresponding MIC). For all antibiotics except vancomycin, Emax (maximal relative efficacy) was close to the lower limit of detection (about a 5-log10-CFU decrease compared to the original inoculum; this was even observed for gentamicin, indicating that this antibiotic remained highly bactericidal against SCVs in spite of its increased MIC). In contrast, vancomycin was clearly less effective against the two SCV strains (menD and hemB) with Emax values at ∼2.5-log10 CFU only (P < 0.05 by analysis of variance [ANOVA] compared with the other strains) but it showed a maximal relative efficacy similar to that of the other antibiotics when tested against the wild-type (WT) strain, the hemB-complemented strain (hemBgc), or the menD strain in the presence of MSB (menDs).
Moving now to intracellular bacteria, the menD mutant (i) grew considerably more slowly than all the other strains (Emin ranging between 0.5- and 1.5-log10-CFU increase versus approximately 3 log10 CFU increase for the other strains or for the same strain in the presence of MSB [menDs]) and (ii) showed a Cs somewhat lower (3- to 16-fold) than those for the other strains. A second key observation is that for all strains and for all antibiotics except moxifloxacin and oritavancin, Emax never reached more than a 2-log10-CFU decrease compared to the original inoculum, which is considerably less than that in broth (Emax values of the menD mutant strain were, however, slightly more negative than those of the other strains for daptomycin and gentamicin). Moreover, with gentamicin, both the hemB mutant and the genetically complemented hemB mutant differed from the menD mutant in medium supplemented with MSB (the latter shows a Cs as low as that of the menD strain, which can be ascribed to the fact that MSB supplementation does not decrease its MIC [P < 0.001 by ANOVA]). In all those cases, a monophasic Hill equation could be fitted to the data. In contrast, the shape of the responses to moxifloxacin and oritavancin for all strains (including the menD mutant) was clearly biphasic, as previously described for oritavancin with a thymidine-dependent SCV (38). A first plateau could be identified at ∼1-log10 CFU decrease (compared to the original inoculum) for an extracellular concentration of about 10× the MIC (and accounting for about half of the total response for oritavancin and about three-fourths for moxifloxacin) followed by a second response that reached 2.5 to 3.5 log10 decrease from the initial inoculum at the maximal concentration tested. Lastly, Cs values were close to those observed extracellularly, except for rifampin (probably in relation to its very low MIC at acidic pH).
Table 3 illustrates for each antibiotic the efficacy that could be reached when extracellular or intracellular bacteria are exposed to a range of concentrations mimicking those than can be achieved in human serum upon treatment with conventional dosages. All drugs were bactericidal against extracellular bacteria, except vancomycin against the menD mutant. In contrast, only oritavancin, moxifloxacin, and rifampin were able to reduce the intracellular inoculum of more than 0.8 log10 CFU for all strains over a range of concentrations mimicking free trough and peak in human serum.
Table 3.
Antibiotic efficacy at clinically relevant concentrations (free trough value [fCmin] and free peak value [fCmax]) observed in the serum of patient receiving conventional dosages
| Antibiotic | Daily dose | Pharmacokinetic parametera |
Extracellular activityb |
Intracellular activityb |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Free fraction (%) | Cmin (total/free) | Cmax (total/free) | Strains | E at fCmin | E at fCmax | Strain(s) | E at fCmin | E at fCmax | ||
| Vancomycin | 15 mg/kg of body wt BIDd | 45 | 8/3.6 | 50/22.5 | WT, menDs strain, hemBgc strain | −2.33 | −4.53 | WT, menDs strain, hemB strain, hemBgc strain | −0.15 | −0.55 |
| menD strain, hemB strain | −1.24 | −2.10 | menD strain | −0.63 | −0.72 | |||||
| Daptomycin | 6 mg/kg/day | 10 | 7/0.7 | 94/9.4 | All strains | −2.38 | −4.96 | WT, menDs strain, hemB strain, hemBgc strain | −0.13 | −0.50 |
| menD strain | −1.08 | −1.54 | ||||||||
| Gentamicin | 6 mg/kg/day | >70 | 2/1.4 | 18/13 | All strains | −2.09 | −4.75 | WT, hemB strain, hemBgc strain | −0.56 | −0.74 |
| menD strain | −0.16 | −1.44 | ||||||||
| menDs strain | 0.11 | −0.69 | ||||||||
| Rifampin | 600 mg BID | 20 | 1.2/2.4 | 18/1.6 | All strains | −4.93 | −4.73 | WT, menDs strain, hemB strain, hemBgc strain | −1.11 | −1.07 |
| menD strain | −1.31 | −1.26 | ||||||||
| Moxifloxacin | 400 mg/day | 70 | 1/0.7 | 4/2.8 | All strains | −4.04 | −4.69 | WT, menDs strain, hemB strain, hemBgc strain | −0.77 | −0.94 |
| menD strain | −1.06 | −1.16 | ||||||||
| Oritavancin | 1,200-mg single dose | 15 | NAc | 129/20 | All strains | NA | <−5 | WT, menDs strain, hemB strain, hemBgc strain | NA | −0.81 |
| menD strain | −1.80 | |||||||||
Values collected in the summary of product characteristics of the corresponding drugs; for oritavancin, data are from reference 10.
Intrapolated from the equations of the concentration-response curves shown in Fig. 4 and 5 (with concentrations converted into mg/liter).
NA, not applicable. Trough concentrations are not relevant since oritavancin will be administered as a single, 1,200-mg dose for treatment of skin and soft tissue infections thanks to its long terminal half-life in plasma (t1/2 = 393 h [43]).
BID, twice a day.
DISCUSSION
The present study is the first to systematically evaluate the activity of antibiotics against the intracellular forms of hemB and menD mutants mimicking the SCV phenotype using a pharmacodynamic approach. A series of novel observations has been made, with respect to both the intracellular infection of monocytes by these strains and antibiotic activity.
Considering initial intracellular infection by itself, we found that the internalization rate was lower for both SCVs than for their parental strain with normal phenotype. A similar behavior was reported for a menB SCV mutant in the same model of THP-1 cells (46), as well as for a series of SCVs collected from cystic fibrosis patients, which were less phagocytized by polymorphonuclear leukocytes (PMN) than were their normal-phenotype counterparts (45). The effect is probably not related to differences in the abilities of SCVs and parental strains to adhere to the macrophage cell surface, because the COL strain used here does not express a series of surface proteins playing a role in adhesion (62). This is not specific to COL, which was initially a clinical isolate, since a huge variability in adhesion properties has also been documented for other clinical isolates of S. aureus (3, 23, 49, 58). Our data, therefore, may suggest that the differences in intracellular inocula observed are probably due to a less avid phagocytosis of SCVs. We know, for example, that the genes involved in capsule synthesis are upregulated in the menD mutant (24), which may contribute to reducing its internalization. Interestingly, also, we found that the intracellular survival of the hemB mutant was similar to that of the parental strain while that of the menD mutant was much lower. Because the growth of the latter was reversed upon menadione bisulfite supplementation, we may suggest that the cellular medium contains the nutrients needed for counterbalancing the slow growth of the hemin-dependent strain but not that of the menadione-dependent strain. These nutrients may possibly come from the culture medium, which contains transferrin that may act as an alternative hemic iron transporter. Other reports suggest that supplementation of the medium by hemin or menadione actually reduces the capacity of the SCVs to persist within the cells by increasing toxin production, thereby favoring its capacity to escape from phagolysosomes (5, 21, 66). While these studies have all been performed in nonprofessional phagocytes where internalized S. aureus cells seem to reach the cytosol, our experiments were made with macrophages where we show here that SCVs remain localized in vacuoles of phagocytes after 24 h of infection. This suggests that the nature of the host may be critical.
Considering antibiotic activity against extracellular bacteria, we show, as anticipated (40), that gentamicin is less active against SCVs than against the normal-phenotype strain, the difference being particularly impressive at acidic pHs. This can be explained by a decrease of susceptibility to aminoglycosides in SCVs, which is known to result from a low electrical potential across the plasma membrane (ΔΨ) within the bacteria (36), the latter being further decreased in acidic medium (15, 34). We also noted a slight increase in moxifloxacin MIC against SCVs compared to wild-type bacteria, but this may not be generalized as it is not observed in surveys of clinical isolates of SCVs (19). More conspicuously, these changes were reversible upon supplementation by hemin but not by menadione. This contrasts with the fact that genetically complemented menadione-dependent clinical isolates with mutations in the menB gene have been shown to fully recover their susceptibility to gentamicin (25). Yet, previous studies showed that supplementation does not necessarily restore gentamicin activity (36), suggesting either that mutations in menD are less easily compensated for by menadione supplementation than are mutations in menB (MenD is involved in an earlier step of menadione synthesis than is MenB [20]) or that other defects affecting gentamicin transport do exist in the mutant that we used. We cannot exclude, however, the possibility that the bisulfite form of menadione is less active in vitro than in vivo due to a limited metabolism. Concentration-effect studies single out vancomycin as the only antibiotic which loses its bactericidal character against SCVs while its MIC remains unchanged (thus turning vancomycin into a bacteriostatic drug). This effect could result from the fact that cell wall synthesis is reduced in SCVs (40). In contrast, daptomycin, aminoglycosides, fluoroquinolones, oritavancin, and, to a lesser extent, rifampin have been shown to remain bactericidal against extracellular slow-growing bacteria (11, 33, 37, 54).
Considering intracellular activities, we demonstrate overall a reduced maximal efficacy for all tested antibiotics, compared to what can be obtained in broth. This is in line with what we have repeatedly described for S. aureus of different origins and resistance phenotypes and for most antibiotics tested in this in vitro model of THP-1 monocytes as well as in other models of phagocytic and nonphagocytic cells (7, 27, 29, 31, 38). The present data, however, expand those observations in two main directions.
First, and most strikingly, we see that the impairment of intracellular growth exhibited by the menD mutant is not accompanied by a decrease of its susceptibility to antibiotics when examined in concentration-dependent experiments. Thus, even though the amplitude of the response (as defined by the Emin-to-Emax span) is reduced, this is entirely due to the decrease in Emin. This is consistent with the fact that the bactericidal activity of all antibiotics tested (except for vancomycin) was maintained against this strain when grown in broth in spite of its lower growth rate and is in sharp contrast to what has been originally shown for Escherichia coli exposed to β-lactams, for which killing rates are reduced in inverse proportion to their generation time (57). We have not studied β-lactams here because of the resistance phenotype of our strains, but the lower response of vancomycin in broth may point to effects specific to cell wall synthesis inhibitors. Yet, our present data question the general hypothesis raised to explain the decreased susceptibility of intracellular S. aureus toward most antibiotics, namely, that their growth rate is impaired in phagolysosomes compared to broth and other extracellular milieus (4, 8, 51, 59). Further studies will need to establish whether our observations are specific to the menD strain and/or can be extended to other slow-growing strains. They also will need to differentiate among the other potential causes, as the overall susceptibility may be dependent on a combination of several environmental as well as bacterial factors (see reference 59 for a review).
A second and perhaps even more striking observation is the shape of the response exhibited by all strains to moxifloxacin and oritavancin. While the modeling performed here may be of limited value because of the restricted number of independent data points, it nevertheless suggests that these two drugs might be able to eradicate intracellular pathogens much more effectively than other antibiotics if their extracellular concentration is further increased. While possibly clinically irrelevant (because this feature could involve drug concentrations that cannot reasonably be obtained in the extracellular milieus in patients), it raises interesting perspectives in terms of a dual mode of action for these drugs. A biphasic shape of the bacterial response to an increase in concentration has already been seen with telavancin (a lipoglycopeptide with structural similarities with oritavancin [61]) for methicillin-susceptible S. aureus (MSSA) and MRSA (6) and with oritavancin for a thymidine-dependent SCV (38). In both cases, this has been ascribed to the known dual mode of action of these drugs that probably involves an impairment of the building-up of the peptidoglycan at low concentrations and membrane-destabilizing effects at higher concentrations (see reference 60 for a review). Conversely, we can offer no explanation for moxifloxacin at this stage, as this will require more extensive studies and detailed comparisons with other fluoroquinolones and other strains. Notably, however, a biphasic shape was also observed when studying the intracellular activity of delafloxacin, a very potent investigational fluoroquinolone (30), against S. aureus. In this case, as in the present study, a biphasic response was observed when extending the range of concentrations to high multiples of the MIC (typically 1,000×, which was possible for delafloxacin in view of its very low MICs, but explains why it escaped our attention in previous studies with other fluoroquinolones).
Concentrating on the SCVs, the present data, combined with those from our previous study using a thymidine-dependent strain (38), suggest that the intracellular behaviors and susceptibilities of thymidine-, hemin-, and menadione-dependent SCVs to antibiotics are rather different. Our data reveal that antibiotics show the same profile of intracellular activity against the hemin-dependent SCV as against a normal-phenotype strain. They also display a similar intracellular maximal efficacy (as defined by the decrease in CFU from the initial inoculum at high concentration) against a wild-type and a menadione-dependent SCV but a much lower Emin, which is reversed upon medium supplementation in menadione bisulfite and therefore attributable to its slower growth. This contrasts with our previous observations using a thymidine-dependent SCV, against which antibiotic activity (measured at a fixed concentration mimicking the human maximum concentration in serum [Cmax]) remained lower than against a normal-phenotype strain, even when its intracellular growth was restored by thymidine supplementation. These differences in drug profiles of activity further show that there is no direct correlation between growth rate and susceptibility to antibiotics intracellularly.
As a conclusion, our work has thus shown that the intracellular fates of the two types of mutants are highly dissimilar, with specific consequences for antibiotic activity. Thus, the hemB mutant that grows intracellularly like the parental strain also responds similarly to antibiotics; the menD mutant, the growth of which is impaired in cells, sees its intracellular growth controlled by lower antibiotic concentrations (higher antibiotic potency) but with no change in maximal efficacy. This may be highly relevant when considering the effects that can be achieved at clinically relevant concentrations. Yet, a potential limitation of the present study is that it was performed with a single strain of each phenotype obtained by genetic engineering. Extending it to clinical isolates would be of clear interest, especially because SCVs are now recognized as a cause of persistence and recurrence in many infections, including osteomyelitis, device-associated infections, and pulmonary infections in CF patients (42). However, our model offers the advantage of comparing strains sharing the same genetic background, which is a first, necessary step when performing the type of systematic pharmacological comparison we undertook here. Taking this limitation into account, the present data suggest that oritavancin and, to a lesser extent, moxifloxacin (for strains that remain susceptible; intracellular breakpoint of susceptibility, MIC, ≤0.125 mg/liter [28]) may offer the most promising activity against these particular forms of intracellular infections, showing a high activity at clinically relevant concentrations. Notably, fluoroquinolones or lipoglycopeptides do not appear in the current recommendations of the Infectious Diseases Society of America for the management of MRSA infections (32). They would therefore deserve further investigations using clinical isolates and in vivo models.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to M. C. Cambier for dedicated technical assistance and to J. Verrax (Toxicology and Cancer Biology Research Group, LDRI, Brussels, Belgium) for useful discussions.
L.G.G. is a Boursière of the Belgian Fonds pour la Formation à la Recherche dans l'Industrie et dans l'Agriculture (FRIA). S.L. and F.V.B. are Chargé de Recherche and Maître de Recherches of the Belgian Fonds de la Recherche Scientifique (FRS-FNRS), respectively. This work was supported by the Fonds de la Recherche Scientifique Médicale (FRSM; grant 3.4639.09) and by grants-in-aid from the Fonds Alphonse et Jean Forton, Targanta Therapeutics (a wholly owned subsidiary of The Medicines Company, Parsippany, NJ), and Bayer Healthcare Belgium.
Footnotes
Published ahead of print 7 May 2012
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1. Arhin FF, et al. 2008. Effect of polysorbate 80 on oritavancin binding to plastic surfaces: implications for susceptibility testing. Antimicrob. Agents Chemother. 52:1597–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Atalla H, Gyles C, Mallard B. 2010. Persistence of a Staphylococcus aureus small colony variants (S. aureus SCV) within bovine mammary epithelial cells. Vet. Microbiol. 143:319–328 [DOI] [PubMed] [Google Scholar]
- 3. Baba-Moussa L, et al. 2008. Virulence factors produced by strains of Staphylococcus aureus isolated from urinary tract infections. J. Hosp. Infect. 68:32–38 [DOI] [PubMed] [Google Scholar]
- 4. Baltch AL, Ritz WJ, Bopp LH, Michelsen P, Smith RP. 2008. Activities of daptomycin and comparative antimicrobials, singly and in combination, against extracellular and intracellular Staphylococcus aureus and its stable small-colony variant in human monocyte-derived macrophages and in broth. Antimicrob. Agents Chemother. 52:1829–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Balwit JM, van Langevelde P, Vann JM, Proctor RA. 1994. Gentamicin-resistant menadione and hemin auxotrophic Staphylococcus aureus persist within cultured endothelial cells. J. Infect. Dis. 170:1033–1037 [DOI] [PubMed] [Google Scholar]
- 6. Barcia-Macay M, Lemaire S, Mingeot-Leclercq MP, Tulkens PM, Van Bambeke F. 2006. Evaluation of the extracellular and intracellular activities (human THP-1 macrophages) of telavancin versus vancomycin against methicillin-susceptible, methicillin-resistant, vancomycin-intermediate and vancomycin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 58:1177–1184 [DOI] [PubMed] [Google Scholar]
- 7. Barcia-Macay M, Seral C, Mingeot-Leclercq MP, Tulkens PM, Van Bambeke F. 2006. Pharmacodynamic evaluation of the intracellular activities of antibiotics against Staphylococcus aureus in a model of THP-1 macrophages. Antimicrob. Agents Chemother. 50:841–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barza M. 1994. Challenges to antibiotic activity in tissue. Clin. Infect. Dis. 19:910–915 [DOI] [PubMed] [Google Scholar]
- 9. Bates DM, et al. 2003. Staphylococcus aureus menD and hemB mutants are as infective as the parent strains, but the menadione biosynthetic mutant persists within the kidney. J. Infect. Dis. 187:1654–1661 [DOI] [PubMed] [Google Scholar]
- 10. Belley A, et al. 2011. Comparison of a single dose of oritavancin to daily dosing regimens of daptomycin or vancomycin against methicillin-resistant Staphylococcus aureus in an in vitro pharmacokinetic/pharmacodynamic model, poster A1-021. Abstr. 51th Intersci. Conf. Antimicrob. Agents Chemother., Chicago, IL [Google Scholar]
- 11. Belley A, et al. 2009. Oritavancin kills stationary-phase and biofilm Staphylococcus aureus cells in vitro. Antimicrob. Agents Chemother. 53:918–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Besier S, et al. 2007. Prevalence and clinical significance of Staphylococcus aureus small-colony variants in cystic fibrosis lung disease. J. Clin. Microbiol. 45:168–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Clinical and Laboratory Standards Institute 2010. Performance standards for antimicrobial susceptibility testing. Twentieth informational supplement (MS100-S20) (29). Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 14. Dyke KG. 1969. Penicillinase production and intrinsic resistance to penicillins in methicillin-resistant cultures of Staphylococcus aureus. J. Med. Microbiol. 2:261–278 [DOI] [PubMed] [Google Scholar]
- 15. Eisenberg ES, Mandel LJ, Kaback HR, Miller MH. 1984. Quantitative association between electrical potential across the cytoplasmic membrane and early gentamicin uptake and killing in Staphylococcus aureus. J. Bacteriol. 157:863–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fuchs PC, Barry AL, Brown SD. 2000. Daptomycin susceptibility tests: interpretive criteria, quality control, and effect of calcium on in vitro tests. Diagn. Microbiol. Infect. Dis. 38:51–58 [DOI] [PubMed] [Google Scholar]
- 17. Ghigo E, et al. 2002. Coxiella burnetii survival in THP-1 monocytes involves the impairment of phagosome maturation: IFN-gamma mediates its restoration and bacterial killing. J. Immunol. 169:4488–4495 [DOI] [PubMed] [Google Scholar]
- 18. Gilligan PH, Gage PA, Welch DF, Muszynski MJ, Wait KR. 1987. Prevalence of thymidine-dependent Staphylococcus aureus in patients with cystic fibrosis. J. Clin. Microbiol. 25:1258–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Idelevich EA, et al. 2011. Comparative in vitro activity of finafloxacin against staphylococci displaying normal and small colony variant phenotypes. J. Antimicrob. Chemother. 66:2809–2813 [DOI] [PubMed] [Google Scholar]
- 20. Jiang M, et al. 2007. Menaquinone biosynthesis in Escherichia coli: identification of 2-succinyl-5-enolpyruvyl-6-hydroxy-3-cyclohexene-1-carboxylate as a novel intermediate and re-evaluation of MenD activity. Biochemistry 46:10979–10989 [DOI] [PubMed] [Google Scholar]
- 21. Kahl B, et al. 1998. Persistent infection with small colony variant strains of Staphylococcus aureus in patients with cystic fibrosis. J. Infect. Dis. 177:1023–1029 [DOI] [PubMed] [Google Scholar]
- 22. Kahl BC, et al. 2003. Population dynamics of persistent Staphylococcus aureus isolated from the airways of cystic fibrosis patients during a 6-year prospective study. J. Clin. Microbiol. 41:4424–4427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Karauzum H, et al. 2008. Comparison of adhesion and virulence of two predominant hospital-acquired methicillin-resistant Staphylococcus aureus clones and clonal methicillin-susceptible S. aureus isolates. Infect. Immun. 76:5133–5138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kohler C, et al. 2008. A defect in menadione biosynthesis induces global changes in gene expression in Staphylococcus aureus. J. Bacteriol. 190:6351–6364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lannergard J, et al. 2008. Identification of the genetic basis for clinical menadione-auxotrophic small-colony variant isolates of Staphylococcus aureus. Antimicrob. Agents Chemother. 52:4017–4022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lecaroz C, Blanco-Prieto MJ, Burrell MA, Gamazo C. 2006. Intracellular killing of Brucella melitensis in human macrophages with microsphere-encapsulated gentamicin. J. Antimicrob. Chemother. 58:549–556 [DOI] [PubMed] [Google Scholar]
- 27. Lemaire S, et al. 2009. Activities of ceftobiprole and other cephalosporins against extracellular and intracellular (THP-1 macrophages and keratinocytes) forms of methicillin-susceptible and methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 53:2289–2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lemaire S, et al. 2011. Activity of moxifloxacin against intracellular community-acquired methicillin-resistant Staphylococcus aureus: comparison with clindamycin, linezolid and co-trimoxazole and attempt at defining an intracellular susceptibility breakpoint. J. Antimicrob. Chemother. 66:596–607 [DOI] [PubMed] [Google Scholar]
- 29. Lemaire S, et al. 2010. Cellular pharmacodynamics of the novel biaryloxazolidinone radezolid: studies with infected phagocytic and nonphagocytic cells, using Staphylococcus aureus, Staphylococcus epidermidis, Listeria monocytogenes, and Legionella pneumophila. Antimicrob. Agents Chemother. 54:2549–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lemaire S, Tulkens PM, Van Bambeke F. 2011. Contrasting effects of acidic pH on the extracellular and intracellular activities of the anti-gram-positive fluoroquinolones moxifloxacin and delafloxacin against Staphylococcus aureus. Antimicrob. Agents Chemother. 55:649–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lemaire S, Van Bambeke F, Tulkens PM. 2011. Activity of finafloxacin, a novel fluoroquinolone with increased activity at acid pH, towards extracellular and intracellular Staphylococcus aureus, Listeria monocytogenes and Legionella pneumophila. Int. J. Antimicrob. Agents 38:52–59 [DOI] [PubMed] [Google Scholar]
- 32. Liu C, et al. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin. Infect. Dis. 52:285–292 [DOI] [PubMed] [Google Scholar]
- 33. Mascio CT, Alder JD, Silverman JA. 2007. Bactericidal action of daptomycin against stationary-phase and nondividing Staphylococcus aureus cells. Antimicrob. Agents Chemother. 51:4255–4260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mates SM, et al. 1982. Membrane potential and gentamicin uptake in Staphylococcus aureus. Proc. Natl. Acad. Sci. U. S. A. 79:6693–6697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McNamara PJ, Proctor RA. 2000. Staphylococcus aureus small colony variants, electron transport and persistent infections. Int. J. Antimicrob. Agents 14:117–122 [DOI] [PubMed] [Google Scholar]
- 36. Miller MH, Edberg SC, Mandel LJ, Behar CF, Steigbigel NH. 1980. Gentamicin uptake in wild-type and aminoglycoside-resistant small-colony mutants of Staphylococcus aureus. Antimicrob. Agents Chemother. 18:722–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Murillo O, et al. 2006. Efficacy of high doses of levofloxacin in experimental foreign-body infection by methicillin-susceptible Staphylococcus aureus. Antimicrob. Agents Chemother. 50:4011–4017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nguyen HA, et al. 2009. Intracellular activity of antibiotics in a model of human THP-1 macrophages infected by a Staphylococcus aureus small-colony variant strain isolated from a cystic fibrosis patient: pharmacodynamic evaluation and comparison with isogenic normal-phenotype and revertant strains. Antimicrob. Agents Chemother. 53:1434–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nguyen HA, et al. 2009. Intracellular activity of antibiotics in a model of human THP-1 macrophages infected by a Staphylococcus aureus small-colony variant strain isolated from a cystic fibrosis patient: study of antibiotic combinations. Antimicrob. Agents Chemother. 53:1443–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Proctor RA, et al. 1998. Staphylococcal small colony variants have novel mechanisms for antibiotic resistance. Clin. Infect. Dis. 27(Suppl 1):S68–S74 [DOI] [PubMed] [Google Scholar]
- 41. Proctor RA, van Langevelde P, Kristjansson M, Maslow JN, Arbeit RD. 1995. Persistent and relapsing infections associated with small-colony variants of Staphylococcus aureus. Clin. Infect. Dis. 20:95–102 [DOI] [PubMed] [Google Scholar]
- 42. Proctor RA, et al. 2006. Small colony variants: a pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat. Rev. Microbiol. 4:295–305 [DOI] [PubMed] [Google Scholar]
- 43. Rubino CM, et al. 2009. Oritavancin population pharmacokinetics in healthy subjects and patients with complicated skin and skin structure infections or bacteremia. Antimicrob. Agents Chemother. 53:4422–4428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sabath LD, Wallace SJ, Gerstein DA. 1972. Suppression of intrinsic resistance to methicillin and other penicillins in Staphylococcus aureus. Antimicrob. Agents Chemother. 2:350–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sadowska B, et al. 2002. Characteristics of Staphylococcus aureus, isolated from airways of cystic fibrosis patients, and their small colony variants. FEMS Immunol. Med. Microbiol. 32:191–197 [DOI] [PubMed] [Google Scholar]
- 46. Sandberg A, et al. 2011. Intra- and extracellular activities of dicloxacillin and linezolid against a clinical Staphylococcus aureus strain with a small-colony-variant phenotype in an in vitro model of THP-1 macrophages and an in vivo mouse peritonitis model. Antimicrob. Agents Chemother. 55:1443–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schwende H, Fitzke E, Ambs P, Dieter P. 1996. Differences in the state of differentiation of THP-1 cells induced by phorbol ester and 1,25-dihydroxyvitamin D3. J. Leukoc. Biol. 59:555–561 [PubMed] [Google Scholar]
- 48. Scorneaux B, Ouadrhiri Y, Anzalone G, Tulkens PM. 1996. Effect of recombinant human gamma interferon on intracellular activities of antibiotics against Listeria monocytogenes in the human macrophage cell line THP-1. Antimicrob. Agents Chemother. 40:1225–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Seidl K, et al. 2011. Combinatorial phenotypic signatures distinguish persistent from resolving methicillin-resistant Staphylococcus aureus bacteremia isolates. Antimicrob. Agents Chemother. 55:575–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sendi P, Proctor RA. 2009. Staphylococcus aureus as an intracellular pathogen: the role of small colony variants. Trends Microbiol. 17:54–58 [DOI] [PubMed] [Google Scholar]
- 51. Seral C, Van Bambeke F, Tulkens PM. 2003. Quantitative analysis of gentamicin, azithromycin, telithromycin, ciprofloxacin, moxifloxacin, and oritavancin (LY333328) activities against intracellular Staphylococcus aureus in mouse J774 macrophages. Antimicrob. Agents Chemother. 47:2283–2292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stokes RW, Doxsee D. 1999. The receptor-mediated uptake, survival, replication, and drug sensitivity of Mycobacterium tuberculosis within the macrophage-like cell line THP-1: a comparison with human monocyte-derived macrophages. Cell. Immunol. 197:1–9 [DOI] [PubMed] [Google Scholar]
- 53. Takemura H, et al. 2000. Evaluation of a human monocytic cell line THP-1 model for assay of the intracellular activities of antimicrobial agents against Legionella pneumophila. J. Antimicrob. Chemother. 46:589–594 [DOI] [PubMed] [Google Scholar]
- 54. Trampuz A, et al. 2007. Efficacy of a novel rifamycin derivative, ABI-0043, against Staphylococcus aureus in an experimental model of foreign-body infection. Antimicrob. Agents Chemother. 51:2540–2545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tsuchiya S, et al. 1980. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int. J. Cancer 26:171–176 [DOI] [PubMed] [Google Scholar]
- 56. Tuchscherr L, et al. 2011. Staphylococcus aureus phenotype switching: an effective bacterial strategy to escape host immune response and establish a chronic infection. EMBO Mol. Med. 3:129–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tuomanen E, Cozens R, Tosch W, Zak O, Tomasz A. 1986. The rate of killing of Escherichia coli by beta-lactam antibiotics is strictly proportional to the rate of bacterial growth. J. Gen. Microbiol. 132:1297–1304 [DOI] [PubMed] [Google Scholar]
- 58. Uhlemann AC, et al. 2012. Identification of a highly transmissible animal-independent Staphylococcus aureus ST398 clone with distinct genomic and cell adhesion properties. mBio 3(2):e00027–12 doi:10.1128/mBio.00027-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Van Bambeke F, Barcia-Macay M, Lemaire S, Tulkens PM. 2006. Cellular pharmacodynamics and pharmacokinetics of antibiotics: current views and perspectives. Curr. Opin. Drug Discov. Dev. 9:218–230 [PubMed] [Google Scholar]
- 60. Van Bambeke F, Mingeot-Leclercq MP, Struelens MJ, Tulkens PM. 2008. The bacterial envelope as a target for novel anti-MRSA antibiotics. Trends Pharmacol. Sci. 29:124–134 [DOI] [PubMed] [Google Scholar]
- 61. Van Bambeke F, Van Laethem Y, Courvalin P, Tulkens PM. 2004. Glycopeptide antibiotics: from conventional molecules to new derivatives. Drugs 64:913–936 [DOI] [PubMed] [Google Scholar]
- 62. Vaudaux P, et al. 2002. Increased expression of clumping factor and fibronectin-binding proteins by hemB mutants of Staphylococcus aureus expressing small colony variant phenotypes. Infect. Immun. 70:5428–5437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Vesga O, et al. 1996. Staphylococcus aureus small colony variants are induced by the endothelial cell intracellular milieu. J. Infect. Dis. 173:739–742 [DOI] [PubMed] [Google Scholar]
- 64. von Eiff C. 2008. Staphylococcus aureus small colony variants: a challenge to microbiologists and clinicians. Int. J. Antimicrob. Agents 31:507–510 [DOI] [PubMed] [Google Scholar]
- 65. von Eiff C, et al. 2001. Intracellular persistence of Staphylococcus aureus small-colony variants within keratinocytes: a cause for antibiotic treatment failure in a patient with Darier's disease. Clin. Infect. Dis. 32:1643–1647 [DOI] [PubMed] [Google Scholar]
- 66. von Eiff C, et al. 1997. A site-directed Staphylococcus aureus hemB mutant is a small-colony variant which persists intracellularly. J. Bacteriol. 179:4706–4712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wendte JM, Ponnusamy D, Reiber D, Blair JL, Clinkenbeard KD. 2011. In vitro efficacy of antibiotics commonly used to treat human plague against intracellular Yersinia pestis. Antimicrob. Agents Chemother. 55:3752–3757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Yamaguchi H, et al. 2002. A Chlamydia pneumoniae infection model using established human lymphocyte cell lines. FEMS Microbiol. Lett. 216:229–234 [DOI] [PubMed] [Google Scholar]
- 69. Zhou H, et al. 2012. Genome-wide RNAi screen in IFN-gamma-treated human macrophages identifies genes mediating resistance to the intracellular pathogen Francisella tularensis. PLoS One 7:e31752 doi:10.1371/journal.pone.0031752 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.