Abstract
The genus Mycobacterium comprises slow-growing species with generation times ranging from hours to weeks. The protracted incubation time before colonies appear on solid culture medium can result in overgrowth by faster-growing microorganisms. To prevent contamination, the solid media used in laboratories and clinics for cultivation of mycobacteria contain the arylmethane compound malachite green, which has broad-spectrum antimicrobial activity. Malachite green has no impact on the plating efficiency of mycobacteria when cells are grown under normal conditions. However, we found that malachite green interfered with colony formation when bacteria were preexposed to antibiotics targeting cell wall biogenesis (isoniazid, ethionamide, ethambutol). This inhibitory effect of malachite green was not observed when bacteria were preexposed to antibiotics targeting cellular processes other than cell wall biogenesis (rifampin, moxifloxacin, streptomycin). Sputum specimens from tuberculosis patients are routinely evaluated on solid culture medium containing high concentrations of malachite green. This practice could lead to underestimation of bacterial loads and overestimation of chemotherapeutic efficacy.
INTRODUCTION
Mycobacterium tuberculosis grows very slowly, with a population doubling time of ∼22 h. Consequently, detection of M. tuberculosis by outgrowth of CFU on solid culture medium requires weeks or months (17). Despite being labor-intensive and time-consuming, enumeration of CFU by plating sputum cultures on solid medium continues to be the “gold standard” for evaluating the effectiveness of antituberculosis chemotherapy (6, 19). Contamination by faster-growing microorganisms is prevented by supplementing the medium with compounds that suppress the growth of common species in the human flora.
In clinical practice, sputum samples from tuberculosis patients are routinely plated on Lowenstein-Jensen or Middlebrook 7H11 agar. These media contain high concentrations of malachite green, a diamino-triphenylmethane dye with broad-spectrum antimicrobial activity against Gram-positive and Gram-negative bacteria (1). The mechanistic basis of malachite green's antimicrobial activity is not understood, nor is it clear why mycobacteria can survive and replicate in the presence of high concentrations of this compound (1, 12).
This paper shows that malachite green interferes with the recovery of mycobacteria on solid culture medium following exposure to certain antibiotics. This inhibitory effect was specific to antibiotics that target cell wall biogenesis (isoniazid, ethionamide, ethambutol) and was not observed with antibiotics that target other cellular processes (rifampin, moxifloxacin, streptomycin). These observations may have implications for clinical practices because underestimation of bacterial loads in patients undergoing chemotherapy could result in overestimation of therapeutic efficacy.
MATERIALS AND METHODS
Bacteria and culture conditions.
Wild-type Mycobacterium smegmatis (strain mc2155) and M. tuberculosis (strain Erdman) were stored at −80°C in 15% glycerol. Bacteria were grown with aeration at 37°C in Middlebrook 7H9 (Difco) liquid culture medium containing 0.5% (wt/vol) bovine albumin serum fraction V (PAA), 0.08% NaCl, 0.05% Tween 80 (Sigma), 0.5% glycerol, and 0.2% glucose. CFU were enumerated by plating bacteria on Luria-Bertani (Sigma) solid culture medium or Middlebrook 7H10 (Difco) solid culture medium containing 10% oleic acid-albumin-dextrose-catalase (OADC) (Difco) and 0.5% glycerol. Difco OADC supplement contains 0.5 g/liter oleic acid, 50 g/liter bovine serum albumin fraction V, 20 g/liter glucose, 40 mg/liter catalase, and 8.5 g/liter sodium chloride. Reconstituted 7H10rec solid culture medium was prepared from individual components (Sigma) according to the directions of the manufacturer of commercial Middlebrook 7H10 medium (Difco). Middlebrook 7H9 (Difco) solid culture medium was prepared by adding 1.5% Bacto agar (Difco) to 7H9 liquid culture medium. The numbers of CFU were scored after incubation of plates at 37°C.
Antibiotics.
All antibiotics were from Sigma with the exception of moxifloxacin, which was generously provided by Stewart Cole (Swiss Federal Institute of Technology, Lausanne, Switzerland). Concentrated stocks were prepared and stored at −20°C: isoniazid (10 mg/ml in water), ethionamide (150 mg/ml in dimethyl sulfoxide [DMSO]), ethambutol (10 mg/ml in water), rifampin (100 mg/ml in DMSO), moxifloxacin (10 mg/ml in water), chloramphenicol (10 mg/ml in ethanol) and streptomycin (10 mg/ml in water). Malachite green (oxalate salt) was from Sigma. Concentrated stocks of malachite green (2.5 mg/ml in water) were prepared and stored at 4°C.
Killing assays.
Bacteria were grown with aeration at 37°C in 7H9 broth to mid-exponential phase (optical density at 600 nm [OD600], ∼0.5) and then diluted in fresh Middlebrook 7H9 broth to an OD600 of ∼0.05 and split. Incubation was continued after the addition of antibiotics at 10× the MIC. At specified time points, aliquots of cultures were withdrawn, washed with fresh 7H9 broth (no antibiotic), serially diluted in 7H9 broth, and plated on solid culture medium. Plates were incubated at 37°C and CFU were enumerated after 3 to 4 days (M. smegmatis) or 3 to 4 weeks (M. tuberculosis). Plates were then returned to 37°C and rescored after prolonged incubation to ensure that late-emerging CFU were not missed.
Malachite green MIC determination.
The MIC of malachite green for M. smegmatis was determined using the broth microdilution assay (2), with concentrations of malachite green ranging from 0.03 μg/ml to 250 μg/ml.
Reactive oxygen species (ROS) scavengers.
For experiments with thiourea and mannitol, bacteria were grown with aeration at 37°C in Middlebrook 7H9 liquid culture medium containing 0.5% (wt/vol) bovine albumin serum fraction V (PAA), 0.08% NaCl, 0.05% Tween 80 (Sigma), 0.5% glycerol, and 0.2% glucose. Isoniazid-treated bacteria were plated for CFU enumeration on Luria-Bertani (Sigma) solid culture medium with or without 0.25 μg/ml malachite green supplemented with 150 μM thiourea (Sigma) or 40 μM mannitol (Sigma). For experiments with catalase, bacteria were grown with aeration at 37°C in 7H9 liquid culture medium containing 0.5% glycerol and 10% OADC (Difco) as a source of catalase. Isoniazid-treated bacteria were plated for CFU enumeration on Middlebrook 7H10 solid culture medium containing 0.5% glycerol and 10% OADC (Difco) as a source of catalase.
RESULTS
Although malachite green does not affect the plating efficiency of mycobacteria grown under standard conditions, we found that it impeded the recovery of CFU from cultures that were preexposed to certain antibiotics. Our initial studies focused on the fast-growing nonpathogenic species Mycobacterium smegmatis (Fig. 1). Cells were grown at 37°C to mid-exponential phase in Middlebrook 7H9 liquid culture medium (15) and diluted 10-fold in fresh 7H9 broth. Incubation was continued after the addition of isoniazid at 50 μg/ml, which is 10× the MIC. Isoniazid inhibits de novo biosynthesis of cell wall mycolic acids (22). Samples were withdrawn at the indicated time points after isoniazid addition, washed, serially diluted, and plated for enumeration of CFU on standard Middlebrook 7H10 solid culture medium, which contains 0.25 μg/ml malachite green (15), or Luria-Bertani (LB) agar without malachite green. Prior to isoniazid exposure, recovery of CFU was equivalent on 7H10 agar and LB agar (Fig. 1A). Following isoniazid exposure, plating efficiency was reduced by ∼100-fold on 7H10 agar compared to LB agar (Fig. 1A). These observations suggest that much of the apparent “killing” activity attributed to isoniazid was actually due to postexposure inhibition of colony formation by some component(s) of 7H10 agar.
Fig 1.
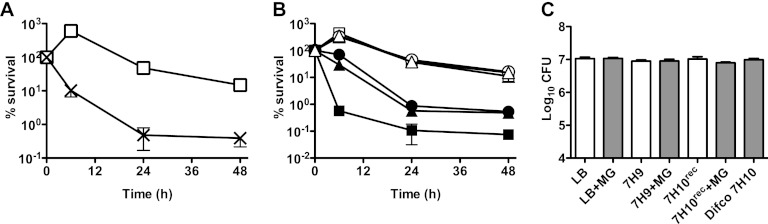
Malachite green interferes with postantibiotic recovery of M. smegmatis on solid culture medium. (A and B) M. smegmatis was grown to mid-exponential phase in Middlebrook 7H9 broth and incubation was continued after the addition of 50 μg/ml isoniazid (10× MIC). At the indicated time points, CFU were enumerated by plating washed and serially diluted culture aliquots on solid culture medium. Percent survival was calculated as (CFU postantibiotic)/(CFU preantibiotic) × 100. Data shown are mean values ± standard errors of the means of results from three independent experiments. (A) Bacteria were plated on LB agar (squares) or Difco Middlebrook 7H10 agar (crosses). (B) Bacteria were plated on LB agar (squares), 7H9 agar (circles), or reconstituted 7H10rec agar (triangles) with (filled symbols) or without (empty symbols) 0.25 μg/ml malachite green. (C) Bacteria were grown to mid-exponential phase in 7H9 broth. CFU were enumerated by plating washed and serially diluted culture aliquots on LB agar, 7H9 agar, reconstituted 7H10rec agar, or Difco 7H10 agar with (gray bars) or without (white bars) 0.25 μg/ml malachite green. Data shown are mean values ± standard errors of the means of results from 10 independent experiments.
Middlebrook 7H10 solid culture medium is a complex synthetic medium containing essential elements and supplements, including bovine serum albumin, oleic acid, catalase, glucose, and glycerol (15). Omission of individual supplements from reconstituted 7H10 agar (7H10rec) had no effect on the plating efficiency of cells that were preexposed to isoniazid (data not shown). In contrast, omission of malachite green from reconstituted 7H10rec agar increased the plating efficiency to the same level as LB agar (Fig. 1B). Conversely, the addition of 0.25 μg/ml malachite green to LB agar or Middlebrook 7H9 solid culture medium reduced the plating efficiency of isoniazid-exposed bacteria by 100- to 1,000-fold (Fig. 1B).
To confirm that the inhibitory effect of malachite green was specific to isoniazid-exposed bacteria, we plated exponential-phase liquid cultures of M. smegmatis on 7H10rec agar, 7H9 agar, and LB agar with and without 0.25 μg/ml malachite green (Fig. 1C). In all three cases, we found that the addition of malachite green had no impact on the plating efficiency of cells that were cultured without isoniazid. Consistent with these observations, we found that the MIC of malachite green for M. smegmatis was ∼30 μg/ml, which is 120-fold higher than the concentration in 7H10 agar (0.25 μg/ml).
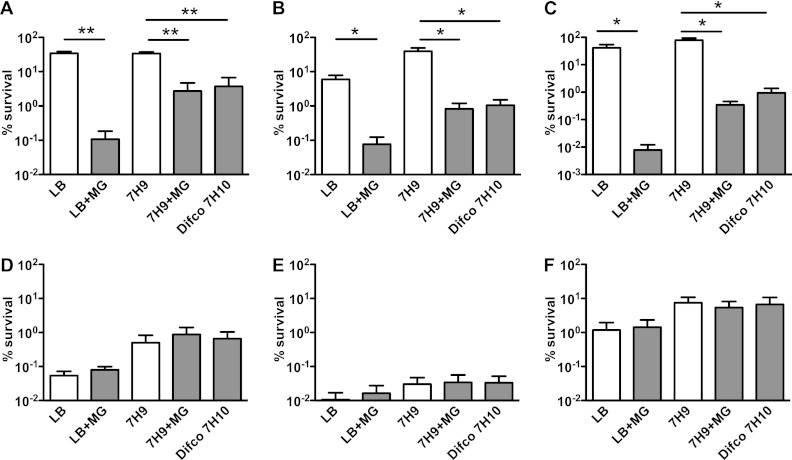
To determine whether the inhibitory effect of malachite green was specific to isoniazid, we tested several other antimycobacterial compounds: ethionamide, which inhibits cell wall mycolic acid synthesis; ethambutol, which inhibits cell wall arabinogalactan synthesis; rifampin, which inhibits RNA polymerase; moxifloxacin, which inhibits DNA gyrase; and chloramphenicol, which inhibits protein synthesis (Fig. 2). M. smegmatis was grown at 37°C to mid-exponential phase in Middlebrook 7H9 broth, diluted 10-fold in fresh 7H9 broth, and split. Incubation was continued for another 24 h after the addition of individual antibiotics at 10× MIC. Samples were withdrawn, washed, serially diluted, and plated on Difco Middlebrook 7H10 agar (0.25 μg/ml malachite green), reconstituted 7H10rec agar with or without 0.25 μg/ml malachite green, and LB agar with or without 0.25 μg/ml malachite green. Plating efficiency on agar containing malachite green was reduced by 100- to 1,000-fold when cells were preexposed to isoniazid, ethionamide, or ethambutol, which inhibit the biosynthesis of cell wall components. Malachite green had no impact on the plating efficiency of cells that were preexposed to rifampin, moxifloxacin, or chloramphenicol, which inhibit cellular processes other than cell wall biogenesis. Recovery of CFU following exposure to rifampin or moxifloxacin was lower on LB agar than on Difco 7H10 or 7H10rec agar, irrespective of the presence or absence of malachite green. This difference was probably due to the presence of bovine serum albumin in Difco 7H10 and 7H10rec agar, because the addition of albumin to LB agar eliminated the difference (Fig. 3A). Malachite green has been shown to trigger the production of reactive oxygen species (ROS) (18), suggesting that ROS might mediate malachite green's growth-inhibitory effect. However, we found that recovery of antibiotic-exposed bacteria on solid culture medium containing malachite green was not improved by supplementation of the medium with ROS scavengers such as thiourea, mannitol, or catalase (Fig. 3B and C) (9, 13).
Fig 2.
Inhibition of postantibiotic recovery by malachite green is specific to antibiotics that target cell wall biogenesis. (A to F) M. smegmatis was grown to mid-exponential phase in Middlebrook 7H9 broth, and incubation was continued for another 24 h after the addition of antibiotics at 10× MIC: (A) 50 μg/ml isoniazid; (B) 200 μg/ml ethionamide; (C) 5 μg/ml ethambutol; (D) 200 μg/ml rifampin; (E) 0.4 μg/ml moxifloxacin; (F) 200 μg/ml chloramphenicol. CFU were enumerated by plating washed and serially diluted culture aliquots on LB agar, Middlebrook 7H9 agar, or Difco 7H10 agar with (gray bars) or without (white bars) 0.25 μg/ml malachite green. Percent survival was calculated as (CFU postantibiotic)/(CFU preantibiotic) × 100. Data shown are mean values ± standard errors of the means of results from three independent experiments. *, P < 0.05; **, P < 0.005 by Student's two-tailed t test.
Fig 3.
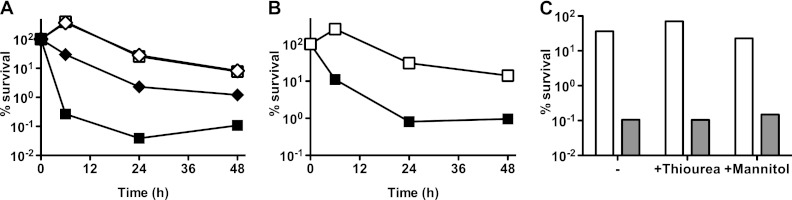
Effect of albumin or ROS scavengers on inhibition of postantibiotic recovery by malachite green. (A to C) M. smegmatis was grown to mid-exponential phase in Middlebrook 7H9 broth (A and C) or in 7H9 broth containing 10% OADC (B) and incubation was continued after the addition of 50 μg/ml isoniazid. At the indicated time points, CFU were enumerated by plating washed and serially diluted culture aliquots on solid culture medium. Percent survival was calculated as (CFU postantibiotic)/(CFU preantibiotic) × 100. (A) Cells were plated on LB agar (squares) or LB agar supplemented with 0.5% (wt/vol) bovine serum albumin (diamonds) with (filled symbols) or without (empty symbols) 0.25 μg/ml malachite green. (B) Cells were plated on Middlebrook 7H10 agar supplemented with 10% OADC as a source of catalase with (filled squares) or without (empty squares) 0.25 μg/ml malachite green. (C) After 24 h of isoniazid exposure, cells were plated on LB agar supplemented with 150 μM thiourea or 40 μM mannitol with (gray bars) or without (white bars) 0.25 μg/ml malachite green. Experiments were performed two times with similar results. Representative data from one experiment are shown.
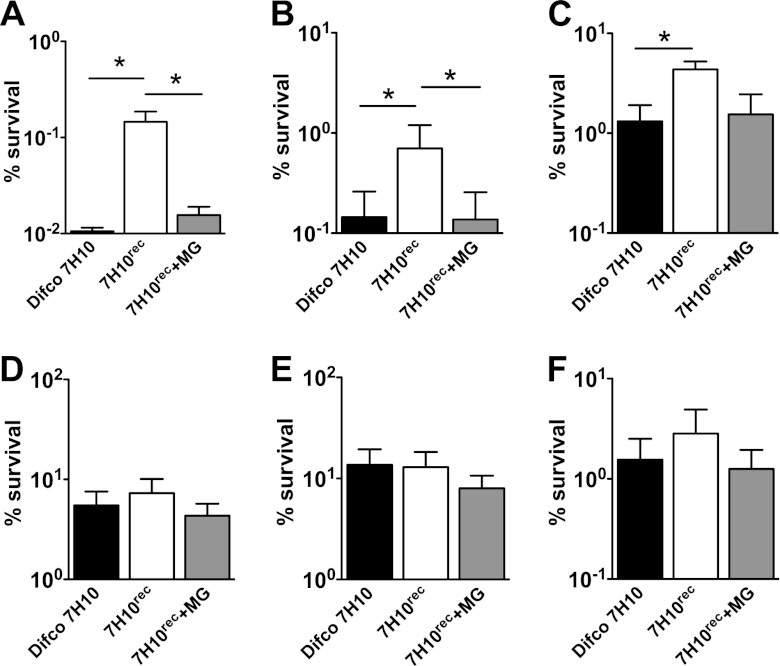
We extended our observations to the slow-growing pathogenic species Mycobacterium tuberculosis (Fig. 4). Cells were grown at 37°C to mid-exponential phase in Middlebrook 7H9 broth, diluted 10-fold in fresh 7H9 broth, and split. Incubation was continued for another 48 h after the addition of individual antibiotics at 10× MIC. Samples were withdrawn, washed, serially diluted, and plated on Difco Middlebrook 7H10 agar containing malachite green or reconstituted 7H10rec agar with or without malachite green. Consistent with our results in M. smegmatis, CFU formation by bacteria that were exposed to cell wall targeting antibiotics (isoniazid, ethionamide, or ethambutol) was ∼10-fold lower on solid medium containing malachite green than on medium without malachite green. Malachite green had no impact on the plating efficiency of bacteria that were preexposed to antibiotic targeting cellular processes other than cell wall biogenesis (rifampin, moxifloxacin, or streptomycin, which inhibits protein synthesis). We used streptomycin rather than chloramphenicol in these experiments because M. tuberculosis is inherently resistant to chloramphenicol.
Fig 4.
Malachite green interferes with postantibiotic recovery of M. tuberculosis on solid culture medium. (A to F) M. tuberculosis was grown to mid-exponential phase in Middlebrook 7H9 broth, and incubation was continued for another 48 h after the addition of antibiotics at 10× MIC: (A) 0.3 μg/ml isoniazid; (B) 10 μg/ml ethionamide; (C) 5 μg/ml ethambutol; (D) 10 μg/ml rifampin; (E) 5 μg/ml moxifloxacin; (F) 4 μg/ml streptomycin. CFU were enumerated by plating washed and serially diluted culture aliquots on Difco Middlebrook 7H10 agar (black bars) or reconstituted 7H10rec agar with (gray bars) or without (white bars) 0.25 μg/ml malachite green. Percent survival was calculated as (CFU postantibiotic)/(CFU preantibiotic) × 100. Data shown are mean values ± standard errors of the means of results from three independent experiments. *, P < 0.05 by Student's two-tailed t test. Differences in CFU for ethambutol-treated cells plated on 7H10rec agar with and without malachite green were reproducible but did not achieve statistical significance.
DISCUSSION
We propose three possible explanations for our observations that malachite green interferes with colony formation by mycobacteria following exposure to antibiotics that target cell wall biogenesis. First, cell wall defects caused by isoniazid, ethionamide, or ethambutol might affect the localization or activity of enzymes that inactivate malachite green. Cell extracts of mycobacteria have been shown to decolorize malachite green (21); decolorizing activity is enriched in cell wall extracts (12) and this activity has been linked to the production of coenzyme F420 (10). Second, increased permeability of cells exposed to cell wall targeting antibiotics could facilitate the access of malachite green to its molecular target(s). Consistent with this interpretation, two recent reports demonstrated that mutant strains of mycobacteria with increased cell wall permeability are hypersensitive to malachite green (3, 4). Third, antibiotic-mediated killing has been linked to the production of reactive oxygen species (ROS) in Gram-negative and Gram-positive bacteria (7), and malachite green has been shown to trigger ROS production via an unknown mechanism (18). Thus, ROS might potentiate the killing of antibiotic-exposed mycobacteria by malachite green, although why this effect should be specific to antibiotics that target cell wall biogenesis is not clear. Also, we found that recovery of antibiotic-exposed bacteria on solid culture medium containing malachite green was not enhanced by supplementation of the medium with ROS scavengers. The impact of malachite green on postantibiotic recovery of bacterial CFU was more pronounced for M. smegmatis than for M. tuberculosis. Although we do not understand the molecular basis of this effect, we speculate that species-specific differences in growth rate or cell wall permeability might contribute.
Previous studies highlighted discrepancies between mycobacterial numbers determined by counting CFU on solid medium compared to “most probable numbers” determined by subculturing in liquid medium (5, 16). This effect was attributed to the presence of “injured” subpopulations of bacteria that were capable of growing in liquid culture medium but incapable of forming colonies on solid culture medium. Consistent with this interpretation, several studies have demonstrated the superior performance of liquid culture medium compared to solid culture medium in recovery of mycobacteria from clinical specimens (11, 14, 20). It has also been reported that anaerobically adapted mycobacteria cannot form colonies on Lowenstein-Jensen medium (which contains 250 μg/ml malachite green) but regain the ability to grow on this medium following adaptation to aerobic conditions (8). We speculate that malachite green might have interfered with the recovery of “injured” bacteria in these studies and could account for the discrepancies observed in bacterial enumeration using liquid medium compared to solid medium.
In many countries where tuberculosis is endemic, diagnosis and drug susceptibility testing are routinely performed by plating sputum specimens on Lowenstein-Jensen medium. Our observations suggest that this clinical practice could lead to underestimation of bacterial loads in patients who have been treated with antibiotics targeting cell wall biogenesis. If this is correct, then interference by malachite green could result in routine overestimation of chemotherapeutic efficacy. Similarly, experiments to evaluate the therapeutic efficacy of experimental antituberculosis compounds in animal models typically involve plating organ homogenates for CFU enumeration on Middlebrook 7H11 agar (which contains 1.0 μg/ml malachite green). This practice could lead to overestimation of the in vivo activity of new compounds targeting cell wall biogenesis and failure to identify the optimal dosing regimen to maximize killing and minimize the selection of resistant mutants.
ACKNOWLEDGMENT
This work was supported by intramural funding from the School of Life Sciences, Swiss Federal Institute of Technology in Lausanne, Switzerland (EPFL).
Footnotes
Published ahead of print 23 April 2012
REFERENCES
- 1. Alderman DJ. 1985. Malachite green—a review. J. Fish Dis. 8:289–298 [Google Scholar]
- 2. Andrews JM. 2001. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 48(Suppl. 1):5–16 [DOI] [PubMed] [Google Scholar]
- 3. Banaei N, et al. 2009. Lipoprotein processing is essential for resistance of Mycobacterium tuberculosis to malachite green. Antimicrob. Agents Chemother. 53:3799–3802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bianco MV, et al. 2011. Role of P27-P55 operon from Mycobacterium tuberculosis in the resistance to toxic compounds. BMC Infect. Dis. 11:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dhillon J, Lowrie DB, Mitchison DA. 2004. Mycobacterium tuberculosis from chronic murine infections that grows in liquid but not on solid medium. BMC Infect. Dis. 4:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Donald PR, et al. 1997. The early bactericidal activity of isoniazid related to its dose size in pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 156:895–900 [DOI] [PubMed] [Google Scholar]
- 7. Dwyer DJ, Kohanski MA, Collins JJ. 2009. Role of reactive oxygen species in antibiotic action and resistance. Curr. Opin. Microbiol. 12:482–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gillespie J, Barton LL, Rypka EW. 1986. Phenotypic changes in mycobacteria grown in oxygen-limited conditions. J. Med. Microbiol. 21:251–255 [DOI] [PubMed] [Google Scholar]
- 9. Gregory EM, Fridovich I. 1974. Oxygen metabolism in Lactobacillus plantarum. J. Bacteriol. 117:166–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guerra-Lopez D, Daniels L, Rawat M. 2007. Mycobacterium smegmatis mc2 155 fbiC and MSMEG_2392 are involved in triphenylmethane dye decolorization and coenzyme F420 biosynthesis. Microbiology 153:2724–2732 [DOI] [PubMed] [Google Scholar]
- 11. Idigoras P, Beristain X, Iturzaeta A, Vicente D, Perez-Trallero E. 2000. Comparison of the automated nonradiometric Bactec MGIT 960 system with Lowenstein-Jensen, Coletsos, and Middlebrook 7H11 solid media for recovery of mycobacteria. Eur. J. Clin. Microbiol. Infect. Dis. 19:350–354 [DOI] [PubMed] [Google Scholar]
- 12. Jones JJ, Falkinham JO., III 2003. Decolorization of malachite green and crystal violet by waterborne pathogenic mycobacteria. Antimicrob. Agents Chemother. 47:2323–2326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ. 2007. A common mechanism of cellular death induced by bactericidal antibiotics. Cell 130:797–810 [DOI] [PubMed] [Google Scholar]
- 14. Lee JJ, et al. 2003. Comparative evaluation of the BACTEC MGIT 960 system with solid medium for isolation of mycobacteria. Int. J. Tuberc. Lung Dis. 7:569–574 [PubMed] [Google Scholar]
- 15. Middlebrook G, Cohn ML. 1958. Bacteriology of tuberculosis: laboratory methods. Am. J. Public Health 48:844–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. O'Sullivan DM, et al. 2007. Evaluation of liquid culture for quantitation of Mycobacterium tuberculosis in murine models. Vaccine 25:8203–8205 [DOI] [PubMed] [Google Scholar]
- 17. Palomino JC, Martin A, Von Groll A, Portaels F. 2008. Rapid culture-based methods for drug-resistance detection in Mycobacterium tuberculosis. J. Microbiol. Methods 75:161–166 [DOI] [PubMed] [Google Scholar]
- 18. Panandiker A, Maru GB, Rao KV. 1994. Dose-response effects of malachite green on free radical formation, lipid peroxidation and DNA damage in Syrian hamster embryo cells and their modulation by antioxidants. Carcinogenesis 15:2445–2448 [DOI] [PubMed] [Google Scholar]
- 19. Sirgel FA, Wiid IJ, van Helden PD. 2009. Measuring minimum inhibitory concentrations in mycobacteria. Methods Mol. Biol. 465:173–186 [DOI] [PubMed] [Google Scholar]
- 20. Srisuwanvilai LO, et al. 2008. Performance of the Bactec MGIT 960 compared with solid media for detection of Mycobacterium in Bangkok, Thailand. Diagn. Microbiol. Infect. Dis. 61:402–407 [DOI] [PubMed] [Google Scholar]
- 21. Tarnok I, Czanik P. 1959. Malachite green reducing enzyme in Mycobacteria. Nature 183:549–550 [DOI] [PubMed] [Google Scholar]
- 22. Vilchèze C, Jacobs WR., Jr 2007. The mechanism of isoniazid killing: clarity through the scope of genetics. Annu. Rev. Microbiol. 61:35–50 [DOI] [PubMed] [Google Scholar]